Abstract
The essential role of Toll-like receptors (TLR) in innate immune responses to bacterial pathogens is increasingly recognized, but very little is known about the role of TLRs in host defense against infections with eukaryotic pathogens. For the present study, we investigated whether TLRs contribute to the innate and acquired immune response to infection with the intracellular protozoan parasite Leishmania major. Our results show that TLR4 contributes to the control of parasite growth in both phases of the immune response. We also addressed the mechanism that results in killing or growth of the intracellular parasites. Control of parasite replication correlates with the early induction of inducible nitric oxide synthase in TLR4-competent mice, whereas increased parasite survival in host cells from TLR4-deficient mice correlates with a higher activity of arginase, an enzyme known to promote parasite growth. This is the first study showing that TLR4 contributes to the effective control of Leishmania infection in vivo.
The leishmaniases are a spectrum of vector-borne parasitic diseases that are a major international public health problem, affecting the lives of millions of people worldwide (49). Experimental infection of mice with Leishmania major is widely used as a model for host resistance or susceptibility. The majority of inbred strains of mice (C57BL/6 and CBA) can control L. major infections, and only mice from a few strains (BALB/c) develop a progressive, nonhealing disease (12, 28, 44, 50). The outcomes of Leishmania infections are determined by adaptive T-helper (Th) cell responses and their interactions with parasitized host cells, usually macrophages. Th1 responses are associated with healing and parasite killing, whereas Th2 responses are associated with nonhealing diseases and uncontrolled parasite growth (50).
Depending on their activation state, macrophages can either host or kill Leishmania. The balance of the activities of inducible nitric oxide synthase (iNOS) and arginase is of crucial importance to parasite fate. These two enzymes are competitively regulated by the cytokines secreted by Th1 and Th2 cells: Th1 cytokines induce iNOS, whereas Th2 cytokines induce arginase (32, 34, 35). The induction of iNOS leads to oxidation of the amino acid l-arginine and the subsequent production of citrulline and nitric oxide (NO). Synthesis of NO has been shown to correlate with the killing of Leishmania parasites both in vitro and in vivo (5, 17, 27, 29, 55), and mice lacking iNOS fail to control L. major infections in vivo (62). Th2 cytokines, on the other hand, promote an alternative activation of macrophages, resulting in reduced killing ability and in the induction of high arginase levels (14, 16, 34). Arginase catalyzes the hydrolysis of l-arginine to urea and l-ornithine, and the latter can be used by the parasite for the synthesis of polyamines, which are essential for the growth of Leishmania (22).
Innate immunity coordinates the inflammatory response to pathogens, and the contribution of Toll-like receptors (TLRs) to this response is becoming widely recognized. TLRs are triggered by pathogen-associated molecular pattern molecules (PAMPs), which are characteristic of various groups of pathogens. Mammalian cells can express up to 10 different TLRs (48), which share an intracellular domain, called Toll-IL-1R (64), and signal through the myeloid differentiation protein 88 (MyD88) (30). The MyD88 signaling pathway results in the nuclear translocation of NF-κB and the expression of cytokine genes. L. major activates interleukin-1α (IL-1α) in macrophages through a MyD88-dependent pathway in vitro (19), and genetically resistant mice lacking the MyD88 adapter protein develop progressive lesions and a polarized Th2 response (36). The PAMPs recognized by some of the TLRs have been identified. For example, TLR2 recognizes peptidoglycan and bacterial lipoproteins on gram-positive bacteria and mycobacteria (2, 6, 56, 60, 66) and is also activated by glycosylphosphatidylinositol anchors and glycoinositolphospholipids from Trypanosoma cruzi (9). Enterobacterial lipopolysaccharide (LPS) is the ligand for TLR4, and the activation of this receptor is critical for the response to gram-negative bacteria (21, 41). In contrast to the ample evidence for the recognition of bacterial PAMPs, very little work has been done on the role of TLRs in the host response to infection with eukaryotic parasites. Although MyD88 signaling is important for the healing of L. major infections (36), there are no reports about TLR engagement by the intracellular parasite Leishmania, and the contribution of TLRs to innate leishmanicidal responses is unknown. Our previous work has suggested a role for TLR4 in the control of L. major infections (33). We showed that C57BL/10ScCr mice, which carry a homozygous null mutation in the tlr4 gene (41), were unable to resolve cutaneous lesions and to restrict parasite growth (33). However, these mice carry an additional mutation, resulting in impaired responsiveness to IL-12 (31, 42). Therefore, to evaluate the contribution of TLR4 alone to the host defense against L. major infection, we used the progenitor strain of the C57BL/10ScCr mice, C57BL/10ScN, which carries an identical deletion of TLR4 (TLR40/0) but has intact IL-12 responsiveness (31, 42, 43). We investigated innate and adaptive immune responses to L. major infection in TLR40/0 mice and compared these to responses in wild-type mice. The results of our study provide compelling evidence that TLR4 engagement is required for efficient parasite control by innate and adaptive immune responses.
MATERIALS AND METHODS
Mice.
C57BL/10ScSn (wild-type), C57BL/10ScN (TLR40/0), and BALB/c mice were bred under specific-pathogen-free conditions in the animal facilities at the Max-Planck-Institut für Immunbiologie, Freiburg, Germany. C57BL/10ScN mice have a homozygous deletion of 74,723 bp at the tlr4 locus which removes all three exons (41, 42). Six- to 10-week-old animals of both sexes were used for this study.
Parasites and infection.
L. major LV39 (MRHO/SU/59/P strain) was maintained in a virulent state by monthly passaging in mice (25). For infections, 2 × 106 stationary-phase parasites were injected subcutaneously into the hind foot pads of mice. The lesions were measured by determining the increase in the foot pad thickness compared to the uninfected contralateral foot pad with a dial gauge caliper (Kröplin Schnelltaster, Schlüchtern, Germany).
To determine whether L. major promastigotes express LPS-like activity, the parasites were analyzed by a Limulus test for endotoxin activity. The results showed that the concentration of L. major promastigotes used for infection (2 × 106 per mouse) did not cause detectable LPS activity (<1 pg). The parasite growth medium also contained no detectable endotoxin activity.
Macrophages.
Bone marrow was obtained by flushing the femurs of naïve 5- to 6-week-old mice. Bone marrow precursor cells were cultured as previously described (33) in hydrophobic Teflon bags (Biofolie 25, Heraeus, Hanau, Germany) in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum, 5% horse serum, and the supernatant of L929 fibroblasts at a final concentration of 15% (vol/vol) as a source of colony-stimulating factors, which drive cell proliferation toward a pure population of bone-marrow-derived macrophages (BMMφ). After 9 to 10 days of culturing, macrophages were harvested and 5 × 105 cells ml−1 were plated and stimulated in the presence or absence of 20 U of gamma interferon (IFN-γ) ml−1, 200 U of tumor necrosis factor alpha (TNF-α) ml−1, 20 U of IL-4 ml−1, 1 μg of LPS ml−1, and 25 × 105 L. major parasites ml−1.
Determination of arginase activity.
Arginase activity was measured in macrophage lysates as previously described (34). Briefly, cells were lysed with 100 μl of 0.1% Triton X-100. After 30 min on a shaker, 100 μl of 25 mM Tris-HCl was added. To 100 μl of this lysate, 10 μl of 10 mM MnCl2 was added, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was conducted by incubating the lysate with 100 μl of 0.5 M l-arginine (pH 9.7) at 37°C for 15 to 20 min. The reaction was stopped with 800 μl of H2SO4 (96%)-H3PO4- (85%)-H2O (1/3/7 [vol/vol/vol]). The urea concentration was measured at 540 nm after the addition of 40 μl of α-isonitrosopropiophenone (dissolved in 100% ethanol) followed by heating at 95°C for 30 min. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1 μmol of urea per min.
Determination of in vitro killing and survival of L. major parasites.
Mature BMMφ (5 × 105 ml−1) were plated and infected 4 h later with 25 × 105 L. major parasites ml−1 in the presence of IFN-γ (20 U ml−1) and TNF-α (200 U ml−1) (Th1 conditions) or IL-4 (20 U ml−1) (Th2 conditions). After 48 h, the macrophages were washed and lysed (33), and a limiting dilution assay was performed (25, 58) to determine the number of viable parasites.
RT-PCR analysis.
For reverse transcription (RT)-PCR, total RNAs were extracted from mouse tissues and macrophages by use of TriReagent (Sigma) according to the manufacturer's instructions. Total RNAs were treated with DNase I (Ambion) and then reverse transcribed with an oligo(dT) primer (Promega) and Omniscript reverse transcriptase (Qiagen). cDNA samples were standardized, based on PCR amplification of the housekeeping gene β-actin. TLR4, arginase 1, and iNOS were amplified by PCR as described previously (1, 63, 65) and were analyzed by densitometry using a UVP bioimaging system and Labworks analysis software.
Determination of parasite load.
The number of living L. major parasites in infected tissues was determined with the parasite limiting dilution assay (25, 58). Briefly, serial dilutions of the footpad homogenate were distributed in replicate wells, and the plates were incubated at 26°C. After 10 to 14 days, the results were read microscopically and the number of viable parasites in the tissue was determined as previously described (25).
Cytokine measurements.
Cells (5 × 106 ml−1) from the lymph nodes draining the lesions of individual mice were restimulated with 1 × 106 L. major promastigotes ml−1. Forty-eight hours later, the culture supernatants were harvested and cytokines and chemokines were measured by an enzyme-linked immunosorbent assay (ELISA) performed according to the suppliers' protocols. Detection limits were 20 pg ml−1 for IL-10, 7.8 pg ml−1 for IL-13, 2 pg ml−1 for IL-6, 1 U ml−1 for IFN-γ, and 4 pg ml−1 for monocyte chemoattractant protein 1 (MCP-1).
Flow cytometric analysis.
For flow cytometric analysis, lymphoid cells were stimulated as described above. Six days later, the cells were harvested and a Ficoll gradient was created. The detection of intracellular cytokine was performed as described previously (40). Briefly, 1.5 × 106 cells were stimulated for 4 hours with 50 ng of phorbol 12-myristate 13-acetate (Sigma) and 500 ng of ionomycin (Calbiochem), or as a control, in the presence of complete medium alone, with 10 μg of brefeldin A (Sigma) added during the last 2 hours. Before surface labeling was done with an anti-CD4 antibody (clone H129.19 or RM4-5; PharMingen), cells were preincubated with 1 μg of the rat anti-mouse monoclonal antibody CD32/CD16 (FcγII/III receptor) (PharMingen) to reduce nonspecific binding. Cells were washed, fixed with 2% formaldehyde (Sigma), and permeabilized with 0.5% saponin (Sigma) before anti-cytokine antibodies or isotype controls were added (anti-IL-4, clone BVD4-1D11; anti-IFN-γ, clone XMG1.2; anti-IL-10, clone JES5-16E3; and appropriately labeled rat immunoglobulin G1 [PharMingen]). Detection of the intracellular cytokines was done with an EPICS XL instrument (Beckman Coulter), and data were analyzed with Beckman Coulter Expo32 software.
Statistical analyses.
Experimental results were analyzed with GraphPad PRISM, version 2.0 (San Diego, Calif.), using a two-tailed Mann-Whitney test, and differences were considered statistically significant at P values of <0.05.
RESULTS
Contribution of TLR4 to the control of parasite growth in vivo.
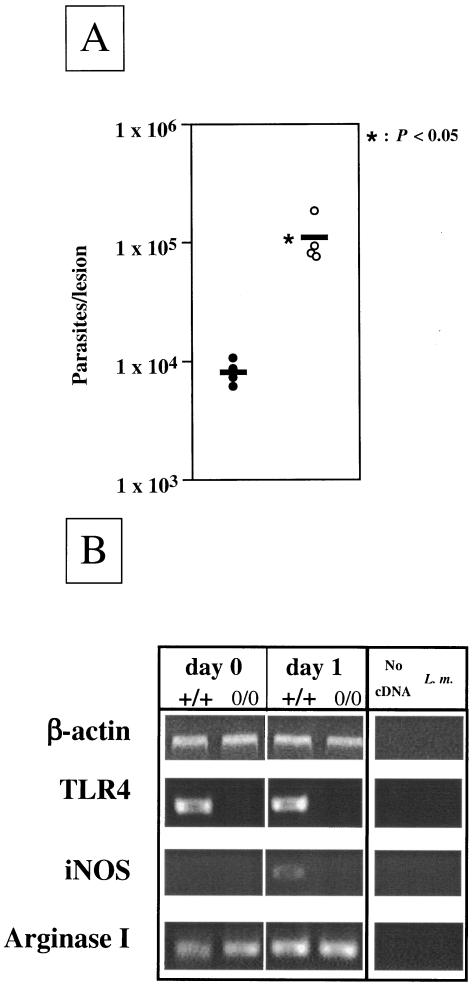
To determine whether the presence of TLR4 contributes to the control of L. major parasites in vivo, we infected wild-type and TLR40/0 mice with L. major promastigotes and assessed the parasite loads at the local sites of infection. The results presented in Fig. 1A show that 24 h after infection, there was a significantly higher number (P < 0.05) of parasites present in the lesions of TLR40/0 mice than in those of wild-type mice. Interestingly, iNOS mRNA expression was detected in the footpads of the wild-type mice at 1 day postinfection but was not detectable at the sites of infection of TLR40/0 mice (Fig. 1B). Arginase 1 mRNA was detectable in both groups of mice (Fig. 1B). TLR4 mRNA was detected in the footpads of naïve and infected wild-type mice (Fig. 1B), indicating that TLR4-expressing cells are present at the local sites of infection. However, the phenotype of the TLR4-expressing cells in vivo remains to be determined.
FIG. 1.
Parasite loads and protein expression in mice. (A) Parasite loads in the footpads of TLR4-competent and TLR4-deficient mice 24 h after L. major infection. Groups of wild-type (n = 4; filled circles) and TLR4-deficient (n = 4; open circles) mice were infected with 2 × 106 L. major promastigotes. At 1 day postinfection, the numbers of viable parasites in the infected footpads were determined. Data show the results of one representative experiment of three independent experiments. Each symbol represents one mouse, and the horizontal black bars represent the means for four individual mice. *, P < 0.05. (B) Expression of iNOS, arginase, and TLR4 mRNAs in the foot pads of L. major-infected TLR4-competent and TLR4-deficient mice. The footpads of wild-type (+/+) and TLR4-deficient (0/0) mice were infected with 2 × 106 L. major parasites. After 24 h, gene expression was analyzed in the footpads of naïve and infected mice by semiquantitative RT-PCR. Samples were standardized by densitometric comparison of the amplification of the housekeeping gene β-actin.
Thus, our results demonstrate that TLR4 is required for more efficient parasite control during the innate immune response to L. major infection.
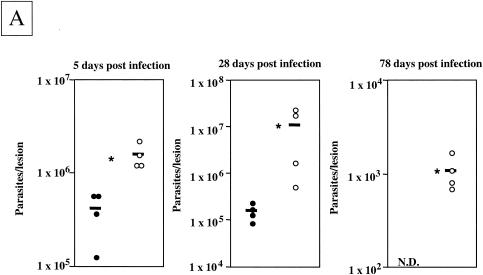
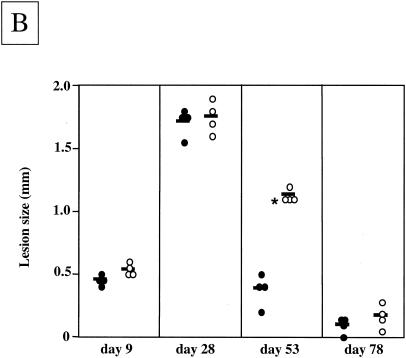
To assess the contribution of TLR4 to the L. major-specific response, we determined the parasite load at 5 days postinfection, at a time when the healing phenotype of the TLR4-competent mice is unequivocally established (28 days) (33) and at a time when TLR4-competent mice had completely resolved their lesions (11 weeks). The lesions of L. major-infected TLR40/0 mice contained significantly larger parasite loads 5, 28, and 78 days after infection (Fig. 2A). We must note that TLR40/0 mice harbored 13.4- and 3.8-fold more parasites than the TLR4-competent mice 1 and 5 days after infection, respectively. Interestingly, 5 days after infection, the parasite loads had increased 49.2-fold for wild-type mice, whereas for TLR40/0 mice the increase was 14.2-fold. These differences in initial parasite control are likely due to more efficient immediate defense systems in TLR4-competent mice. While the parasite loads in the footpads of TLR40/0 mice were still increasing at 28 days postinfection, they had started to decrease in wild-type mice (Fig. 2A). At 11 weeks postinfection, even though TLR40/0 mice had resolved their lesions, they still harbored significant amounts of parasites, whereas no parasites were detectable in the lesions of TLR4-competent mice. These data are in agreement with our previous findings (33) and confirm that TLR4-competent C57BL/10ScSn mice are able to control parasite growth and to resolve lesions. In contrast, TLR40/0 mice not only had significantly larger parasite loads throughout the course of infection, but they were also less efficient at resolving the lesions and still had significantly larger cutaneous lesions and parasite loads after 2 months of infection, at a time when the lesions of the control mice had healed (Fig. 2B).
FIG. 2.
Parasite loads and lesion sizes during infection. (A) Parasite loads in the footpads of TLR4-competent and TLR4-deficient mice during the course of L. major infection. Groups of wild-type (filled circles) and TLR40/0 (open circles) mice were infected with 2 × 106 L. major promastigotes. At 5, 28, and 78 days postinfection, the numbers of viable parasites in the infected footpads were determined by a limiting dilution assay. Each symbol represents one mouse, and the horizontal black bars represent the means for four individual mice. *, P < 0.05. N.D., not detectable. (B) Lesion sizes in TLR4-competent and TLR4-deficient mice during L. major infection. Groups of wild-type (n = 4; filled circles) and TLR40/0 (n = 4; open circles) mice were infected with 2 × 106 L. major promastigotes. Values represent the lesion sizes of individual mice at the indicated times after infection. Each symbol represents one mouse, and the horizontal black bars represent the means for four individual mice. *, P < 0.05.
These results show that TLR40/0 mice cannot efficiently control parasite replication and resolve cutaneous lesions. Therefore, the activation of TLR4 is not only crucial for the innate immune response, but it is also essential for a successful adaptive immune response to L. major parasites.
Influence of TLR4 on parasite-specific cytokine and chemokine production.
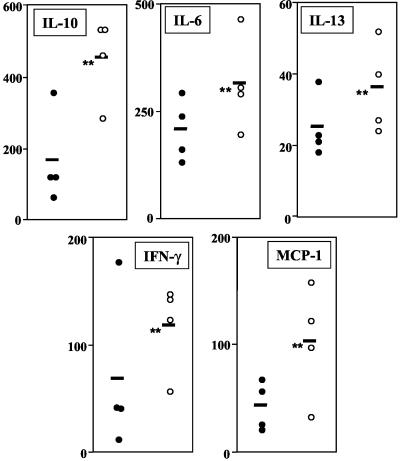
The levels of L. major-specific cytokines and chemokines secreted by cells in the draining lymph nodes of wild-type and TLR40/0 mice were determined at 4 weeks postinfection. The results presented in Fig. 3 show that lymph node cells from TLR40/0 mice produced higher levels of IL-10 in response to antigenic restimulation than did cells from infected wild-type mice. Levels of IL-6 and IL-13 secreted by lymph node cells from L. major-infected TLR40/0 mice were also higher, but IL-4 was not detectable. The levels of L. major-specific IFN-γ were higher in the supernatants of lymph node cells cultured from TLR40/0 mice than in those from wild-type mice (Fig. 3). Lymph node cells from L. major-infected TLR40/0 mice secreted more MCP-1 than did those from wild-type mice (Fig. 3). Thus, even though the differences were not statistically significant (P > 0.05) 4 weeks after infection, lymph node cells from L. major-infected TLR40/0 mice secreted higher levels of Th1 and Th2 cytokines than did those from infected wild-type mice. Similar results were obtained by determining the frequency of cytokine-expressing CD4+ T cells by intracellular cytokine staining. Four weeks after infection, there was a slightly higher frequency of IFN-γ-, IL-4-, and IL-10-expressing CD4+ T cells in the lymph nodes draining the lesions of TLR40/0 mice than in those of control mice, and the mean fluorescence intensities were also slightly higher (P > 0.05) (Table 1) in response to antigenic restimulation in vitro. The cytokine levels detected directly ex vivo were below the detection limit (1%).
FIG. 3.
Cytokine and chemokine production by lymph node cells from L. major-infected wild-type and TLR40/0 mice. Groups of wild-type (filled circles) and TLR40/0 (open circles) mice were infected with 2 × 106 L. major promastigotes in one hind footpad. At 4 weeks postinfection, 5 × 106 popliteal lymph node cells were restimulated with 106 L. major parasites. Supernatants were harvested after 48 h and tested for their cytokine contents by ELISA. Each symbol represents one mouse, and the horizontal black bars represent the means for four individual mice. **, P > 0.05.
TABLE 1.
Intracellular cytokine staining in lymph node cells from L. major-infected wild-type and TLR40/0 micea
| Mouse strain | % CD4+ cells | % CD4+ IFN-γ+ cells | MFIb | % CD4+ IL-4+ cells | MFIb | % CD4+ IL-10+ cells | MFIb |
|---|---|---|---|---|---|---|---|
| Wild-type | 50.3 ± 2.0 | 6.1 ± 1.2 | 5.4 ± 0.2 | 1.3 ± 0.1 | 4.5 ± 0.2 | 2.3 ± 0.7 | 4.0 ± 0.2 |
| TLR40/0 | 52.1 ± 2.8 | 7.7 ± 3.3 | 6.2 ± 1.5 | 1.9 ± 0.7 | 5.6 ± 1.0 | 2.8 ± 1.1 | 4.6 ± 0.2 |
Groups of wild-type and TLR40/0 mice were infected with 2 × 106 L. major promastigotes in one hind footpad. At 4 weeks postinfection, 5 × 106 popliteal lymph node cells were stimulated with 106 L. major parasites. Six days later, a Ficoll gradient was performed and the cells were restimulated with PMA and ionomycin in the presence of brefeldin-A, as described in Materials and Methods. The frequencies of IFN-γ-, IL-4-, and IL-10-expressing CD4+ T cells were determined by flow cytometry, and the values represent averages ± standard deviations for four mice. The results of one of two similar experiments are presented.
MFI, mean fluorescence intensity.
Parasite survival and arginase activity in BMMφ from TLR40/0 and wild-type mice.
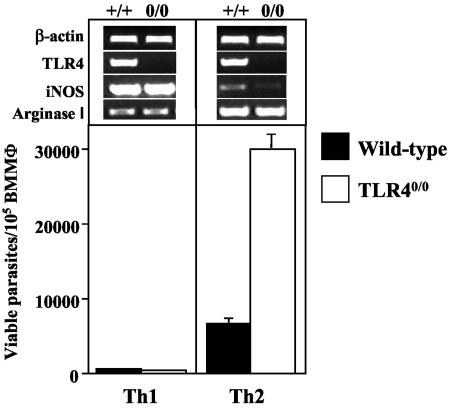
We showed in Fig. 1 and 2 that TLR40/0 mice harbor larger parasite loads in their infected foot pads than do wild-type mice. To assess whether the macrophages from TLR40/0 mice provide a more permissive environment for parasite growth, we determined whether the absence of TLR4 influences the survival of parasites in BMMφ in vitro. The results presented in Fig. 4 show that L. major growth is strongly enhanced in BMMφ activated with Th2 cytokines (alternatively activated BMMφ) from TLR40/0 mice. Thus, TLR4-competent macrophages provide a less permissive environment for Leishmania, controlling the growth of intracellular parasites more efficiently than TLR40/0 macrophages. In the absence of exogenous cytokines, macrophages from TLR40/0 mice supported parasite growth more efficiently (1.5- to 3.5-fold more parasites survived) (data not illustrated) than did those from wild-type mice. Furthermore, these results confirm that the activation of macrophages with type 1 cytokines results in parasite killing, whereas activation with type 2 cytokines promotes parasite growth. We conclude from the differential survival of L. major parasites in wild-type and TLR40/0 macrophages that TLR4 contributes to an efficient host defense against Leishmania parasites.
FIG. 4.
Parasite survival in macrophages from TLR40/0 and wild-type mice. BMMφ (5 × 105 ml−1) from naïve wild-type (black bars) and TLR40/0 (open bars) mice were infected with 25 × 105 L. major parasites ml−1 in the presence of IFN-γ (20 U ml−1) and TNF-α (200 U ml−1) (Th1 conditions) or IL-4 (20 U ml−1) (Th2 conditions). After 48 h of incubation, macrophages were lysed and a limiting dilution assay was performed to determine the number of viable parasites. Data show the results of one representative experiment of four independent experiments. In addition, gene expression was analyzed by semiquantitative RT-PCR. Samples were standardized by densitometric comparison with the amplification of the housekeeping gene β-actin. Data show the results of one representative experiment of two independent experiments.
Since iNOS and arginase activity have been associated with parasite killing and parasite growth, respectively, we determined their relative expression levels in L. major-infected macrophages from wild-type and TLR40/0 mice. The activation of L. major-infected macrophages by IL-4 induced increased arginase 1 mRNA expression, whereas activation with type 1 cytokines (IFN-γ plus TNF-α) upregulated iNOS mRNA expression (Fig. 4). The weak expression of arginase 1 in macrophages activated with type 1 cytokines is due to the autocrine production of IL-10 (35). Similar results were obtained by analyzing iNOS mRNA expression with an RNase protection assay and by measuring the levels of nitric oxide in the culture supernatants (data not shown). Control stimulation with LPS upregulated both arginase 1 and iNOS mRNAs in macrophages from wild-type mice, but not in those from TLR40/0 mice (data not shown).
Thus, the differential survival of Leishmania parasites in macrophages (Fig. 4) correlates with the induction of iNOS and arginase mRNAs in these cells.
In vitro arginase activity in L. major-infected macrophages from TLR40/0 and wild-type mice.
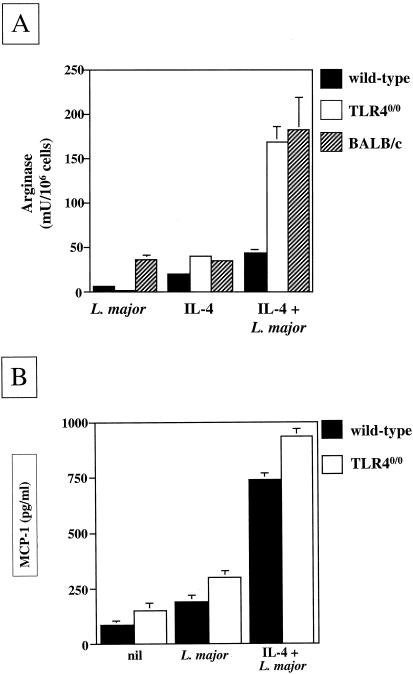
Since increased arginase levels have been shown to promote the multiplication of Leishmania parasites (22) and since TLR40/0 macrophages are more permissive for Leishmania growth (Fig. 4), we determined the enzymatic activity of arginase in macrophages from wild-type and TLR40/0 mice. The results presented in Fig. 5A show that the stimulation of BMMφ in vitro with IL-4 alone induced similar arginase activities in wild-type and TLR40/0 mice. However, the simultaneous infection of these macrophages with L. major parasites clearly creates synergism with IL-4 in vitro, resulting in the induction of 3.8-fold more arginase activity in macrophages from TLR40/0 mice than in those from wild-type mice. The arginase activity in parasitized macrophages from TLR40/0 mice in vitro was comparable to that induced in macrophages from the nonhealer BALB/c strain (Fig. 5A). The simultaneous activation of L. major-infected macrophages with IL-10 and IL-4 enhanced the arginase activity even further (for TLR40/0 mice, 1,443 mU/106 cells; for the wild type, 622 mU/106 cells; data not illustrated). L. major parasites themselves constitutively express arginase activity (8); however, it is unlikely that the parasite arginase activity contributed to the values shown in Fig. 5A, since the arginase activity of 2.5 × 106 L. major promastigotes ml−1 was below the limit of detection (data not shown).
FIG. 5.
(A) Differential induction of arginase activity in L. major-infected macrophages from TLR4-competent or TLR4-deficient mice. BMMφ (5 × 105 ml−1) from naïve wild-type (black bars), TLR40/0 (white bars), and BALB/c (hatched bars) mice were cultured in the presence and/or absence of 25 × 105 L. major parasites ml−1 and IL-4 (20 U ml−1). After 48 h, the arginase activity in macrophage lysates was measured. Data show the results of one representative experiment of five independent experiments. (B) MCP production by L. major-infected macrophages from TLR4-competent or TLR4-deficient mice. BMMφ (5 × 105 ml−1) from naïve wild-type (black bars) and TLR40/0 (white bars) mice were cultured in the presence or absence of 25 × 105 L. major parasites ml−1 and IL-4 (20 U ml−1). Supernatants were harvested after 48 h and tested for their MCP-1 contents by ELISA. Data show the results of one representative experiment of three independent experiments.
These results demonstrate that in the absence of TLR4, alternatively activated L. major-infected macrophages express more arginase activity in vitro, which is likely to promote the polyamine synthesis required for parasite growth. Thus, the activation of parasitized macrophages with type 2 cytokines not only induces more arginase activity in the absence of TLR4, but also results in enhanced parasite growth.
Induction of cytokine and chemokine production in L. major-infected macrophages.
To identify the cytokines and chemokines produced as a consequence of parasite interactions with macrophages, we tested the culture supernatants for the presence of IL-1α, IL-6, IL-10, IL-12 p70, and MCP-1. The infection of macrophages from wild-type and TLR40/0 mice with L. major for 48 h did not result in detectable cytokine production (data not shown). The stimulation of infected macrophages with type 1 cytokines only induced IL-6 production (for wild-type mice, 171 ± 36 pg ml−1; for TLR40/0 mice, 215 ± 69 pg ml−1), whereas stimulation with type 2 cytokines did not induce any detectable cytokine production (data not shown). These results confirm that L. major infection results in a poor activation of macrophages, a feature that helps the parasites to escape immune defense mechanisms. The chemokine MCP-1 was constitutively produced by naïve macrophages, and infection with L. major resulted in slightly increased levels. IL-4 formed synergism with the parasites to induce an increase in MCP-1 production, which was further enhanced in the absence of TLR4 (Fig. 5B).
DISCUSSION
The results of our study demonstrate unequivocally that TLR4 plays a role in the host defense against parasitic protozoa. We show that after infection with L. major, TLR4 contributes to both innate and adaptive immune responses: TLR40/0 mice are clearly less efficient at controlling parasite growth at the local site of infection in the early (days 1 and 5) as well as the late (weeks 4 and 11) phases of infection. The more efficient parasite control of TLR4-competent mice 1 day after infection correlated with the expression of iNOS at the local site of infection. TLR40/0 mice not only had larger parasite burdens, but they were also less efficient in the resolution of cutaneous lesions. Indeed, about 2 months after infection, the cutaneous lesions were healed in the TLR4-competent mice, whereas TLR40/0 mice still displayed pronounced foot pad swelling. In agreement with the increased parasite growth in the footpads of TLR40/0 mice, alternatively activated macrophages were more permissive for parasite growth in the absence of TLR4 in vitro. This increased parasite proliferation was associated with more arginase activity; indeed, our results show that IL-4 formed synergism with L. major in the induction of enhanced arginase activity in TLR40/0 macrophages, indicating that TLR4 signaling contributes to the regulation of l-arginine metabolism. The growth of Leishmania parasites within the macrophage environment is dependent on their ability to ensure the provision of nutrients. Arginase catalyzes the hydrolysis of l-arginine into urea and l-ornithine, and the latter is used by parasites to generate polyamines, which are essential for their proliferation. A correlation between intracellular parasite growth and arginase induction was also observed when infected alternatively activated macrophages from nonhealer and healer strains of mice were compared in vitro: parasite growth and arginase levels were significantly higher in macrophages from susceptible BALB/c mice than in those from resistant C57BL/6 mice (22). In a Schistosoma mansoni infection model, the inducible expression and activation of arginase 1 in vivo directly correlated with a type 2 cytokine response, and arginase activity was functionally related to pathology in the granulomas induced by schistosome eggs (20). Several studies with mice have indicated that the balance between arginase and iNOS is an important mechanism for controlling macrophage function (16, 32, 34). Indeed, the expression of iNOS or arginase in vivo has been suggested to be a better predictor of the pathology induced by schistosome eggs than the Th1 or Th2 cytokine levels (20). There is ample evidence that the iNOS pathway is crucial for the killing of Leishmania parasites (5, 17, 27, 29, 55, 62). Here we showed that increased parasite killing in wild-type mice correlates with iNOS expression in L. major-infected macrophages and in the footpads of TLR4-competent mice infected for 24 h. These data confirm that TLR4 signaling contributes to the induction of the iNOS pathway (52, 59). Interestingly, the engagement of TLR4 by LPS results in the induction of IFN-α/β (54), and the early expression of iNOS is dependent on IFN-α/β (11). Since IFN-α/β has a protective role in experimental leishmaniasis (4), it is tempting to speculate that the beneficial effect of TLR4 in the control of L. major infection is in part associated with TLR4-mediated induction of early IFN-α/β.
Of the cytokines that were elevated in the absence of TLR4 after in vivo infection, the antigen-specific production of IL-10 is of particular interest. The severity of visceral leishmaniasis is strongly associated with increased IL-10 levels (24, 50), while CD4+ T cells from L. major-infected nonhealer mice express high levels of IL-10 mRNA (45) and IL-10-deficient mice from a nonhealer background can control L. major infections (3, 23). Since IL-10 acts primarily on activated macrophages to decrease the secretion of proinflammatory cytokines and to prevent parasite killing (23), it is likely that the increased levels of IL-10 in L. major-infected TLR40/0 mice contribute to the observed reduction in host defense. Here we demonstrated that IL-10 forms synergism with IL-4 and L. major to increase arginase 1 activity in vitro. This is in agreement with previous work showing that arginase 1 expression is induced by IL-4 and IL-13 (35) and that IL-10 strongly forms synergism with Th2 cytokines for the induction of arginase in vitro (34). In addition, IL-10 alone induces low levels of this enzyme (32). Global gene expression analysis has shown that IL-10 influences not only arginase 1, but also arginase 2, induction in macrophages (26) and that IL-10 also causes an upregulation of IL-4Rα expression (26). Thus, a functional consequence of the exposure of macrophages to IL-10 is an enhanced sensitivity to IL-4 and IL-13, with both cytokines promoting the alternative activation of macrophages, which express higher levels of arginase and thus promote the growth of intracellular pathogens. By the same criteria, the increased levels of IL-10 in L. major-infected TLR40/0 mice are likely to enhance macrophage responsiveness to Th2 cytokines, thereby enhancing the permissiveness of these host cells for parasite growth. Thus, our results demonstrating that alternatively activated parasitized macrophages from TLR4-deficient mice express increased arginase activity in vitro (Fig. 5) and are more permissive for parasite growth in vitro (Fig. 2) suggest that TLR4-mediated signaling modulates the effector function of parasitized macrophages in favor of the pathway resulting in parasite killing. However, increased arginase activity in alternatively activated, parasitized macrophages in response to IL-4 in vitro does not necessarily reflect the complex mechanisms underlying the increased parasite survival rate in the absence of TLR4 in vivo.
The detection of increased levels of MCP-1 in the draining lymph nodes of TLR40/0 mice at a time when the acquired immune response is unequivocally established suggests a negative correlation with parasite killing. MCP-1 influences innate immunity through its effects on the recruitment of monocytes and adaptive immunity by its effects on T-helper cells, specifically by controlling the polarization of Th2 cells (18, 61). Indeed, MCP-1-deficient mice from a nonhealer background are resistant to L. major infections and unable to mount a Th2 response (18). Our results support these observations, as higher levels of MCP-1 in TLR40/0 mice correlate with a more pronounced type 2 response, less efficient control of parasite replication, and lesion resolution. Although a pathogenic role for MCP-1 has been shown previously (10, 15) and although MCP-1 has been implicated in Th2 polarization, beneficial effects of MCP-1 in cutaneous leishmaniasis have also been reported. Infection with L. major has been shown to induce a rapid transient induction of MCP-1 1 day after infection in mice from healer strains (61), and MCP-1 has been reported to form synergism with IFN-γ to promote parasite killing in vitro (46). Mice that are from a genetically resistant background but are unable to express the chemokine receptor CCR2, the ligand for MCP-1, are susceptible to L. major infection (51), while increased levels of MCP-1 are found in the lesions of patients with self-healing disease (47).
Our data show that TLR4 signaling helps parasitized macrophages to kill intracellular L. major more efficiently and to control arginase activity, an effect that might be ascribed to the balance of Th1 and Th2 responses. Indeed, signaling through TLRs has been associated with the induction of Th1 responses and a lack of signaling has been associated with the induction of Th2 responses (39, 53). Although we showed that TLR4 contributes to the efficient control of L. major parasites during both innate and adaptive immune responses, we cannot exclude the possibility that the natural mutation in the tlr4 locus (37, 42, 43) leads to altered homeostatic responses affecting early parasite survival. Indeed, it cannot be excluded that mechanisms of early defense, such as complement activation (49), are altered in TLR40/0 mice compared to wild-type mice. We have obtained evidence that cell recruitment to the site of parasite inoculation is different for TLR4-competent and TLR40/0 mice 10 hours after infection (P. Kropf, N. Freudenberg, C. Kalis, M. Modolell, S. Herath, C. Galanos, M. Freundenberg, and I. Müller, submitted for publication). This initial difference in cell recruitment could contribute to the more efficient invasion of host cells 1 day after infection. Even though the parasite load is consistently higher in the absence of TLR4, it is interesting that the dynamics of parasite replication between days 1 and 5 are different. These differences in parasite growth could be due to (i) more efficient invasion of the host cells, (ii) more efficient growth of the parasites, or (iii) more efficient killing of the parasites.
In addition, in macrophages from TLR40/0 mice, there may be more competition for polyamines, which is essential for their growth due to the higher parasite number in these cells at day 1. Furthermore, it is also possible that cooperation between different TLRs or between TLR4 and parasite-specific receptors (7, 13) is required, and we are currently addressing these issues. Regardless of the underlying mechanism, we have unequivocal evidence that the increased parasite load in the TLR40/0 mice used in the present study was indeed due to the absence of TLR4, as the insertion of a TLR4 transgene in these mice enabled them to control parasite replication as efficiently as the wild-type mice (Kropf et al., submitted).
Based on observations in bacteria, it can be hypothesized that TLRs recognize PAMPs associated with Leishmania parasites and that these interactions are essential for the implementation and maintenance of active innate and adaptive immune responses to infection. However, at least in vitro, L. major parasites do not have a direct effect on the activation of TLR4 in a dual luciferase reporter system (H. P. Price and D. F. Smith, unpublished data). Although at a first glance these data seem to indicate that TLR4 does not interact with parasite molecules in vitro, they may also indicate that in vivo multiple innate immune recognition receptors interact during pathogen recognition (7, 13) and that in vitro transfection systems do not necessarily reflect the complexity of host-parasite interactions in vivo. Because TLR4 not only interacts with pathogen-associated PAMPs, but can also recognize endogenous ligands such as heat shock proteins (38) and components of the extracellular matrix (57), we cannot yet conclude whether TLR4 activation after L. major infection is due to the recognition of parasite PAMPs or an interaction with endogenous ligands. TLRs may represent novel targets for the activation of the adaptive immune response and TLR agonists may be useful for the prevention and treatment of leishmaniasis.
Acknowledgments
We thank V. Weber, H. Stübig, and N. Goos for expert technical assistance and B. Askonas and C. Bangham for helpful discussions and critical reading of the manuscript.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), The Wellcome Trust, The St. Mary's Development Trust, and die Deutsche Forschungsgemeinschaft.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abdallahi, O. M. S., H. Bensalem, R. Augier, M. Diagana, M. De Regi, and B. Gharib. 2001. Arginase expression in peritoneal macrophages and increase in circulating polyamine levels in mice infected with Schistosoma mansoni. Cell. Mol. Life Sci. 58:1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R.-B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907-916. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R.-B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. L. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G. D., J. Herre, D. L. Williams, J. A. Willment, A. S. J. Marshall, and S. Gordon. 2003. Dectin-1 mediates biological effects of β-glucans. J. Exp. Med. 197:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo, P. E., J. A. Coelho, G. Moraes, and E. N. Figueiredo. 1978. Trypanosoma spp., Leishmania spp. and Leptomonas spp.: enzymes of ornithine-arginine metabolism. Exp. Parasitol. 46:141-144. [DOI] [PubMed] [Google Scholar]
- 9.Campos, M. A. S., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 10.Chensue, S. W., K. S. Warmington, J. H. Ruth, P. S. Sanghi, P. Lincoln, and S. L. Kunkel. 1996. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation. J. Immunol. 157:4602-4608. [PubMed] [Google Scholar]
- 11.Diefenbach, A., H. Schindler, N. Donhauser, E. Lorenz, T. Laskay, J. MacMicking, M. Röllinghoff, I. Gresser, and C. Bogdan. 1998. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8:77-87. [DOI] [PubMed] [Google Scholar]
- 12.Etges, R., and I. Müller. 1998. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J. Mol. Med. 76:372-390. [DOI] [PubMed] [Google Scholar]
- 13.Gantner, B., R. M. Simmons, S. J. Çanavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory response by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137-142. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo, J.-A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. C. Wells, A. Proudfoot, A.-C. Martinez, T. Bjerke, A. J. Coyle, and J.-C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188:157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 17.Green, S. J., C. A. Nacy, and M. S. Meltzer. 1991. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J. Leukoc. Biol. 50:93-103. [DOI] [PubMed] [Google Scholar]
- 18.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 19.Hawn, T. R., A. Ozinsky, D. M. Underhill, F. S. Buckner, S. Akira, and A. Aderem. 2002. Leishmania major activates IL-1α expression in macrophages through a MyD88-dependent pathway. Microbes Infect. 4:763-771. [DOI] [PubMed] [Google Scholar]
- 20.Hesse, M., M. Modolell, A. C. La Flamme, M. Schito, J. M. Fuentes, A. W. Cheever, E. J. Pearce, and T. A. Wynn. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type1/type2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533-6544. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino, K., O. Takeuchi, T. Kawai, J. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 22.Iniesta, V., L. C. Gomez-Nieto, I. Molano, A. Mohedano, J. Carcelen, C. Miron, C. Alonso, and I. Corraliza. 2002. Arginase I induction in macrophages, triggered by Th2-type cytokines supports the growth of intracellular Leishmania parasites. Parasite Immunol. 24:113-118. [DOI] [PubMed] [Google Scholar]
- 23.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 24.Kaye, P. M., A. J. Curry, and J. M. Blackwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J. Immunol. 146:2763-2770. [PubMed] [Google Scholar]
- 25.Kropf, P., K. Brunson, R. Etges, and I. Müller. 1998. The leishmaniasis model. Methods Microbiol. 25:419-458. [Google Scholar]
- 26.Lang, R., D. Patel, J. J. Morris, R. L. Rutschman, and P. J. Murray. 2002. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169:2253-2263. [DOI] [PubMed] [Google Scholar]
- 27.Liew, F. Y., S. Millot, C. Parkinson, R. M. J. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from arginine. J. Immunol. 144:4794-4797. [PubMed] [Google Scholar]
- 28.Liew, F. Y., and C. A. O'Donnell. 1993. Immunology of leishmaniasis. Adv. Parasitol. 32:162-259. [DOI] [PubMed] [Google Scholar]
- 29.Mauel, J., A. Ransijn, and Y. Buchmüller-Rouiller. 1991. Killing of Leishmania parasites in activated murine macrophages is based on an l-arginine-dependent process that produces nitrogen derivatives. J. Leukoc. Biol. 49:73-82. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. Janeway. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 31.Merlin, T., A. Sing, P. J. Nielsen, C. Galanos, and M. A. Freudenberg. 2001. Inherited IL-12 unresponsiveness contributes to the high LPS resistance of the Lpsd C57BL/10ScCr mouse. J. Immunol. 166:566-573. [DOI] [PubMed] [Google Scholar]
- 32.Modolell, M., I. M. Corraliza, F. Link, G. Soler, and K. Eichmann. 1995. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 25:1101-1104. [DOI] [PubMed] [Google Scholar]
- 33.Müller, I., M. Freudenberg, P. Kropf, A. Kiderlen, and C. Galanos. 1997. Leishmania major infection in C57BL/10 mice differing at the Lps locus: a new non-healing phenotype. Med. Microbiol. Immunol. 186:75-81. [DOI] [PubMed] [Google Scholar]
- 34.Munder, M., K. Eichmann, and M. Modolell. 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160:5347-5354. [PubMed] [Google Scholar]
- 35.Munder, M., K. Eichmann, J. M. Moran, F. Centeno, G. Soler, and M. Modolell. 1999. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 163:3771-3777. [PubMed] [Google Scholar]
- 36.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237-4241. [DOI] [PubMed] [Google Scholar]
- 37.Nicolas, L., S. Sidjanski, J.-H. Colle, and G. Milon. 2002. Leishmania major reaches distant cutaneous sites where it persists transiently while persisting durably in the primary dermal site and its draining lymph node: a study with laboratory mice. Infect. Immun. 68:6561-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 39.O'Neill, L. A. J. 2002. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 23:296-300. [DOI] [PubMed] [Google Scholar]
- 40.Pala, P., T. Hussell, and P. J. M. Openshaw. 2000. Flow cytometric measurement of intracellular cytokines. J. Immunol. Methods 243:107-124. [DOI] [PubMed] [Google Scholar]
- 41.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 42.Poltorak, A., T. Merlin, P. J. Nielsen, O. Sandra, I. Smirnova, I. Schupp, T. Boehm, C. Galanos, and M. A. Freudenberg. 2001. A point mutation in the IL-12R β2 gene underlies the IL-12 unresponsiveness of Lps-defective C57BL/10ScCr mice. J. Immunol. 167:2106-2111. [DOI] [PubMed] [Google Scholar]
- 43.Poltorak, A., I. Smirnova, R. Clisch, and B. Beutler. 2000. Limits of a deletion spanning Tlr4 in C57BL/10ScCr mice. J. Endotoxin Res. 6:51-56. [DOI] [PubMed] [Google Scholar]
- 44.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 45.Reiner, S. L., S. Zheng, Z.-E. Wang, L. Stowring, and R. M. Locksley. 1994. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 179:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritter, U., and H. Moll. 2000. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-γ and is antagonized by IL-4. Eur. J. Immunol. 30:3111-3120. [DOI] [PubMed] [Google Scholar]
- 47.Ritter, U., H. Moll, T. Laskay, E. Brocker, O. Velazco, I. Becker, and R. Gillitzer. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699-709. [DOI] [PubMed] [Google Scholar]
- 48.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacks, D., and A. Sher. 2002. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 3:1041-1047. [DOI] [PubMed] [Google Scholar]
- 50.Sacks, D. L., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 51.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR) 2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells: absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schilling, D., K. Thomas, K. Nixdorff, S. N. Vogel, and M. J. Fenton. 2002. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 production in macrophages. J. Immunol. 169:5874-5880. [DOI] [PubMed] [Google Scholar]
- 53.Schnare, M., G. M. Barton, A. Czopik Holt, K. Takeda, S. Akria, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 54.Sing, A., T. Merlin, H.-P. Knopf, P. J. Nielsen, H. Loppnow, C. Galanos, and M. A. Frendenberg. 2000. Bacterial induction of beta interferon in mice is a function of the LPS component. Infect. Immun. 68:1600-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenger, S., H. Thuring, M. Röllinghoff, and C. Bogdan. 1994. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J. Exp. Med. 180:783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 57.Termeer, C., F. Bendix, K. Sleemann, C. Fieber, U. Voith, T. Ahrens, K. Miyake, M. Freudenberg, C. Galanos, and J. C. Simon. 2002. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 59.Toshchakov, V., B. W. Jones, P.-Y. Perera, K. Thomas, M. J. Cody, I. Zhang, B. R. G. Williams, J. Major, T. A. Hamilton, M. J. Fenton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 60.Underhill, D. M., A. Oszinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 61.Vester, B., K. Müller, W. Solbach, and T. Laskay. 1999. Early gene expression of NK-cell activating chemokines in mice resistant to Leishmania major. Infect. Immun. 67:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei, X., I. G. Charles, A. Smith, J. Ure, G. Feng, F. Huang, D. Xu, W. Müller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 63.Wolfs, T. G. A. M., W. A. Buurman, A. van Schadewijk, B. de Vries, M. A. R. C. Daemen, P. S. Hiemstra, and C. van't Veer. 2002. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J. Immunol. 168:1286-1293. [DOI] [PubMed] [Google Scholar]
- 64.Xu, Y., X. Tao, B. Shen, T. Horng, R. Medzhitov, J. L. Manley, and L. Tong. 2000. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408:111-115. [DOI] [PubMed] [Google Scholar]
- 65.Yamaoka, J., T. Kume, A. Akaike, and Miyachi Y. 2000. Suppressive effect of zinc ion on iNOS expression induced by interferon-γ or tumor necrosis factor-α in murine keratinocytes. J. Dermatol. Sci. 23:27-35. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golgenbock. 1999. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]