Abstract
Staphylococcus aureus is a common cause of intramammary infections, which frequently become chronic, associated with the ability of the bacteria to produce biofilm. Here, we report a relationship between the ability to produce chronic bovine mastitis and biofilm formation. We have classified bovine mastitis S. aureus isolates into three groups based on the presence of particular genetic elements required for biofilm formation: group 1 (ica+ bap+), group 2 (ica+, bap negative), and group 3 (ica negative, bap negative). Overall, animals naturally infected with group 1 and 2 isolates had a lower milk somatic cell count than those infected with isolates of group 3. In addition, Bap-positive isolates were significantly more able to colonize and persist in the bovine mammary gland in vivo and were less susceptible to antibiotic treatments when forming biofilms in vitro. Analysis of the structural bap gene revealed the existence of alternate forms of expression of the Bap protein in S. aureus isolates obtained under field conditions throughout the animal's life. The presence of anti-Bap antibodies in serum samples taken from animals with confirmed S. aureus infections indicated the production of Bap during infection. Furthermore, disruption of the ica operon in a bap-positive strain had no effect on in vitro biofilm formation, a finding which strongly suggested that Bap could compensate for the deficiency of the PIA/PNAG product (a biofilm matrix polysaccharide). Altogether, these results demonstrate that, in the bovine intramammary gland, the presence of Bap may facilitate a biofilm formation connected with the persistence of S. aureus.
The gram-positive bacterium Staphylococcus aureus is an adaptable, opportunistic pathogen whose ability to persist and multiply in a variety of environments causes a wide spectrum of diseases in both humans and animals. In humans, S. aureus is the causative agent of many infections, ranging from superficial skin suppurations to life-threatening septicemias associated with visceral or bone infections. Successful treatment is often hindered by the increasing prevalence of methicillin-resistant strains and by antibiotic inefficacy against the bacteria involved in chronic infections.
In lactating animal females, S. aureus is a common cause of intramammary infections (IMI), frequently leading to chronic mastitis. Because this type of infection is very difficult to eradicate with antibiotic therapy, a premature culling of animals, involving substantial production losses, is the only efficient strategy to control this type of mastitis. S. aureus IMI is usually initiated either by colonization of the teat canal with bacteria derived from infected epidermis or by an influx of contaminated milk entering the gland, a problem due to such deficient management practices as a poorly adjusted vacuum in milking machines. Bacteria adapt and multiply in the milk, gaining access to the upper part of the gland. It has been proposed that bacteria adhere to the ductular and alveolar epithelium in the gland and begin production of toxins. These adhered bacteria trigger macrophage activation and neutrophil migration from the blood into the milk (a situation resulting in an increase in the somatic cell count [SCC]), inflammation of the mammary gland, impairment of the host immune system, and epithelial cell damage. As a result, bacteria reach the basal subepithelial cell layers, bind fibrinogen and other host receptor proteins, and finally establish an infection which often becomes chronic (14).
Many chronic infections are associated with a bacterial growth in the form of adherent colonies surrounded by a large exopolysaccharide matrix, constituting a biofilm (for reviews, see references 5, 6, and 11). Because of their aggregate size, biofilms are not susceptible to macrophage phagocytosis and become resistant to some antibiotics (2, 29, 30).
The implication of biofilms in chronic infections has triggered an increasing interest in the characterization of genes involved in biofilm formation (15). The icaADBC cluster, an operon present in Staphylococcus epidermidis (18) and S. aureus (7), participates in biofilm formation by encoding proteins involved in the synthesis of a biofilm matrix polysaccharide (named PIA/PNAG) composed of linear β-1-6-linked N-acetylglucosamine residues (23, 25). Recently, we have identified a surface protein, Bap (for biofilm associated protein), also implicated in bovine S. aureus biofilm formation (8). The bap gene encodes a large protein of 2,276 amino acids (aa) and promotes both primary attachment to inert surfaces and intercellular adhesion; in contrast, PIA/PNAG seems to be mainly involved in intercellular adhesion. Bap is structurally related to the alpha C protein of group B streptococci and has a core C region (aa 948 to 2139) consisting of 13 identical 258-nucleotide tandem repeat units encoding reiterations of an 86-aa sequence (C repeats). The C repeat region accounts for 52% of the Bap protein, and each repeat unit shows a high sequence identity with other repeat units of this protein. The bap gene is contained in a mobile pathogenicity island (35), and so far it has only been found in bovine mastitis isolates.
The purpose of this study was to investigate whether biofilm production in S. aureus strains from bovine mastitis is a pathogenic factor contributing to that mastitis. We report that bap-positive S. aureus strains show an elevated capacity to infect and persist in the mammary gland. Further, we find that, throughout the course of an infection, Bap frequently undergoes changes in the number of repeats within the C repeat region.
MATERIALS AND METHODS
Bacterial isolates.
S. aureus isolates were collected from 195 subclinical mastitis-infected cows belonging to 13 dairy herds of the Spanish province of Valencia. One herd (herd E) was involved in a longitudinal survey of the population dynamics of IMI. In this herd, milk samples were collected at 3-month intervals from udders which were found to be naturally infected with S. aureus and which yielded milk with a high SCC. In this farm, milk was also collected on two occasions from all the animals, independent of their SCC score. Bacteria were cultured from the milk samples according to National Mastitis Council standards (16) and were identified as S. aureus by standard procedures, including Gram staining, catalase and coagulase tests (22), and API-Staph (bioMerieux) and were stored at −80°C until use. The criteria we established to consider an animal as infected was the isolation from that animal of the same clonal type of S. aureus in two consecutive milk samples. For comparison, 50 clinical human isolates from Valencia were analyzed.
For determination of antibiotic resistance, four representative bovine S. aureus isolates from herd E were chosen: V329 (highly adherent and with a rough colony morphology on Congo red agar), harboring the bap and icaADBC genes (8); m556, an isogenic transposon insertional Bap-mutant of V329, with a smooth colony morphology on Congo red agar and harboring icaADBC (8); V299 (a bap-negative icaADBC-positive strain); and V315 (a bap-negative and icaADBC-negative strain).
The criterion applied to detect subclinical mastitis was based on the SCC obtained with a Fossomatic 90 cell counter (A/S N Foss Electric, Hillerød, Denmark). The threshold between noninfected and infected was set at 200,000 cells/ml (10), a low threshold established to avoid false-negative results. Based on SCC, isolates were classified into four categories: category A, very low SCC (<200 × 103 cells/ml); category B, low-medium SCC (between 200 × 103 and 750 × 103 cells/ml); category C, high SCC (between 750 × 103 and 1,500 × 103 cells/ml); and category D, very high SCC (over 1,500 × 103 cells/ml). The California mastitis test (CMT) was also performed regularly as a control for mastitis diagnosis in the field.
Molecular typing of S. aureus strains.
PCR amplification of the coa gene was performed as previously described (20). Briefly, the oligonucleotides coa-1m (5′-ATAGAGATGCTGGTACAGG-3′) and coa-2c (5′-GCTTCCGATTGTTCGATGC-3′), encompassing the entire 3′ repeats, were utilized. Each amplification comprised 100 ng of DNA template, 100 pmol of each primer, 200 μM (each) deoxynucleoside triphosphates (dATP, dGTP, dCTP, and dTTP), 1× buffer (Netzyme), 1 mM MgCl2, and 1 U of thermostable DNA polymerase (Netzyme). Water was added to a final volume of 25 μl, and thermal cycling was performed as outlined in reference 20. An initial denaturation step at 94°C for 2 min was followed by 30 cycles of 94°C for 20 s, 57°C for 15 s, and 72°C for 30 s, with a final step at 72°C for 5 min. The size of the PCR products (5-μl aliquot) was analyzed by electrophoresis on 1% (wt/vol) agarose gels.
Restriction endonuclease analysis of the PCR-amplified coa gene was performed as previously described (20). Approximately 500 ng of PCR product was digested with 5 U of restriction endonuclease CfoI (Roche) at 37°C for 2 h and analyzed by electrophoresis on 3% (wt/vol) agarose gels.
Analysis of genes involved in biofilm formation.
S. aureus chromosomal DNA was extracted using standard procedures (32). For PCR analysis, two pairs of primers were designed for amplifications of fragments of the ica and bap genes of S. aureus. The ica primers (icaH-1m, 5′-TATACCTTTCTTCGATGTCG-3′; icaH-7c, 5′-CTTTCGTTATAACAGGCAAG-3′) were designed to amplify part of the icaR and icaA genes of the icaADBC locus. For amplification of the bap gene (AF288402), primers sasp-6m (5′-CCCTATATCGAAGGTGTAGAATTGCAC-3′) and sasp-7c (5′-GCTGTTGAAGTTAATACTGTACCTGC-3′) were utilized. Each PCR was performed in duplicate. The DNA amplifications were carried out using the same procedure as in the molecular typing of the S. aureus strains; the thermal cycling profile consisted of an initial denaturation at 94°C for 2 min, 40 cycles of 94°C for 20 s, 42°C for 20 s, and 72°C for 50 s, with a final step at 72°C for 5 min. The size of the PCR products was analyzed by electrophoresis on 0.8% (wt/vol) agarose gels.
For Southern blot hybridization, chromosomal DNA digested with EcoRI was analyzed by agarose gel electrophoresis. Gels were blotted onto nylon membranes (Hybond-N [0.45-mm-pore-size filters]; Amersham Life Science) using standard methods (32). The PCR product of the amplified ica region was used as a DNA probe. Labeling of the probe and DNA hybridization were performed according to the protocol supplied with the PCR-DIG DNA-labeling and chemiluminescent detection kit (Roche).
Homologous recombination.
Wild-type S. aureus V329 containing plasmid pSC23 (7) was grown overnight in B medium at 30°C with chloramphenicol (10 μg/ml), diluted 1:1,000, grown again at 30°C with antibiotic selection, diluted 1:1,000, and grown at 42°C without antibiotic selection twice, diluted 1:100, and plated on tryptone soy agar (Difco) plates containing tetracycline (2.5 μg/ml). Homologous recombination and plasmid curing of the chloramphenicol-sensitive, tetracycline-resistant colonies were then confirmed by PCR and Southern blotting.
Biofilm formation.
The quantification of biofilm formation on abiotic surfaces was assessed basically as described elsewhere (17). Briefly, S. aureus was grown overnight in tryptone soy broth (TSB) (Difco) supplemented with 0.25% glucose (TSB-glucose). The culture was diluted 1:40 in TSB-glucose, and 200 μl of this cell suspension was used per well to inoculate sterile, 96-well polystyrene microtiter plates (Iwaki). After 18 h of incubation at 37°C, the wells were gently washed three times with 200 μl of sterile phosphate-buffered saline (PBS), air dried in an inverted position, and stained with 0.1% safranin for 30 s. The wells were rinsed again, and the absorbance was determined at 490 nm (Micro-ELISA Autoreader Elx800; Bio-Tek Instruments). Each assay was performed in triplicate in five separate experiments.
Colony morphology was studied on Congo red agar as previously described (4). A positive result (biofilm formation) was indicated by the presence of black or pink colonies with a dry crystalline surface (rough colony phenotype). A deficiency in biofilm formation was indicated by the presence of smooth colonies.
Classical antibiotic susceptibility assays.
The resistance profile (MIC and minimal bactericidal concentration [MBC]) was determined for each isolate by a broth microdilution method, following the recommendations of the National Committee for Clinical Laboratory Standards (31), and the concentration theoretically reached in serum (peak in serum [PS]) was obtained taking as reference the values described by Mensa (27). The three concentrations (MIC, MBC, and PS) were used in the antibiotic susceptibility assay for bacteria in biofilms.
Antibiotic susceptibility assay for bacteria in biofilms.
Following a previously outlined procedure to produce the biofilms (30), 25 μl of stationary-phase bacterial cell cultures grown in TSB (Difco, Detroit) were added to flat-bottom microtiter wells (Iwaki) containing 175 μl of TSB enriched with 2% glucose (2). An incubation period of 6 or 24 h at 37°C followed, depending upon the desired biofilm age; for the 24-h biofilm, the growth medium was renewed at 12 h. After washing the biofilms with distilled water to remove free bacteria, 100 μl of antibiotic and 100 μl of Mueller-Hinton broth were added to finally reach the concentrations specified above (MIC, MBC, and PS). Six antibiotics were tested: cefazolin, cefuroxime, rifampin, tobramycin, vancomycin (Sigma), and ciprofloxacin (Bayer). These antibiotics had been resuspended, diluted in water, filter sterilized (pore size, 0.22 μm), and stored at 4°C before testing. After a 24-h exposure of the biofilm to the antibiotic at 37°C, the supernatant was discarded and viable bacteria were quantified by ATP-bioluminescence (2). Tests were done in triplicate and on three different occasions. Samples not treated with antibiotics were included as controls.
Adhesion to epithelial cells.
Cellular studies were performed by well-known methods (12). An established epithelial cell line, designated 293 (ATCC CRL-1573), was used for all experiments. The 293 cells were grown in Dulbecco's modified Eagle medium (Invitrogen Life Technologies) containing 10% fetal bovine serum (Invitrogen Life Technologies), 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate/ml (Invitrogen Life Technologies). Prior to use, the cells were seeded at 6 × 104 cells/well and grown for 3 days at 37°C with 5% CO2. For the adhesion assays, the cells were grown in 24-well tissue culture plates (Iwaki).
Approximately 16 h prior to the adhesion experiments the 293 cell growth medium was replaced with 0.5 ml of adhesion medium (growth medium without antibiotics or fetal bovine serum). The morning of the experiment, the medium was removed, and the 293 cells were washed once with adhesion medium and given 0.5 ml of fresh adhesion medium. Appropriate wells of 293 cells were then inoculated with 106 CFU of washed S. aureus and incubated at 37°C with 5% CO2. After 2 h, supernatants of the cocultures were removed; the 293 cell monolayers were washed three times with sterile PBS, and further lysed with 0.025% Triton X-100 (Sigma) in sterile distilled water. The cell lysates were serially diluted 10-fold and plated in triplicate on tryptone soy agar plates; the plates were incubated overnight at 37°C, and the CFU were counted. The results of representative experiments are described.
Repeat number variation in the C region of the bap gene.
To assess the repeat number variation in the C region of the bap gene, primers sasp-6m and sasp-9c (5′-CTCTCCACCTTTGTAAGTGGAGAGCC-3′) were used to amplify across the C repeat region of the bap gene. Briefly, 25 μl of the PCR mixture consisted of 250 ng of DNA; a 0.2 μM concentration of each of the forward and reverse primers; a 200 μM concentration each of dATP, dCTP, dGTP, and dTTP; 2.5 mM MgCl2; and 2.5 U of DyNAzyme EXT (Finnzymes) in a 1× reaction buffer. After an initial denaturation step at 95°C for 4 min, the mixture was subjected to 40 cycles of denaturation (at 94°C for 30 s), annealing (at 54°C for 30 s), and extension (at 72°C for 5 min). One-tenth of the amplified reaction mixture was mixed with a gel-loading buffer and electrophoresed on a 0.8% (wt/vol) agarose gel; the reaction products were visualized by ethidium bromide staining.
In order to confirm that the different size observed was a consequence of a different C repeat number, a restriction endonuclease analysis of the PCR-amplified product was performed. Approximately 500 ng of the PCR product was digested with 5 U of the restriction endonuclease PstI (Roche) at 37°C for 2 h. The digested PCR product was analyzed by electrophoresis on 2% (wt/vol) agarose gels.
Recombinant proteins.
Polyhistidine-tagged fusion proteins were produced for use in the immunoblot analyses. S. aureus bap gene and S. epidermidis bhp gene (AY028618) were PCR amplified (the oligonucleotides used being Sasp-2mN [5′-GGGGGGCATATGGGAAATAAACAAGGTTTTTTACC-3′] and Sasp-3cB [5′-GGGGGGATCCCAACCTCGTCAATGGTTAAGTCAGC] for bap and RP62A-5mN [5′-GGAATTCCATATGAAAAATAAACAAGGATTTCTTCC-3′] and RP62A-4c [5′-CGATCTTGACTAATAGGGTTTCC] for bhp, with the NdeI-BamHI sites [underlined]) and cloned downstream from the His tag sequence in the pET-15b vector (Novagen). Purified plasmid DNA from Escherichia coli DH5α cells was used to transform the expression host BL21(DE3), and the fusion protein was produced as specified by the manufacturer (Novagen). After disrupting the cells by sonication, the recombinant protein was purified by immobilized metal affinity chromatography using a cobalt-based resin (Clontech).
Western blot analysis.
The Bap immunoblotting assay was performed as described previously (8). Briefly, protein extracts were prepared and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% separation gel, 4.5% stacking gel), and blotted onto Immobilon P membrane (Millipore). The anti-Bap serum was diluted 1:2,500 with Tris-buffered saline (TBS) (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) and immuno-absorbed with 5% skim milk. As secondary antibodies, alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Sigma) was used for the anti-Bap serum and alkaline phosphatase-conjugated rabbit anti-bovine immunoglobulin G (Sigma) for the bovine sera. Both secondary antibodies were diluted 1:15,000 in TBS-5% skim milk, and the subsequent chemiluminescence reaction (CSPD; Roche) was recorded.
Experimental infection.
The experimental infection was carried out essentially by established methods (9). Twenty to 25 days following parturition, each gland of five healthy lactating ewes was inoculated with 1 ml of a mixed bacterial suspension containing 2 × 103 CFU of the Bap-positive V329 strain and the same amount of the Bap-mutant m556 strain. The lactating mothers were separated from their suckling lambs 2 h before the bacterial inoculation to ensure the presence of milk (as a natural lubricant) in the teat duct at the time of inoculation. After the teats were disinfected with 70% ethanol, the bacterial inoculum was introduced into one gland through a 21-gauge cannula. After inoculation, since suckling favored a removal of the bacteria, the lambs were separated from their mothers for 180 min to increase the bacterial colonization of the mammary gland. Milk samples for the bacteriological analyses were obtained 1, 4, 7, and 14 days after inoculation. Aliquots of the milk samples were placed directly on blood agar and Congo red agar plates. In addition, to exclude the possibility of contamination, bacteria recovered at the end of the experimental period were compared with the parental (inoculated) strains by coagulase DNA typing.
Statistical analysis.
A two-tailed Student's t test was used to determine the differences in biofilm formation between the groups. A nonparametric test (the Mann-Whitney U test) was used to assess significant differences within the groups in bacterial recovery in bovine infections. The log-normalized SCCs for the clinical groups were compared by means of a one-way analysis of variance (1). Comparisons between the antibiotic treatment and the control groups were made with a two-factor analysis of variance (with the help of the Statview program [version 4.0] for Macintosh), using Scheffe's F to present the results. For the analysis of the cure ratio in the experimental infection, a two-by-two contingency table was produced and Fisher's exact test was applied. Differences were considered statistically significant when P was <0.05.
RESULTS
Distribution of the genes involved in biofilm formation among field S. aureus isolates.
A PCR analysis using the icaADBC- and bap-specific primers of 195 bovine mastitis isolates from 13 herds showed that 184 (94.36%) were icaADBC positive, and 50 of these (25.6%) were also bap positive. All the bap-positive isolates were also ica positive. The absence of the icaADBC operon was confirmed by additional Southern blot analysis with an ica-specific probe (data not shown).
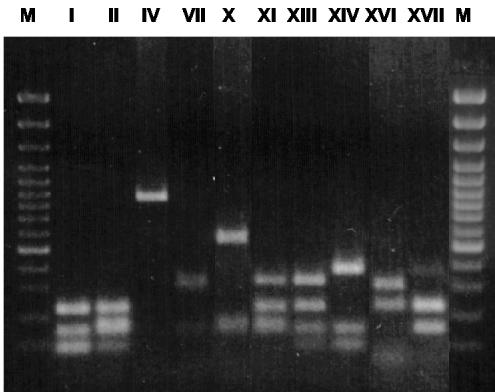
In order to determine whether the different bap or ica positive isolates could be clonally related, we performed a PCR-restriction fragment length polymorphism (RFLP) analysis of the coa gene (20). Ten distinct coa electrophoretic patterns were observed among the 195 isolates studied (Fig. 1). Based on the presence or absence of the two loci under study (bap and ica) and the coa pattern, three groups of isolates were established: group 1 was bap and ica positive, with all the isolates of this group showing an identical coa pattern (pattern I); group 3 was bap and ica negative, with all the isolates of this group also exhibiting an identical coa pattern (pattern IV); finally, group 2 was bap negative and ica positive and displayed different (but never types I and IV) coa patterns.
FIG. 1.
RFLP electrophoretic patterns of PCR-amplified coagulase gene digested with CfoI. The different patterns observed in the analysis of the bovine S. aureus strains are shown in individual lanes. M, molecular weight marker (gene ruler 100-bp DNA ladder plus; Fermentas).
To determine whether the coa electrophoretic patterns observed in the bovine isolates were also common in staphylococci from a different host species, a study of the molecular typing of 50 human S. aureus isolates was carried out; the resultant patterns differed from the bovine isolates (data not shown). This finding suggests a divergence between S. aureus isolates of human and bovine origin.
Biofilm formation capacity of S. aureus isolates.
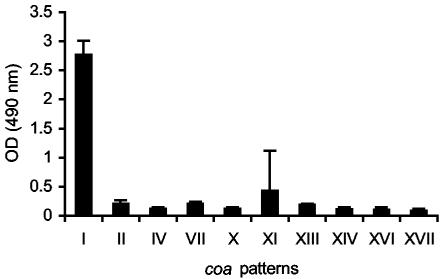
Taking into account the involvement of the ica and bap genes in biofilm formation, we compared the three groups of S. aureus mastitis isolates, with regard to biofilm formation capacities. Only group 1 isolates (bap positive, ica positive, coa pattern I) were strong biofilm producers (P < 0.001 [Fig. 2]). Interestingly, group 2 isolates, all icaADBC positives, were less able to form biofilms in our experimental conditions, an inhibition suggesting that genes other than the icaADBC play a major role in the in vitro biofilm development process of bovine S. aureus isolates. This intriguing result encouraged us to determine whether the Bap protein was sufficient to induce in vitro biofilm formation in the absence of the ica operon product. We generated by allelic exchange a deletion of the ica operon in an isolate of group 1 (V329). The resulting mutant strain, named JP38, showed a biofilm formation capacity and Congo red agar morphology identical to the wild-type isolate, demonstrating that in the absence of PIA/PNAG, the product of the ica operon, the expression of the Bap protein is sufficient to induce biofilm production on abiotic surfaces (data not shown).
FIG. 2.
Biofilm formation study using S. aureus strains. The types of S. aureus shown in Fig. 1 were grown overnight in polystyrene microtiter wells in TSB supplemented with 0.25% glucose. The cells that adhered to the plate after washing were then visualized by staining with safranin. Data represent the mean values ± standard deviations of triplicate determinations of the increase of bacterial adherence. Significant differences in adherence (P < 0.01) were noted between the means of strains belonging to coa pattern I and strains belonging to the other described coa patterns.
bap and ica association with a lower SCC and a higher S. aureus prevalence.
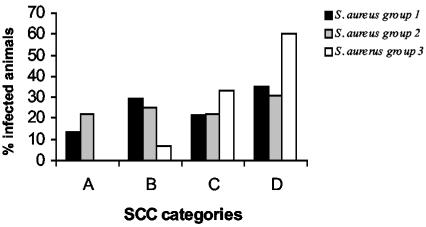
Two studies were carried out to analyze this association. In the first study, we determined the SCC (geometric and arithmetic means) in milk from animals infected with group 1, group 2, or group 3 isolates, as well as in uninfected control animals. For this purpose, we analyzed 212 glands from animals from one herd (herd E). Considering the SCC categories established (categories A to D), the mean SCC of animals infected with isolates of groups 1 and 2 was high (category C), but was very high for animals infected with isolates of group 3 (category D; P < 0.05 [Table 1]). Accordingly, the SCC values of 13.5% of the animals infected with isolates of group 1 and 22% of the animals infected with isolates of group 2 were so low that no evidence of infection was obtained based on SCC diagnosis (Fig. 3). Similar results were observed with the CMT (data not shown), indicating that there were cows naturally infected with S. aureus in groups 1 and 2 which could not be identified as infected animals by indirect classical methods (SCC and CMT). This problem was demonstrated by an isolation of the same clonal type of S. aureus in two consecutive milkings from the same animal. Evidently, these indirect classical methods have a low sensitivity for the detection of infections caused by isolates of groups 1 and 2. These results also demonstrate that the biofilm-producing isolates (bap and ica positives) may be unable to cause a significant inflammation (a neutrophil influx into the milk) and point out the need for performing a bacteriological analysis rather than a SCC to detect infection by bap- and ica-positive isolates in milk with a low SCC.
TABLE 1.
Differences between the three S. aureus groups in SCC in milk
| Animal group | No. of glands analyzed | No. of samples analyzed | SCC (103 cells/ml)
|
||
|---|---|---|---|---|---|
| Log SCC (mean ± SE) | GMa | AMb (mean ± SD) | |||
| S. aureus | |||||
| Group 1 | 36 | 90 | 5.88 ± 0.50 | 758 | 1,330 ± 1,666 |
| Group 2 | 32 | 77 | 5.82 ± 0.60 | 660 | 1,077 ± 1,244 |
| Group 3 | 16 | 67 | 6.2 ± 0.80 | 1,585 | 3,469 ± 5,766 |
| Mastitis free | 128 | 128 | 4.74 ± 0.04 | 53 | 95 ± 107 |
GM, geometric mean.
AM, arithmetic mean.
FIG. 3.
Percentage of animals infected with S. aureus isolates of groups 1 (bap+ ica+), 2 (bap negative, ica+), and 3 (bap negative, ica negative) among the four SCC categories: very low SCC (A), low-medium SCC (B), high SCC (C), and very high SCC (D).
We then investigated in a second study the possible consequence of omitting the bacteriological analysis and carrying out only SCC testing on a farm where isolates able to form biofilm are present. To accomplish this, we classified all the animals of herd E (450 cows) according to the SCC categories on two occasions, three months apart; later, we performed a bacteriological analysis, as well as bap and ica typing in the same milk samples. On the first occasion, the incidence of mastitis (milk with >200 SCC/ml) produced by S. aureus in the herd was 31, 31, and 38% for groups 1, 2, and 3, respectively. However, 44 of the noninfected cows according to the SCC criteria (milk with <200 SCC/ml) were found to be infected by bacteriological analyses (22 by bap- and ica-positive isolates and the remaining 22 with ica-positive isolates). On the second occasion, the rate of occurrence of mastitis (milk with >200 SCC/ml) caused by S. aureus in the herd was 36, 50, and 14% for groups 1, 2, and 3, respectively (the prevalence having changed at least in part because some infected animals were sacrificed). However, 24 of the noninfected cows according to the SCC criteria (milk with <200 SCC/ml) were found to be infected by the bacteriological tests (18 by bap- and ica-positive isolates and the remaining 6 with ica-positive isolates). These results further indicate that cows with a low SCC represent a reservoir of bacteria with the capacity to form biofilms and allow the persistence in the farm of isolates of a low early pathogenicity, a situation obstructing the eradication of S. aureus in SCC-based mastitis control programs. Thus, biofilm formation constitutes a pathogenesis mechanism that helps the microorganism to survive in the mammary gland and to persist on the farm.
To discover whether the different strains' capacities to infect the mammary gland was a consequence of a varying abilities to adhere to epithelial cells present in that gland, their adhesion to 293 cells was studied in vitro. No differences were observed between the three different strains of S. aureus analyzed from herd E (strains V329, V299, and V315, corresponding to groups 1, 2, and 3; data not shown).
Decrease in antibiotic killing effect associated with the presence of bap and icaADBC genes.
It is well-established that biofilm-grown cells have an increased resistance to antimicrobial agents. In order to determine whether biofilm formation could be one of the reasons for therapy failure in bovine mastitis, in vitro antimicrobial susceptibility tests were performed using one representative isolate of each group: V329 (group 1), V299 (group 2), and V315 (group 3). As the biofilm aged from 6 to 24 h, a general decrease in the killing effect was observed in the three isolates. The killing effect of antibiotics was lowest in the isolate with the highest capacity to form biofilms (group 1, bap positive), progressively increased in the isolate harboring ica but not bap (group 2) and was highest in the isolate lacking both genes (group 3) (Table 2). In confirmation of the biofilm-associated antibiotic resistance, this difference in susceptibility disappeared when the MIC and MBC were studied in classical broth microdilution tests (data not shown).
TABLE 2.
Effect of 24-h antibiotic treatments on TSB-grown biofilmsa
| Antibiotic | Isolate | Biofilm bacteria survival (percentage CFU recovered relative to untreated control) at different antibiotic concentrations
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | Bacterium survivalb in biofilm at:
|
MBC (μg/ml) | Bacterium survivalb in biofilm at:
|
PS (μg/ml) | Bacterium survivalb in biofilm at:
|
|||||
| 6 h | 24 h | 6 h | 24 h | 6 h | 24 h | |||||
| Cefazolin | V329 | 0.12 | 28.8* | 83.7 | 2 | 30.9* | 56.2 | 121 | 30.9* | 72.4 |
| m556 | 0.12 | 28.8* | 91.2 | 2 | 27.5* | 66.0 | 121 | 16.9* | 91.2 | |
| V299 | 1 | 28.8* | 60.2 | 2 | 31.6* | 100 | 121 | 2.6* | 100 | |
| V315 | 1 | 13.1* | 58.8 | 2 | 2.8* | 69.1 | 121 | 1.2* | 9.1* | |
| Cefuroxime | V329 | 1 | 39.8* | 54.9 | 4 | 37.1* | 54.9 | 98 | 28.8* | 74.1 |
| m556 | 1 | 3.71* | 16.9* | 4 | 3.7* | 20.8* | 98 | 4.4* | 19.0* | |
| V299 | 2 | 18.6* | 45.7* | 4 | 12.5* | 47.8* | 98 | 13.4* | 53.7* | |
| V315 | 4 | 13.8* | 15.1* | 32 | 15.1* | 17.7* | 98 | 3.1* | 19.0* | |
| Ciprofloxacin | V329 | 0.12 | 75.8 | 72.4 | 1 | 100 | 58.8 | 2 | 45.7* | 69.1 |
| m556 | 0.12 | 69.1 | 95.4 | 1 | 100 | 91.2 | 2 | 37.1* | 95.4 | |
| V299 | 0.5 | 10.2* | 61.6 | 1 | 7.2* | 85.1 | 2 | 3.3* | 100 | |
| V315 | 0.5 | 8.12* | 53.7* | 1 | 7.5* | 61.6 | 2 | 1.3* | 52.4* | |
| Rifampin | V329 | 0.12 | 77.6 | 87.0 | 0.5 | 100 | 77.6 | 10 | 2.4* | 7.5* |
| m556 | 0.12 | 52.4* | 100 | 0.5 | 25.1* | 75.8 | 10 | 1.1* | 7.07* | |
| V299 | 0.5 | 20.4* | 77.6 | 0.5 | 20.8* | 77.6 | 10 | 2.5* | 12.8* | |
| V315 | 0.5 | 5.37* | 28.1* | 0.5 | 5.3* | 28.1* | 10 | 1.8* | 12.0* | |
| Tobramycin | V329 | 0.25 | 54.9 | 56.2 | 0.5 | 54.9 | 54.9 | 8 | 43.6* | 74.1 |
| m556 | 0.25 | 54.9 | 29.5* | 0.5 | 53.7* | 38.0* | 8 | 35.4* | 38.0* | |
| V299 | 4 | 52.4* | 45.7* | 4 | 52.4* | 45.7* | 8 | 36.3* | 52.4* | |
| V315 | 1 | 25.1* | 27.5* | 2 | 15.8* | 28.8* | 8 | 7.9* | 35.4* | |
| Vancomycin | V329 | 2 | 85.1 | 64.5 | 16 | 100 | 87.0 | 40 | 41.6* | 70.7 |
| m556 | 2 | 69.1 | 100 | 16 | 32.3* | 34.6* | 40 | 11.2* | 26.3* | |
| V299 | 2 | 95.4 | 72.4 | 2 | 95.4 | 72.4 | 40 | 16.9* | 95.4 | |
| V315 | 1 | 77.6 | 81.2 | 2 | 77.6 | 83.1 | 40 | 7.5* | 56.2 | |
S. aureus isolates V329 (group 1), m556 (Bap mutant), V299 (group 2), and V315 (group 3) were used. Antibiotic concentrations used were the MIC and MBC, both obtained by classical broth microdilution, and the concentration theoretically reached in serum (PS).
Measured as percentage of bacterial CFU surviving in the treated samples relative to untreated control samples. Higher percentage of survival means lower antibiotic killing effect and lower probability of significant differences in bacterial recovery with respect to untreated controls. Higher survival is generally observed in older (24-h) biofilms. *, significant viability decrease (P < 0.05) in the treated versus the untreated control samples in an analysis of variance.
A rise in biofilm formation and antibiotic resistance was also observed in isolate V329 (group 1) when compared with its isogenic bap mutant (m556) (Table 2), a change further substantiating the role of bap on the decrease in the antibiotic killing effect. These results strongly suggest that bap-mediated biofilm formation and not the presence of bap itself is responsible for antibiotic resistance.
Bovine sera contain anti-Bap antibodies.
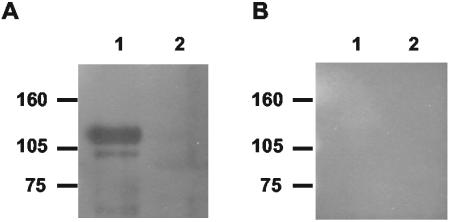
To determine if the Bap protein is expressed in vivo, the specific antibody production against Bap in naturally infected cows was analyzed. A recombinant polypeptide corresponding to the amino-terminal region of Bap was produced as a soluble hexahistidine-tagged protein in E. coli BL21(DE3) and purified by affinity chromatography (8). Additionally, a recombinant polypeptide corresponding to the amino-terminal region of S. epidermidis Bhp was produced as a negative control. The purity (>95%) of the isolated recombinant proteins was verified by SDS-PAGE (data not shown). Serum samples from animals naturally infected with S. aureus strains were used to probe a Western blot of the recombinant polypeptides of Bap and Bhp. Two out of seven sera analyzed from animals infected with bap-positive S. aureus strains showed antibodies to the Bap polypeptide (Fig. 4), whereas the three sera analyzed from animals infected with bap-negative S. aureus strains were unreactive. This result suggests that the Bap protein is expressed during S. aureus infections.
FIG. 4.
Western immunoblot of S. aureus Bap (line 1) and S. epidermidis Bhp (line 2) polypeptides with serum samples collected from two animals with S. aureus mastitis. (A) Serum from a representative animal among those infected with a bap-positive S. aureus strain. A similar result was obtained with the other positive sample. (B) Serum representative of those obtained from animals infected with a bap-negative S. aureus strain.
Effect of Bap on persistence of S. aureus in an intramammary gland infection model.
Both mammary glands of each of five animals were experimentally infected to determine the effect of the presence of Bap on bacterial persistence in ewes' mastitis. Equal numbers of the Bap-positive strain V329 and the Bap-negative strain m556 were inoculated together in each animal. As summarized in Table 3, on day 1 postinoculation, the animals were infected predominantly with m556 (P < 0.05). Subsequently (days 4, 7, and 14 postinoculation), seven out of eight mammary glands were infected predominantly with the V329 strain (Bap-positive; P < 0.05). Thus, the presence of Bap allows the bacteria to persist longer in the mammary glands of the infected animals.
TABLE 3.
Effect of Bap on persistence of S. aureus in an intramammary gland infection model
| Animal | % Persistencea at postinoculation day:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1
|
4
|
7
|
14
|
|||||
| L | R | L | R | L | R | L | R | |
| I | 50 | 50 | 100 | 90 | 100 | 100 | 100 | 99 |
| II | NI | 1 | NI | 50 | NI | 80 | NI | 98 |
| III | 1 | 0 | 95 | 0 | 95 | 0 | 100 | 0 |
| IV | 30 | 30 | 80 | 95 | 100 | 100 | 100 | 100 |
| V | NI | 1 | NI | 100 | NI | 100 | NI | 100 |
Percentage of the V329 (Bap-positive) strain in the mixed population. Milk samples from the left (L) or right (R) mammary glands were plated on Congo red agar, and the percentage of rough colonies was calculated. NI, not infected.
Tandem repeat variation in the C region of bap.
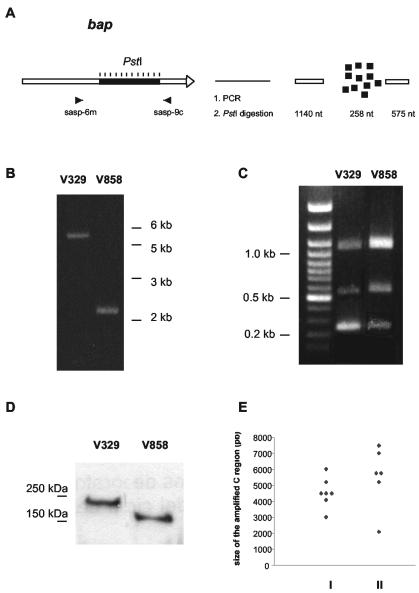
The presence of tandem, highly conserved sequences within the C region of the bap gene suggested that different staphylococcal isolates may exhibit variations in the number of repeats as a result of homologous recombination. To determine if that variation occurred in vivo, we amplified the C region of the bap gene from different isolates using the primers sasp-6m and sasp-9c (Fig. 5A). Subsequently, each of the PCR-amplified products was restricted with PstI (which cuts once within each C repeat unit) and the restriction products were analyzed on 2% agarose gel electrophoresis. The PCR amplifications across the C repeat region revealed substantial variation in the number of C repeats among the bap-positive isolates. We first compared two of these Bap-positive isolates (V329 and V858), and observed a variation in the size of the C region (Fig. 5B). Evidently, this difference in size corresponded to multiples of the 258 bp (C repeats), because only three restriction fragments of the expected sizes (1, 140, 258, and 575 bp) were observed, and the corresponding band intensity in ethidium bromide-stained gels demonstrated an overrepresentation of the repeating 258-bp fragment (Fig. 5C). Thus, the deduced number of C repeats was 13 for the V329 strain and 1 for the V858 strain. A SDS-PAGE and Western blot analysis of these strains (strain V329 with 13 C repeats, and strain V858 with 1 C repeat) confirmed the variation in the number of the C repeats by indicating the presence of Bap proteins with different mobilities (Fig. 5D). Further studies on additional bap-positive strains from the same and other farms confirmed the variation in the size of the C region, with the number of deduced C repeat units varying from 1 to 16 (Fig. 5E).
FIG. 5.
(A) Schematic representation of the method used to determine the variation in the size of the C region among bap-positive strains. PCR amplification (B) and PstI digestion of the amplified product (C) of the C region of the V329 (13 C repeats) and V858 (1 C repeat) strains. (D) Results of the SDS-PAGE and Western blot analysis of the V329 and V858 strains analyzed in panels B and C showing the different size of the Bap protein. (E) Representation of the variation in the size of the C region among bap-positive strains from different farms (I) or among bap-positive strains from one individual farm (II).
In order to determine whether the number of C repeats present in Bap was related to the functionality of the protein to produce biofilm, a comparative study using a standard in vitro biofilm assay was performed on microtiter plates. No association was found between the number of C repeat units within the bap gene and biofilm formation in the Bap-positive isolates under the in vitro conditions applied (data not shown).
Stability in the number of C repeats in vivo.
A longitudinal study was carried out in order to determine whether the number of C repeats in the bacterial bap gene changed during lactation. Milk from three animals naturally infected with isolates of group 1 (bap positive, ica positive, coa pattern I) was collected over a 6-month period. Analysis of the C repeat region revealed that the number of C repeats in the bap gene changed throughout the study period in isolates obtained from two of these animals (data not shown). In their bap, ica, and coa patterns, the isolates of the three animals presented the phenotype of the bacteria originally recovered from the animal. Although no evidence for sample contamination or reinfections of these three animals was obtained, the possibility cannot be excluded. In contrast, no changes in the number of repeats were observed when V329 was subcultured in a laboratory medium over a 2-year period (data not shown), a finding strongly suggesting that interaction with the host may be a driving force for the natural selection of bacteria carrying variant Bap proteins that differ in the number of C repeats.
DISCUSSION
S. aureus bovine mastitis remains a substantial problem for milk producers worldwide, despite several decades of research aimed at controlling the disease. It is widely accepted that mammary gland infection by highly toxigenic strains promotes the migration of blood neutrophils to the alveolar lumen, consequently increasing the milk SCC and the severity of mastitis. In previous work, Baselga et al. observed that the severity of ruminant mastitis decreased but the bacterial capacity to colonize the mammary gland increased when the infection was caused by a mucoid (slime producer) rather than a nonmucoid S. aureus isolate (4). We later found that the mucoid isolate used in that study carried bap as well as icaADBC genes (our unpublished results), both involved in biofilm formation.
In agreement with our previous results, this study showed that the presence of the two main genetic loci involved in the biofilm formation process, bap and icaADBC, favored the appearance of mild infection (13.5 to 22% of the udders) and led to a very low SCC. This situation had important consequences in milk production at the farm level, as mastitis control inadvertently allowed the misclassification of cows as uninfected when they were in fact infected with ica- and bap-positive bacteria. These microorganisms remained undetected in the udder and the infected cows were not submitted to antibiotic treatment, so that a further spread and persistence of mastitis in the farm with consequent production losses could take place. The low SCC associated with a high mammary gland colonization by some bap-positive ica-positive isolates also suggests a reduced toxin production during these infections. This reduction could be explained by the finding that bap is carried by SaPIbov2, an S. aureus pathogenicity island in which the toxin region has been replaced by a putative composite transposon, including the biofilm-associated protein (bap) gene, an ABC transporter operon and a transposase (35). The finding that the bap and toxin genes are located in the same chromosomal region suggests that both genes are involved in two opposite strategies of host-bacterium interactions. On one hand, bacteria may form biofilms with little or no obvious harmful effect on the host which are therefore frequently undetected. Alternatively, non-biofilm-forming bacteria may produce toxins, become more pathogenic in the short term, and cause damage to the host tissues. This may result in an exposure of host adhesins which enable the bacteria to adhere by interacting with host receptor proteins and to spread to other body sites.
The early detection of infection should be coupled with appropriate antimicrobial therapy (28). In this work, we report experimental evidence that the antibiotic killing effect of some antibiotics on aged biofilms greatly decreases if the biofilms are of bap-positive strains (strong biofilm producers). This decrease might be enhanced in cows suffering from mastitis, as it is known that, compared with TSB, milk enhances biofilm formation (2). Antibiotics to control chronic bovine mastitis are usually chosen on the basis of conventional in vitro diffusion and dilution evaluation methods and without taking into account the role of biofilm formation on resistance. As high concentrations of many known antibiotics are required to kill biofilm bacteria (3, 30) and lead only to a partial killing effect, there is a need to find antimicrobials that are efficient against biofilm bacteria.
The biofilm structure may depend on the nature of the molecules involved in biofilm formation. Bap comprises a novel family of proteins, named BAP (for biofilm associated proteins), which are important for biofilm formation in both gram-positive and gram-negative bacteria. Members of this family have been described in S. aureus (Bap) (8), S. epidermidis (Bap and Bhp) (our unpublished results), Enterococcus faecalis (Esp) (33, 34), Burkholderia cepacia (Bap) (21), Pseudomonas putida (mus20) (13), and Salmonella enterica serovar Typhimurium (Stm2689) (26). All members of the BAP family share the following characteristics: (i) a high molecular weight; (ii) a signal sequence for extracellular secretion; and (iii) a core domain of repeats, whose number varies among different isolates. The variation in the length of the Bap C region detected in this work suggests that the C repeat region may undergo changes throughout the course of an infection. Interestingly, a decreased number of repeats affected neither the functionality of the protein nor the biofilm formation capacity. We hypothesize that the C region of the Bap might have a structural function, helping to maintain an elongated protein conformation at the cell surface. The phase-switching differences in the C repeat numbers could also be related to an evasion from the immune response, as observed in the structurally related alpha C protein (24), which undergoes antigenic variation. The analysis of the amino-terminal region of Bap and Esp reveals the presence of calcium binding motifs and dimerization domains, which could promote the interaction of these proteins with biofilm exopolysaccharides and the intercellular adhesion (two Bap molecules of different bacteria could undergo dimerization), or could interfere with the staphylococcal adhesion to surfaces and host molecules (9).
It is likely that the presence of Bap reduced infectivity in the short term by blocking early adherence dependent on host-MSCRAMM interaction (9) but had the opposite effect in late adherence, allowing longer bacterial persistence in the mammary gland. The prevalence of the Bap protein itself among the S. aureus mastitis isolates analyzed changed depending on the herd and geographic area. Remarkably, Bap can be found (usually in a small proportion) among bovine mastitis isolates of different species, including S. aureus, S. epidermidis, Staphylococcus chromogenes, Staphylococcus hycus, and Staphylococcus xylosus, but it is absent from the human Staphylococcus spp. tested so far (our unpublished results). This difference between the human and bovine isolates, together with differences between both isolate sources in PCR-RFLP patterns of the coa gene, suggest that bovine and human S. aureus isolates are not clonally related and that specific host-dependent pathogenic factors may have evolved independently in both species. This diversity between isolates from different hosts corroborates the results of Herron et al. (19), who in a preliminary analysis of the genome of a common clone of bovine S. aureus showed the presence of numerous genes and sequences that differentiate this bovine isolate from previously characterized human S. aureus strains. These differences indicate that a rational and effective strategy to control IMI caused by bovine-specific isolates may be advantageous.
Acknowledgments
We express our gratitude to F. Götz for providing us plasmid pSC23. We also thank Jesse Wright for critically reading the manuscript.
This work was supported by grants BIO99-0285 and BIO2002-04542-C02-01 from the Comisión Interministerial de Ciencia y Tecnología (C.I.C.Y.T.) and grants from the Cardenal Herrera-CEU University and Conselleria de Agricultura, Pesca i Alimentació (Generalitat Valenciana) to J.R.P. Fellowship support for Carme Cucarella, María Ángeles Tormo, and Pilar Trotonda from the Cardenal Herrera-CEU University and for Carles Úbeda from the Conselleria de Agricultura, Pesca i Alimentació (Generalitat Valenciana) is gratefully acknowledged.
Editor: V. J. DiRita
REFERENCES
- 1.Ali, A. K., and G. E. Shook. 1980. An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 63:487-490. [Google Scholar]
- 2.Amorena, B., E. Gracia, M. Monzón, J. Leiva, C. Oteiza, M. Pérez, J. L. Alabart, and J. Hernández-Yago. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J. Antimicrob. Chemother. 44:43-55. [DOI] [PubMed] [Google Scholar]
- 3.Anwar, H., J. L. Strap, and J. W. Costerton. 1992. Establishment of aging biofilms: possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 36:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baselga, R., I. Albizu, M. de la Cruz, E. del Cacho, M. Barberan, and B. Amorena. 1993. Phase variation of slime production in Staphylococcus aureus: implication in colonization and virulence. Infect. Immun. 61:4857-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucarella, C., M. A. Tormo, E. Knecht, B. Amorena, I. Lasa, T. J. Foster, and J. R. Penades. 2002. Expression of the biofilm-associated protein interferes with host protein receptors of Staphylococcus aureus and alters the infective process. Infect. Immun. 70:3180-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohoo, I. R., and K. E. Leslie. 1993. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med. 10:225-237. [Google Scholar]
- 11.Donlan, R. M. 2000. Role of biofilms in antimicrobial resistance. ASAIO J. 46:S47-S52. [DOI] [PubMed] [Google Scholar]
- 12.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa-Urgel, M., A. Salido, and J. L. Ramos. 2000. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J. Bacteriol. 182:2363-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 15.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 16.Harmon, R. J., R. J. Eberhart, D. E. Jasper, B. E. Langlois, and R. A. Wilson. 1990. Microbiological procedures for the diagnosis of bovine udder infection. National Mastitis Council, Arlington, Va.
- 17.Heilmann, C., C. Gerke, F. Perdreau-Remington, and F. Götz. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect. Immun. 64:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 19.Herron, L. L., R. Chakravarty, C. Dwan, J. R. Fitzgerald, J. M. Musser, E. Retzel, and V. Kapur. 2002. Genome sequence survey identifies unique sequences and key virulence genes with unusual rates of amino acid substitution in bovine Staphylococcus aureus. Infect. Immun. 70:3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hookey, J. V., J. F. Richardson, and B. D. Cookson. 1998. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. J. Clin. Microbiol. 36:1083-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber, B., K. Riedel, M. Kothe, M. Givskov, S. Molin, and L. Eberl. 2002. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol. Microbiol. 46:411-426. [DOI] [PubMed] [Google Scholar]
- 22.Kloos, W. E., and K. H. Schleifer. 1975. Simplified scheme for routine identification of human Staphylococcus species. J. Clin. Microbiol. 1:82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madoff, L. C., J. L. Michel, E. W. Gong, D. E. Kling, and D. L. Kasper. 1996. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc. Natl. Acad. Sci. USA 93:4131-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maira-Litran, T., A. Kropec, C. Abeygunawardana, J. Joyce, G. Mark III, D. A. Goldmann, and G. B. Pier. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 27.Mensa, J. 1998. Guía terapéutica antimicrobiana, 8th ed. Masson, Barcelona, Spain.
- 28.Milner, P., K. L. Page, and J. E. Hillerton. 1997. The effects of early antibiotic treatment following diagnosis of mastitis detected by a change in the electrical conductivity of milk. J. Dairy Sci. 80:859-863. [DOI] [PubMed] [Google Scholar]
- 29.Monzon, M., C. Oteiza, J. Leiva, M. Lamata, and B. Amorena. 2002. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 44:319-324. [DOI] [PubMed] [Google Scholar]
- 30.Monzon, M., C. Oteiza, J. Leiva, and B. Amorena. 2001. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 48:793-801. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 5th ed. Approved standards M7-A5. Volume 20. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penades, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubeda, C., M. A. Tormo, C. Cucarella, P. Trotonda, T. J. Foster, I. Lasa, and J. R. Penades. 2003. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol. Microbiol. 49:193-210. [DOI] [PubMed] [Google Scholar]