Abstract
Secreted phospholipase B (PLB) activity promotes the survival and replication of Cryptococcus neoformans in macrophages in vitro. We therefore investigated the role of mononuclear phagocytes and cryptococcal PLB in the dissemination of infection in a mouse model, using C. neoformans var. grubii wild-type strain H99, a PLB1 deletion mutant (Δplb1), and a reconstituted strain (Δplb1rec). PLB facilitated the entry of endotracheally administered cryptococci into lung IM. PLB was also required for lymphatic spread from the lung to regional lymph nodes and for entry into the blood. Langhans-type giant cells containing budding cryptococci were seen free in the lymphatic sinuses of hilar nodes of H99- and Δplb1rec-infected mice, suggesting that they may have a role in the dissemination of cryptococcal infection. The transfer of infected lung macrophages to recipient mice by tail vein injections demonstrated that these cells can facilitate hematogenous dissemination of cryptococci to the brain, independent of cryptococcal PLB secretion. PLB activities of cryptococci isolated from lung macrophages or infected brains were not persistently increased. We conclude that mononuclear phagocytes are a vehicle for cryptococcal dissemination and that PLB activity is necessary for the initiation of interstitial pulmonary infections and for dissemination from the lung via the lymphatics and blood. PLB is not, however, essential for the establishment of neurological infections when cryptococci are presented within, or after passage through, mononuclear phagocytes.
Cryptococcus neoformans is a common cause of potentially fatal fungal meningoencephalitis, especially in immunocompromised patients. Primary infections are acquired by inhalation of infectious propagules from environmental sources (1). However, mechanisms by which C. neoformans establishes pulmonary disease and disseminates to the central nervous system (CNS) are not understood.
Recent studies using murine models and macrophage-like cell lines have implicated secreted phospholipase B (PLB), the protein produced by the PLB1 gene (5), in intracellular survival, growth, and replication of C. neoformans within macrophages (5, 7, 15). Furthermore, the persistence of cryptococcal infections has been correlated with the presence of viable cryptococci within macrophages (8, 10). Cryptococcal PLB also enhances pulmonary infections, possibly by inhibiting the development of a protective immune response in the lung, and is required for dissemination to pulmonary lymph nodes and the brain (15). It has been proposed that PLB initiates invasion of the lung interstitium by cryptococci since phospholipids in the pulmonary surfactant and the outer leaflet of mammalian cell membranes are preferred substrates of the enzyme (3, 17). The mechanisms by which cryptococcosis is established in the CNS are unknown, although it has been suggested that cryptococci cross the blood-brain barrier within monocytes or after the penetration of endothelial cells (4) and that CNS infection is associated with survival and replication of cryptococci within microglia (13).
The demonstration of viable cryptococci in lung macrophages (5, 15), blood monocytes, and microglia (4) led us to postulate that mononuclear phagocytes are the vehicle for the spread of infection from the lungs to the brain. For the present study, we investigated the role of cryptococcal PLB and host mononuclear phagocytes in the initiation and dissemination of infection in a BALB/c mouse model, using PLB1 deletion mutant (Δplb1), reconstituted (Δplb1rec), and wild-type strains of C. neoformans var. grubii strain H99.
MATERIALS AND METHODS
Cryptococcal strains and culture.
Isogenic strains of C. neoformans var. grubii, namely H99 (wild type), HCM5 (Δplb1 mutant), and HCM15 (Δplb1rec [reconstituted strain]), were generously provided by Gary Cox, Duke University, Durham, N.C. The HCM5 strain contained a single insertion of a construct in which the URA5 gene had been inserted into PLB1. All three strains exhibited similar growth rates at 37°C and similar capsule formation, laccase, and urease activities (5). The absence of secreted phospholipase activity in Δplb1 and the restoration of extracellular phospholipase activity in Δplb1rec were confirmed by a radiometric assay (3). C. neoformans var. grubii strain BL-1a was produced by multiple passages in vitro of the clinical isolate BL-1. It exhibited attenuated virulence in an intravenous mouse model and reduced lysophospholipase (LPL) and PLB activities compared with the parent strain. Mice inoculated intravenously with BL-1a did not develop CNS infections and all animals remained healthy after 21 days, in contrast to BL-1-infected animals, which had developed meningoencephalitis and were ill or had died. The phospholipase activities of BL-1a (see Table 5) were approximately 2.5% of the LPL activity (40 μmol of substrate degraded mg−1 min−1) and 1.8% of the PLB activity (1 μmol of substrate degraded mg−1 min−1) of the parental BL-1 strain (19).
TABLE 5.
Phospholipase activities of strain BL-1a cultured from donor and recipient mice
| Treatment | Activity (nmol/mg of protein/min)a
|
||
|---|---|---|---|
| LPL | LPTA | PLB | |
| Before inoculation | 1,192 ± 499 | 606 ± 202 | 18.0 ± 18.0 |
| Donor LM at 6 days | 1,908 ± 356 | 690 ± 91 | 8.0 ± 4.6 |
| Donor brains at 6 days | 1,801 ± 666 | 811 ± 294 | 23.7 ± 14.4 |
| Donor brains at 11 days | 1,390 ± 642 | 385 ± 292 | 8.3 ± 3.7 |
| Recipient brains as in 4(B) | 1,035 ± 391 | 287 ± 149 | 1.7 ± 1.6 |
| Recipient brains as in 4(D) | 1,745 ± 614b | 521 ± 167 | 10.3 ± 5.5 |
The experimental protocol was the same as in Table 4. All activities are expressed as nanomoles of substrate degraded (PLB and LPL) or product formed (LPTA) mg protein−1 min−1 (means and SEM of three assays).
P = 0.04 by the paired two-tail t test compared to activity before inoculation.
Cryptococcal isolates were grown to confluence on Sabouraud's dextrose (SAB) agar at 30°C and then subcultured in yeast nitrogen broth containing 1% glucose for 24 h at 37°C, with gentle agitation. Prior to endotracheal inoculation, cells were washed twice with sterile, nonpyrogenic saline, counted in a hemocytometer, and adjusted to a concentration of 3 × 107 cells in 50 μl of calcium- and magnesium-free phosphate-buffered saline [PBS(−)].
Murine endotracheal inoculation.
Female BALB/c mice (9 to 12 weeks old and specific pathogen free), obtained from either the Animal Research Centre (Perth, Australia) or the animal facility at Monash University, Melbourne, Australia, were anesthetized by intraperitoneal injection of 1.25 mg of ketamine and 0.2 mg of xylazine per 25 g of body weight. Mice were positioned supine, their tongues were drawn forward, and a 5-cm 19-gauge Cholangiocath (Becton Dickinson) was passed through the vocal cords into the proximal trachea. The cryptococcal suspension (50 μl containing 3 × 107 cells), or PBS(−) for control mice (sham infected), was instilled through the catheter by using a 1-ml syringe. This was followed by 250 μl of air to ensure the dispersal of all organisms into the lungs. Mice were allowed to recover, housed in filter-top cases, and given food and water ad libitum.
Determination of cryptococcal virulence.
Mice were examined daily for evidence of cryptococcal disease (diminished appetite or water intake, ruffled fur, weight loss, reduced activity, abnormal behavior, and cranial bulging) and mortality. Cryptococcal CFU were quantified on spread plates prepared from serial dilutions of homogenates of whole lungs, lymph nodes, and brains.
Kinetics of appearance of cryptococci in mononuclear phagocytes from different body sites. (i) Harvesting and purification of mononuclear phagocytes.
Blood and other tissues were obtained from test and control mice (three per group) 1, 3, 7, and 10 to 14 days after cryptococcal infection. Three separate experiments were performed.
(a) Blood monocytes.
Mice were anesthetized, and peripheral blood was obtained by exsanguination after cardiac puncturing. Heparinized blood samples were diluted with an equal volume of PBS(−), layered over Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden), and centrifuged at 400 × g for 30 min at room temperature. The interface layer containing monocytes from each mouse was washed twice in PBS(−). No neutrophils were seen in smears of this layer, and no more than 10% of the monocytes were lost to the neutrophil pellet at the bottom of the tube. Monocytes were then transferred in 2 ml of RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum and 2 mM glutamine to one well of a six-well tissue culture flask and incubated at 37°C in an atmosphere of 5% CO2 in air for 90 min. Nonadherent cells were then removed by washing with PBS(−). The adherent peripheral blood monocytes (PBM) were removed from the wells and harvested by trypsinization followed by scraping with a rubber policeman.
(b) AM.
After being subjected to cardiac puncture, animals were euthanized by asphyxiation with CO2. Alveolar macrophages (AM) were obtained by bronchoalveolar lavage (BAL). Lungs were subjected to lavage five times in situ with 3 ml of Hanks balanced salt solution containing 0.2% bovine serum albumin, the fluid was centrifuged for 5 min at 400 × g, and the cells were plated in RPMI medium as described for blood monocytes.
(c) Lung (interstitial) macrophages.
After lavage, lungs were removed, weighed, and placed in 20 ml of RPMI medium. Interstitial lung macrophages (IM) were released by agitating the lung tissue with sterile 5-mm-diameter glass beads in a Stuart flask shaker at the maximum speed for 30 min. The resulting homogenate was transferred to a 25-ml tissue culture flask (Sarstedt, Adelaide, Australia) and incubated at 37°C for 90 min in an atmosphere of 5% CO2 in air. IM cell preparations, obtained as for blood monocytes, were characterized morphologically by differential counting of Giemsa-stained smears and by flow cytometry using surface markers. T lymphocytes, B lymphocytes, and granulocytes were identified by staining with fluorescence-labeled antibodies to the CD3 ɛ chain, CD19, and Gran-1, respectively. Monocytes and dendritic cells were identified with antibodies to CD11b, CD11c, and CD8a. Data were collected on a FACScan flow cytometer (Becton Dickinson), and analysis was performed with CellQuest software (Becton Dickinson).
(ii) Quantification of cryptococci.
Phagocyte-associated cryptococci were quantified by counting of the total mononuclear phagocytes obtained after purification from the BAL fluid, lungs, or blood of each mouse in a hemocytometer, plating of serial dilutions of these onto SAB agar, and incubation for 72 h at 30°C to allow for the determination of CFU. The number of cryptococci per phagocyte was calculated by dividing the total number of phagocytes recovered by the number of cryptococci cultured from them. Brains and lymph nodes (hilar, mesenteric, cervical, retroperitoneal, and axillary) were harvested, weighed, and homogenized in sterile saline with a minipestle. Aliquots of the homogenates were serially diluted and plated onto SAB agar to determine the number of CFU of C. neoformans per gram of brain tissue or per whole lymph node.
(iii) Histochemistry.
In the first of the kinetic experiments, lungs and hilar, cervical, mesenteric, retroperitoneal, and axillary lymph nodes were collected at the specified times from each of three mice inoculated with H99, Δplb1, or Δplb1rec. Tissues were fixed in formalin (10%) in neutral buffered saline, blocked in paraffin, sectioned, and stained with periodic acid-Schiff reagent (PAS) or hematoxylin and eosin. An additional experiment was performed with eight mice that were euthanatized 14 days after infection. Lungs, lymph nodes, and intact skulls were harvested. The skulls were decalcified (confirmed by radiography) with EDTA prior to embedding. Sections through the skull, including the brain and meninges, were prepared and stained.
Adoptive transfer experiments.
Three adoptive transfer experiments were performed. In the first, donor mice (six per group) were infected by endotracheal instillation of 3 × 107 cryptococci (H99, Δplb1, or Δplb1rec). Saline was instilled into control animals. On day 6, total lung macrophages (LM; combined AM and IM) and PBM were isolated as described above. Adherent cells were scraped from the flasks with a rubber policeman (trypsin was not used, so that macrophage viability by trypan blue exclusion was maintained at >90%). The phagocytes obtained from each body site from the six donor mice were pooled (1-ml volume) and counted in a hemocytometer. Estimates of the cryptococcal content of the pooled phagocytes were obtained from SAB plates spread with aliquots (25 μl) of undiluted PBM or LM suspension. Aliquots of the remaining phagocytes (200 μl) were injected into the tail veins of recipient mice (three per group). These mice were euthanatized 5 days later. Lungs, spleens, lymph nodes, and brains were harvested, weighed, homogenized, and plated undiluted onto SAB agar to estimate cryptococcal loads.
A second transfer experiment was performed by the same methodology (without the inclusion of BAL), but using only strains H99 and Δplb1. Cryptococci (number of CFU) were quantified for brain and lung homogenates obtained from groups of donor mice at 6 days postinfection and from recipient mice 5 days after the transfer of infected phagocytes by serial dilution on SAB agar plates.
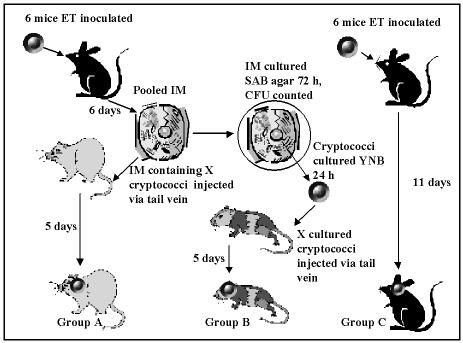
A third experiment was designed to study the effects of adoptive transfer on virulence and phospholipase activity. The experimental method is outlined in cartoon form in Fig. 1 and was performed with strains Δplb1 and BL-1a. For each strain, 12 donor mice were infected by endotracheal instillation of 3 × 107 cells. One group of six mice was culled after 11 days, and cryptococci were quantified for lung and brain homogenates (Fig. 1, group C). The other group of six mice was culled on day 6, and 100-μl samples of the whole lung (without prior BAL) and brain homogenates from each mouse were serially diluted and plated onto SAB agar for the determination of cryptococcal CFU. IM were then isolated from the whole lung homogenates as described above and were pooled (800 μl). Immediately after a sample (200 μl) of the pooled IM was serially diluted and plated onto SAB agar, recipient mice (n = 3) were injected intravenously with the remaining IM (Fig. 1, group A) and culled at day 5 for the quantification of cryptococci in lung and brain homogenates. Meanwhile, cryptococcal colonies from the IM samples cultured on SAB plates for 72 h at 30°C were counted to determine the number of cryptococci which had been injected into the group A mice (Fig. 1). Colonies from the plates were then subcultured in yeast nutrient broth containing 1% glucose for 24 h at 35°C, centrifuged, washed in saline, and counted with a hemocytometer. The same number of cryptococci (Fig. 1) as that determined to be present in the IM injected into the group A mice was injected into each of another group of four recipient mice (group B). These mice were culled 5 days later, and cryptococci were quantified in lung and brain homogenates.
FIG. 1.
Experimental protocol comparing the effects on virulence and phospholipase activity of inoculation with cryptococci inside macrophages or cultured from them. ET, endotracheal; IM, interstitial lung macrophages. BL-1a and Δplb1 strains were tested separately with this protocol.
Statistics.
Statistics were calculated with GraphPad InStat, version 3. The tests used and P values obtained are indicated in the text, the tables, or the legends to the figures. P values of <0.05 were considered significant.
RESULTS
Effect of PLB on virulence of C. neoformans in mice.
All mice inoculated with wild-type (H99) or recombinant (plb1rec) strains were ill by day 7 and either died of cryptococcosis or were euthanatized because of illness between days 10 and 14. For simplicity, time course data for days 10 to 14 are recorded as day 14. Noninfected controls and mice inoculated with Δplb1 or BL-1a all remained well up to the time of euthanasia on day 10 to 14.
Kinetics of cryptococcal infection.
The kinetics of infection of mononuclear phagocytes in lungs, blood, lymph nodes, and brains were determined in triplicate experiments.
(i) Effect of PLB on initiation and establishment of infection in pulmonary macrophages.
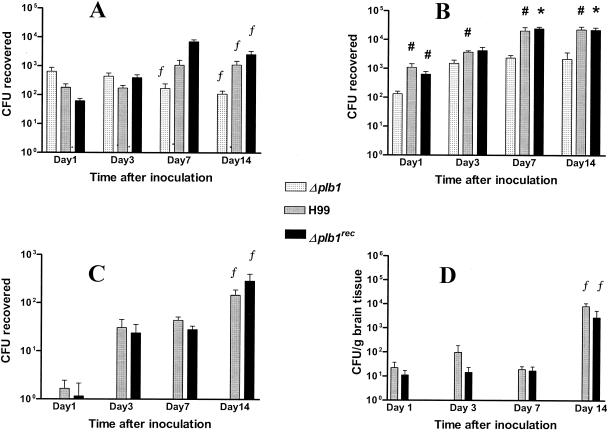
Within 24 h of endotracheal infection, there was a trend (not statistically significant) towards higher numbers of Δplb1 organisms in AM obtained by lavage than of wild-type and reconstituted strains (Fig. 2A). However, significantly fewer Δplb1 cells than H99 and Δplbrec cells were recovered from IM (P < 0.05) (Fig. 2B). When the numbers of CFU recovered at day 1 from AM and IM were combined, they were similar for the three groups of infected mice (Fig. 2A and B). Similarly, the total numbers of IM per mouse at day 1 were similar for all infected groups and none was significantly different from sham-infected controls (Table 1).
FIG. 2.
Kinetics of appearance of cryptococcal CFU in AM (A), IM (B), PBM (C), and brain tissues (D). Organs and blood were harvested from mice after endotracheal inoculation with 3 × 107 cells of the H99, Δplb1rec, and Δplb1 cryptococcal strains. Note that a log scale was used to report CFU. Data represent the means ± standard errors of the means of three experiments (two for day 14) using three mice for each strain per time point. Statistics were calculated by combining data from all experiments. No CFU were recovered from blood monocytes or brain tissues from mice inoculated with Δplb1. Significant differences from Δplb1 are noted as follows: #, P < 0.05; *, P < 0.005 by the paired two-tail t test; #*, P < 0.05 by the Mann-Whitney U test, except for H99 on day 7, for which P = 0.06. f, significantly different from day 1 (P < 0.05 by the unpaired two-tail t test and the Mann-Whitney U test).
TABLE 1.
Numbers of IM isolated over the time course of infection with C. neoformans
| Strain | No. of IM per mouse (mean [104] [SEM]) at indicated daya
|
|||
|---|---|---|---|---|
| 1 | 3 | 7 | 14 | |
| Control | 8.64 (5.61) | 6.74 (5.43) | 4.82 (3.02) | 6.82 (3.89) |
| Δplb1 | 8.83 (6.74) | 24.32 (16.19) | 30.75 (9.24)b,c | 13.57 (7.75) |
| H99 | 10.00 (4.41) | 12.87 (4.99)b | 34.04 (10.32)b,c | 44.97 (16.65)d |
| Δplb1rec | 7.75 (3.27) | 35.83 (19.81) | 62.50 (15.75)b,c | 64.05 (19.02)b,c,d |
Data given are from two experiments (n = 6).
Compared with value for sham-infected controls on the same day, P < 0.05 by the Mann-Whitney U test.
Compared with value for sham-infected controls on day 1, P < 0.05 by the Mann-Whitney U test.
P < 0.05 compared with value for Δplb1 by the Mann-Whitney U test.
Over the 14 days postinfection, the cryptococcal load of Δplb1 in AM gradually fell, reaching statistical significance by day 7 (compared with day 1). In contrast, that of H99 and Δplb1rec increased significantly in AM by day 14 (Fig. 2A). Similarly, significantly fewer Δplb1 cells were isolated from IM at each time point, and cryptococcal loads reached a plateau earlier (3 days versus 7 days) than in mice infected with H99 and (except for day 3) Δplb1rec (Fig. 2B). The numbers of IM isolated from infected animals were highly variable but were higher than those of the controls at each time point, though this reached statistical significance with all three strains at day 7 only (Table 1). By day 14, more IM were recovered from the PLB-competent strains than from the deletion mutant (Table 1).
When cryptococcal numbers per phagocyte were calculated, the cryptococcal contents of AM increased consistently over the four time points for both test groups with a functional PLB1 gene (H99 and Δplbrec), although the increase did not achieve statistical significance. However, for Δplb1 the reverse was true, with a significant decrease in numbers per AM on day 14 compared with day 1 (P = 0.004 by Mann-Whitney U test) (data not shown). There was a gradual increase in the average number of cryptococci per IM over 3 to 14 days in all groups of infected mice, but no significant differences were noted between the groups at each time point.
(ii) Effect of PLB on kinetics of cryptococcal infection of blood monocytes.
No Δplb1 cells were cultured from blood leukocyte or plasma fractions over the 14 days postinoculation. By day 3, significant numbers of H99 and Δplb1rec were recovered from PBM. These numbers increased further, reaching statistical significance compared to day 1 at day 14 (Fig. 2C). By day 14, there was also a significant increase in the number of H99 and Δplb1rec cells per monocyte (data not shown) compared with day 1, suggesting intracellular replication (P = 0.036 and 0.016, respectively, by the Mann-Whitney U test).
By day 14, 30% of all cryptococci in the blood were recovered from the plasma fraction, 20% were recovered from the isolated monocyte fraction, and the remainder were recovered from the cell pellet (consisting of neutrophils, some monocytes, eosinophils, lymphocytes, cell debris, etc). The cell pellet contained four times as many leukocytes as the monocyte fraction, indicating that cryptococci were concentrated in monocytes (data not shown).
(iii) Role of PLB in dissemination to lymph nodes.
Cryptococci were quantified by culturing of lymph node homogenates rather than by culturing of isolated macrophages (data not shown). No Δplb1 mutant was cultured from any of the nodes at days 7 and 14, although a few CFU were cultured from cervical and retroperitoneal nodes on day 1. These were not detected histologically. By day 7, and especially on day 14, large numbers of H99 and Δplb1rec cells were cultured from hilar, cervical, and mesenteric nodes (data not shown). Some H99 cells were also cultured from axillary nodes. Nodes from sham-infected control mice were all culture negative.
(iv) Kinetics of establishment of cerebral cryptococcosis.
The brains of all mice inoculated with Δplb1 were culture negative. The kinetics of dissemination of H99 and Δplb1rec to the CNS are shown in Fig. 2D. Cryptococcal loads remained below 102 CFU/g until day 7, and at day 14 there was a >200-fold increase. By comparison with Fig. 2A, B, and C, it can be seen that the establishment of CNS infection was delayed compared with that in the lungs and blood. Clinical signs of neurological infection (cranial bulging) were evident in mice infected with H99 between days 10 and 14 and in mice infected with Δplb1rec by day 14.
Histopathology of lung sections.
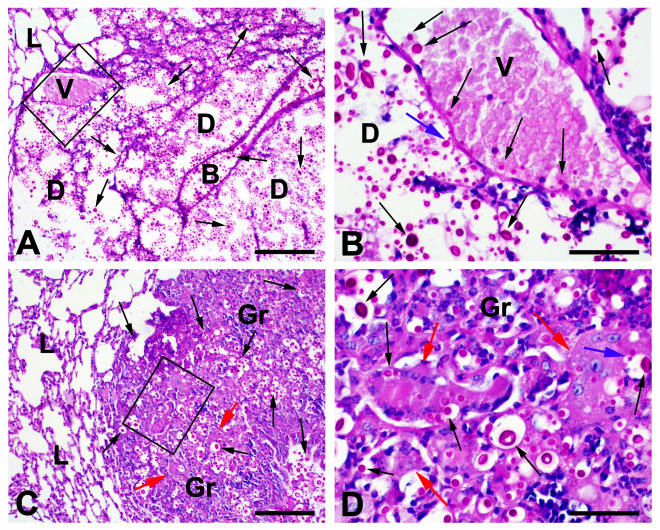
Lung sections from the above experiments confirmed the results shown in Fig. 2B. Control animals were normal upon histological examination. Fourteen days after infection, a few granulomas, which contained scanty intracellular cryptococci with occasional budding, were present in Δplb1-infected mice. Much larger granulomas, consisting primarily of epithelioid macrophages with occasional Langhans-type giant cells, were present in mice infected with H99 or Δplb1rec. Cryptococci were invariably present, often within the giant and epithelioid cells (Fig. 3). Large areas of extracellular budding yeasts associated with the destruction of lung tissue (called “cryptococcomas”) that were separate from the areas of granulomatous response were the dominant feature of infection in 9 of 11 mice inoculated with the wild-type strain H99, and intravascular cryptococci were present in these areas in 7 mice. Extensive granulomas were present in the remaining two mice. Pulmonary cryptococcomas were also noted in the Δplb1rec-infected mice, with associated intravascular cryptococci in sections from one of these mice (Fig. 3B).
FIG. 3.
PAS-stained paraffin sections of lung tissue harvested from mice 14 days after infection with H99. Cryptococci were evident as strongly PAS-positive round cells (black arrows), often surrounded by a capsule and occasionally budding (blue arrows). (A) Although the alveolar structure of the lung (L) could be identified in many sites, this was lost in areas containing large numbers of extracellular cryptococci, which apparently caused tissue destruction (D). Major structures such as bronchioles (B) were often retained. (B) At a higher magnification of areas showing tissue destruction, occasional cryptococci within blood vessels (V) were noted. (C) Areas of granulomatous reaction (Gr) containing organisms within epithelioid macrophages and Langhans-type giant cells were also seen (red arrows). (D) At a higher magnification, budding cryptococci (blue arrows) were noted in some giant cells. Bars = 150 μm for panels A and C and 60 μm for panels B and D.
Histopathology of lymph node cryptococcosis.
Histopathology confirmed that the extent of nodal infection increased over time in mice inoculated with PLB-competent cryptococci. Cryptococci were never seen in any section of nodes from the Δplb1-infected group. H99 appeared in cervical lymph nodes in small numbers at day 3 and increased substantially by day 14 (Fig. 4). Small numbers were visualized in mesenteric nodes on day 14 (not shown). These histopathological findings were consistent with the large numbers of CFU cultured from mesenteric and cervical nodes. Cryptococci were never detected histologically in sections of the axillary or retroperitoneal lymph nodes, which were used as control sites.
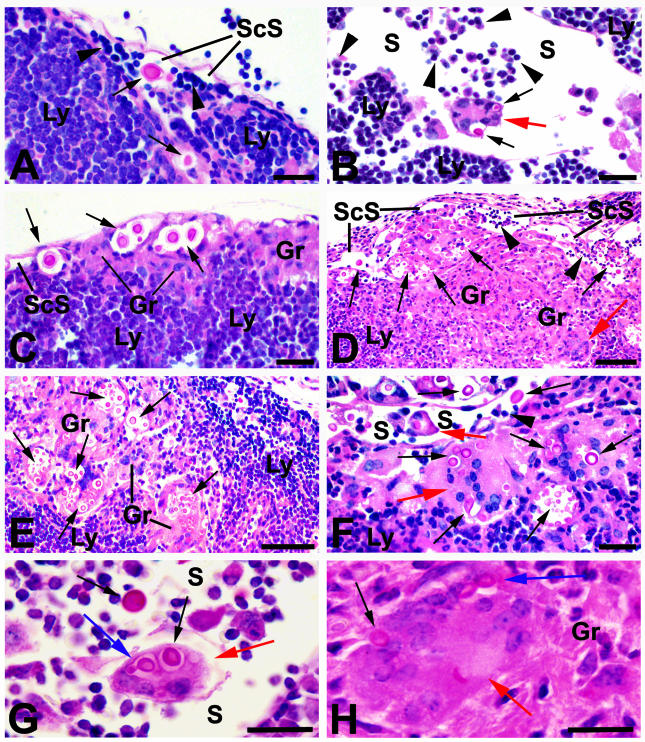
FIG. 4.
PAS-stained sections of hilar and cervical lymph nodes collected from mice 14 days after infection with strain H99. (A) The lymph node stroma (Ly), heavily infiltrated with lymphocytes, is readily apparent and is separated from subcapsular lymphatic sinuses (ScS) containing recirculating lymphocytes (arrowheads) by a layer of endothelium. Cryptococci (black arrows) were often present free within the subcapsular and other lymphatic spaces (S). (B) Cryptococci were also found in Langhans-type giant cells (red arrows), some of which were free within the lymphatic spaces (S) in panels B, F, and G. (C) Small granulomas (Gr) comprised of epithelioid macrophages with phagocytosed cryptococci were present in subcapsular sites in some areas. In others, these granulomatous lesions occupied large portions of the peripheral lymph node tissues (D) as well as more central zones of nodes (E). Langhans-type giant cells (red arrows) containing cryptococci were also found among the epithelioid macrophages in these lesions (D, F, and H). Budding cryptococci (blue arrows) were present in Langhans-type giant cells (red arrows), free within lymphatic sinuses (G), and within granulomas (H). Bars = 50 μm for panels A, B, C, and F; 100 μm for panels D and E; and 25 μm for panels G and H.
By 14 days after infection, granulomas were present in sections of the hilar lymph nodes of eight of the nine H99-infected mice examined (Fig. 4) and of all mice which were infected with Δplb1rec. Similarly, cryptococci were seen free in hilar lymphatic spaces and lymph node pulp of seven of the nine H99-infected mice examined and of all nine examined after infection with Δplb1rec. Budding cryptococci were noted in half of the H99-infected mice but in only one Δplb1rec-infected animal.
Langhans-type giant cells, often with intracellular cryptococci, were noted in the lymphatic spaces of hilar nodes of one H99-infected mouse at day 7 and of seven of the nine mice examined at day 14, as well as at day 14 in a single animal infected with Δplb1rec (Fig. 4). These cells appeared to be free in the lymph and often contained budding cryptococci (Fig. 4).
Histopathology of cerebral cryptococcosis.
Δplb1-infected mice remained healthy and no cryptococci were seen in brain sections. Distinct foci of cryptococcal infection (cryptococcomas) containing budding yeasts were seen in one-third of the animals infected with H99 at day 14. Single extracellular organisms were noted in nearby capillaries (Fig. 5A and B) and in venules within and on the surface of the brain (Fig. 5C and D).
FIG. 5.
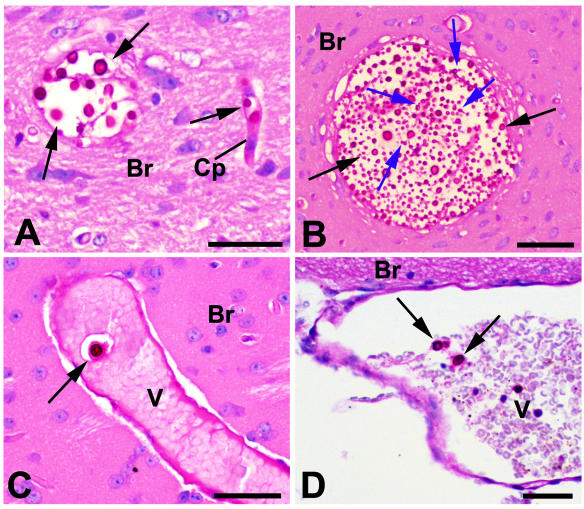
PAS-stained paraffin sections of brain tissue collected from mice after 14 days of infection with H99. Cryptococci appear as strongly PAS-positive cells (black arrows), often surrounded by a capsule and occasionally budding (blue arrows). (A and B) Brain tissue (Br) contained foci of cryptococci; organisms were also found in capillaries (Cp). (C) Venules (V) containing cryptococci were present within the brain tissue, as well as on the surface (D). Bars = 50 μm.
Adoptive transfer experiments.
Because both PLB and mononuclear phagocytes appeared to play a role in the dissemination of cryptococci from lungs via blood and lymphatics to the brains of infected animals, we wanted to determine if infection could be transferred from one mouse to another via infected macrophages. In the first experiment, donor mice were infected by endotracheal inoculation of H99, Δplb1, and Δplb1rec and were sacrificed 6 days after inoculation. This time point was chosen because lung and blood infection, but little cerebral infection, was well established by then (Fig. 2B). Pooled infected PBM and LM were then transferred by tail vein injection into recipient mice. Five days later, the animals were sacrificed, as we hypothesized that cerebral infection might have been established at this time in animals infected with the wild-type and reconstituted strains, but not the deletion mutant (Fig. 2D, days 10 to 14). In recipient mice inoculated with PBM, cryptococci were isolated from lungs, spleen, brains, and nodes of mice infected with H99 and from lungs and spleens of mice infected with Δplb1rec (Table 2). As expected, organs harvested from recipient mice injected with PBM from Δplb1-infected animals were culture negative, except for a few yeast cells isolated from the spleen of one recipient animal only (Table 2). Surprisingly, large numbers of cryptococci were cultured from the lungs, brains, and spleens of all recipient mice inoculated with LM containing intracellular cryptococci, including H99, Δplb1rec, and Δplb1 (Table 2). In the mice injected with LM containing H99 and Δplb1rec, but not Δplb1, cryptococci were also present in lymph nodes.
TABLE 2.
Cryptococcal infection in tissues of recipient mice after transfer of PBM and LM from donor micea
| Strain | No. of donor cells (107 ml−1)b
|
CFU cultured from donor cells (104 ml−1)
|
No. of infected recipient mice/total no. of recipient mice
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung
|
Spleen
|
Brain
|
Lymph nodesc
|
|||||||||
| PBM | LM | PBM | LM | PBM | LM | PBM | LM | PBM | LM | PBM | LM | |
| Control | 0.35 | 0.70 | 0 | 0 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Δplb1 | 0.52 | 0.31 | 0 | 8.37 | 0/3 | 2/3 | 1/3 | 3/3 | 0/3 | 3/3 | 0/3 | 0/3 |
| H99 | 0.52 | 1.33 | 0.02 | 7.18 | 2/3 | 3/3 | 2/3 | 3/3 | 1/3 | 3/3 | 1/3 | 3/3 |
| Δplb1rec | 0.45 | 0.88 | 2.47 | 14.05 | 3/3 | 3/3 | 3/3 | 3/3 | 0/3 | 3/3 | 0/3 | 2/3 |
Donor mice (n = 6) were infected by endotracheal instillation of 3 × 107 cells. PBM and LM were purified as described in Materials and Methods after the animals were sacrificed at 6 days. Aliquots of the pooled phagocyte suspension (200 μl per mouse) were then transferred to the recipient mice (n = 3) by tail vein injection. Five days later, mice were sacrificed and fungal burdens in recipient organs were examined on SAB agar plates.
The numbers of monocytes and macrophages pooled from six donor mice are shown.
Includes hilar, cervical, and mesenteric lymph nodes.
Cryptococcal loads in the lungs and brains of donor mice and recipient mice after the transfer of IM and PBM from groups of five mice infected endotracheally with Δplb1 and H99 are shown in Table 3 (second adoptive transfer experiment). Large loads of H99 were present in brains of recipient mice receiving infected IM (1,100-fold increase over the inoculated IM CFU) compared with those receiving PBM (only 25-fold increase over the inoculated CFU) or compared with the brains of donor mice harvested 6 days after endotracheal instillation of cryptococci. Substantial numbers of Δplb1 were also recovered from the brains of recipient mice inoculated with PBM, although this was not increased relative to the inoculated CFU.
TABLE 3.
Transfer of cryptococcal infection to recipient mice via infected mononuclear phagocytes from blood and lungs of donor micea
| Strain | Cryptococcal load (mean CFU/g of tissue ± SEM) in mouse tissue (no. of mice with brain infection/total no. of mice)
|
|||||
|---|---|---|---|---|---|---|
| Donor mouse tissueb
|
Recipient mouse brainb
|
|||||
| IM | PBM | Lungs | Brains | PBM injection | IM injection | |
| Δplb1 | 6.35 × 103c | 0 | 2.3 × 106 ± 0.9 × 106 | 0 | 0 (0/3) | 3.77 × 103 ± 1.94 × 103 (3/3) |
| H99 | 8.15 × 103c | 1.80 × 103c | 32.7 × 106 ± 3.7 × 106d | 1.07 × 103 ± 0.58 × 103 | 44.78 × 103 ± 26.03 × 103 (3/3) | 8.96 × 106 ± 5.19 × 106e (3/3) |
Inoculation and transfer protocols were similar to those described in Table 1.
Results are expressed for five mice after 6 days (donor mice) and three mice after 5 days (recipient mice).
Data are expressed as CFU/200 μl of inoculum, pooled from five mice.
Significantly different from Δplb1 (P < 0.0001 by the unpaired two-tail t test and P = 0.008 by the Mann-Whitney U test).
Significantly different from H99 donor brains by the Mann-Whitney U test (P = 0.04).
Effect of in vivo passaging of cryptococci through macrophages on virulence.
The data in Tables 2 and 3 suggested that cryptococcal virulence was increased during intracellular existence in macrophages. As PLB is a virulence factor, it could also be upregulated. These hypotheses were tested with an adoptive transfer experiment using the experimental protocol shown in Fig. 1. Since no cryptococci were cultured from PBM after endotracheal inoculation of Δplb1, IM were used as a source of “naturally” infected mononuclear phagocytes for adoptive transfer. The phospholipase deletion mutant was compared with BL-1a, the laboratory-passaged strain which had lost much of the secreted PLB activity and virulence of the parent BL-1, but still carried the PLB1 gene (see Materials and Methods). In two preliminary experiments, with each using three mice per group, we established that BL-1a caused substantial cerebral infection after the intravenous transfer of infected IM to recipient mice, but not after direct injection into the tail vein (data not shown).
Counting of >1,000 Giemsa-stained cells from the adherent IM preparations from BL-1a and Δplb1-infected mice revealed that most of the cells (84 to 89.5%) were mononuclear and phenotypically resembled activated macrophages. Some neutrophils (8.4 to 15%) and lymphocytes (1 to 1.6%) were also present. Preliminary flow cytometric analysis also indicated that the majority of the cells resembled myeloid lineage cells, based on forward and side scatter measurements and CD11b and CD11c staining (data not shown), with only 7 to 14% neutrophils (Gran-1 positive) and 5 to 8% CD3-positive T lymphocytes. No CD19-positive B lymphocytes were observed. This preliminary analysis suggested that the pulmonary infiltration in response to the cryptococcal infection at day 6 consists overwhelmingly of macrophages, although further phenotypic and flow cytometric analyses are required to definitely exclude the presence of dendritic cells.
The results of the quantitative experiment are summarized in Table 4. Six days after endotracheal inoculation, more cryptococci were isolated from IM of donor mice inoculated with strain BL-1a than with Δplb1, and BL-1a was cultured from the brain tissue of one of six mice, whereas Δplb1 was not isolated from any mice (Table 4). Cerebral infection was established in recipient mice within 5 days of intravenous injection of freshly isolated pulmonary IM containing BL-1a (3,016 CFU per mouse) or Δplb1 (904 CFU per mouse) (Table 4).
TABLE 4.
Dissemination of cryptococcal infection via infected lung IM compared with cryptococci cultured from thema
| Strain | No. of IM in donor mice at 6 days, prior to transfer | Cryptococcal CFU in pooled donor IM | CFU in donor brains | Cryptococcal load (mean CFU/g of tissue ± SEM) in mouse tissue (no. of infected mice/total no. of mice)
|
Cryptococcal load (mean CFU/g of tissue ± SEM) in mouse tissue (no. of infected mice/total no. of mice)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| 5 days after IM transfers by tail vein injection (recipients)b
|
11 days after endotracheal infection (donors)c
|
5 days after tail vein injection, cultured from IM (recipients)d
|
|||||||
| Lung | Brain | Lung | Brain | Lung | Brain | ||||
| Δplb1 | 2.3 × 106 | 3.62 × 103 | 0 | 4.17 × 103 ± 2.41 × 103 (3/3) | 0.32 × 103 ± 0.18 × 103 (2/3) | 30.13 × 106 ± 8.36 × 106 (5/5) | —h | 0.19 × 103 ± 0.1 × 103 (3/4) | 9.11 × 103 ± 5.74 × 103 (3/4) |
| BL-1a | 1.4 × 106 | 12.06 × 103 | —h | 11.48 × 103 ± 10.90 × 103 (2/3) | 77.22 × 103 ± 21.89 × × 103 (3/3)e | 72.52 × 106 ± 13.36 × 106 (5/5)f | 0.93 × 103 ± 0.65 × 103 (3/5)g | —h | 3.74 × 103 ± 2.98 × 103 (4/4)g |
Donor mice were inoculated endotracheally with 3 × 107 cells. Recipient mice were inoculated with IM containing 904 CFU of Δplb1 or 3,016 CFU of BL-1a or with the same number of cryptococci derived from these IM.
Data are for three mice.
Data are for five mice.
Data are for four mice.
Significantly different from Δplb1 (P < 0.05 by two-tailed unpaired t test).
Significantly different by the Mann-Whitney U test.
Significant (P ≤ 0.01) difference from the same cryptococci in the same tissue in recipient mice 5 days after IM transfer by the two-tailed unpaired t test and also (P ≤ 0.05) by the Mann-Whitney U test. In control experiments, no cryptococci were cultured from brains of animals injected intravenously with either BL-1a or Δplb1 strains.
—, CFU were present in only one mouse, and mean data are not available.
In contrast, no cryptococci were cultured from brains of mice injected intravenously with 106 CFU of Δplb1 or BL-1a (stock strains, not passaged through mice) for as long as 20 days after infection (data not shown). Eleven days after endotracheal instillation of either strain, most cryptococci were confined to the lungs, and a few colonies of Δplb1 were cultured from the brain of one mouse only (Table 4).
To check if the presence of macrophages was necessary for the upregulation of virulence, we injected another group of recipient mice intravenously with cryptococci cultured from the IM used for injection for the previous experiment (Table 4). The same number of cryptococci were used as described above, i.e., 3,016 CFU of BL-1a and 904 CFU of Δplb1 per mouse. Relatively few cryptococci of either BL-1a or Δplb1 were recovered from lungs of recipient mice. The load of BL-1a in brain tissue was significantly higher (21-fold) after the direct injection of infected macrophages than after injection of cryptococci cultured from them (Table 4), suggesting that the presentation of BL-1a to the CNS within mononuclear phagocytes is more effective for the establishment of cerebral infection. In contrast, the reverse appeared to be true for Δplb1, but the difference between direct injection of macrophages infected with this strain and injection of cryptococci cultured from them was not statistically significant.
Effect of murine passaging on cryptococcal phospholipase activities.
To establish whether the increased activity of cryptococcal PLB contributed to the apparent increase in virulence of strain BL-1a after passage through macrophages, we calculated the specific activities of the three phospholipase activities secreted by BL-1a cultured from donor macrophages and donor and recipient brain tissues. A trend towards increased LPL activity was noted after one to two passages through mice (Table 5). Except for cryptococci cultured from donor brains after 6 days, LPTA and PLB activities tended to be similar to or reduced compared to activities before inoculation (not statistically significant). As a control, we confirmed that Δplb1 cells secreted no measurable phospholipase activities at any stage.
DISCUSSION
Our data indicate that secreted PLB is involved in the initiation and development of pulmonary cryptococcosis and that it is essential for the entry of cryptococci into the pulmonary lymphatics and the blood, but not the CNS. We have also shown that mononuclear phagocytes are an effective vehicle for hematogenous dissemination and establishment of CNS infections. The importance of PLB and monocytes/macrophages in the different stages of pathogenesis of cryptococcosis has not been defined previously.
PLB and macrophages in establishment of pulmonary infection.
The establishment of pulmonary cryptococcosis after intratracheal inoculation or inhalation of C. neoformans is well described for murine models employing immunologically intact mice and strains with selective immune defects (1). The outcome is dependent on the mouse strain and is also affected by a suite of cryptococcal virulence determinants (6, 7, 9, 11, 15). We used immunologically intact BALB/c mice and showed for the first time that PLB facilitates the entry of cryptococci from the airways into the pulmonary interstitium, since significantly fewer cryptococci of the Δplb1 strain were cultured from IM at 24 h postinfection. An alternative explanation, such as enhanced intracellular killing of PLB-deficient cryptococci by IM or multiplication of PLB-competent strains within them, is unlikely. At 24 h, there was no difference in the total numbers of cryptococci isolated from AM and IM for the three groups of mice, nor were there differences in the numbers of cryptococci per IM. Furthermore, the total numbers of IM per mouse were the same as for uninfected controls. The trend toward higher counts of Δplb1 in AM at day 1 is of interest since we and others have shown that this mutant is phagocytosed to the same extent as wild-type H99 by macrophage-like cell lines (5, 15). It is possible that PLB-competent strains enter the lung parenchyma more efficiently and that PLB-deficient strains remain in the airways and are phagocytosed in situ. The exact mechanism of invasion from alveolar spaces to the lung interstitium is unknown.
PLB and evolution of pulmonary infection.
For the first time, we showed that intracellular multiplication within AM, first noted 3 days after infection with PLB-expressing strains, is PLB dependent, as evidenced by the substantial increase in the cryptococcal loads of H99 and Δplbrec in AM by day 14 and by increased numbers of cryptococci per macrophage, compared with a dramatic fall in Δplb1 numbers. Reduced cryptococcal budding and growth of Δplb1 were observed previously in vitro with two macrophage-like cell lines (5, 15).
Our kinetic and histopathological data indicate that the recruitment of IM into the lungs occurred earlier, and was more extensive, in mice infected with the H99 and Δplb1rec strains than with the Δplb1 mutant. A recent report of host cell infiltration into the lung after tracheal injection of cryptococci showed similar trends (15). In mice inoculated with H99 and Δplb1rec, cryptococcomas consisting of masses of budding cryptococci of variable sizes, associated with lung destruction and little inflammatory response, interspersed with areas of large granulomas, were present. These features have been described for other mouse models of cryptococcal infection (7, 15). In addition, we observed budding cryptococci within giant cells. In contrast, only small, scattered granulomas containing yeasts with occasional budding and no cryptococcomas were evident in Δplb1-infected mice, confirming that PLB promotes the survival and growth of cryptococci within the lung (15).
Dissemination of cryptococcal infection from the lung.
Infection with Δplb1 remained confined to the lungs, whereas by days 1 to 3, strains H99 and Δplb1rec were isolated from blood monocytes, plasma, peritoneal macrophages (not shown), and cerebral tissue and H99 was visualized in sections of hilar lymph nodes from day 3. These data indicate that PLB is essential for hematogenous dissemination of cryptococci from the lung and for lymphatic spread from the lung to regional lymph nodes. It is notable that with a different mouse strain, Noverr et al. (15) cultured small numbers of Δplb1 from lymph nodes of mice after 3 weeks, although this was not confirmed by histology and may have represented contamination from infected lungs.
We have shown for the first time that mononuclear phagocytes are a vehicle for hematogenous dissemination of cryptococcal infections and that infection can be transferred from one animal to another by mononuclear phagocytes. Using one cryptococcal strain, H99, we have evidence that macrophages of pulmonary origin are more effective in transferring the infection than peripheral blood monocytes, though this remains to be proven. Another respiratory pathogen, Chlamydia pneumoniae, which can disseminate systemically to produce neurologic and cardiovascular syndromes, was transmitted from donor mice to recipient mice via infected macrophages, with the establishment of infection in the lungs, thymus, spleen, and/or abdominal lymph nodes (14).
A novel finding in the present study was the presence of multinucleated Langhans-type giant cells containing budding H99 free in the lymphatic sinuses of hilar nodes in a number of animals. It is possible that these cells are a vehicle for the transport of cryptococci into efferent lymphatics and thence to the thoracic duct and the venous circulation. There is evidence that other microorganisms are carried from the lungs within AM or IM (or both) to bronchiolar-associated lymphoid tissue (14, 16). Infected macrophages then traverse the mediastinal lymph nodes and reach the left subclavian vein via the thoracic duct (14, 18). At the same time, there is evidence that hilar nodes are important in initiating the immune response to cryptococcal infection and limiting dissemination (12). Hill (11) described giant cells as sedentary and as a mechanism for the sequestration of serotype A cryptococci within the respiratory tract.
Hematogenous dissemination of cryptococcal infection to the CNS.
Since PLB was essential for dissemination from the lung to the blood, the failure to establish CNS cryptococcosis after endotracheal inoculation did not indicate whether mononuclear phagocytes and/or PLB is required for invasion of the CNS. The adoptive transfer experiments showed that two cryptococcal strains, one expressing very low levels of PLB despite the presence of the gene and the other lacking a functional gene (PLB negative), could establish CNS infection if presented within, as well as cultured from, mononuclear phagocytes, but not when injected directly into the venous circulation. This suggests that mononuclear phagocytes can act as a vehicle for dissemination of cryptococci to the CNS by a PLB-independent mechanism. It was shown previously that PLB is necessary for the establishment of CNS infections by intravenous injection of free organisms (2, 19). It is notable that cryptococci cultured out of infected pulmonary macrophages also established CNS infections at a much lower level for BL-1a, but at a higher level (though not statistically significant) for Δplb1, than those injected within macrophages (Table 4). Replication within macrophages has been shown to be retarded in the deletion mutant (5, 15), which could explain why fewer Δplb1 than BL-1a cells, relative to inoculum size, became established in the brain after direct inoculation of IM (Table 4). The removal of these activated macrophages by culturing on SAB agar could allow for increases in brain CFU from the deletion mutant after inoculation (Table 4). The residual PLB activity in BL-1a, on the other hand, may be sufficient to produce eicosanoids to downregulate the macrophage function and promote cryptococcal growth (15). Clearly, in both cryptococcal strains the regulation of virulence factors other than PLB must be involved. These have not been identified.
We quantified PLB activity produced by BL-1a cells isolated from mononuclear phagocytes since it is known that cryptococcal virulence determinants may be upregulated during passaging through the donor animal in association with a well-known, but poorly understood, phenomenon called phenotypic switching (9). However, there was only a small, transient increase in the activity of phospholipases, which is consistent with our previous findings (L. Wright, unpublished data) that up to four passages through mice are required to restore the secreted PLB activity of BL-1a to the levels of BL-1 (19).
It has been postulated that cryptococci cross the blood-brain barrier within monocytes (the so-called Trojan horse) or via endothelial cells (4). The present study, while indicating that cryptococci may cross the blood-brain barrier by a process which does not require PLB, does not distinguish between these possibilities. However, it was noted previously (19) that the hydrolytic PLB activity most likely to facilitate penetration of the brain from the blood is actually inhibited in the presence of serum at pH 7.4, and therefore unphagocytosed cryptococci are unlikely to invade successfully by a PLB-dependent mechanism. After delivery into the brain tissues, the phagocytosed cryptococci, with PLB active in the acidic environment of the phagosome, could escape from the phagocytes, acidify their environment by acetic acid production (20), and continue using PLB to assist in tissue destruction. These processes are the subjects of further investigation.
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia grants 990738 and 211040.
We thank Lisa Sedger (Centre for Virus Research, Westmead Millennium Institute) for assistance with the flow cytometry of IM preparations.
Editor: T. R. Kozel
REFERENCES
- 1.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 2.Chen, S. C. A., M. Muller, J. Z. Zhou, L. C. Wright, and T. C. Sorrell. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J. Infect. Dis. 175:414-420. [DOI] [PubMed] [Google Scholar]
- 3.Chen, S. C. A., L. C. Wright, R. T. Santangelo, M. Muller, V. R. Moran, P. W. Kuchel, and T. C. Sorrell. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect. Immun. 65:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrétien, F., O. Lortholary, I. Kansau, S. Neuville, F. Gray, and F. Dromer. 2002. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 186:522-530. [DOI] [PubMed] [Google Scholar]
- 5.Cox, G. M., H. C. Mc Dade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 6.Curtis, J. L., G. B. Huffnagle, G. H. Chen, M. L. Warnock, M. R. Gyetko, R. A. Mc Donald, P. J. Scott, and G. B. Toews. 1994. Experimental murine pulmonary cryptococcosis. Differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab. Investig. 71:113-126. [PubMed] [Google Scholar]
- 7.Feldmesser, M., Y. Krass, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmesser, M., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 9.Fries, B. C., C. P. Taborda, E. Serfass, and A. Casadevall. 2001. Phenotypic switching occurs in vivo and influences the outcome of infection. J. Clin. Investig. 108:1577-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He, W., A. Casadevall, S. C. Lee, and D. L. Goldman. 2003. Phagocytic activity and monocyte chemotactic protein expression by pulmonary macrophages in persistent pulmonary cryptococcosis. Infect. Immun. 71:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill, J. O. 1992. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J. Exp. Med. 175:1685-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huffnagle, G. B., and M. F. Lipscomb. 1998. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 149:387-396. [DOI] [PubMed] [Google Scholar]
- 13.Lee, S. C., Y. Kress, M. Zhao, D. W. Dickson, and A. Casadevall. 1995. Cryptococcus neoformans survives and replicates in human microglia. Lab. Investig. 73:871-879. [PubMed] [Google Scholar]
- 14.Moazed, T. C., K. Cho-chou, J. T. Grayston, and L. A. Campbell. 1998. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J. Infect. Dis. 177:1322-1325. [DOI] [PubMed] [Google Scholar]
- 15.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roser, B. 1970. The origins, kinetics and fate of macrophage populations. J. Reticuloendothelial Soc. 8:139-161. [PubMed] [Google Scholar]
- 17.Santangelo, R. T., M. H. Nouri-Sorkhabi, T. C. Sorrell, M. Cagney, S. C. Chen, P. W. Kuchel, and L. C. Wright. 1999. Biochemical and functional characterization of secreted phospholipases from Cryptococcus neoformans in their naturally occurring state. J. Med. Microbiol. 48:731-740. [DOI] [PubMed] [Google Scholar]
- 18.Tilney, N. L. 1971. Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109:369-383. [PMC free article] [PubMed] [Google Scholar]
- 19.Wright, L. C., S. C. A. Chen, C. F. Wilson, M. F. Simpanya, R. Blackstock, G. M. Cox, J. W. Murphy, and T. C. Sorrell. 2002. Strain-dependent effects of environmental signals on the production of extracellular phospholipase by Cryptococcus neoformans. FEMS Microbiol. Lett. 209:175-181. [DOI] [PubMed] [Google Scholar]
- 20.Wright, L. C., W. Bubb, J. Davidson, R. Santangelo, M. Krockenberger, U. Himmelreich, and T. C. Sorrell. 2002. Metabolites released by Cryptococcus neoformans var. neoformans and var. gattii differentially affect human neutrophil function. Microbes Infect. 4:1427-1438. [DOI] [PubMed] [Google Scholar]