Abstract
A genome-wide association study of educational attainment was conducted in a discovery sample of 101,069 individuals and a replication sample of 25,490. Three independent SNPs are genome-wide significant (rs9320913, rs11584700, rs4851266), and all three replicate. Estimated effects sizes are small (R2 ≈ 0.02%), approximately 1 month of schooling per allele. A linear polygenic score from all measured SNPs accounts for ≈ 2% of the variance in both educational attainment and cognitive function. Genes in the region of the loci have previously been associated with health, cognitive, and central nervous system phenotypes, and bioinformatics analyses suggest the involvement of the anterior caudate nucleus. These findings provide promising candidate SNPs for follow-up work, and our effect size estimates can anchor power analyses in social-science genetics.
Twin and family studies suggest that a broad range of psychological traits (1), economic preferences (2–4), and social and economic outcomes (5) are moderately heritable. Discovery of genetic variants associated with such traits leads to insights regarding the biological pathways underlying human behavior. If the predictive power of a set of genetic variants considered jointly is sufficiently large, then a “risk score” that aggregates their effects could be useful to control for genetic factors that are otherwise unobserved, or to identify populations with certain genetic propensities, for example in the context of medical intervention (6).
To date, however, few if any robust associations between specific genetic variants and social-scientific outcomes have been identified likely because existing work [for review see (7)] has relied on samples that are too small [for discussion, see (4, 6, 8, 9)]. In this paper, we apply to a complex behavioral trait—educational attainment—an approach to gene discovery that has been successfully applied to medical and physical phenotypes (10), namely meta-analyzing data from multiple samples. The phenotype of educational attainment is available in many samples with genotyped subjects (5). Educational attainment is influenced by many known environmental factors, including public policies. Educational attainment is strongly associated with social outcomes, and there is a well-documented health-education gradient (5, 11). Estimates suggest that around 40% of the variance in educational attainment is explained by genetic factors (5). Furthermore, educational attainment is moderately correlated with other heritable characteristics (1), including cognitive function (12) and personality traits related to persistence and self-discipline (13).
To create a harmonized measure of educational attainment, we coded study-specific measures using the International Standard Classification of Education (ISCED 1997) scale (14). We analyzed a quantitative variable defined as an individual’s years of schooling (EduYears) and a binary variable for college completion (College). College may be more comparable across countries, whereas EduYears contains more information about individual differences within countries.
A genome-wide association study (GWAS) meta-analysis was performed across 42 cohorts in the discovery phase. The overall discovery sample comprises 101,069 individuals for EduYears and 95,427 for College. Analyses were performed at the cohort level according to a pre-specified analysis plan, which restricted the sample to Caucasians (to help reduce stratification concerns). Educational attainment was measured at an age at which subjects were very likely to have completed their education [over 95% of the sample was at least 30; (5)]. On average, subjects have 13.3 years of schooling, and 23.1% have a college degree. To enable pooling of GWAS results, all studies conducted analyses with data imputed to the HapMap 2 CEU (r22.b36) reference set. To guard against population stratification, the first four principal components of the genotypic data were included as controls in all the cohort-level analyses. All study-specific GWAS results were quality controlled, cross-checked, and meta-analyzed using single genomic control and a sample-size weighting scheme at three independent analysis centers.
At the cohort level, there is little evidence of general inflation of p-values. As in previous GWA studies of complex traits (15), the Q-Q plot of the meta-analysis exhibits strong inflation. This inflation is not driven by specific cohorts and is expected for a highly polygenic phenotype even in the absence of population stratification (16).
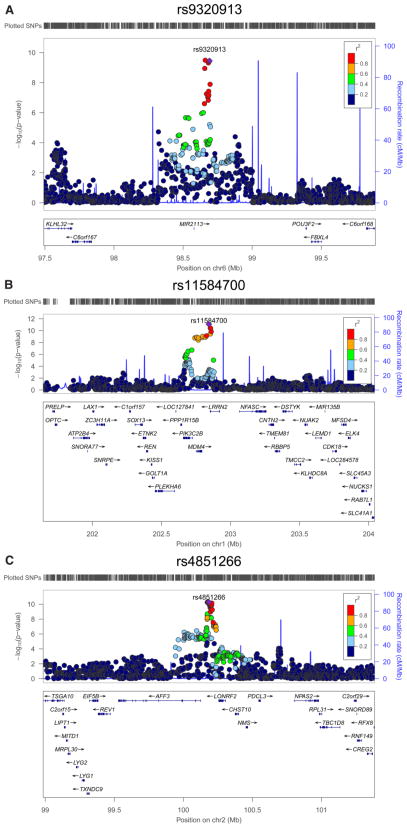
From the discovery phase, we identified one genome-wide significant locus (rs9320913, p = 4.2 × 10−9) and three suggestive loci (defined as p < 10−6) for EduYears. For College, we identified two genome-wide significant loci (rs11584700, p = 2.1 × 10−9, and rs4851266, p = 2.2 × 10−9) and an additional four suggestive loci (Table 1). We conducted replication analyses in 12 additional, independent cohorts that became available after the completion of the discovery meta-analysis, using the same pre-specified analysis plan. For both EduYears and College, the replication sample comprises 25,490 individuals.
Table 1.
The results of the GWAS meta-analysis for the independent signals reaching p < 10−6 in the discovery stage.
| SNP | Chr | Position (bp) | Nearest gene | Effective allele | Frequency | Discovery stage
|
Replication stage
|
Combined stage
|
Combined stage – sex-specific
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta/OR | P-value | I2 | Phet | Beta/OR | P-value | Beta/OR | P-value | Phet | Beta/OR (Males) | P-value (Males) | Beta/OR (Females) | P-value (Females) | ||||||
| EduYears | ||||||||||||||||||
| rs9320913 | 6 | 98691454 | LOC100129158 | A | 0.483 | 0.106 | 4.19×10−9 | 18.3 | 0.097 | 0.077 | 0.012 | 0.101 | 3.50×10−10 | 0.350 | 0.095 | 1.87×10−4 | 0.100 | 1.43×10−6 |
| rs3783006 | 13 | 97909210 | STK24 | C | 0.454 | 0.096 | 2.29×10−7 | 0 | 0.982 | 0.056 | 0.055 | 0.088 | 8.45×10−8 | 0.959 | 0.064 | 1.44×10−2 | 0.108 | 3.35×10−7 |
| rs8049439 | 16 | 28745016 | ATXN2L | T | 0.581 | 0.090 | 7.12×10−7 | 10.7 | 0.229 | 0.065 | 0.026 | 0.086 | 1.15×10−7 | 0.205 | 0.097 | 1.43×10−4 | 0.078 | 1.90×10−4 |
| rs13188378 | 5 | 101958587 | SLCO6A1 | A | 0.878 | −0.136 | 7.49×10−7 | 0 | 0.791 | 0.091 | 0.914 | −0.097 | 1.37×10−4 | 0.646 | −0.134 | 8.21×10−3 | −0.080 | 5.92×10−3 |
| College | ||||||||||||||||||
| rs11584700 | 1 | 202843606 | LRRN2 | A | 0.780 | 0.921 | 2.07×10−9 | 13.8 | 0.179 | 0.912 | 4.86×10−4 | 0.919 | 8.24×10−12 | 0.221 | 0.934 | 6.11×10−4 | 0.911 | 2.12×10−9 |
| rs4851266 | 2 | 100184911 | LOC150577 | T | 0.396 | 1.050 | 2.20×10−9 | 23.7 | 0.049 | 1.049 | 0.003 | 1.050 | 5.33×10−11 | 0.072 | 1.054 | 1.55×10−5 | 1.052 | 6.74×10−8 |
| rs2054125 | 2 | 199093966 | PLCL1 | T | 0.064 | 1.468 | 5.55×10−8 | 7 | 0.325 | 1.098 | 0.225 | 1.376 | 2.12×10−7 | 0.268 | 1.264 | 1.74×10−2 | 1.503 | 1.95×10−7 |
| rs3227 | 6 | 33770273 | ITPR3 | C | 0.498 | 1.043 | 6.02×10−8 | 5 | 0.363 | 1.010 | 0.280 | 1.037 | 3.24×10−7 | 0.415 | 1.046 | 9.44×10−5 | 1.029 | 1.37×10−3 |
| rs4073894 | 7 | 104254200 | LHFPL3 | A | 0.207 | 1.076 | 4.41×10−7 | 0 | 0.765 | 1.003 | 0.467 | 1.062 | 5.55×10−6 | 0.513 | 1.050 | 2.18×10−2 | 1.073 | 1.74×10−5 |
| rs12640626 | 4 | 176863266 | GPM6A | A | 0.580 | 1.041 | 4.94×10−7 | 10.9 | 0.234 | 1.000 | 0.495 | 1.034 | 7.48×10−6 | 0.420 | 1.038 | 1.59×10−3 | 1.031 | 7.61×10−4 |
The rows in bold are the independent signals reaching p < 5 × 10−8 in the discovery stage. “Frequency” refers to allele-frequency in the combined-stage meta-analysis. “Beta/OR” refers to the effect size in the EduYears analysis and to the Odds Ratio in the College analysis. All p-values are from the sample-size-weighted meta-analysis (fixed effects). The p-value in the replication stage meta-analysis was calculated from a one-sided test. I2 represents the % heterogeneity of effect size between the discovery stage studies. phet is the heterogeneity p-value.
For each of the ten loci that reached at least suggestive significance, we brought forward for replication the SNP with the lowest p-value. The three genome-wide significant SNPs replicate at the Bonferroni-adjusted 5% level, with point estimates of the same sign and similar magnitude (Fig. 1 and Table 1). The seven loci that did not reach genome-wide significance did not replicate (the effect went in the anticipated direction in 5 out of 7 cases). The meta-analytic findings are not driven by extreme results in a small number of cohorts (see phet in Table 1), by cohorts from a specific geographic region (figs. S7 to S15), or by a single sex (figs. S3 to S6). Given the high correlation between EduYears and College (5), it is unsurprising that the set of SNPs with low p-values exhibit considerable overlap in the two analyses (tables S8 and S9).
Fig. 1.
Regional association plots of replicated loci associated with educational attainment [(A): rs9320913, (B): rs11584700, (C): rs4851266]. The plots are centered on the SNPs with the lowest p-values in the discovery stage (purple diamond). The R2 values are from the CEU HapMap 2 samples. The CEU HapMap 2 recombination rates are indicated with a blue line on the right-hand y-axis. The figures were created with LocusZoom (http://csg.sph.umich.edu/locuszoom/).
The observed effect sizes of the three replicated individual SNPs are small [see (5) for discussion]. For EduYears, the strongest effect identified (rs9320913) explains 0.022% of phenotypic variance in the replication sample. This R2 corresponds to a difference of ~1 months of schooling per allele. For college completion, the SNP with the strongest estimated effect (rs11584700) has an odds ratio of 0.912 in the replication sample, equivalent to a 1.8 percentage-point difference per allele in the frequency of completing college.
We subsequently conducted a “combined stage” meta-analysis, including both the discovery and replication samples. This analysis revealed additional genome-wide significant SNPs: four for EduYears and three for College. Three of these newly genome-wide significant SNPs (rs1487441, rs11584700, rs4851264) are in linkage disequilibrium with the replicated SNPs. The remaining four are located in different loci and warrant replication attempts in future research: rs7309, a 3′UTR variant in TANK; rs11687170, close to GBX2; rs1056667, a 3′UTR variant in BTN1A1; and rs13401104 in ASB18.
Using the results of the combined meta-analyses of discovery and replication cohorts, we conducted a series of complementary and exploratory supplemental analyses to aid in interpreting and contextualizing the results: gene-based association tests; eQTL analyses of brain and blood tissue data; pathway analysis; functional annotation searches; enrichment analysis for cell-type-specific overlap with H3K4me3 chromatin marks; and predictions of likely gene function using gene-expression data. Table S20 summarizes promising candidate loci identified through follow-up analyses (5). Two regions in particular showed convergent evidence from functional annotation, blood cis-eQTL analyses, and gene-based tests: chromosome 1q32 (including LRRN2, MDM4, and PIK3C2B) and chromosome 6 near the Major Histocompatibility Complex (MHC). We also find evidence that in anterior caudate cells, there is enrichment of H3K4me3 chromatin marks (believed to be more common in active regulatory regions) in the genomic regions implicated by our analyses (fig. S20). Many of the implicated genes have previously been associated with health, central nervous system, or cognitive-process phenotypes in either human-GWAS or model-animal studies (table S22). Gene co-expression analysis revealed that several implicated genes (including BSN, GBX2, LRRN2, and PIK3C2B) are likely involved in pathways related to cognitive processes (such as learning and long-term memory) and neuronal development or function (table S21).
Although the effects of individual SNPs on educational attainment are small, many of their potential uses in social science depend on their combined explanatory power. To evaluate the combined explanatory power, we constructed a linear polygenic score (5) for each of our two education measures using the meta-analysis results (combining discovery and replication), excluding one cohort. We tested these scores for association with educational attainment in the excluded cohort. We constructed the scores using SNPs whose nominal p-values fall below a certain threshold, ranging from 5 × 10−8 (only the genome-wide significant SNPs were included) to 1 (all SNPs were included).
We replicated this procedure with two of the largest cohorts in the study, both of which are family-based samples (QIMR and STR). The results suggest that educational attainment is a highly polygenic trait (Fig. 2 and table S23): the amount of variance accounted for increases as the p-value threshold becomes less conservative (i.e., includes more SNPs). The linear polygenic score from all measured SNPs accounts for ≈ 2% (p = 1.0 × 10−29) of the variance in EduYears in the STR sample and ≈ 3% (p = 7.1 × 10−24) in the QIMR sample.
Fig. 2.
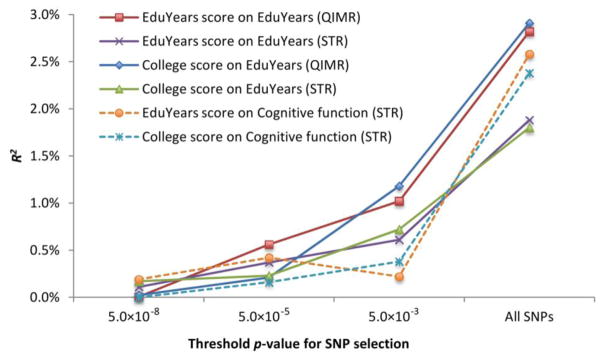
Solid lines show results from regressions of EduYears on linear polygenic scores in a set of unrelated individuals from the QIMR (N = 3526) and STR (N = 6770) cohorts. Dashed lines show results from regressions of Cognitive function on linear polygenic scores in a sample from STR (N = 1419). The scores are constructed from the meta-analysis for either EduYears or College, excluding the QIMR and STR cohorts.
To explore one of the many potential mediating endophenotypes, we examined how much the same polygenic scores (constructed to explain EduYears or College) could explain individual differences in cognitive function. While it would have been preferable to explore a richer set of mediators, this variable was available in STR, a dataset where we had access to the individual-level genotypic data. Cognitive function had been measured in a subset of males using the Swedish Enlistment Battery (used for conscription) (5, 17). The estimated R2 ≈ 2.5% (p < 1.0 × 10−8) for cognitive function is actually slightly larger than the fraction of variance in educational attainment captured by the score in the STR sample. One possible interpretation is that some of the SNPs used to construct the score matter for education through their stronger, more direct effects on cognitive function (5). A mediation analysis (table S24) provides tentative evidence consistent with this interpretation.
The polygenic score remains associated with educational attainment and cognitive function in within-family analyses (table S25). Thus, these results appear robust to possible population stratification.
If the size of the training sample used to estimate the linear polygenic score increased, the explanatory power of the score in the prediction sample would be larger because the coefficients used for constructing the score would be estimated with less error. In (5), we report projections of this increase. We also assess, at various levels of explanatory power, the benefits from using the score as a control variable in a randomized educational intervention (5). An asymptotic upper bound for the explanatory power of a linear polygenic score is the additive genetic variance across individuals captured by current SNP microarrays. Using combined data from STR and QIMR, we estimate that this upper bound is 22.4% (S.E. = 4.2%) in these samples (5) (table S12).
Placed in the context of the GWAS literature (10), our largest estimated SNP effect size of 0.02% is over an order of magnitude smaller than those observed for height and BMI: 0.4% (15) and 0.3% (18) respectively. While our linear polygenic score for education achieves an R2 of 2% estimated from a sample of 120,000, a score for height reached 10% estimated from a sample of 180,000 (15), and a score for BMI using only the top 32 SNPs reached 1.4% (18). Taken together, our findings suggest that the genetic architecture of complex behavioral traits is far more diffuse than that of complex physical traits.
Existing claims of “candidate gene” associations with complex social-science traits have reported widely varying effect sizes—many with R2 values more than one hundred times larger than those we find (4, 6). For complex social-science phenotypes that are likely to have a genetic architecture similar to educational attainment, our estimate of 0.02% can serve as a benchmark for conducting power analyses and evaluating the plausibility of existing findings in the literature.
The few GWAS studies conducted to date in social-science genetics have not found genome-wide significant SNPs that replicate consistently (19, 20). One commonly proposed solution is to gather better measures of the phenotypes in more environmentally homogenous samples. Our findings demonstrate the feasibility of a complementary approach: identify a phenotype that, although more distal from genetic influences, is available in a much larger sample [see (5) for a simple theoretical framework and power analysis]. The genetic variants uncovered by this “proxy-phenotype” methodology can then serve as a set of empirically-based candidate genes in follow-up work, such as tests for associations with well-measured endophenotypes (e.g., personality, cognitive function), research on gene-environment interactions, or explorations of biological pathways.
In social-science genetics, researchers must be especially vigilant to avoid misinterpretations. One of the many concerns is that a genetic association will be mischaracterized as “the gene for X,” encouraging misperceptions that genetically influenced phenotypes are immune to environmental intervention [for rebuttals, see (21, 22)] and misperceptions that individual SNPs have large effects (which our evidence contradicts). If properly interpreted, identifying SNPs and constructing polygenic scores are steps toward usefully incorporating genetic data into social-science research.
Supplementary Material
Acknowledgments
This research was carried out under the auspices of the Social Science Genetic Association Consortium (SSGAC), a cooperative enterprise among medical researchers and social scientists that coordinates genetic association studies for social science variables. Data for our analyses come from many studies and organizations, some of which are subject to an MTA (5). Results from the meta-analysis are available at the website of the consortium, www.ssgac.org. The formation of the SSGAC was made possible by an EAGER grant from the NSF and a supplemental grant from the NIH/OBSSR (SES-1064089). This research was also funded in part by the Söderbergh Foundation (E9/11), the NIA/NIH through grants P01-AG005842, P01-AG005842-20S2, P30-AG012810, and T32-AG000186-23 and the Intramural Research Program of the NIA/NIH. For a full list of acknowledgments, see (5).
Footnotes
www.sciencemag.org/cgi/content/full/science.1235488/DC1
Materials and Methods
References and Notes
- 1.Plomin R, DeFries J, Knopik V, Neiderhiser J. Behavioral Genetics. Vol. 6. Worth Publishers; 2013. p. 560. [Google Scholar]
- 2.Cesarini D, Dawes CT, Johannesson M, Lichtenstein P, Wallace B. Genetic variation in preferences for giving and risk taking. Q J Econ. 2009;124:809. doi: 10.1162/qjec.2009.124.2.809. [DOI] [Google Scholar]
- 3.Benjamin DJ, et al. The genetic architecture of economic and political preferences. Proc Natl Acad Sci USA. 2012;109:8026. doi: 10.1073/pnas.1120666109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp JP, et al. Molecular genetics and economics. J Econ Perspect. 2011;25:57. doi: 10.1257/jep.25.4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Please see the supplementary materials on Science Online.
- 6.Benjamin DJ, et al. The promises and pitfalls of genoeconomics. Annu Rev Econ. 2012;4:627. doi: 10.1146/annurev-economics-080511-110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65:831. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenbach JP, et al. European Union Working Group on Socioeconomic Inequalities in Health, Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 12.Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13. doi: 10.1016/j.intell.2006.02.001. [DOI] [Google Scholar]
- 13.Heckman JJ, Rubinstein Y. The importance of noncognitive skills: Lessons from the GED testing program. Am Econ Rev. 2001;91:145. doi: 10.1257/aer.91.2.145. [DOI] [Google Scholar]
- 14.UNESCO Institute for Statistics. International Standard Classification of Education. 2006. [Google Scholar]
- 15.Lango Allen H, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, et al. GIANT Consortium, Genomic inflation factors under polygenic inheritance. Eur J Hum Genet. 2011;19:807. doi: 10.1038/ejhg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlstedt B. Cognitive Abilities: Aspects of Structure, Process and Measurement. Acta Universitatis Gothoburgensis; Göteborg, Sweden: 2000. [Google Scholar]
- 18.Speliotes EK, et al. MAGIC; Procardis Consortium, Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Moor MH, et al. Meta-analysis of genome-wide association studies for personality. Mol Psychiatry. 2012;17:337. doi: 10.1038/mp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benyamin B, et al. Wellcome Trust Case Control Consortium 2 (WTCCC2), Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.184. doi: 10.1038/mp.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jencks C. Heredity, environment, and public policy reconsidered. Am Sociol Rev. 1980;45:723. doi: 10.2307/2094892. [DOI] [PubMed] [Google Scholar]
- 22.Goldberger AS. Heritability. Economica. 1979;46:327. doi: 10.2307/2553675. [DOI] [Google Scholar]
- 23.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84:210. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servin B, Stephens M. Imputation-based analysis of association studies: Candidate regions and quantitative traits. PLoS Genet. 2007;3:e114. doi: 10.1371/journal.pgen.0030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997. doi: 10.1111/j.0006-341X.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 29.Willer CJ, Li Y, Abecasis GR. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SCAN. SNP and CNV Annotation Database. 2012 www.scandb.org/
- 31.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 32.de Bakker PIW, et al. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet. 2008;17:R122. doi: 10.1093/hmg/ddn288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taubman P. Earnings, education, genetics, and environment. J Hum Resour. 1976;11:447. doi: 10.2307/145426. [DOI] [PubMed] [Google Scholar]
- 34.Branigan AR, McCallum KJ, Freese J. Variation in the heritability of educational attainment: An international meta-analysis. Northwestern University Institute for Policy Research Working Paper. 2013;13-09 [Google Scholar]
- 35.Cesarini D. Essays on Genetic Variation and Economic Behavior. Massachusetts Institute of Technology; 2010. [Google Scholar]
- 36.Lichtenstein P, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 37.Turkheimer E. Three laws of behavior genetics and what they mean. Curr Dir Psychol Sci. 2000;9:160. doi: 10.1111/1467-8721.00084. [DOI] [Google Scholar]
- 38.Yang J, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross CE, Wu C. The links between education and health. Am Sociol Rev. 1995;60:719. doi: 10.2307/2096319. [DOI] [Google Scholar]
- 40.Cutler DM, Lleras-Muney A. In: Making Americans Healthier: Social and Economic Policy as Health Policy. House J, Schoeni R, Kaplan G, Pollack H, editors. Russell Sage Foundation; New York: 2008. [Google Scholar]
- 41.Johnson W, et al. Does education confer a culture of healthy behavior? Smoking and drinking patterns in Danish twins. Am J Epidemiol. 2011;173:55. doi: 10.1093/aje/kwq333. [DOI] [PubMed] [Google Scholar]
- 42.Johnson W, et al. Education reduces the effects of genetic susceptibilities to poor physical health. Int J Epidemiol. 2010;39:406. doi: 10.1093/ije/dyp314. [DOI] [PubMed] [Google Scholar]
- 43.Vermeiren AP, et al. Do genetic factors contribute to the relation between education and metabolic risk factors in young adults? A twin study. Eur J Public Health. 2012 doi: 10.1093/eurpub/cks167. [DOI] [PubMed] [Google Scholar]
- 44.Lleras-Muney A. The relationship between education and adult mortality in the United States. Rev Econ Stat. 2005;72:189. doi: 10.1111/0034-6527.00329. [DOI] [Google Scholar]
- 45.Lager ACJ, Torssander J. Causal effect of education on mortality in a quasi-experiment on 1.2 million Swedes. Proc Natl Acad Sci USA. 2012;109:8461. doi: 10.1073/pnas.1105839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arendt JN. Does education cause better health? A panel data analysis using school reforms for identification. Econ Educ Rev. 2005;24:149. doi: 10.1016/j.econedurev.2004.04.008. [DOI] [Google Scholar]
- 47.Illig T, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42:137. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu JZ, et al. AMFS Investigators, A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR. Estimation of pleiotropy between complex diseases using using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics. 2012;28:2540. doi: 10.1093/bioinformatics/bts474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trynka G, et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet. 2013;45:124. doi: 10.1038/ng.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cvejic A, et al. SMIM1 underlies the Vel blood group and influences red blood cell traits. Nat Genet. 2013;45:542. doi: 10.1038/ng.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreae LC, Lumsden A, Gilthorpe JD. Chick Lrrn2, a novel downstream effector of Hoxb1 and Shh, functions in the selective targeting of rhombomere 4 motor neurons. Neural Dev. 2009;4:27. doi: 10.1186/1749-8104-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinzen EL, et al. Tissue-specific genetic control of splicing: Implications for the study of complex traits. PLoS Biol. 2008;6:e1. doi: 10.1371/journal.pbio.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webster JA, et al. NACC-Neuropathology Group, Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84:445. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fehrmann RSN, et al. Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet. 2011;7:e1002197. doi: 10.1371/journal.pgen.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelis M, et al. Genetic structure of Europeans: A view from the North-East. PLoS ONE. 2009;4:e5472. doi: 10.1371/journal.pone.0005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westra HJ, et al. MixupMapper: Correcting sample mix-ups in genome-wide datasets increases power to detect small genetic effects. Bioinformatics. 2011;27:2104. doi: 10.1093/bioinformatics/btr323. [DOI] [PubMed] [Google Scholar]
- 58.Lee PH, O’Dushlaine C, Thomas B, Purcell SM. INRICH: Interval-based enrichment analysis for genome-wide association studies. Bioinformatics. 2012;28:1797. doi: 10.1093/bioinformatics/bts191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashburner M, et al. The Gene Ontology Consortium, Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25:25. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koch CM, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Need AC, et al. A genome-wide study of common SNPs and CNVs in cognitive performance in the CANTAB. Hum Mol Genet. 2009;18:4650. doi: 10.1093/hmg/ddp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Logue MW, et al. Multi-Institutional Research on Alzheimer Genetic Epidemiology (MIRAGE) Study Group, A comprehensive genetic association study of Alzheimer disease in African Americans. Arch Neurol. 2011;68:1569. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burroughs-Garcia J, Sittaramane V, Chandrasekhar A, Waters ST. Evolutionarily conserved function of Gbx2 in anterior hindbrain development. Dev Dyn. 2011;240:828. doi: 10.1002/dvdy.22589. [DOI] [PubMed] [Google Scholar]
- 64.Wassarman KM, et al. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development. 1997;124:2923. doi: 10.1242/dev.124.15.2923. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Chatterjee M, Li JY. The mouse homeobox gene Gbx2 is required for the development of cholinergic interneurons in the striatum. J Neurosci. 2010;30:14824. doi: 10.1523/JNEUROSCI.3742-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muers M. Complex disease: Ups and downs at the MHC. Nat Rev Genet. 2011;12:456. doi: 10.1038/nrg3021. [DOI] [PubMed] [Google Scholar]
- 67.Migliorini D, et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol. 2002;22:5527. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Altrock WD, et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787. doi: 10.1016/S0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 70.Burton PR, et al. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parkes M, et al. Wellcome Trust Case Control Consortium. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrett JC, et al. UK IBD Genetics Consortium; Wellcome Trust Case Control Consortium 2, Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franke A, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barrett JC, et al. NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium, Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955. doi: 10.1038/ng.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jostins L, et al. International IBD Genetics Consortium (IIBDGC), Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGovern DP, et al. NIDDK IBD Genetics Consortium, Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson CA, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imielinski M, et al. Western Regional Alliance for Pediatric IBD; International IBD Genetics Consortium; NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium, Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stahl EA, et al. BIRAC Consortium; YEAR Consortium, Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferguson A, Sedgwick DM, Drummond J. Morbidity of juvenile onset inflammatory bowel disease: Effects on education and employment in early adult life. Gut. 1994;35:665. doi: 10.1136/gut.35.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackner LM, Sisson DP, Crandall WV. Review: Psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004;29:243. doi: 10.1093/jpepsy/jsh027. [DOI] [PubMed] [Google Scholar]
- 82.Frazer KA, et al. International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J, et al. Genetic Investigation of ANthropometric Traits (GIANT) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369, S1. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daetwyler HD, Villanueva B, Woolliams JA. Accuracy of predicting the genetic risk of disease using a genome-wide approach. PLoS ONE. 2008;3:e3395. doi: 10.1371/journal.pone.0003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayes BJ, Visscher PM, Goddard ME. Increased accuracy of artificial selection by using the realized relationship matrix. Genet Res. 2009;91:47. doi: 10.1017/S0016672308009981. [DOI] [PubMed] [Google Scholar]
- 87.Goddard ME, Wray NR, Verbyla K, Visscher PM. Estimating effects and making predictions from genome-wide marker data. Stat Sci. 2009;24:517. doi: 10.1214/09-STS306. [DOI] [Google Scholar]
- 88.Visscher PM, Yang J, Goddard ME. A commentary on ‘common SNPs explain a large proportion of the heritability for human height’ by Yang et al. (2010) Twin Res Hum Genet. 2010;13:517. doi: 10.1375/twin.13.6.517. [DOI] [PubMed] [Google Scholar]
- 89.Fryer RG. Financial incentives and student achievement: Evidence from randomized trials. Q J Econ. 2011;126:1755. doi: 10.1093/qje/qjr045. [DOI] [Google Scholar]
- 90.Heckman J, Moon SH, Pinto R, Savelyev P, Yavitz A. Analyzing social experiments as implemented: A reexamination of the evidence from the HighScope Perry Preschool Program. Quant Econ. 2010;1:1. doi: 10.3982/QE8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eckenrode J, et al. Long-term effects of prenatal and infancy nurse home visitation on the life course of youths: 19-year follow-up of a randomized trial. Arch Pediatr Adolesc Med. 2010;164:9. doi: 10.1001/archpediatrics.2009.240. [DOI] [PubMed] [Google Scholar]
- 92.Masse LN, Barnett WS. Cost-Effectiveness and Educational Policy. Larchmont, NY: Eye on Education, Inc; 2002. A benefit-cost analysis of the Abecedarian early childhood intervention; pp. 157–173. [Google Scholar]
- 93.Heckman JJ, Moon SH, Pinto R, Savelyev PA, Yavitz A. The rate of return to the HighScope Perry Preschool Program. J Public Econ. 2010;94:114. doi: 10.1016/j.jpubeco.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harris TB, et al. Age, Gene/Environment Susceptibility–Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fraser A, et al. Cohort profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2012;42:97. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt R, et al. Assessment of cerebrovascular risk profiles in healthy persons: Definition of research goals and the Austrian Stroke Prevention Study (ASPS) Neuroepidemiology. 1994;13:308. doi: 10.1159/000110396. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: Three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53:132. doi: 10.1212/WNL.53.1.132. [DOI] [PubMed] [Google Scholar]
- 98.Shock NW, et al. Normal human aging: The Baltimore Longitudinal Study of Aging. NIH Publication. 1984:84–2450. [Google Scholar]
- 99.Einarsdóttir K, et al. Linkage disequilibrium mapping of CHEK2: Common variation and breast cancer risk. PLoS Med. 2006;3:e168. doi: 10.1371/journal.pmed.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang ET, Hedelin M, Adami HO, Grönberg H, Bälter KA. Alcohol drinking and risk of localized versus advanced and sporadic versus familial prostate cancer in Sweden. Cancer Causes Control. 2005;16:275. doi: 10.1007/s10552-004-3364-2. [DOI] [PubMed] [Google Scholar]
- 101.Hedelin M, et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: The cancer prostate Sweden study (Sweden) Cancer Causes Control. 2006;17:169. doi: 10.1007/s10552-005-0342-2. [DOI] [PubMed] [Google Scholar]
- 102.Lindmark F, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. J Natl Cancer Inst. 2004;96:1248. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 103.Firmann M, et al. The CoLaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudan I, et al. “10001 Dalmatians:” Croatia launches its national biobank. Croat Med J. 2009;50:4. doi: 10.3325/cmj.2009.50.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ehret GB, et al. International Consortium for Blood Pressure Genome-Wide Association Studies; CARDIoGRAM consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen consortium; CHARGE-HF consortium, Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sleegers K, et al. Cerebrovascular risk factors do not contribute to genetic variance of cognitive function: The ERF study. Neurobiol Aging. 2007;28:735. doi: 10.1016/j.neurobiolaging.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 107.Sayed-Tabatabaei FA, et al. Heritability of the function and structure of the arterial wall: Findings of the Erasmus Rucphen Family (ERF) study. Stroke. 2005;36:2351. doi: 10.1161/01.STR.0000185719.66735.dd. [DOI] [PubMed] [Google Scholar]
- 108.Vartiainen E, et al. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39:504. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- 109.Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: Studies on Finnish twins and twin families. Twin Res. 2002;5:366. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- 110.Purcell SM, et al. International Schizophrenia Consortium, Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.FBPP Investigators. Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39:3. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 112.Harris TB, et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci. 2000;904:462. doi: 10.1111/j.1749-6632.2000.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 113.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 114.Ferrucci L, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 115.Wichmann HE, Gieger C, Illig R. MONICA/KORA Study Group, KORA-gen - Resource for population genetics, controls and a broad specturm of disease phenotypes. Gesundheitswesen. 2005;67:26. doi: 10.1055/s-2005-858226. [DOI] [PubMed] [Google Scholar]
- 116.Stolk RP, et al. Universal risk factors for multifactorial diseases. LifeLines: A three-generation population-based study. Eur J Epidemiol. 2008;23:67. doi: 10.1007/s10654-007-9204-4. [DOI] [PubMed] [Google Scholar]
- 117.Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86:130. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- 118.Deary IJ, et al. The Lothian Birth Cohort 1936: A study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Magnus P, et al. MoBa Study Group, Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 120.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435. doi: 10.1080/j.1600-0412.2000.079006435.x. [DOI] [PubMed] [Google Scholar]
- 121.Penninx BWJH, et al. NESDA Research Consortium, The Netherlands Study of Depression and Anxiety (NESDA): Rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rantakallio P. Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scand. 1969;193(suppl):193, 1. [PubMed] [Google Scholar]
- 123.Sabatti C, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin NW, et al. Educational attainment: A genome wide association study in 9538 Australians. PLoS ONE. 2011;6:e20128. doi: 10.1371/journal.pone.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Estrada K, et al. GRIMP: A web- and grid-based tool for high-speed analysis of large-scale genome-wide association using imputed data. Bioinformatics. 2009;25:2750. doi: 10.1093/bioinformatics/btp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hofman A, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol. 2011;26:657. doi: 10.1007/s10654-011-9610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bennett DA, et al. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bierut LJ, et al. Gene, Environment Association Studies Consortium, A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA. 2010;107:5082. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pilia G, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Völzke H, et al. Cohort profile: The study of health in Pomerania. Int J Epidemiol. 2011;40:294. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 131.Magnusson PKE, et al. The Swedish Twin Registry: Establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16:317. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- 132.Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and Healthy Ageing Twin Study. Int J Epidemiol. 2013;42:76. doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raitakari OT, et al. Cohort profile: The cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37:1220. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- 134.Pfaffenrath V, et al. Regional variations in the prevalence of migraine and tension-type headache applying the new IHS criteria: The German DMKG Headache Study. Cephalalgia. 2009;29:48. doi: 10.1111/j.1468-2982.2008.01699.x. [DOI] [PubMed] [Google Scholar]
- 135.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255:1121. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 136.Aromaa A. Health and functional capacity in Finland: Baseline results of the Health 2000 health examination survey. Kansanterveyslaitos Folkhälsoinstitutet National Public Health Institute Kansanterveyslaitoksen Julkaisuja B12. 2004 [Google Scholar]
- 137.McEvoy M, et al. Cohort profile: The Hunter Community Study. Int J Epidemiol. 2010;39:1452. doi: 10.1093/ije/dyp343. [DOI] [PubMed] [Google Scholar]
- 138.Weir D. Biosocial Surveys. In: Weinstein M, Vaupel JW, Wachter KW, editors. Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys. Vol. 78. 2007. chap. 4. [Google Scholar]
- 139.Miller MB, et al. The Minnesota Center for Twin and Family Research genome-wide association study. Twin Res Hum Genet. 2012;15:767. doi: 10.1017/thg.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee JH, Cheng R, Graff-Radford N, Foroud T, Mayeux R. National Institute on Aging Late-Onset Alzheimer’s Disease Family Study Group, Analyses of the National Institute on Aging late-onset Alzheimer’s disease family study: Implication of additional loci. Arch Neurol. 2008;65:1518. doi: 10.1001/archneur.65.11.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boomsma DI, et al. Netherlands Twin Register: From twins to twin families. Twin Res Hum Genet. 2006;9:849. doi: 10.1375/twin.9.6.849. [DOI] [PubMed] [Google Scholar]
- 142.McQuillan R, et al. Runs of homozygosity in European populations. Am J Hum Genet. 2008;83:359. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Theodoraki EV, et al. Fibrinogen beta variants confer protection against coronary artery disease in a Greek case-control study. BMC Med Genet. 2010;11:28. doi: 10.1186/1471-2350-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mukherjee S, et al. Cohort profile: The Western Australian Sleep Health Study. Sleep Breath. 2012;16:205. doi: 10.1007/s11325-011-0491-3. [DOI] [PubMed] [Google Scholar]
- 145.Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behav Genet. 1996;26:89. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- 146.Miller P, Mulvey C, Martin N. The return to schooling: Estimates from a sample of young Australian twins. Labour Econ. 2006;13:571. doi: 10.1016/j.labeco.2004.10.008. [DOI] [Google Scholar]
- 147.Silventoinen K, Krueger RF, Bouchard TJ, Jr, Kaprio J, McGue M. Heritability of body height and educational attainment in an international context: Comparison of adult twins in Minnesota and Finland. Am J Hum Biol. 2004;16:544. doi: 10.1002/ajhb.20060. [DOI] [PubMed] [Google Scholar]
- 148.Heath AC, et al. Education policy and the heritability of educational attainment. Nature. 1985;314:734. doi: 10.1038/314734a0. [DOI] [PubMed] [Google Scholar]
- 149.Isacsson G. Estimating the economic return to educational levels using data on twins. J Appl Econ. 2004;19:99. doi: 10.1002/jae.724. [DOI] [Google Scholar]
- 150.Taubman P. The determinants of earnings: Genetics, family, and other environments: A study of white male twins. Am Econ Rev. 1976;66:858. [Google Scholar]
- 151.Lykken DT, Bouchard TJ, Jr, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Genet Med Gemellol (Roma) 1990;39:35. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- 152.Behrman JR, Taubman P, Wales T. Kinometrics: Determinants of Socioeconomic Success Within and Between Families. North-Holland Publishing Company; New York: 1977. p. 35. [Google Scholar]
- 153.Soler Artigas M, et al. GIANT consortium, Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thye T, et al. African TB Genetics Consortium; Wellcome Trust Case Control Consortium. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet. 2010;42:739. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shaffer JR, et al. GWAS of dental caries patterns in the permanent dentition. J Dent Res. 2013;92:38. doi: 10.1177/0022034512463579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eeles RA, et al. UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology; UK ProtecT Study Collaborators; PRACTICAL Consortium, Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pajewski NM, et al. A genome-wide association study of host genetic determinants of the antibody response to Anthrax Vaccine Adsorbed. Vaccine. 2012;30:4778. doi: 10.1016/j.vaccine.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sandholm N, et al. DCCT/EDIC Research Group, New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 2012;8:e1002921. doi: 10.1371/journal.pgen.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Benyamin B, et al. Variants in TF and HFE explain ~40% of genetic variation in serum-transferrin levels. Am J Hum Genet. 2009;84:60. doi: 10.1016/j.ajhg.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qayyum R, et al. A meta-analysis and genome-wide association study of platelet count and mean platelet volume in african americans. PLoS Genet. 2012;8:e1002491. doi: 10.1371/journal.pgen.1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gieger C, et al. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fox CS, et al. GIANT Consortium; MAGIC Consortium; GLGC Consortium, Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet. 2012;8:e1002695. doi: 10.1371/journal.pgen.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kolz M, et al. EUROSPAN Consortium; ENGAGE Consortium; PROCARDIS Consortium; KORA Study; WTCCC, Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Man M, et al. Beyond single-marker analyses: Mining whole genome scans for insights into treatment responses in severe sepsis. Pharmacogenomics J. 2012;13:218. doi: 10.1038/tpj.2012.1. [DOI] [PubMed] [Google Scholar]
- 165.Landers JE, et al. Reduced expression of the Kinesin-Associated Protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2009;106:9004. doi: 10.1073/pnas.0812937106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cotsapas C, et al. GIANT Consortium, Common body mass index-associated variants confer risk of extreme obesity. Hum Mol Genet. 2009;18:3502. doi: 10.1093/hmg/ddp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakabayashi K, et al. Identification of independent risk loci for Graves’ disease within the MHC in the Japanese population. J Hum Genet. 2011;56:772. doi: 10.1038/jhg.2011.99. [DOI] [PubMed] [Google Scholar]
- 168.Kestenbaum B, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol. 2010;21:1223. doi: 10.1681/ASN.2009111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barber MJ, et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS ONE. 2010;5:e9763. doi: 10.1371/journal.pone.0009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yashin AI, Wu D, Arbeev KG, Ukraintseva SV. Joint influence of small-effect genetic variants on human longevity. Aging. 2010;2:612. doi: 10.18632/aging.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 172.Willer CJ, et al. Wellcome Trust Case Control Consortium; Genetic Investigation of ANthropometric Traits Consortium, Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 175.van der Loos MJHM, et al. The molecular genetic architecture of self-employment. PLoS ONE. 2013;8:e60542. doi: 10.1371/journal.pone.0060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.