Abstract
An emphasis on cellular immunity against Rickettsia has led to neglect of analysis of the role of antibody. The availability of an excellent mouse model of spotted fever rickettsiosis enabled investigation of a potential role of antibody in immunity to Rickettsia conorii. C3H severe combined immunodeficiency (SCID) mice were passively transfused with monoclonal antibodies against rickettsial outer membrane protein A (OmpA), OmpB, or lipopolysaccharide (LPS), polyclonal anti-R. conorii serum, Fab fragments of polyclonal antiserum, or no antibodies and then challenged 48 h later with 10 50% lethal doses (LD50) of R. conorii. All mice that received monoclonal antibodies against OmpA and two of four mice that received monoclonal antibodies against OmpB or polyclonal antisera were completely protected, but the recipients of anti-LPS antibodies or the Fab fragments were not protected. Polyclonal antibody treatment of C3H SCID mice that had been infected with 10 LD50 of R. conorii 4 or 5 days earlier prolonged the life of the infected mice from 10.4 to 22.5 days and resulted in decreased levels of infectious rickettsiae in the spleen and liver 24 and 48 h later. Treatment with protective antibodies resulted in the development of large aggregates of R. conorii antigens in splenic macrophages and intraphagolysosomal rickettsial death and digestion. The kinetics of development of antibodies to R. conorii determined by immunoblotting revealed antibodies to LPS on day 6 and antibodies to OmpA and OmpB on day 12, when recovery from the infection had already occurred. Antibodies to particular epitopes of OmpA and OmpB may protect against reinfection, but they may not play a key role in immunity against primary infection. Antibodies might be useful for treating infections with antibiotic-resistant organisms, and some B-cell epitopes should be included in a subunit vaccine.
Conventional wisdom led scientists interested in immunity to rickettsiae to focus on cellular immune mechanisms. It was postulated that obligately intracellular bacteria residing free in the cytosol of endothelial cells are not accessible to the effects of antibodies. For spotted fever group (SFG) rickettsiae, this possibility seemed particularly likely owing to the ability of these organisms to spread from cell to cell via host actin-based mobility (13), thus avoiding exposure to extracellular antibodies.
The development of valid animal models of rickettsiosis with disseminated endothelial infection, critical organ involvement including the brain and lungs, and pathological lesions resembling those observed with human Rocky Mountain spotted fever, boutonneuse fever, murine typhus, and epidemic typhus enabled advances in the experimental investigation of mechanisms of immunity to rickettsiae (11, 27, 28). In fact, activation of endothelial cells by cytokines (gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]) results in inhibition of rickettsial growth and survival of experimentally infected animals (7). In vitro studies demonstrated that IFN-γ and TNF-α stimulate the synthesis of rickettsicidal nitric oxide (NO) by inducible nitric oxide synthase in murine endothelial cells (26).
Human endothelial cells, macrophages, and hepatocytes activated by IFN-γ, TNF-α, interleukin-1β, and RANTES also inhibit the growth and survival of Rickettsia conorii by a variety of effects, including NO-dependent, reactive-oxygen-species-dependent, and/or tryptophan-degradation-dependent mechanisms (9). In animal models NK cells are activated early in the course of rickettsial infection and also dampen rickettsial growth by production of IFN-γ (2). CD8 T lymphocytes are essential for the clearance of rickettsiae via major histocompatibility complex class I-restricted cytotoxic T-lymphocyte activity (8, 25). Thus, two decades of investigation of cellular immunity to rickettsiae established the mechanistic basis for its importance (23). However, the potential role of humoral immunity to rickettsiae has not been critically evaluated. The availability of a well-characterized mouse model of SFG rickettsiosis enabled the potential role of antibodies to R. conorii and its surface antigens in protective immunity to be studied. To our surprise, not only did polyclonal antibodies to R. conorii and monoclonal antibodies to major outer membrane protein A (OmpA) and OmpB protect severe combined immunodeficiency (SCID) mice challenged with a lethal dose of R. conorii, but also antibodies administered to SCID mice 4 or 5 days after establishment of infection significantly reduced the infectious rickettsial contents in the spleen, lungs, and liver 24 and 48 h later and prolonged the life of the recipients by an average of 12 days.
MATERIALS AND METHODS
Rickettsia.
R. conorii strain Malish 7 (= ATCC VR-613), a human isolate from South Africa, was obtained from the American Type Culture Collection (Manassas, Va.) and was cloned by plaque purification in our laboratory. The 50% lethal dose (LD50) of a 10% yolk sac suspension stock was 4.6 × 103 PFU/mouse for wild-type C3H/HeN mice.
Antibodies.
Polyclonal hyperimmune serum was obtained from mice immunized by infection with a sublethal dose of R. conorii (1,000 PFU/mouse) followed by a high-dose booster immunization (1 × 105 PFU/mouse), 10 days after which the sera were collected. Ascites fluids containing monoclonal antibodies U16 (anti-OmpA), U7, U12, U14, and U20 (anti-OmpB), and U28 (anti-lipopolysaccharide [LPS]) were obtained from hybridomas developed in our laboratory from mice immunized with R. conorii by fusion of immune spleen cells and myeloma SP 2/0 Ag14 cells (ATCC CRL 8006) (24). The immune serum and the monoclonal antibodies were purified by precipitation of the immunoglobulin fraction with 50% saturated ammonium sulfate. Ascites fluid induced with myeloma SP 2/0 fusion partner cells was used as a negative control. The immunofluorescent antibody titers of the antibodies against R. conorii were as follows: polyclonal serum, U12, and U16, 1:2,560; U14 and U20, 1:5,120; and U7 and U28, 1:1,280. Fab fragments of antibodies were prepared with a Fab fragment kit (Pierce, Rockford, Ill.), and F(ab′)2 fragments were prepared with a Pure (Fab′)2 preparation kit (Pierce) used according to the instructions of the manufacturer.
Passive protection.
Eighty SCID C3H mice were divided into 11 groups. Each mouse received 0.5 ml intravenously and 1 ml intraperitoneally of polyclonal hyperimmune serum, monoclonal antibody U7, U12, U14, U16, U20, or U28, SP 2/0 ascites fluid, or normal serum or received no serum. Experiments were performed with three mice per group, and for most antibodies three repetitions were performed; the exceptions were monoclonal antibodies U7, U12, and U20, which were evaluated in only one experiment. Forty-eight hours after inoculation of the antibody, the mice were challenged intravenously with 10 LD50 of R. conorii. The mice were observed daily for 4 weeks, and the mortality was recorded. Other groups of eight and three mice received Fab and F(ab′)2 fragments of polyclonal immune serum, respectively.
Protection of mice with previously established R. conorii infection by passive polyclonal antibody.
In the first experiment, 10 SCID C3H mice were inoculated with 10 LD50 of R. conorii. On day 5 the mice were separated into two groups. Each mouse in the first group was given 0.5 ml of immune immunoglobulin against R. conorii intravenously and 1.0 ml intraperitoneally. Each mouse in the other group of five mice received the same dose of normal mouse serum; this group served as a control group. Twenty-four hours later two mice from each group were sacrificed. One half of the spleen, one half of the liver, and one half of the lungs from each mouse were harvested in Eagle's minimum essential medium (GIBCO, Grand Island, N.Y.) containing 1% bovine calf serum and used for a plaque assay as described previously (24) to measure the quantity of infectious rickettsiae in each organ. The other half of each of the organs was fixed in 4% neutral formaldehyde for immunohistologic detection of rickettsiae. The remaining three mice in each group were observed daily for illness and death. The experiments were performed three times. In the third experiment, the mice were sacrificed 48 h after inoculation of antibody, and the contents of infective rickettsiae in the organs were determined by plaque assay titration.
Immunohistology.
C3H/HeN SCID mice were inoculated intravenously with 100 LD50 of R. conorii (2.6 × 103 PFU/mouse). Six days later, when the mice showed signs of illness, polyclonal anti-R. conorii serum or normal mouse serum (0.5 ml intravenously and 1.0 ml intraperitoneally for each mouse) was administered. At 8, 24, 32, and 48 h after the antibody treatment, the mice were sacrificed, and parts of the spleens, livers, lungs, kidneys, brains, testes, and peritesticular adnexae were fixed in 4% neutral formaldehyde, embedded in paraffin, and sectioned (thickness, 5 μm). Tissue sections were stained immunohistochemically by using an automated DAKO Autostainer universal staining system (DAKO Corporation, Carpinteria, Calif.). Briefly, the sections were deparaffinized by heating the glass slides at 70°C for 20 min, followed by immersion in three xylene baths (5 min each). The slides were then rehydrated by immersion in a series of alcohol baths with alcohol concentrations ranging from 100 to 80% (3 min each). The slides were then washed in distilled water, placed in the stainer for processing, and incubated with an endogenous peroxidase inhibitor solution containing 3% hydrogen peroxide in methyl alcohol for 10 min at 37°C; this was followed by incubation with an avidin-biotin blocking solution for 10 min, as recommended by the manufacturer (Vector Laboratories, Burlingame, Calif.). Tissue sections were then digested with DAKO Ready-to-Use proteinase K (DAKO Corporation) for 10 min at room temperature and incubated for 30 min with rabbit anti-R. conorii polyclonal antibody at 37°C; this was followed by three washes in phosphate-buffered saline. A goat biotinylated anti-rabbit immunoglobulin G(heavy and light chains) antibody (Vector Laboratories) was then incubated with the slides for 15 min at a 1:800 dilution and 37°C. The slides were then washed in phosphate-buffered saline and incubated with streptavidin-horseradish peroxidase conjugate (DAKO Corporation) for 15 min at 37°C and then with DAKO liquid diaminobenzidine solution (DAKO Corporation) for 5 min at 37°C. Counterstaining with hematoxylin was performed, and coverslips were added to the slides. Normal rabbit serum was used as the primary antibody for a negative control. For quantitation purposes, the glass slides were examined with an Olympus microscope with a ×20 objective (total magnification, ×200), and all microscopic fields of the spleen and liver were examined. Clusters of rickettsiae were counted in each field. Statistical analysis was performed by using the t test or the Mann-Whitney rank sum test as indicated below (SigmaStat, version 2.0; SPSS Inc., Chicago, Ill.).
Evaluation of the direct effect of complement on R. conorii.
Renografin density gradient-purified rickettsiae were incubated with fresh mouse serum containing complement, heat-inactivated (56°C, 30 min) mouse serum, which inactivated complement activity, or sucrose-phosphate-glutamate buffer (218 mM sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM monosodium l-glutamic acid; pH 7.0) for 1 h at 37 or 4°C and then fixed and processed for electron microscopy.
Fifty microliters of Renografin density gradient-purified R. conorii diluted 1:3 in sucrose-phosphate-glutamate buffer was mixed with fresh or heat-inactivated (56°C, 30 min) normal mouse serum and incubated at 37°C in a water bath for 1 h; 0.9 ml of minimal essential medium containing 1% bovine calf serum was added to this mixture, and serial 10-fold dilutions were prepared. Each dilution was inoculated onto monolayers of Vero cells (0.2 ml/well) in duplicate to determine the rickettsial infectivity titer by a plaque assay as described previously (28).
Electron microscopy.
To evaluate the effect of antibody on rickettsial infection by electron microscopy, portions of the spleens and livers of the mice sacrificed for immunohistochemical studies were fixed for electron microscopy. The tissues were cut into 1-mm3 cubes and fixed in Ito's fixative, a mixture of 1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% trinitrophenol, and 0.03% CaCl2 in 0.05 M cacodylate buffer at pH 7.3 (14). The tissues were postfixed in 1% osmium tetroxide for 1 h and stained en bloc in 1% uranyl acetate-0.1 M maleate buffer (pH 5.2) for 30 min at 60°C. Samples were subjected to a series of dehydration steps by using ethanol and propylene oxide, embedded in Poly/Bed 812 epoxy resin, and polymerized at 60°C overnight. Ultrathin sections were cut with a Reichert Ultracut S ultramicrotome, placed on copper grids, and subsequently stained with uranyl acetate and lead citrate. Sections were examined with a Phillips CM 100 electron microscope or a Phillips 201 electron microscope operated at 60 kV.
Western immunoblot determination of the kinetics of development of antibodies to antigens of R. conorii.
A sublethal dose of R. conorii was inoculated intravenously into 40 C3H/HeN mice. On days 6, 12, 18, and 30 after infection, 10 mice were anesthetized, and blood was collected from each mouse and processed individually. After separation of the sera, the samples were kept at −20°C until they were examined for the presence of antibodies to antigens of R. conorii by Western immunoblotting. The precast NuPAGE NOVEX bis-Tris minigels and the reagents and solutions used for electrophoresis and semidry blotting were obtained from Invitrogen (Carlsbad, Calif.). Electrophoresis was performed at 100 V for 1.5 h, and blotting was performed at 20 V for 20 min.
RESULTS
Passive protection by antibody present before rickettsial challenge.
In preliminary experiments, it was observed that hyperimmune serum provided effective protection against a lethal dose of rickettsiae. To evaluate the possibility that a concomitantly developing cellular immune response might have contributed substantially to this outcome, SCID mice were utilized in subsequent experiments. The survival data confirmed that hyperimmune serum and some of the monoclonal antibodies that recognized only one conformational epitope on the surface of rickettsial OmpA or OmpB protected against a high lethal dose (10 LD50) of rickettsiae (Table 1). One anti-OmpA monoclonal antibody (U16) and two anti-OmpB monoclonal antibodies (U14 and U20) conferred complete protection. The anti-LPS monoclonal antibody (U28) and one of the monoclonal antibodies against OmpB (U7) were not protective. Monoclonal antibody U12 against OmpB conferred only partial protection as it prolonged the survival of the mice significantly (P = 0.006), but all of the mice eventually died. We concluded that only some of the monoclonal antibodies provided passive protection against rickettsial challenge. The normal serum or myeloma cell-induced ascites fluid from naïve mice provided no protection. Thus, the possible role of innate immunity (e.g., NK cells, macrophages, or complement) was not critical in these experiments with SCID mice. From these results, we confirmed that passive transfer of antibody prevented rickettsial illness and death in SCID mice that were not capable of developing acquired T- or B-lymphocyte-mediated immunity. The protective effect was Fc receptor dependent; Fab and F(ab)2 fragments from hyperimmune serum did not confer protection.
TABLE 1.
Passive antibody protection of C3H SCID mice infected with 10 LD50 of R. conorii
| Group | No. of mice | % Sur- vival | Day of death
|
|
|---|---|---|---|---|
| Range | Mean ± SD | |||
| Polyclonal hyperimmune serum | 9 | 100 | ||
| Polyclonal anti-R. conorii Fab | 8 | 0 | 9-13 | 10.1 ± 1.2 |
| Polyclonal anti-R. conorii F(ab′)2 | 3 | 0 | 13-14 | 13.7 ± 0.6 |
| Normal serum | 9 | 0 | 9-13 | 11.4 ± 1.6 |
| Anti-OmpA monoclonal antibody U16 | 9 | 100 | ||
| Anti-OmpB monoclonal antibody U14 | 9 | 100 | ||
| Anti-OmpB monoclonal antibody U20 | 3 | 100 | ||
| Anti-OmpB monoclonal antibody U7 | 3 | 0 | 10-13 | 11.3 ± 1.5 |
| Anti-OmpB monoclonal antibody U12 | 3 | 0 | 15 | 15 ± 0.0a |
| Anti-LPS monoclonal antibody U28 | 9 | 0 | 12-14 | 12.3 ± 1.1 |
| SP 2/0 ascites fluid | 9 | 0 | 9-13 | 12.5 ± 1.2 |
| No serum | 9 | 0 | 13 | 13 ± 0.0 |
Survival was prolonged significantly (P = 0.006) compared with survival after normal ascites fluid treatment.
Passive transfer of antibody to R. conorii substantially prolonged the course of mice with established infection.
Ten 50% lethal doses of R. conorii caused SCID C3H mice to become ill on day 5 or 6 (i.e., the mice had ruffled fur, a hunched posture, reduced movement, and partially closed eyelids) and to die on day 13. To determine whether the antibody had a protective effect if it was administered near the end of the incubation period or at the early stage after the appearance of symptoms due to rickettsial infection, SCID C3H mice were given antibody on day 4 or 5 after rickettsial infection. All of the experimentally treated and control mice became ill and died. The mice in the control group died on days 9 to 12 (mean, day 10.4 ± 1.1), and the mice in the experimentally treated group died on days 19 to 30 (mean, day 22.5 ± 4.1). Thus, the survival period for the antibody-treated mice was significantly longer than that for the controls (P < 0.001). The rickettsial loads of the antibody-treated animals, which were expressed in numbers of PFU per gram of organ in three repeated experiments, were significantly less than the rickettsial loads of the mice in the control group (Table 2). These results confirmed that administration of antibody during the early stage of illness could act as a host defense against rickettsial infection. The quantities of infective rickettsiae in the spleens, livers, and lungs of the experimentally treated mice were significantly less than the quantities of infective rickettsiae in the organs of the control mice. However, all the SCID mice that received antibody subsequently died, presumably after the decay of passively transferred antibodies, even though the survival period was substantially longer.
TABLE 2.
Comparison of rickettsial contents in immune serum-treated and normal serum-treated C3H SCID mice infected with 10 LD50 of R. conorii
| Expt | Organ | Amt of infectious R. conorii (104 PFU/g of organ)
|
Day of antibody inoculation after rickettsial inoculation | P valuea | |
|---|---|---|---|---|---|
| Immune serum treated | Normal serum treated | ||||
| 1 (24 h) | Spleen | 128.1 ± 30.3 | 2,937.5 ± 187.5 | 5 | 0.007 |
| Liver | 380 ± 5.7 | 838.2 ± 44.1 | 0.005 | ||
| Lungs | 2,138.9 ± 27.8 | 3,262.9 ± 24.3 | <0.001 | ||
| 2 (24 h) | Spleen | 156.3 ± 31.3 | 392.9 ± 35.7 | 4 | 0.02 |
| Liver | 34.9 ± 11.6 | 112.9 ± 16.1 | 0.031 | ||
| Lungs | 229.2 ± 62.5 | 232.1 ± 17.9 | 0.169 | ||
| 3 (48 h) | Spleen | 98.2 ± 8.9 | 1,229.1 ± 20.8 | 4 | 0.001 |
| Liver | 32.9 ± 0.7 | 323.0 ± 40.1 | 0.009 | ||
| Lungs | 63.8 ± 1.3 | 135.4 ± 10.4 | 0.011 | ||
Most of the P values determined by Student's t-test were <0.05; the only exception was the P value for the experiment 2 lung results, which was 0.169.
Immunohistochemical observations.
In the spleens, the numbers of rickettsial clusters were similar at 8 h (P = 0.086), but the numbers of clusters of rickettsiae were statistically significantly higher in the antibody recipients at 24 and 32 h (Fig. 1). The numbers of rickettsial clusters at these two time points were higher in the animals treated with rickettsia-specific antibodies than in the animals treated with normal serum (P < 0.001 for both time points). Interestingly, at 48 h, the numbers of rickettsial clusters in the spleens of animals treated with specific antibodies against rickettsiae were markedly decreased, suggesting that there was almost total clearance of the rickettsiae from the infected animals. In contrast, the spleens of animals treated with normal serum contained large numbers of confluent rickettsial clusters at 48 h, confirming that rickettsial replication occurred. On the other hand, at 8 h the numbers of rickettsial clusters in the livers of animals treated with antirickettsial antibodies were lower than the numbers in animals treated with normal serum (P < 0.001). At 24 h, the numbers of rickettsial clusters were higher in the livers of animals treated with antirickettsial antibodies (P < 0.016). At 32 and 48 h, the differences between the two groups were not statistically significant (P = 0.790 and P = 0.842, respectively). In the antibody-treated animals, the clusters of rickettsial antigen appeared to be amorphous, in contrast to the clusters in control animals, in which typical rickettsial morphology was observed (Fig. 1).
FIG. 1.
Immunohistochemical staining of R. conorii in the spleens of mice 32 h after treatment with polyclonal mouse anti-R. conorii serum (A and C) or normal mouse serum (B and D). The antibody-treated mice (A) contained a greater quantity of rickettsial antigen in their spleens than the normal serum-treated controls contained (B). The rickettsial antigen in splenic macrophages of antibody-treated mice (C) comprised amorphous rickettsial antigen and fragmented particles smaller than rickettsiae (short and long arrows). Rickettsial antigen in the spleens of normal serum-treated mice (D) included individual, morphologically typical rickettsiae (arrowhead). (A and B) Magnification, ×200; (C and D) magnification, ×1,000.
Fate of rickettsiae as determined by ultrastructural observations.
Ultrastructurally, at 32 h the majority of rickettsiae were found in the spleens of infected mice after antibody treatment or in livers after normal serum treatment. The rickettsiae in the normal serum-treated control mice were primarily found within Kupffer cells. The majority of these rickettsiae were in the cytosol of infected cells and had a normal rickettsial morphology, including the characteristic electron-lucent zone immediately adjacent to the outer membrane (Fig. 2). Only one rickettsial organism with morphology consistent with rickettsial damage was identified within a phagolysosome-like vacuole of a Kupffer cell in mice that did not receive passive antibody.
FIG. 2.
Electron photomicrograph of the liver of a mouse infected with R. conorii 32 h after treatment with nonimmune serum. Several viable rickettsiae (arrows) are free in the cytosol. The arrowhead indicates a rickettsia enlarged in the inset. Bar = 0.5 μm. (Inset) Viable rickettsia with typical bacillary morphology, including normal ultrastructure of the cytoplasm, nucleoid, cell wall (arrowhead), and cytoplasmic membrane (arrow). Bar = 0.5 μm.
Conversely, splenic rickettsiae in the polyclonal antibody-treated mice were found primarily in macrophages at 32 h after antibody treatment. The majority of these rickettsiae were present either singly or in groups within phagolysosome-like vacuoles (Fig. 3). These vacuoles appeared to be in various stages of maturation, as smaller membrane-bound vacuoles with lysosome-like morphology were often observed in the process of fusing with the larger rickettsia-containing vacuoles. Phagolysosomes contained rickettsiae that had ultrastructural characteristics consistent with death and different stages of digestion. These morphological characteristics included the presence of electron-dense rickettsial cytoplasm and nucleoplasm and electron-lucent zones within the nucleoplasm, loss of the rickettsial bacillary morphology, increased membrane waviness, distorted periplasmic space, and breaks in the cell wall and cytoplasmic membrane.
FIG. 3.
Electron photomicrograph of three morphologically nonviable rickettsiae (large arrows) within a phagolysosome (asterisk) of a splenic macrophage in an R. conorii-infected SCID mouse 32 h after polyclonal immune antibody treatment. There are distortions of the cell wall (small arrows), dilated periplasmic space (p), loss of the typical bacillary morphology, and vesicles shed from the cell wall (arrowheads). Bar = 0.5 μm.
Absence of ultrastructural rickettsial damage or rickettsicidal activity associated with direct action of complement.
The ultrastructural appearance of R. conorii exposed to the fresh components of the complement system, the ultrastructural appearance of R. conorii exposed to serum in which the complement system was inactivated, and the ultrastructural appearance of rickettsiae maintained in the optimal buffer system were indistinguishable at both 37 and 4°C. All of the rickettsiae appeared to be completely normal. No damaged rickettsiae were observed. The cell walls of the rickettsiae were intact.
The number of plaques in rickettsiae incubated with fresh serum and the number of plaques in rickettsiae incubated with serum in which complement was inactivated were 150 × 104 ± 14 × 104 and 162.5 × 104 ± 3.5 × 104, respectively. There was no significant difference between the two groups (P = 0.348). There was no antirickettsial effect related to the activity of complement.
Kinetics of the development of antibodies to OmpA, OmpB, and LPS of mice infected with a sublethal dose of R. conorii.
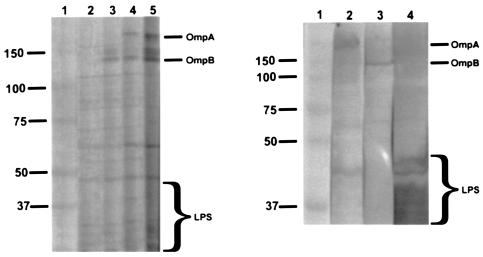
On day 6 of infection of naïve C3H/HeN mice, Western immunoblots demonstrated that the sera of all 10 mice contained antibodies only to the LPS bands of R. conorii. On day 12, antibodies to OmpA and OmpB were observed (Fig. 4). The antibody reactivity with OmpA and OmpB was stronger on day 18 and strongest on day 30.
FIG. 4.
Western immunoblots of R. conorii antigens reactive with sera collected serially from mice on days 6 (lane 2), 12 (lane 3), 18 (lane 4), and 30 (lane 5) after infection (left panel) and with monoclonal antibodies to OmpA (lane 2), OmpB (lane 3), and LPS (lane 4) (right panel). Lanes 1 contains molecular size standards (37 to 150 kDa).
DISCUSSION
Antibody to R. conorii can make a crucial difference in the outcome of SFG rickettsiosis. Passive transfer of polyclonal antibodies or monoclonal antibodies to OmpA or OmpB converted a lethal outcome in untreated control mice to 100% survival in the absence of adaptive immunity associated with T and B lymphocytes. Extrapolation of these data to the presence of circulating antibodies to protective epitopes in a person exposed to the bite of an SFG rickettsia-infected tick led to the prediction that disease would likely be prevented. The ameliorative effect of treatment of SCID mice on day 4 or 5 after infection with a lethal dose of R. conorii is even more remarkable. The life of SCID mice with an established infection was prolonged by a mean of 12 days, a period of time during which an immunocompetent host should have generated an effective cell-mediated immune response, including IFN-γ and cytotoxic T lymphocytes, to kill intracellular rickettsiae and clear the infected cells. The effect of treating the mice with antibodies at the onset of illness resembles the possible events in a person whose antibodies have become undetectable years after recovery from SFG rickettsiosis but who develops an anamnestic humoral immune response upon reinfection. It is highly likely that the antibodies play a protective role in this situation. Indeed, well-documented reinfections with SFG rickettsiae are vanishingly rare, if they occur at all. Moreover, in the preantibiotic era, some patients with Rocky Mountain spotted fever were treated with rabbit polyclonal antibodies against Rickettsia rickettsii (22). The effectiveness appeared to depend on the interval between the onset of symptoms and the initiation of treatment. Of 52 patients with Rocky Mountain spotted fever who were treated with immune serum on or before the third day of rash, only 2 (3.8%) died, compared with an expected 18.8% fatality rate calculated from historic records adjusted for age, a statistically significant difference. None of the 40 patients who were 39 years old or younger and were treated with immune serum died. The two treated patients who died were males who were 66 and 72 years old, an age- and gender-specific group with a particularly high fatality rate. A dose-dependent protective effect of immune serum was also observed in experimentally infected guinea pigs and rhesus monkeys (21, 22).
The mechanism of protection by antibody in our experiments was opsonization dependent, as Fab and F(ab′)2 fragments of protective antibodies did not have a beneficial effect. The presence of antibodies resulted in phagocytic removal of R. conorii by macrophages as observed by immunohistochemistry, in rickettsial killing as documented by the reduction in the number of viable R. conorii cells in spleens and livers at 24 and 48 h after administration of the antibodies, and in intraphagolysosomal death and digestion of rickettsiae in macrophages of antibody-treated mice as observed ultrastructurally. In contrast, rickettsiae escaped from the phagosomes and multiplied in the cytosol of cells of untreated animals. Thus, antibody-exposed rickettsiae were taken up, retained within phagosomes, and killed by macrophages rather than entering endothelial cells, escaping from the phagosomes into the cytosol, and replicating.
A comparison of the immunohistochemical and rickettsial titration results is instructive. Many of the rickettsial clusters represented organisms retained within phagosomes and phagolysosomes. In the spleens of mice treated with antibodies 24 h earlier, there were more clusters of rickettsial antigen than there were in the normal serum recipients, yet there were fewer viable rickettsiae in the spleens of the antibody-treated mice at this time. Clearly, the time that it took for the antigen to be digested was less than the time required for viability to have been lost. The amorphous quality of the rickettsial antigen in the antibody-treated mice also indicated that the rickettsiae were no longer intact. By 48 h the quantity of antigen was decreased to the limit of detection by immunohistochemistry in the antibody-treated animals, which correlated with rickettsial killing and digestion. In contrast, growth of R. conorii was observed by both immunohistochemistry and rickettsial titration in the spleens of the normal serum-treated animals at 48 h. Similar results were obtained for the livers of antibody-treated mice at 24 h. Differences between the immunohistochemical results obtained for the spleens and the livers at 8, 32, and 48 h may reflect the presence of a larger portion of endothelial cells in the livers than in the spleens. Interestingly, the rickettsial titers were lower not only in the livers and spleens but also in the lungs, indicating that there was protection of the key target cells, namely, the endothelial cells.
No evidence was obtained for a direct effect of complement on R. conorii, and mice treated with normal serum, monoclonal antibodies against LPS, two monoclonal antibodies against OmpB, and Fab fragments died of overwhelming rickettsial infection despite the presence of complement. Thus, complement does not appear to play a critical role in protective immunity against rickettsiae. Nevertheless, it is likely that complement plays an adjunct role in enhancing the killing of opsonized rickettsiae by macrophages (12).
Analysis of the kinetics of production of antibodies by Western immunoblotting revealed that on day 6, when the immune response curtails the increase in growth of rickettsiae, no antibodies to the protective antigens, OmpA and OmpB, had been produced or the concentration of antibodies to OmpA and OmpB was below the limit of detection by immunoblotting. The only antibodies detected at this critical point were nonprotective as they were directed against LPS. On day 12, after recovery and clearance of the organisms from the tissues (28), antibodies against OmpA and OmpB appeared. These kinetics suggest that antibodies are unlikely to play an important role in the protective immunity responsible for recovery from a primary infection. The possibility of a minor role in a primary infection and a larger role in a secondary challenge infection is greater than the possibility of an apparent role of polyclonal antibody that develops during infection by another intracellular bacterium, Listeria monocytogenes, against which passive transfer of immune serum provides no protection (19).
The general explosion of observations of intracellular bacteria against which protective antibodies have been identified is remarkable (3-6, 15, 18, 20). A comparison of the bacterial antigenic targets of antibodies that confer protection against intracellular bacteria revealed two general categories, surface-exposed antigens and secreted proteins. There is considerable diversity of surface-exposed antigens, and these antigens include proteins, such as rickettsial OmpA and OmpB and a 28-kDa protein of Ehrlichia chaffeensis, carbohydrates, such as arabinomannan of Mycobacterium tuberculosis, and LPS, such as the LPS of Brucella melitensis and Brucella abortus (4, 16-18, 29). Monoclonal antibodies to mycobacterial arabinomannan direct M. tuberculosis to a different cell entry pathway (18), which leads to activation of protective immune mechanisms that result in increased survival of the animals and greater containment of similar quantities of bacteria in granulomas (20). Clearly, antirickettsial mechanisms mediated by antibodies differ from antimycobacterial mechanisms in terms of the reduction in rickettsial viability. The explanation for the difference between the protective effect of antibodies to smooth LPS epitopes of Brucella and the lack of protection of anti-LPS antibodies against R. conorii remains to be elucidated. Moreover, the similarity of the effect of antibodies to listeriolysin O of L. monocytogenes and the effect of antibodies to R. conorii, both of which are associated with failure of the bacteria to escape from the phagosomes, is not understood at the mechanistic level for rickettsiae, for which the mechanism of phagosomal escape has yet to be determined (6).
It should be noted that the effects of antibodies to rickettsiae have been studied by using a method that has since been discredited as a model for rickettsial infection or immunity, namely, toxicity of a large dose of intravenously inoculated rickettsiae leading to death within 24 h associated with pathological effects of TNF-α (1, 10; Feng and Walker, unpublished data). It is possible that the similarities of some of the results of protection from infection and from toxicity related to the interactions of macrophages and rickettsiae opsonized with Fc-containing antibodies to some conformational epitopes of OmpA and OmpB and the lack thereof for antibodies to LPS share, in part, a mechanistic basis. However, we do not believe that the toxicity phenomenon offers insight into mechanisms of immunity to rickettsiae.
In vitro investigation of the effect of antibodies against Rickettsia typhi on infection of human macrophages demonstrated that there was enhanced phagocytosis and intracellular killing rather than growth (12). Guinea pig complement further increased macrophage engulfment of antibody-opsonized rickettsiae. We are currently investigating the mechanisms of the effects of the protective and nonprotective monoclonal antibodies in vitro on rickettsial infections of both endothelium and macrophages. Inclusion of the B-cell antigens that stimulate protective antibodies should be a useful adjunct to the CD4 and CD8 T-lymphocyte-stimulating antigens of Rickettsia for development of protective subunit vaccines.
Acknowledgments
We thank Kelly Cassity, Marvel Denard, and Susan Butler for assistance with preparation of the manuscript and Violet C. Han for expert assistance with electron microscopy.
This research was supported by grant AI 21242 from the National Institute of Allergy and Infectious Diseases.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anacker, R. L., R. H. List, R. E. Mann, S. F. Hayes, and L. A. Thomas. 1985. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J. Infect. Dis. 151:1052-1060. [DOI] [PubMed] [Google Scholar]
- 2.Billings, A. N., H.-M. Feng, J. P. Olano, and D. H. Walker. 2001. Rickettsial infection in murine models activates an early anti-rickettsial effect mediated by NK cells and associated with production of gamma interferon. Am. J. Trop. Med. Hyg. 65:52-56. [DOI] [PubMed] [Google Scholar]
- 3.Brieland, J. K., L. A. Heath, G. B. Huffnagle, D. G. Remick, M. S. McClain, M. C. Hurley, R. K. Kunkel, J. C. Fantone, and C. Engleberg. 1996. Humoral immunity and regulation of intrapulmonary growth of Legionella pneumophila in the immunocompetent host. J. Immunol. 157:5002-5008. [PubMed] [Google Scholar]
- 4.Cloeckaert, A., I. Jacques, P. de Wergifosse, G. Dubray, and J. N. Limet. 1992. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect. Immun. 60:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Cutting edge: paradigm revisted: antibody provides resistance to Listeria infection. J. Immunol. 163:4087-4090. [PubMed] [Google Scholar]
- 6.Edelson, B. T., and E. R. Unanue. 2001. Intracellular antibody neutralizes Listeria growth. Immunity 14:503-512. [DOI] [PubMed] [Google Scholar]
- 7.Feng, H.-M., V. L. Popov, and D. H. Walker. 1994. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 62:1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, H.-M., V. L. Popov, G. Yuoh, and D. H. Walker. 1997. Role of T-lymphocyte subsets in immunity of spotted fever group rickettsiae. J. Immunol. 158:5314-5320. [PubMed] [Google Scholar]
- 9.Feng, H.-M., and D. H. Walker. 2000. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68:6729-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, H.-M., and D. H. Walker. 2003. Cross-protection between distantly related spotted fever group rickettsiae. Vaccine 21:3901-3905. [DOI] [PubMed] [Google Scholar]
- 11.Feng, H.-M., J. Wen, and D. H. Walker. 1993. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am. J. Pathol. 142:1471-1482. [PMC free article] [PubMed] [Google Scholar]
- 12.Gambrill, M. R., and C. L. Wisseman, Jr. 1973. Mechanisms of immunity in typhus infections. III. Influence of human immune serum and complement on the fate of Rickettsia mooseri within human macrophages. Infect. Immun. 8:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzen, R. A., S. F. Hayes, M. G. Peacock, and T. Hackstadt. 1993. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect. Immun. 61:1926-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito, S., and Y. Rikihisa. 1981. Techniques for electron microscopy of rickettsiae, p. 213-227. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 15.Koesling, J., T. Aebischer, C. Falch, R. Schülein, and C. Dehio. 2001. Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J. Immunol. 167:11-14. [DOI] [PubMed] [Google Scholar]
- 16.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 17.Li, J. S.-Y., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 18.Malik, Z. A., G. M. Denning, and D. J. Kusner. 2000. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191:287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki, K., and M. B. Mackaness. 1964. The passive transfer of acquired resistance to Listeria monocytogenes. J. Exp. Med. 120:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teitelbaum, R., A. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topping, N. H. 1940. Rocky Mountain spotted fever. Treatment of infected laboratory animals with immune rabbit serum. Public Health Rep. 55:41-46. [Google Scholar]
- 22.Topping, N. H. 1943. Rocky Mountain spotted fever: further experience in the therapeutic use of immune rabbit serum. Public Health Rep. 58:757-775. [Google Scholar]
- 23.Valbuena, G., H.-M. Feng, and D. H. Walker. 2002. Mechanisms of immunity against rickettsiae. New perspectives and opportunities offered by unusual intracellular parasites. Microbes Infect. 4:625-633. [DOI] [PubMed] [Google Scholar]
- 24.Walker, D. H., S. D. Hudnall, W. K. Szaniawski, and H.-M. Feng. 1999. Monoclonal antibody-based immunohistochemical diagnosis of rickettsialpox: the macrophage is the principal target. Mod. Pathol. 12:529-533. [PubMed] [Google Scholar]
- 25.Walker, D. H., J. P. Olano, and H.-M. Feng. 2001. Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect. Immun. 69:1841-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker, D. H., V. L. Popov, P. A. Crocquet-Valdes, C. J. R. Welsh, and H.-M. Feng. 1997. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab. Investig. 76:129-138. [PubMed] [Google Scholar]
- 27.Walker, D. H., V. L. Popov, and H.-M. Feng. 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. 80:1361-1372. [DOI] [PubMed] [Google Scholar]
- 28.Walker, D. H., V. L. Popov, J. Wen, and H.-M. Feng. 1994. Rickettsia conorii infection of C3H/HeN mice: a model of endothelial-target rickettsiosis. Lab. Investig. 70:358-368. [PubMed] [Google Scholar]
- 29.Winslow, G. M., E. Yager, K. Shilo, E. Volk, A. Reilly, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]