Abstract
Active immunization with Porphyromonas gingivalis whole-cell preparations has been shown to prevent P. gingivalis infection and oral bone loss. Employing passive antibody transfer and opsonization, we demonstrate with this study that immunization-elicited P. gingivalis-specific immunoglobulin G facilitates clearance of P. gingivalis in a subcutaneous chamber model and prevents P. gingivalis-elicited oral bone loss.
Epidemiological data indicate that by age 35 approximately 30% of the U.S. adult population suffers from periodontal disease (17), a chronic inflammatory disease of the hard and soft tissues supporting the teeth that in severe cases leads to tooth loss. Most cases of this disease are caused by the anaerobic bacterial pathogen Porphyromonas gingivalis (13, 18). Numerous studies have assessed the serum antibody profiles of patients with periodontitis. High levels of P. gingivalis-specific immunoglobulin G (IgG) are commonly observed (12, 19), yet a variable host response to this organism appears to exist (1, 11, 14, 16). However, despite this potent antibody response the host is apparently unable to clear this chronic infection. Notwithstanding this fact, Wilton et al. (20) reported that sera obtained from patients with periodontal disease contain antibodies that promote opsonophagocytosis of P. gingivalis by human neutrophils.
Previous reports suggest that vaccination affords protection from P. gingivalis infection (3, 5-7, 9, 10, 15). Gibson et al. (7) demonstrated that immunization of mice with heat-killed P. gingivalis elicited high levels of specific IgG and protected mice from subsequent oral bone loss elicited by this organism. Employing both murine subcutaneous chamber and oral challenge models, we demonstrate in this study that prevention of P. gingivalis infection occurs with immunization-elicited P. gingivalis-specific IgG.
P. gingivalis strain A7436 was used in all of our studies. This organism was initially cultivated on anaerobic blood agar plates, and bacteria were havested by centrifugation and grown in brain heart infusion (BHI) broth overnight. P. gingivalis organisms were collected and suspended in fresh BHI broth to a final cell density of 109 CFU/ml (7). Heat-killed P. gingivalis and formaldehyde-fixed P. gingivalis were prepared as described previously (7). BALB/C mice (Jackson Laboratories, Bar Harbor, Maine) were immunized subcutaneously with heat-killed P. gingivalis whole-organism preparations three times per week for 3 weeks, and the mice were exsanguinated. Serum was applied to a Protein G column (Amersham Pharmacia Biotech, Uppsala, Sweden) and total IgG was eluted (approximately 50 mg); the result was designated P. gingivalis-specific IgG. Using enzyme-linked immunosorbent assay, we observed that the titer of the P. gingivalis-specific IgG was 1:50,000 (data not shown). A commercially obtained mouse IgG (irrelevant IgG [IRR-IgG]; Sigma, St. Louis, Mo.) was used as an IgG control. For passive transfer studies, mice received an injection of 100 μl of P. gingivalis-specific IgG or IRR-IgG (1 mg/ml) in the subcutaneous chamber 24 h prior to P. gingivalis challenge. In addition, P. gingivalis was opsonized with either 100 μg of P. gingivalis-specific IgG or IRR-IgG per ml. The effect of opsonization on P. gingivalis viability was assessed by determination of CFU per milliliter and did not affect P. gingivalis viability.
Initially, we assessed the protective capacity of P. gingivalis-specific IgG by using a murine subcutaneous chamber model (4). Mice were separated into six groups (n = 8 mice/group). Group 1 received 0.1 ml of sterile BHI broth only (vehicle), group 2 received 0.1 ml of P. gingivalis in vehicle (∼108 CFU), groups 3 and 4 received passive transfer of P. gingivalis-specific IgG or IRR-IgG, respectively, and groups 5 and 6 were challenged with P. gingivalis opsonized with P. gingivalis-specific IgG or IRR-IgG. Following challenge, mice were examined daily for signs of infection, including cachexia (defined as ruffled hair), hunched bodies, weakness, and lethargy, as well as for the presence of primary and secondary lesions. Animals in group 2 exhibited signs of severe cachexia and presented with chamber lesions that eventually resulted in chamber rejection by day 10 (Table 1), while mice in group 1 appeared healthy. Passive immunization (group 3) or opsonization of P. gingivalis with P. gingivalis-specific IgG (group 5) protected mice from all signs of P. gingivalis infection (Table 1). In contrast, animals from groups 4 and 6 were not protected and resembled mice from group 2 (Table 1).
TABLE 1.
Gross pathological observations of mice passively immunized or opsonized with P. gingivalis-specific IgG and challenged with P. gingivalis in a murine subcutaneous chamber modela
| Group | Expt parameter
|
Result
|
|||
|---|---|---|---|---|---|
| Passive transfer | Opsonization | Challenge with | Lesionsb | Cachexiac | |
| 1 | None | None | None | None (0/8) | None |
| 2 | None | None | P. gingivalis A7436 | ++++ (2/8) | Moderate |
| 3 | P. gingivalis-specific IgG | None | P. gingivalis A7436 | None (0/8) | None |
| 4 | IRR-IgG | None | P. gingivalis A7436 | ++++ (4/8) | Severe |
| 5 | None | P. gingivalis-specific IgG | P. gingivalis A7436 | None (0/8) | None |
| 6 | None | IRR-IgG | P. gingivalis A7436 | ++++ (3/8) | Severe |
Groups of animals received vehicle only (none), 100 μg of murine P. gingivalis-specific IgG, or 100 μg of IRR-IgG 24 h prior to chamber challenge or were challenged with P. gingivalis opsonized with 100 μg of either P. gingivalis-specific IgG or IRR-IgG.
The severity of the lesions was determined by using an arbitrary four-plus scale whereby lesions spreading from the area of challenge are considered the most severe. After 10 days, mice were observed for evidence of chamber rejection, and the number in parentheses is the number of mice with chamber rejection.
Cachexia was expressed as none, moderate, or severe and was determined as a function of animal hunching, ruffled hair, and lethargy.
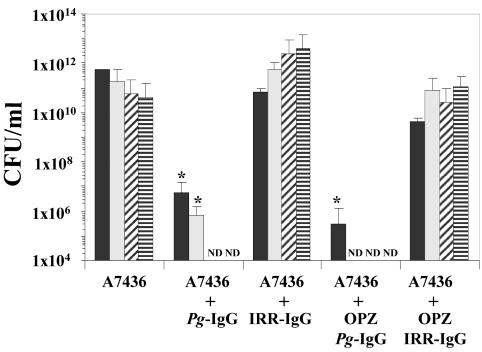
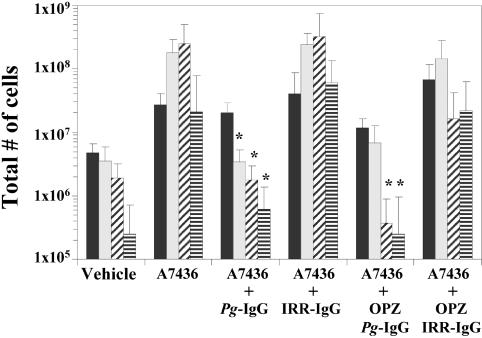
A sample of chamber fluid (50 μl) was collected from each animal at 1, 3, 7, and 10 days post-P. gingivalis challenge to assess bacterial replication and the inflammatory response. A 10-μl aliquot of each chamber fluid sample was serially 10-fold diluted in 1% peptone and plated onto anaerobic blood agar plates, and the number of CFU of P. gingivalis per milliliter was determined. Chamber fluid samples obtained from mice in group 2 demonstrated that P. gingivalis was capable of replication and persistence throughout the 10-day period (Fig. 1). Analysis of chamber fluid samples obtained from group 3 mice revealed that P. gingivalis was readily cleared, with no viable organisms recovered by day 7 (Fig. 1). Animals from group 5 also efficiently cleared P. gingivalis. Mice in groups 4 and 6 failed to clear P. gingivalis and resembled animals that had been challenged with P. gingivalis alone (Fig. 1). These data demonstrate that passive transfer of immunization-elicited P. gingivalis-specific IgG facilitates clearance of P. gingivalis in a virulence model. An additional 10-μl aliquot from each chamber fluid sample was used to determine the total inflammatory cell count by trypan blue staining. Animals from group 1 possessed relatively few inflammatory cells. By day 3, the levels of inflammatory cells present in chamber fluids of mice in group 2 were elevated and remained higher throughout the remainder of the experiment (Fig. 2). The inflammatory cell responses of mice from groups 3 and 5 were reduced by day 3 and resembled those of mice in group 1 at days 7 and 10, while mice from groups 4 and 6 possessed levels of inflammatory cells that resembled those of mice in group 2 (Fig. 2). These data support the supposition that P. gingivalis infection is controlled in mice that received P. gingivalis-specific IgG, as the cellular inflammatory response of mice challenged with P. gingivalis is resolved following clearance of bacteria.
FIG. 1.
Chamber fluid levels of P. gingivalis expressed in terms of CFU per milliliter. Chamber fluids were collected at 1 (solid bar), 3 (speckled bar), 7 (diagonal hatched bar), and 10 (horizontal hatched bar) days postchallenge from groups of mice that were challenged with P. gingivalis (A7436), P. gingivalis following passive antibody transfer of P. gingivalis-specific IgG (Pg-IgG) or IRR-IgG antibody, or P. gingivalis opsonized with P. gingivalis-specific IgG (OPZ Pg-IgG) or IRR-IgG (OPZ IRR-IgG). *, P < 0.05 compared with matched IRR-immunized groups by the Student t test; ND, P. gingivalis not detected in chamber fluid samples.
FIG. 2.
Chamber fluid levels of inflammatory cells as determined by trypan blue vital dye counts. Chamber fluid samples obtained on 1 (solid bar), 3 (speckled bar), 7 (diagonal hatched bar), and 10 (horizontal hatched bar) days postchallenge from groups of mice that were challenged with P. gingivalis (A7436), P. gingivalis following passive antibody transfer of P. gingivalis-specific (P. gingivalis-specific IgG) or irrelevant (IRR-IgG) antibody, or P. gingivalis opsonized with P. gingivalis-specific IgG (OPZ Pg-IgG) or IRR-IgG (OPZ IRR-IgG). The samples were diluted 1:10 with trypan blue vital dye, and the total number of inflammatory cells was determined by using hemocytometer counts. *, P < 0.05 compared with matched IRR-immunized groups by the Student t test.
We also employed a modified version of the fluorescent microscopic opsonophagocytosis assay of Kalmer et al. (8) and Cutler et al. (2) to assess the interaction of the inflammatory cells and P. gingivalis in the murine chamber fluid samples. In brief, a 10-μl aliquot of each chamber fluid sample was diluted to 1:50 in sterile phosphate-buffered saline; was incubated with 4′6′-diamidino-2-phenylindole (DAPI; 5 μg/ml, Sigma), propidium iodine (5 μg/ml, Sigma), and acridine orange (2.5 mM, Sigma); and was cytocentrifuged onto a glass slide. The polymorphonuclear leukocyte (PMN) viability, the ratio of the number of P. gingivalis organisms to the number of PMN (P. gingivalis/PMN), and the percentage of PMNs with at least one P. gingivalis organism were determined in blinded fashion. Mice challenged with P. gingivalis had approximately 20% dead PMNs present in the chamber fluid samples at day 1 postchallenge. Interestingly, the percentage of dead PMNs present in chamber fluid samples of mice from groups 3 and 5 was significantly reduced compared with that of group 2 animals (P < 0.05, data not shown). Passive transfer (group 4) or opsonization with IRR-IgG (group 6) failed to prevent PMN death in response to P. gingivalis challenge, as the PMN viability results resembled those of mice in group 2.
We also recorded the number of P. gingivalis/PMN as well as the percentage of phagocytosis from the murine chamber fluid samples (Table 2). At day 1, we observed that approximately half of the PMNs in the chamber fluid samples of group 2 mice possessed bacteria and that each had approximately five associated P. gingivalis organisms. Unexpectedly, the mice from group 3 possessed fewer P. gingivalis/PMN and had a reduced percentage of phagocytosis compared with that of group 4 (Table 2). Opsonization of P. gingivalis with P. gingivalis-specific IgG (group 5) resulted in an increased number of P. gingivalis/PMN compared to that found in group 2; however, this level was only approximately half the number of P. gingivalis/PMN compared to that of the group 6 mice. At day 3, we observed that approximately 40% of the PMNs were actively participating in phagocytosis of P. gingivalis, with nearly four bacteria per PMN in group 2. Chamber fluids of the mice in group 3 presented with more bacteria within each PMN compared with results for group 2 or group 4 (Table 2). Similarly, we observed that group 5 facilitated P. gingivalis uptake by PMNs compared with results for the IRR-IgG opsonization group (group 6). These data support our supposition that P. gingivalis is actively taken up by PMNs in the presence of specific IgG.
TABLE 2.
Assessment of the interaction of P. gingivalis with murine inflammatory cells in murine chamber fluids by fluorescent microscopya
| Group | Data for day 1
|
Data for day 3
|
||
|---|---|---|---|---|
| P. gingivalis/ PMNb | % Phagocytosisc | P. gingivalis/ PMN | % Phagocytosis | |
| 1 | NDd | ND | ND | ND |
| 2 | 4.81 ± 4.35 | 55.39 ± 42.29 | 3.90 ± 3.68 | 42.41 ± 20.45 |
| 3 | 0.24 ± 0.19 | 4.33 ± 4.91 | 5.62 ± 7.81 | 37.35 ± 27.93 |
| 4 | 8.08 ± 7.95 | 59.15 ± 30.45 | 2.82 ± 1.91 | 44.34 ± 17.41 |
| 5 | 7.12 ± 7.27 | 53.65 ± 39.66 | 5.66 ± 4.20 | 52.32 ± 14.85 |
| 6 | 14.81 ± 4.5 | 40.79 ± 44.08 | 1.53 ± 1.32 | 30.92 ± 21.94 |
Groups of mice (n = 8 animals per group) were challenged subcutaneously with P. gingivalis, and chamber fluid samples were harvested at 1 and 3 days after challenge. Group 1 received vehicle only, group 2 was challenged with P. gingivalis, group 3 received P. gingivalis-specific IgG injection into the chamber 24 h prior to challenge, group 4 received IRR-IgG injection prior to challenge, group 5 was challenged with P. gingivalis opsonized with P. gingivalis-specific IgG, and group 6 was challenged with IRR-IgG-opsonized P. gingivalis.
P. gingivalis/PMN was determined as the total number of live and dead bacteria present within at least 100 live PMNs.
% Phagocytosis was determined as the number of viable PMNs that have at least one associated bacterium divided by the total number of PMNs counted, all multiplied by 100.
ND, not determined.
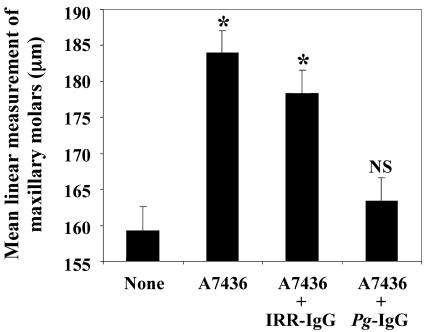
Finally, the murine oral challenge model was employed to assess the impact of P. gingivalis-specific IgG opsonization on P. gingivalis-elicited oral bone loss (7). Eight mice were included in each group. Group 1 was not treated and served as the controls, group 2 was gavaged with 100 μl of live P. gingivalis in carboxymethyl cellulose, group 3 was gavaged with P. gingivalis opsonized with IRR-IgG, and group 4 was gavaged with P. gingivalis opsonized with P. gingivalis-specific IgG. Mice in group 2 developed significant maxillary molar bone loss compared with that of group 1 (P < 0.05; Fig. 3). Mice in group 4 were protected from oral bone loss (P < 0.05) and resembled group 1 mice (P > 0.2). Mice in group 3 were not protected from oral bone loss and were significantly different from mice in group 4 (P < 0.05; Fig. 3). These data demonstrate that opsonization of P. gingivalis with specific IgG inhibits P. gingivalis-elicited oral bone loss.
FIG. 3.
Opsonization of P. gingivalis with P. gingivalis-specific IgG prior to oral challenge inhibits P. gingivalis-elicited oral bone loss. Groups of BALB/C mice were either unchallenged (None), were orally challenged with P. gingivalis (A7436), or were gavaged with P. gingivalis opsonized with control IRR-IgG or P. gingivalis-specific IgG. Linear measurements were obtained from the cementum-enamel junction to the alveolar bone crest from the maxillary molars at 14 landmark sites per mouse. *, P < 0.05 by the Student t test versus control mice (None); NS, not significant versus control (None).
To our knowledge, passive antibody transfer studies using both prevention of infection and oral bone loss as end points have not been performed to show that immunization-elicited IgG alone mediates clearance of P. gingivalis in naïve animals. By using both a murine subcutaneous chamber model and an oral bone loss model, the present study demonstrates that immunization-elicited P. gingivalis-specific IgG prevents subsequent P. gingivalis infection. We are continuing to understand the contribution of immunization-elicited IgG to specific P. gingivalis antigens for vaccinology to prevent P. gingivalis-mediated periodontal disease.
Acknowledgments
This work was supported by NIH grants F32-DE05326 (F.C.G.) and R01-DE12517 (C.A.G.).
Editor: A. D. O'Brien
REFERENCES
- 1.Califano, J. V., R. E. Schifferle, J. C. Gunsolley, A. M. Best, H. A. Schenkein, and J. G. Tew. 1999. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J. Periodontol. 70:730-735. [DOI] [PubMed] [Google Scholar]
- 2.Cutler, C. W., J. R. Kalmar, and R. R. Arnold. 1991. Phagocytosis of virulent Porphyromonas gingivalis by human polymorphonuclear leukocytes requires specific immunoglobulin G. Infect. Immun. 59:2097-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans, R. T., B. Klausen, and R. J. Genco. 1992. Immunization with fimbrial protein and peptide protects against Porphyromonas gingivalis-induced periodontal tissue destruction. Adv. Exp. Med. Biol. 327:255-262. [DOI] [PubMed] [Google Scholar]
- 4.Genco, C. A., C. W. Cutler, D. Kapczynski, K. Maloney, and R. R. Arnold. 1991. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect. Immun. 59:1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genco, C. A., D. R. Kapczynski, C. W. Cutler, R. J. Arko, and R. R. Arnold. 1992. Influence of immunization on Porphyromonas gingivalis colonization and invasion in the mouse chamber model. Infect. Immun. 60:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genco, C. A., B. M. Odusanya, J. Potempa, J. Mikolajczyk-Pawlinska, and J. Travis. 1998. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infect. Immun. 66:4108-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson, F. C., III, and C. A. Genco. 2001. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect. Immun. 69:7959-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalmar, J. R., R. R. Arnold, and T. E. van Dyke. 1987. Direct interaction of Actinobacillus actinomycetemcomitans with normal and defective (LJP) neutrophils. J. Periodontal Res. 22:179-181. [DOI] [PubMed] [Google Scholar]
- 9.Katz, J., K. P. Black, and S. M. Michalek. 1999. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 67:4352-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuboniwa, M., A. Amano, S. Shizukuishi, I. Nakagawa, and S. Hamada. 2001. Specific antibodies to Porphyromonas gingivalis Lys-gingipain by DNA vaccination inhibit bacterial binding to hemoglobin and protect mice from infection. Infect. Immun. 69:2972-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopatin, D. E., and E. Blackburn. 1992. Avidity and titer of immunoglobulin G subclasses to Porphyromonas gingivalis in adult periodontitis patients. Oral Microbiol. Immunol. 7:332-337. [DOI] [PubMed] [Google Scholar]
- 12.Mooney, J., and D. F. Kinane. 1994. Humoral immune responses to Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in adult periodontitis and rapidly progressive periodontitis. Oral Microbiol. Immunol. 9:321-326. [DOI] [PubMed] [Google Scholar]
- 13.Nonnenmacher, C., R. Mutters, and L. F. de Jacoby. 2001. Microbiological characteristics of subgingival microbiota in adult periodontitis, localized juvenile periodontitis and rapidly progressive periodontitis subjects. Clin. Microbiol. Infect. 7:213-217. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien-Simpson, N. M., C. L. Black, P. S. Bhogal, S. M. Cleal, N. Slakeski, T. J. Higgins, and E. C. Reynolds. 2000. Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect. Immun. 68:2704-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien-Simpson, N. M., R. A. Paolini, and E. C. Reynolds. 2000. RgpA-Kgp peptide-based immunogens provide protection against Porphyromonas gingivalis challenge in a murine lesion model. Infect. Immun. 68:4055-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa, T., Y. Kono, M. L. McGhee, J. R. McGhee, J. E. Roberts, S. Hamada, and H. Kiyono. 1991. Porphyromonas gingivalis-specific serum IgG and IgA antibodies originate from immunoglobulin-secreting cells in inflamed gingiva. Clin. Exp. Immunol. 83:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver, R. C., L. J. Brown, and H. Loe. 1998. Periodontal diseases in the United States population. J. Periodontol. 69:269-278. [DOI] [PubMed] [Google Scholar]
- 18.Page, R. C., L. C. Altman, J. L. Ebersole, G. E. Vandesteen, W. H. Dahlberg, B. L. Williams, and S. K. Osterberg. 1983. Rapidly progressive periodontitis. A distinct clinical condition. J. Periodontol. 54:197-209. [DOI] [PubMed] [Google Scholar]
- 19.Whitney, C., J. Ant, B. Moncla, B. Johnson, R. C. Page, and D. Engel. 1992. Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect. Immun. 60:2194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilton, J. M., T. J. Hurst, and J. A. Sterne. 1993. Elevated opsonic activity for Porphyromonas (Bacteroides) gingivalis in serum from patients with a history of destructive periodontal disease: a case control study. J. Clin. Periodontol. 20:563-569. [DOI] [PubMed] [Google Scholar]