Abstract
Optogenetics is a powerful tool that enables spatiotemporal control of neuronal activity and circuits in behaving animals. Here, we describe our protocol for optical activation of neurons in Drosophila larvae. As an example, we discuss the use of optogenetics to activate larval nociceptors and nociception behaviors in the third larval instar. We have previously shown that, using spatially-defined GAL4 drivers and potent UAS-channelrhodopsin-2∷YFP transgenic strains developed in our laboratory, it is possible to manipulate neuronal populations in response to illumination by blue light and to test whether activation of defined neural circuits is sufficient to shape behaviors of interest. Although we have only used the protocol described here in larval stages, the procedure can be adapted to study neurons in adult flies – with the caveat that blue light may not sufficiently penetrate the adult cuticle to stimulate neurons deep in the brain. This procedure takes a week to culture optogenetic animals and about an hour per group for the behavioral assays.
Keywords: Drosophila, optogenetics, channelrhodopsin-2, behavior, nociception
Introduction
A major goal of neurobiology is to understand how the activity of defined sets of neurons controls animal behavior. With its genetic amenability, rich behavioral repertoire, and complex nervous system, Drosophila is an ideal model system to study the neural basis of animal behaviors. In the past two decades, a genetic tool kit has been developed which allows the investigator to either inhibit or activate defined sets of neurons in living intact animals1-11.
Optogenetic tools allow for the control of neurons with light. A variety of optogenetic tools now exist12, but in studies of Drosophila, the Channelrhodopsin-2 (ChR2) light sensitive ion channel has been most successfully utilized. ChR2 is a light-gated cation channel from the green algae, Chlamydomonas reinhardtii13. Upon photoactivation (with ∼480 nm blue light) ChR2 evokes depolarizing inward current on a millisecond timescale. When expressed heterologously in neurons this allows for precise control of neuronal activity with light14, 15. ChR2 is genetically targetable, either by fusion to enhancer elements or through the use of binary expression systems such as GAL4/UAS16, LexA/LexAop17-19 or the Q/QUAS system20, 21. This allows the investigator to control neuronal activity in well defined sets of neurons of behaving animals with both spatial and temporal resolution. Channelrhodpsin-2 was first expressed in neurons of Drosophila by Schroll et al.9 who showed that optogenetic stimulation of neurons could serve as an unconditioned stimulus in olfactory learning assays. In these experiments, larvae formed negative or positive association with odors that had been earlier paired with optogenetic stimulation of dopamine or octopamine neurons respectively.
Optogenetics may also be used to trigger complete behavioral fixed action patterns22. As an example of this application, here we describe our protocol for optogenetic activation of larval nociceptive behavior, which was first described by Hwang et al.22. Upon blue light illumination of larvae expressing ChR2∷YFP, escape behavior is triggered within 30-50 milliseconds of the start of illumination
Overview
Drosophila larvae show a highly stereotypic nocifensive escape locomotion in response to noxious stimuli23. When exhibiting nocifensive behavior, the larva rotates around the anterior-posterior body axis in a corkscrew-like motion. This rolling response is specifically triggered by noxious sensory stimuli such as temperatures greater than 39°C or harsh mechanical stimulation (> 30 mN)23. Indeed, in its natural environment, this is thought to be a defensive behavior against attacks by female parasitoid wasps whose sharp ovipositor is used for penetrating the larval epidermis for the purpose of egg laying22. In Hwang et al. the optogenetic technique with ChR2∷YFP was developed and used to demonstrate that Class IV multidendritic (md) neurons are nociceptive sensory neurons whose activation is sufficient to trigger larval nocifensive escape locomotion22.
The workflow of the protocol described here is shown in Figure 1 and is broken into three main stages:
Figure 1. Workflow for the optogenetic activation of larval nociception.
The chart summarizes procedures and necessary materials for this protocol.
Stage 1: genetic crosses are performed to yield progeny that will be used for optogenetic manipulation; animals expressing ChR2 in neurons of interest are generated by crossing a selected UAS-ChR2 strain to a GAL4 driver strain. In our example for optogenetic activation of larval nociception behaviors, the ppk1.9-GAL4 driver line is crossed to UAS-ChR2∷YFP (Yellow Fluorescent Protein-tagged ChR2) as the ppk1.9-GAL4 driver causes relatively restricted expression of UAS transgenes in larval nociceptive neurons22, 24.
Stage 2: animals expressing ChR2∷YFP are raised in the presence of food containing all-trans-retinal. In the fly model, unlike in mammalian systems, dietary supplementation of the all-trans-retinal co-factor is necessary for the production of functional ChR2∷YFP. It is also critical to keep these experimental animals in the dark since ChR2∷YFP is a photosensitive channel and can be inactivated by excessive exposure to light.
Stage 3: optogenetic animals are tested for their behavioral response to blue light stimulation beneath a fluorescent stereomicroscope. Since ChR2∷YFP is a blue light gated cation channel, stimulation with blue light activates neurons expressing the channel and subsequently triggers behaviors which are governed by the activated neurons. In our example, the animals expressing ChR2∷YFP in Class IV md neurons exhibit nocifensive escape behavior upon blue light stimulation.
Applications
As summarized in Table 1, optogenetic manipulation has been successfully applied to a variety of neurons and in a number of behavioral and physiological assays by Drosophila researchers. For the study of freely behaving animals the protocol described here is most useful for the larval stages which are optically transparent. Because the adult cuticle strongly absorbs blue light, successful applications in adults have been limited to neurons near the surface of the animal, or in electrophysiological preparations where the cuticle has been removed. This technique has also been utilized in the investigation of the signaling pathways that operate in those circuits25. In addition, our robust protocol can easily be used in teaching laboratories26 and for demonstrations of the basic principles of neural circuits. In our experience, students that observe the optogenetically triggered nociception behavior are invariably amazed by the ability to remotely control animal behavior with pulses of light.
Table 1. Cell types and behaviors to which channelrhodopsin-2 have been applied.
| Stage | Cell types | Application/Behavioral assay |
|---|---|---|
| Larva | Dopaminergic neurons | Olfactory learning9 |
| Octopaminergic neurons | Olfactory learning9 | |
| Muscles | Contraction9, 49_ and electrophysiology49 | |
| Motor neurons | Contraction9, 49, basal locomotion41 and electrophysiology9, 49 | |
| Multidendritic sensory neurons Painless-gal4 positive neurons | Nociception behavior22, 25 and light avoidance42 | |
| Painless-gal4 positive neurons | Avoidance behavior28 | |
| Body wall sensory neurons | Basal locomotion41_ | |
| Chordotonal neurons | Thermotaxis50 | |
| Atypical guanylyl cyclase expressing neurons | Hypoxia avoidance51 | |
| Olfactory receptor neurons | Olfactory behaviors52 | |
| Adult | Olfactory receptor neurons | CO2 avoidance53 |
| Dopaminergic neurons | Basal locomotion28 | |
| Giant fiber neurons | Startle response28 | |
| Gustatory receptor neurons | Proboscis extension28, 54 | |
| Gustatory motor neurons | Proboscis extension54 | |
| Acj6-GAL4 positive neurons | Startle response49 | |
| Projection neurons | Electrophysiology55 |
Limitations
The most severe limitation of the ChR2 approach applies to its use in freely behaving adult flies. Because the adult cuticle strongly absorbs blue light, successful applications in adults have been limited to neurons near the surface of the animal, or in electrophysiological preparations where the cuticle has been removed (Table 1). If activation of adult neurons is desired, alternative approaches described below are available and are likely to be preferable to a ChR2 based approach.
ChR2 is not suitable for experiments in which prolonged or repeated strong neuronal activation is required, since the ChR2 channel shows fast adaptation upon photostimulation and recovers slowly under dark conditions27. These characteristics are also consistent with our observations in behavioral assays; robust behavioral responses are seen immediately after the initial light stimulus, but subsequent light exposures trigger responses less frequently.
The availability of suitable GAL4 driver lines may limit the widespread utility of this method for all neurons and all behaviors. A high expression level of ChR2 at the cell surface is required to achieve reliable activation of neurons and behavioral responses28 (see Experimental design for detail). It is also possible that some neurons with low input resistance may not be activated by ChR2. These limitations may be overcome through the use of driver lines which confer sufficient expression of ChR2. In addition to the expression level, in some cases it may be difficult to identify GAL4 lines that target neurons of interest with specific and sparse expression patterns. However, more restricted expression patterns may be generated through the use of intersectional tools. These include the split-GAL4 driver systems29, 30, the GAL80 repressor that inhibits GAL4 activity31, more recently developed binary expression systems18-20 (see alternative approaches below) and recombinase-mediated mosaic systems32.
ChR2 is photosensitive, and experimental animals must be cultured under dark conditions to avoid photoadaptation of the channel. Thus this protocol might be affected by experimental conditions in which animals must be raised in the presence of light.
Alternative methods
Methods complementary to the use of ChR2 are available for temporal and spatial control of neuronal activity in Drosophila. P2X2, a mammalian ionotropic purinoceptor that has no homologue in flies has been used for optical neuronal control in Drosophila8, 33-36. P2X2 evokes depolarizing currents in response to ATP. Through the injection of caged-ATP and UV radiation pulses for the purpose of uncaging the ATP, the P2X2 channel can be controlled in a temporal fashion. Because P2X2 has larger single-channel conductance compared to ChR212, it is useful for applications which need strong cellular depolarization to evoke behavioral responses. This might be the case if only weak GAL4 expression drivers are available or if the neurons of interest have a low input resistance or other intrinsic excitability issues that might limit their control by Channelrhodpsin-2. However, the need for microinjections to supply caged-ATP to individual experimental animals is laborious in comparison to dietary supplementation of all-trans-retinal for ChR2. This becomes a major obstacle to apply the P2X2 approach to large populations of animals. In addition, the lasers necessary for the supply of intense UV radiation are costly in comparison to the light sources needed for blue light activation of ChR2.
“Thermogenetic activation” approaches have also been developed for use in flies. These techniques rely on the expression of thermally sensitive Transient Receptor Potential (thermoTRP) channels. Following expression of the thermoTRP channel in the neuronal subset of interest, neuronal depolarization is then induced by shifting the flies to a temperature that matches the activation threshold of the particular thermoTRP channel. For example, the mammalian TRPM8 channel activates fly neurons in response to cooling10 and the Drosophila TRPA1 channel activates neurons with warming11, 37-40. Because the TRP channels adapt slowly in comparison to ChR241, these manipulations may be more useful for applications which require sustained stimulation of neurons. In addition, since flies have low thermal capacitance, neurons deep in the brain (even in adult animals) can be targeted without difficulty using a thermogenetic approach. ThermoTRPs also do not require supplementations of co-factors or ligands to activate neurons. A downside of using thermoTRPs is that neural activation through control of ambient temperature in the thermogenetic technique is slower, and thus provides lower temporal precision in behavioral manipulation compared to optogenetic approaches using light.
Experimental design
Intensity and wavelength of light
Proper light intensity and wavelength is required for activation of ChR2. Although it has been reported that 525 ± 25 nm green light is capable of activating ChR242, the excitation maximum for ChR2 is 460 ∼ 480 nm13, 28, 43. In our hands, green light is ineffective at eliciting larval nociceptive behaviors from ChR2∷YFP expressing animals. Thus, for the most effective activation, blue light around its excitation maximum spectra should be delivered to the test animals.
UAS-ChR2 transgenic lines
Neuronal and behavioral manipulation by ChR2 requires sufficient expression of the protein and localization to the plasma membrane of targeted cells28. Because the expression level of transgenes can vary among independent transgenic lines due to chromosomal position effects44 it is important to use potent ChR2 transgenic lines to elicit robust optically-manipulated behaviors. We have generated several independent transgenic fly strains with genomic insertions of YFP tagged ChR2 under control of GAL4 binding sites (UAS-ChR2∷YFP). The use of the YFP tagged Channelrhodpsin-2 is advantageous because the visible fluorescence of the YFP can be used to visualize the expression pattern of the transgene. In addition, YFP signal intensity on the tagged ChR2 allowed us to identify insertions which were permissive for robust expression from the transgene (Figure 2a). These strains are freely available to investigators interested in using this technique. A line with a second chromosome insertion (UAS-ChR2∷YFP line C) is commonly used in our laboratory and has potent activity in triggering behavior (Figure 2b). This potency is correlated with the expression level of the YFP tag of the transgene (Figure 2a, b). Other strains with multiple insertions of the UAS-ChR2∷YFP transgene provide even higher levels of expression.
Figure 2. Efficacy of behavioral response compared amongUAS-channelrhodopsin-2 lines.
(a) Comparison of Channelrhodopsin-2∷YFP fluorescence in various UAS-channelrhodopsin-2∷YFP insertion lines generated by Hwang et al. (2007)22. YFP signal intensities are shown as normalized to line C. (b) Frequency of larval nociception behavior seen when ppk1.9-GAL4 was crossed to the various UAS-channelrhodopsin-2∷YFP lines. Note the frequency of triggered nociception behavior correlates with the expression level of Channelrhodopsin∷YFP Reprinted with modifications from Hwang et al. (2007)22 with permission.
New variants of ChR2 have been engineered45, 46, and two of these variants (H134R and ChETA∷YFP) are currently available for use in Drosophila41, 47. The H134R variant has potentially improved performance in activation of neurons due to it's prolonged open state, lowered inactivation kinetics, and enhanced sodium selectivity. Although in vivo experiments using the ChETA variant have yet to be performed in flies, this variant has fast kinetics compared to wild-type ChR248 allowing for more precise control of spike timing.
The protocol described here has been developed for use with the UAS-ChR2∷YFP line C developed in our laboratory. Nevertheless, our protocol can be used without modification for other strains given the caveat that the exact line chosen for the experiment will produce different behavioral response levels (as shown in Figure 2)22.
Alternative binary expression systems
In addition to GAL4/UAS system, more recently introduced binary expression systems such as LexA/lexAop and Q system can be used to drive ChR2. To adapt these newer binary systems, it is necessary to use the appropriate transgenic flies that carry a ChR2 transgene expressed under control of the promoter for the appropriate driver., In the case of the LexA system the lexAop element should be used, in the case of the Q system the QUAS element is needed. At this time, the only alternatives to the GAL4/UAS based Chr2 system are the, QUAS-ChETA strains described above 47. Because of their more recent development, libraries of LexA or QF driver strains are of limited scale in comparison to GAL4 driver libraries. However, with the ease of Drosophila transgenesis it is relatively straightforward to clone the regulatory sequences from GAL4 strains of interest and to generate the desired strains.
Culture condition of experimental animals
Several days of all-trans-retinal feeding are necessary to achieve sufficient activity of ChR2 We recommend at least three days of dietary supplementation. In addition, to avoid photoisomerization of the ChR2 chromophore, experimental animals should be raised in the dark.
Controls
Animals expressing ChR2 raised on food without all-trans-retinal supplementation (ATR-) serve as a control for ChR2-induced behavioral responses. In addition, although most optogenetic behavioral responses in Drosophila require dietary supplementation with all-trans-retinal, light-dependent behavioral responses are sometimes seen even in the absence of all-trans-retinal supplementation (W.D.T. unpublished). In order to control for this possibility, all-trans-retinal fed UAS-ChR2 (UAS-ChR2∷YFP line C in our example) animals in the absence of a driver should be included for comparison.
Materials Equipment
Epifluorescence microscope (Leica MZ16 FA or equivalent) equipped with a camera mount (C-mount).
Mercury burner (LEJ ebq100 isolated)
Mercury bulb (OSRAM mercury short arc HBO 103 W/2) CAUTION Mercury is highly toxic when inhaled or absorbed from skin. The handling of mercury bulbs needs extra attention to avoid breakage and exposure to mercury.
GFP excitation filter (Leica GFP2 480/40)
Halogen light source
Photometer
Martin Microscope MM99 Camera Adapter (Used for attaching the video camera to the C-mount)
Digital video camera (SONY DCR-DVD610 or equivalent)
Tri-pour graduated beaker for fly cages (VWR cat. no. 25384-152)
60 × 15 mm petri dish (BD Falcon cat. no. 25373-085)
Paint brush
Pipet
Rubber bands
0.6ml Microcentrifuge tubes
Fly incubator
Cardboard box
Reagents
Fly strains (in the example below, we use UAS-ChR2∷YFP line C and ppk1.9-GAL4 which are available from the authors on request)
Cornmeal molasses agar fly medium
All-trans-retinal (Sigma cat. no. R2500-100MG, Santa Cruz cat. no. sc-210778) CAUTION All-trans-retinal may cause skin irritation and be harmful if inhaled or ingested. Gloves and handling in safety hood recommended. The solution may stain surfaces if spilled.
100% Ethanol (Pharmaco-AAPER cat. no. 111ACS200)
Dry bakers yeast (Lesaffre Yeast Corporation)
Deionized water
100% apple juice (Supermarket brands)
Agar (Difco cat. no. 0145-17-0)
Sucrose (Mallinckrodt Analytical cat. no. 8360-06)
Methyl 4-hydroxybenzoate (Sigma-Aldrich cat. no. H5501-500g)
Equipment Setup
Fly incubator
Maintain flies in an incubator set to 25°C, 75% relative humidity, 12-hour light/dark cycle.
Egg collection cages
Drill approximately 20 holes into the side and base of the tri-pour graduated beakers. These holes allow for air flow into the fly cages and prevent the build up of excess condensation within the cages.
CRITICAL Not all brands of 60 mm petri dishes will fit properly into the opening of the tri-pour beakers. We recommend the brand listed above when building fly cages from the tri-pour beakers. Many fly laboratories have their own solution for egg/embryo collection cages. Any of these will suffice.
Epifluorescence microscope
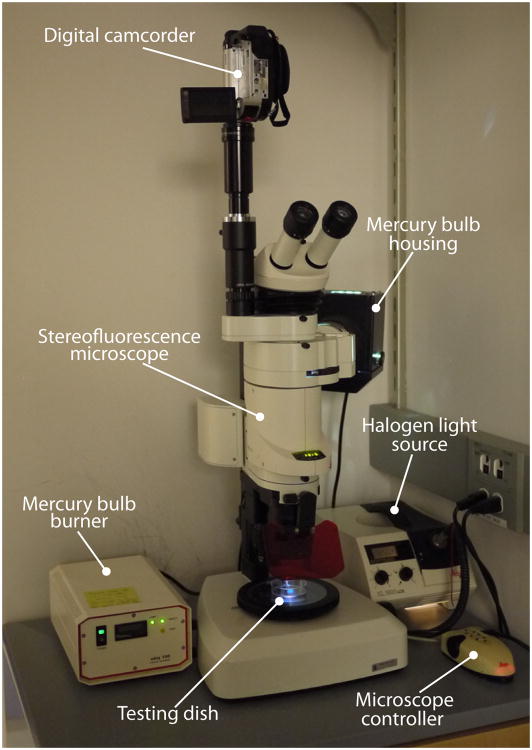
A conventional epifluorescence microscope is used for the delivery of blue light to the optogenetic animals. Our laboratory utilizes a Leica MZ16 FA stereo epifluorescence microscope for optogenetic activation experiments (Figure 3). Delivery of blue light for excitation of Channelrhodpsin2∷YFP is through a GFP excitation band pass filter with a 40 nm band of light centered around 480 nm (Leica GFP2 480/40). The mercury light source used in our laboratory (OSRAM, HBO 103 W/2) is powered by the standard housing supplied by Leica (LEJ ebq100). For the purpose of video recording the behavioral responses, we utilize a digital video camera mounted on an MM99 microscope adapter (available from Martin Microscope Company). This provides a relatively inexpensive solution for video recording through the microscope. For the purpose of video recording, animals are weakly illuminated from below (∼ 300 lux) with a halogen light source.
Figure 3. Setup of videorecording and illuminating devices.
The photograph shows the stereofluorescence microscope with a digital video recorder where optogenetic behavioral experiments are performed in our laboratory.
CRITICAL The use of the proper excitation wavelength is critical for evoking behaviors using ChR2.
Reagent Setup
Fly collection Collect virgin females of either UAS-ChR2∷YFP line C or ppk1.9-GAL4. Maintain the collected virgin females in yeasted vials for at least 3 days to allow for oogenesis to take place. Well-fed females will produce a greater yield of eggs in subsequent steps.
Apple juice agar plates Add 30 g agar to 1 L water in a 2 L flask, autoclave for 15 min to completely dissolve agar. Stir the solution continuously and allow it to cool to ∼ 60 °C. Mix 333 ml apple juice, 2 g methyl-4-hydroxybenzoate, and 30 g sucrose in another flask and bring to a boil in a microwave oven. Remove from the oven and mix until the sucrose is completely dissolved. Add the apple juice solution to the molten agar and mix well. Pipette 10 ml of the medium into each 60 mm petri dishes. This recipe makes approximately 140 plates. Leave the covered plates overnight at room temperature (21∼23 °C) to let the agar solidify. Store the plates at 4 °C in sealed plastic bags to prevent desiccation. Plates normally last ∼ 2 months.
100 mM All-trans-retinal stock solution Dissolve 100 mg all-trans-retinal into 3.52 ml 100% EtOH. Aliquot 100 μl to 0.6 ml tubes. Wrap the individual tubes with aluminum foil and store them in a light proof box at -20 °C.
CRITICAL All-trans-retinal is a photosensitive reagent. Minimum exposure to light is recommended.
Procedure
Setting up crosses to generate the optogenetic animals
TIMING 1 min per cross followed by 2 day incubation period.
1. Cross at least 10 virgin females and 5 males of appropriate UAS-ChR2 and GAL4 driver lines in a yeasted fly food vial and allow mating to proceed for 2 days; in the example used here, we cross UAS-ChR2∷YFP line C to the ppk1.9-GAL4 driver line. As both transgenes are present on autosomes, the direction of the cross is unimportant. Set up multiple crosses to obtain a sufficient number of experimental progeny (> 100 animals in total for the example nociception experiment). Homozygous strains for both transgenes are available and if these are used all of the progeny will be of the desired genotype. Alternatively, heterozygous driver animals and UAS-ChR2∷YFP can be used and the YFP fluorescence of the crossed progeny can be scored to test for inheritance of both transgenes.
CRITICAL If scoring of YFP fluorescence is used for the purpose of genotyping, this genotyping step must be performed AFTER step 16. Otherwise, blue light illumination used for scoring of YFP will bleach all-trans-retinal of the ChR2 molecule (and trigger behaviors).
Preparing egg collection plates TIMING ∼ 30 min
2. Remove apple juice plates from storage at 4 °C and allow them to warm to room temperature.
3. Prepare yeast paste containing 500 μM all-trans-retinal. For each apple juice plate: Add 2.5 μl of 100 mM All-trans-retinal per plate to 500 μl deionized water and mix well. Add 0.7 g dried yeast and mix thoroughly with a spatula.
For control egg collection plates lacking all-trans-retinal, add 2.5 μl of 100% EtOH in place of 100 mM all-trans-retinal.
CRITICAL STEP It is important to use freshly prepared yeast paste containing 500 μM all-trans-retinal.
4. Using a spatula, spread the yeast paste around the center of an apple juice plate.
Egg collection TIMING 1 min per cage, followed by 24 hour incubation
5. Anesthetize the mated flies and transfer them to a fly cage (See Equipment setup). Place an egg collection plate snugly into the opening of the cage. Secure the plate to the cage using a rubber band. Save the lid of apple juice agar plate for later use.
6. Lay the cages on their sides until the flies have recovered from anesthesia. This will prevent the adult flies from falling into the yeast paste. Once the flies have recovered, place the cages in a dark box in a fly incubator with the apple juice plate down. Note that the all-trans-retinal fed animals become light sensitive and placing them in the box is used to protect them from the light. Allow the flies to lay eggs for 24 hours and then replace the seeded apple juice agar plate with a freshly yeasted apple juice agar plate.
? TROUBLESHOOTING
Larval culture TIMING 4 day incubation
7. Return the petri dish lids that were saved from step 5 to the seeded egg collection plates. Place the seeded plates in a dark box in the fly incubator until the required developmental stage is reached; in our example, the eggs were allowed to develop for an additional 4 days until they reached the 3rd instar larval stage.
CRITICAL Three days of feeding on all-trans-retinal is necessary to accumulate sufficient activity of the ChR2 to activate larval nociceptive neurons. This prevents the use of this method for the examination of early larval stages. Similarly, successful applications in adult flies require several days of all-trans-retinal dietary supplementation55.
Setting up the microscope and video camera TIMING 20 min
8. Turn on the halogen light source for the microscope. Adjust the halogen light brightness to approximately 300 lux at the microscope stage. Use a hand-held photometer for measurement of the light intensity.
CRITICAL STEP Excessive illumination of experimental animals with halogen light causes decreased behavioral responses to subsequent illumination with blue light (this is presumably caused by photoisomerization of the ChR2∷YFP by prolonged exposure to the halogen light).
9. Switch on the mercury lamp and allow the bulb to warm up for at least 5 min. Select the appropriate GFP excitation filter on the fluorescent microscope. The blue light delivered through the GFP excitation filter is necessary for the activation of Channelrhodpsin-2. In addition, the GFP channel can be used for identifying YFP expressing animals after the behaviors have been observed. Adjust the alignment of mercury bulb to achieve a homogeneous field of illumination.
10. Set the intensity of blue light by adjusting the optical zoom of the microscope. Place a hand-held photometer under the fluorescence microscope. Place a piece of paper with crosshair target on the surface of the light sensor of the light meter, adjust focus. Remove the paper and find an optical zoom setting which produces a measured light intensity of 14 klux when blue light is properly focused on the sensor of the photometer. A lower zoom will provide a lower light intensity. Return focus back to the microscope stage without changing the optical zoom.
CRITICAL STEP Proper blue light intensity is critical for optogenetic experiments with ChR2. Since mercury bulbs decay over use, it is recommended to check and adjust blue light intensity regularly before starting the behavioral assay. Change the bulb according to the usage hours recommended by the manufacturer.
11. Turn on the digital camcorder. Set up zoom, exposure, and white balance settings to prepare for videotaping.
Behavioral assay TIMING ∼ 1 hour per experimental group
12. Larvae must be tested in a shallow aqueous environment to examine optogenetically-induced nocifensive escape behavior. For this purpose, prepare two 60 mm dishes by the addition of 1 ml deionized water. In order to lower the surface tension of the water, add a few particles of dried yeast and allow them to dissolve. Swirl the aqueous solution so that the bottom of the dish is completely covered with a thin layer of water.
13. Remove the larval culture plates (from step 6) from the dark box in the fly incubator. Using a paintbrush, gently transfer larvae from the culture plate to one of the 60-mm petri dishes that were prepared in step 10.
CRITICAL STEP Unnecessary exposure of optogenetic larvae to light can cause poor larval responses. Collection plates with larvae that are waiting to be tested should remain inside the dark box.
? TROUBLESHOOTING
14. After collecting the larvae, gently transfer the larvae one at time to the other 60 mm petri dish prepared in step 10 (testing dish) using a fine paintbrush. Allow the larva to recover until it starts forward peristalsis.
15. Place the testing dish containing the larva under dim halogen illumination on the microscope and begin the videotaping. Deliver a 5 second blue light pulse once to each larvae using the fluorescence shutter of the microscope.
16. Score resulting behavior. Larval nocifensive escape locomotion is defined as a full 360° rotation around the long body axis. The larval responses are scored into one of two categories: Note “roll” if the larva makes a full 360° rotation during 5 seconds of photostimulation, note “no roll” if this rotation or a partial rotation occurs during stimulation. Videotaped behavior can be analyzed at a later time off line.
? TROUBLESHOOTING
17. Genotype the larva using the presence or absence of YFP signal when necessary. Discard the photostimulated larva from the testing dish. It is desirable to test > 100 animals for each experimental group for statistical analysis.
Data analysis TIMING 5 min per group to input data to a spread sheet and ∼ 1 hour for further analysis.
18. Calculate percentages of animals which show nocifensive escape locomotion when stimulated with blue light for each experimental group. Compare data statistically using appropriate statistical analysis for proportional data such as Fisher's exact test, p-value should be adjusted for multiple comparisons (i.e. with Bonferroni correction) if necessary.
Anticipated Results
Representative results are shown in Figure 4. In all-trans-retinal fed animals, larval responses are typically seen in less than 1 second from the initiation of blue light. With 14 klux blue light, 80 to 90% of all-trans-retinal fed larvae show nocifensive escape locomotion in response to photostimulation. In contrast, only 1 to 2% of larvae without all-trans-retinal feeding or UAS-ChR2∷YFP animals without ppk1.9-GAL4 driver show blue light-induced nocifensive escape locomotion.
Figure 4. Optogenetically triggered nociceptive responses in all-trans-retinal fed ppk1.9-GAL4/UAS-channelrhodopsin-2∷YFP and control animals.
Blue light induced nocifensive escape locomotion was seen in 83±2.8% of all-trans-retinal fed larvae expressing Channelrhodopsin-2∷YFP in nociceptive sensory neurons (ppk × UAS-ChR2∷YFP, ATR+; n = 181, p < e2-16 compared to the controls with Fisher's exact test with Boferroni correction). In contrast, neither the all-trans-retinal fed controls (ppk × UAS-ChR2∷YFP, ATR-; n = 112) nor the driverless control with all-trans-retinal supplementation (w1118 × UAS-ChR2∷YFP, ATR+; n = 102) showed nocifensive responses to light stimulation (0.9±0.9% and 2±1.4%, respectively). Error bars represent the standard error. The graph was made based on the data originally published in Hwang et al. (2007) 22 with permission.
Timing
Step 1: 1 min per cross followed by 2 day incubation, preparing fly crosses
Steps 2-4: ∼ 30 min, preparing egg collection plates
Step 5,6: 1 min per cage followed by 24 hour incubation, egg collection
Step 7: 4 day incubation, larval culture
Step 8-11: 20 min, setting up illuminating devices and camcorder
Steps 12-17: ∼ 1 hour per group, behavioral assay
Steps 18: 5 min per group to input data to and ∼ 1 hour for further analysis
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
Table 2. Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 6 | Poor yield of eggs | Flies not acclimatized to fly cages | Repeat egg collections until mated flies have acclimatized to the cages |
| Too few flies. | Increase the number of adult flies in the cages. | ||
| Some strains of flies do not lay well in cages. | Reverse the direction of the cross. Increase the number of flies. | ||
| 13 | Larvae die in egg collection plates | Too much condensation in the lid causes hypoxia | Wipe condensation from the lid after two days of incubation. Dry apple juice plates for additional days. |
| 16 | Larvae don't show nocifensive response | Excessive exposure of all-trans-retinal to light. | Use fresh all-trans-retinal stock |
| Blue light intensity is too low. | Check and adjust light intensity using photometer. | ||
| Hg bulb is old | Change the Hg bulb. | ||
| Hg bulb is not properly focused | Focus Hg bulb to ensure uniform illumination of the field. |
Supplementary Material
The movie shows a representative example of a ppk1.9-GAL4/UAS-channelrhodopsin-2∷YFP larva executing the stereotyped escape locomotion in response to blue light stimulations. The movie was originally published in Hwang et al. (2007)22
Acknowledgments
This work was supported by a grant from the National Institutes of Neurological Disorders and Stroke 5R01NS054899 (W.D.T.). K.H. is a fellow supported by the Japan Society for the Promotion of Science postdoctoral fellowship for research abroad.
Footnotes
Competing Financial Interest Statement: The authors declare they have no competing financial interests.
Author Contribution Statement: W.D.T. conceived of and supervised the project. R.Y.H. performed experiments and generated original data. K.H., R.Y.H. and W.D.T designed the standardized protocol. K.H. and W.D.T. wrote the manuscript.
References
- 1.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 2.White B, Osterwalder T, Keshishian H. Molecular genetic approaches to the targeted suppression of neuronal activity. Curr Biol. 2001;11:R1041–1053. doi: 10.1016/s0960-9822(01)00621-2. [DOI] [PubMed] [Google Scholar]
- 3.Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 4.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 5.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 6.Broughton SJ, Kitamoto T, Greenspan RJ. Excitatory and inhibitory switches for courtship in the brain of Drosophila melanogaster. Curr Biol. 2004;14:538–547. doi: 10.1016/j.cub.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Nitabach MN, et al. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Peabody NC, et al. Characterization of the decision network for wing expansion in Drosophila using targeted expression of the TRPM8 channel. J Neurosci. 2009;29:3343–3353. doi: 10.1523/JNEUROSCI.4241-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miesenbock G. Optogenetic control of cells and circuits. Annu Rev Cell Dev Biol. 2011;27:731–758. doi: 10.1146/annurev-cellbio-100109-104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel G, et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 16.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 17.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagi R, Mayer F, Basler K. Refined LexA transactivators and their use in combination with the Drosophila Gal4 system. Proc Natl Acad Sci U S A. 2010;107:16166–16171. doi: 10.1073/pnas.1005957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter CJ, Luo L. Using the Q system in Drosophila melanogaster. Nat Protoc. 2011;6:1105–1120. doi: 10.1038/nprot.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang RY, et al. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 24.Ainsley JA, et al. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13:1557–1563. doi: 10.1016/s0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulver SR, Hornstein NJ, Land BL, Johnson BR. Optogenetics in the teaching laboratory: using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ. 2011;35:82–91. doi: 10.1152/advan.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegemann P, Moglich A. Channelrhodopsin engineering and exploration of new optogenetic tools. Nat Methods. 2011;8:39–42. doi: 10.1038/nmeth.f.327. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 29.Luan H, et al. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting CY, et al. Focusing transgene expression in Drosophila by coupling Gal4 with a novel split-LexA expression system. Genetics. 2011;188:229–233. doi: 10.1534/genetics.110.126193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- 32.Bohm RA, et al. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci U S A. 2010;107:16378–16383. doi: 10.1073/pnas.1004669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 35.Hu A, Zhang W, Wang Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc Natl Acad Sci U S A. 2010;107:10262–10267. doi: 10.1073/pnas.0914912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Zhang W, Qiao W, Hu A, Wang Z. Functional connectivity and selective odor responses of excitatory local interneurons in Drosophila antennal lobe. Neuron. 2010;67:1021–1033. doi: 10.1016/j.neuron.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS One. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang K, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J Mol Biol. 2008;375:686–694. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 44.Spradling AC, Rubin GM. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983;34:47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- 45.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin JY. A user's guide to channelrhodopsin variants: features, limitations and future developments. Exp Physiol. 2011;96:19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen LK, Stowers RS. A Gateway MultiSite recombination cloning toolkit. PLoS One. 2011;6:e24531. doi: 10.1371/journal.pone.0024531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunaydin LA, et al. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann G, et al. Manipulation of an innate escape response in Drosophila: photoexcitation of acj6 neurons induces the escape response. PLoS One. 2009;4:e5100. doi: 10.1371/journal.pone.0005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vermehren-Schmaedick A, Ainsley JA, Johnson WA, Davies SA, Morton DB. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellmann D, et al. Optogenetically Induced Olfactory Stimulation in Drosophila Larvae Reveals the Neuronal Basis of Odor-Aversion behavior. Front Behav Neurosci. 2010;4:27. doi: 10.3389/fnbeh.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh GS, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 54.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie shows a representative example of a ppk1.9-GAL4/UAS-channelrhodopsin-2∷YFP larva executing the stereotyped escape locomotion in response to blue light stimulations. The movie was originally published in Hwang et al. (2007)22