Abstract
The binding of human secretory immunoglobulin A (SIgA), the primary immunoglobulin in the gut, to Escherichia coli is thought to be dependent on type 1 pili. Type 1 pili are filamentous bacterial surface attachment organelles comprised principally of a single protein, the product of the fimA gene. A minor component of the pilus fiber (the product of the fimH gene, termed the adhesin) mediates attachment to a variety of host cell molecules in a mannose inhibitable interaction that has been extensively described. We found that the aggregation of E. coli K-12 by human secretory IgA (SIgA) was dependent on the presence of the pilus fiber, even in the absence of the mannose specific adhesin or in the presence of 25 mM α-CH3Man. The presence of pilus without adhesin also facilitated SIgA-mediated biofilm formation on polystyrene, although biofilm formation was stronger in the presence of the adhesin. IgM also mediated aggregation and biofilm formation in a manner dependent on pili with or without adhesin. These findings indicate that the pilus fiber, even in the absence of the adhesin, may play a role in biologically important processes. Under conditions in which E. coli was agglutinated by SIgA, the binding of SIgA to E. coli was not increased by the presence of the pili, with or without adhesin. This observation suggests that the pili, with or without adhesin, affect factors such as cell surface rigidity or electrostatic repulsion, which can affect agglutination but which do not necessarily determine the level of bound immunoglobulin.
The ability of secretory immunoglobulin A (SIgA) to bind and agglutinate enteric bacteria is thought to be of critical importance, providing the basis for the immune system's interactions with enteric bacteria (7, 53, 59). SIgA binding and agglutination of enteric bacteria prevents the bacteria from breaching the epithelial barrier, a process termed “immune exclusion” (59, 60). SIgA may also facilitate biofilm formation by the normal flora in the large bowel, a process that may aid in the growth of the normal flora and attenuate growth of pathogenic microorganisms (5). The SIgA-mediated aggregation of enteric bacteria (59) and SIgA-mediated biofilm formation by enteric bacteria have been assessed in vitro (5). Aggregation of bacteria by IgA can be blocked in vitro by antisera specific for the heavy or for the light chains of IgA, indicating that the agglutination is specific for the IgA molecule (59). SIgA-mediated biofilm formation by Escherichia coli in vitro is also specific, since biofilm formation can be mediated by SIgA and by mucin but not by the absence of protein or by IgG, albumin, hemoglobin, secretory chain, or the Fab and Fc domains of SIgA (5).
There are a number of bacterial components that are important in autoaggregation and biofilm formation by E. coli. These components include colanic acid (13), type 1 pili (52), curli (49), and antigen 43 (12, 31). However, the potential role of these molecules in bacterial aggregation and biofilm formation in the gut remains to be determined; curli and colanic acid are not expressed well under conditions found in the mammalian gut (34, 41, 42, 50). In addition, the importance of antigen 43 and type 1 pili for biofilm formation is dependent on culture conditions (12). Further, the biofilm-forming effects of antigen 43 may only be important in the absence of type 1 pili (25). Perhaps most importantly, SIgA can mediate aggregation and biofilm formation by E. coli under conditions in which E. coli does not typically form aggregates or biofilms. The observation that SIgA and mucus, common components of the intestinal milieu, could facilitate biofilm formation suggests that microbes in the gut probably do not need to produce all necessary components for biofilm formation in order to form a biofilm. Such an idea is not unprecedented, as formation of bacterial biofilms by bacteria associated with plant roots is facilitated at least in part by molecules secreted by the plant (6, 15, 20). Thus, if indeed IgA and mucus are involved in biofilm formation or aggregation of bacteria in the gut, it is of substantial interest to determine which bacterial components may be required to facilitate these interactions. At least one study has indicated that the interaction between SIgA and E. coli may depend on type 1 pili (61).
Type 1 pili are filamentous proteinaceous appendages produced by several members of the Enterobacteriaceae. In Escherichia coli, type 1 pili have been conclusively associated with the ability to infect extraintestinal sites (30, 43). However, the involvement of the pili in intestinal colonization is less certain (4, 29, 44). Type 1 pili have been studied extensively with regard to their genetics, biosynthesis, and ability to bind receptor molecules on a variety of eucaryotic cells in a mannose-inhibitable manner (43). Although the pili are made principally of a single protein monomer, the product of the fimA gene, several minor protein components are also incorporated (23, 47). These are most often found at the ends of pili and are organized into fibrillar structures (28). One of the minor components, the product of the fimH gene (FimH, termed the adhesin), binds directly to receptor molecules (32).
A variety of receptors on eucaryotic cells (17, 18, 26) and molecules of interstitial spaces (48, 55) are bound by the adhesin. This binding, and even intermolecular adhesin binding (24), is characterized by its sensitivity to mannose inhibition. In the absence of a functional fimH gene product, piliated E. coli appear to lack all of the colonization and host cell binding properties associated with type 1 piliation (24, 29, 30). A role of the fimbrial fiber itself in bacterium-host interactions (apart from being required for adhesin presentation) has been speculated upon (43) but never supported experimentally (29).
Type 1 pili are recognized by SIgA and mucins (37), molecules present in high abundance along the mucosal barrier. The binding of SIgA to enteric bacteria has been shown to decrease the ability of those bacteria to breach the intestinal barrier (59, 60). Thus, interactions between type 1 pili and SIgA may help reduce chronic inflammation in the intestine and aid in the initial colonization of the host. Indeed, IgA-deficient individuals appear to be colonized with E. coli that exhibit reduced type 1 pilus expression (16).
Natural antibodies, i.e., antibodies occurring without any known history of sensitization to the relevant corresponding antigen, often bind to bacterial antigens and are, at least to some degree, elicited by exposure to the normal microbial flora (8, 56). Based on our current understanding of natural antibodies, it might be thought that the binding of these antibodies, including natural SIgA, to bacteria is dependent on the specificity of the antigen binding site of the antibody. On the other hand, some studies have suggested that the binding of SIgA to E. coli may depend on the mannose-specific interactions between the adhesin of the type 1 pili and mannose residues expressed on SIgA. For example, Moshier et al. found that expression of type 1 pili enhanced adhesion to surfaces coated with SIgA or with mucins (37). This adhesion was inhibited by mannose, a finding consistent with the idea that mucin and SIgA may provide receptors for binding of type 1pili. Wold and colleagues found that the sIgA2-mediated agglutination of E. coli expressing type 1 pili can be inhibited by mannose (agglutination titer reduced from 128 to 2 in the presence of 43 mM α-CH3Man) (61). This finding is expected, since IgA2 carries oligosaccharide receptors for type 1 pili (61). However, Wold et al. also found that, like IgA2-mediated agglutination, IgA1-mediated agglutination of E. coli expressing type 1 pili is inhibited by mannose (agglutination titer reduced from 64 to 4 in the presence of 43 mM α-CH3Man) (61). This finding is unexpected since, in contrast to IgA2, IgA1 apparently does not contain mannose residues that might be recognized by the adhesin of the type 1 pili (1, 2). Thus, it remains unclear to what extent the binding of the mannose specific adhesin to mannose residues on SIgA is important in the binding of SIgA to E. coli.
In the present study, the interactions between human SIgA and various strains of E. coli expressing pili with adhesin, pili without adhesin, or no pilus and no adhesin were evaluated. Measures of this interaction included (i) direct binding between antibody and E. coli, (ii) antibody-mediated aggregation of E. coli, and (iii) antibody-mediated biofilm formation by E. coli. These studies provide strong support for the idea that, in addition to previously described adhesin-dependent interactions, the fiber of the type 1 pili of E. coli has a strong adhesin-independent effect on the aggregation of E. coli by SIgA. This adhesin-independent effect is potentially due to alterations in the surface topology of the E. coli as a result of pilus expression. These findings provide new insight into the mechanisms underlying the interactions between type 1 pili and SIgA and thus into the potential role of type 1 pili in colonization of the gut.
MATERIALS AND METHODS
Materials.
Minimal essential Eagle medium (MEM) and alkaline phosphatase-conjugated, affinity-isolated goat antibodies specific for human α-chain were obtained from Sigma Chemical Co. (St. Louis, Mo.). Sodium pyruvate, HEPES, and nonessential amino acids (MEM-Amino Acids) were obtained from Gibco-BRL (Grand Island, N.Y.). Solvable was purchased from Perkin-Elmer Life Sciences (Boston, Mass.). Uniformly labeled [14C]glucose was obtained from Amersham (Piscataway, N.J.). Wire screen (150 mesh, 94 mm) cell screen filters were obtained from Bellco Glass, Inc. (Vineland, N.J.).
IgM was purified from normal human plasma by using the euglobulin precipitation technique as follows. Outdated human plasma was obtained from the American Red Cross (Charlotte, N.C.) and stored at −85°C until use. After it thawed, the plasma was clarified by centrifugation at 6,500 × g for 30 min at 4°C. The plasma was then diluted with 20 parts of cold distilled water and incubated overnight at 4°C. The mixture was again centrifuged at 6,500 × g for 30 min at 4°C, and the precipitated euglobulin fraction was collected. The pellet was dissolved in phosphate-buffered saline (PBS), and the IgM was separated from the other proteins on a 2.5-cm (internal diameter)-by-90-cm (length) Sepharose CL-4B column. Purified IgM was stored in 10% glycerol and flash frozen until use.
For purification of SIgA, unneeded human milk from anonymous donors was obtained from the Duke University Medical Center Pediatric Intensive Care Unit. Milk from 10 to 20 donors was pooled and stored at −80°C until use. SIgA was purified from the pooled milk by size exclusion chromatography on a 2.5-cm (internal diameter)-by-90-cm (length) Sepharose CL-4B column. Purified SIgA was stored in 10% glycerol and flash frozen until use. Purification on the Sepharose column resulted in a greater loss of IgA2 than of IgA1, since the IgA2 eluted later, resulting in contamination of part of the IgA2 fraction with other milk proteins. The IgA preparation was determined to contain 85% IgA1 and 15% IgA2 based on two independent assessments. First, 84% of the IgA preparation was cleaved with Igase (specific for IgA1). Second, quantitative analysis of the elution profile from the Sepharose column, which contains the IgA1 peak followed by the IgA2 peak (partially resolved), was conducted. The absorbance profile at 280 nm was fitted to Gaussian distributions, and the areas under the curve were calculated by using the program GRAMS/32 (version 5.10; Galactic Industries Corp., Salem, N.H.). Based on this analysis, our preparations contained 80 to 85% IgA1, and 50 to 60% of the IgA2 was discarded during the preparation.
Before use, purified SIgA or IgM was thawed and dialyzed against two changes of PBS, followed by a final dialysis against MEM (for measuring biofilm formation) or against PBS (for measuring aggregation). For measurement of biofilm formation, the purified immunoglobulin was then sterilized by filtration through a 0.2-μm-pore-size filter.
Bacterial strains and growth conditions.
All bacterial strains utilized in the present study were derivatives of E. coli K-12 (Table 1). Strains were propagated in L broth and L agar under conditions previously described (51). For plasmid containing strains, growth medium was supplemented with chloramphenicol (20 μg/ml). For agglutination studies and biofilm formation, bacteria were grown in MEM supplemented with 100 mM HEPES, 0.1 mM MEM-Amino Acids, and 1.0 mM sodium pyruvate prior to use.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant propertiesa | Source and/or reference |
|---|---|---|
| Strains | ||
| MG1655 | F− λ− | 3 |
| ORN225 | MG1655 except fimB′-tetR fimE::IS1 (stably expresses type 1 pili); Hag+ Bag+ | P1 transduction from strain ORN202b |
| ORN226 | ORN225 except fimH′-neoR (stably expresses nonhemagglutinating type 1 pili); Bag+ Hag− | P1 transduction from strain ORN133 (35) |
| ORN227 | MG1655 except Δ(fimBEAICDFGH) ΩneoR; Hag− Bag− | P1 transduction from strain ORN172 (62) |
| ORN201 | thr-1 leuB thi-1 Δ(argF-lac)U169 malAI xyl-7 ara-13 mtl-2 gal-6 rpsL fhuA2 supE44 pilG recA13 Δ(fimBEAICDFGH); Hag− Bag− | 22 |
| Plasmids | ||
| pACYC184 | P15A; Cmr Tcr | 9 |
| pSH2 | pACYC184 fimBEAICDFGH; Cmr (Hag+ Bag+ when resident in strain ORN201) | 21 |
| pORN307 | KpnI-SalI fimH deletion mutant of pSH2; entire fimH gene deleted (Hag− Bag+ when resident in strain ORN201) | 21 |
Hag+, Capable of hemagglutinating guinea pig erythrocytes as described in the text; Bag+, bacterial agglutination in polyclonal rabbit antiserum raised against purified type 1 pili as described in the text; Cmr, chloramphenicol resistant; Tcr, tetracycline resistant.
Strain ORN202 was constructed by first inserting a XhoI DNA fragment containing the tetR gene from Tn10 into the ClaI site in fimB on pSH2 in vitro, creating pORN314. (The ClaI site in fimB had been converted to an XhoI site via linker insertion mutagenesis [45] after partial digestion of pSH2 with ClaI.) Linearization of the plasmid with SalI, followed by transformation ORN117 (ΩfimA′-lacZ fimE::IS1 recBC sbcB) (46), produced a pool of recombinants, some of which had the desired fimE::IS1 fimB′-tetR alleles and were in the locked ON phase (stably transcribing the fimA′-lacZ gene). Such recombinants were identified by phenotypic screening, and the genotype was confirmed by DNA hybridization tests by methods previously described (57). P1 transductions were subsequently used to position the fimE::IS1 fimB′-tetR alleles adjacent to fimA in ORN115 (46), creating the ORN202 donor strain used to construct the stably piliated version of MG1655 (ORN225) used here.
Genetic techniques.
Bacterial transformation with circular plasmid DNA was as described by Lederberg and Cohen (33). Plasmid-encoded alleles were introduced into the E. coli chromosome by recombination after transformation of a recBC sbcB strain of E. coli with linearized plasmid DNA as described by Orndorff et al. (46). Transfer of chromosomal alleles was accomplished via P1 transduction as described by Miller (36) by using P1 vir (46).
Piliation assays.
Piliated E. coli were detected in bacterial agglutination assays that used type 1-specific antiserum (51). Strains producing pili with a functional FimH adhesin were detected in hemagglutination assays with guinea pig erythrocytes as previously described (51). Under the conditions used in our assays, all piliated strains had approximately the same level of piliation per cell as indicated by bacterial agglutination titer and electron microscopic examination of negatively stained preparations (51).
SIgA-mediated agglutination measurements.
Bacteria were grown in minimal medium for 16 h at 37°C in an air-tight 15-ml container. The number of bacteria in each tube was quantified by using a Beckman Coulter Multisizer II Coulter counter with a 50-μm aperture. A correction factor was used to determine absolute numbers of bacteria, since it was found that the counter with a 50-μm aperture detects 1.0% of E. coli (absolute numbers of bacteria based on CFU found by serial dilution). The agglutination reaction was carried out at room temperature with 4.9 × 108 bacteria per ml and 0.5 mg of SIgA/ml in PBS.
The agglutination of bacteria was monitored by using a Coulter Multisizer II counter with a 50-μm aperture. To assess the number of agglutinated bacteria, 100 μl of the mixture of bacteria in 0.5 mg of SIgA/ml was diluted into 10.0 ml of sterile filtered saline. For this dilution, large-orifice graduated pipette tips (USA Scientific, Ocala, Fla.) were used to avoid breaking up aggregates. The numbers of aggregates between 3 to 6 μm and the number >6 μm in diameter were recorded as a function of time. These two size ranges were selected for several reasons. First, it was difficult to monitor the presence of aggregates smaller that 3 μm because the unaggregated bacteria interfered with the measurement of these smaller aggregates. Second, it was decided to monitor the formation of large (>6-μm) aggregates separately because (i) aggregates larger than 6 μm formed later during the agglutination reaction compared to smaller (<6 μm) aggregates; (ii) even though there were small numbers of large aggregates compared to the number of smaller aggregates, each large aggregate represented a substantial amount of bacteria [given the number of bacteria within a spherical aggregate varies approximately as (4/3)Πr3, the volume of a sphere]; and (iii) large aggregates were not formed when the agglutination reaction was not as vigorous. Thus, monitoring the formation of large aggregates (>6 μm) separately provided additional information, whereas the measurement of smaller aggregates (3 to 6 μm) provided an approximate measure of the absolute number of total aggregates.
Biofilm growth measurements.
Biofilms were grown in 12-by-75-mm Becton Dickinson 5-ml polystyrene round-bottom Falcon tubes. To initiate bacterial growth in the tubes, the tubes were incubated for 16 h under aerobic conditions with minimal medium (1.0 ml) that was inoculated with bacteria. Failure of particular strains to grow biofilms was not due simply to failure to adhere during this initial incubation, since planktonic (nonadherent) growth was observed in all tubes with all strains for the duration of all experiments. After the initial 16-h incubation, the medium was slowly drained from the tube, and fresh medium containing 25 nCi of 14C/ml was then slowly added with or without 0.5 mg of SIgA/ml. The steel needle from a 16-gauge, 5.25-in. catheter (BD Angiocath; Becton-Dickinson, Sandy, Utah) coupled to a syringe was used to remove and add liquid to all tubes. To add or remove liquid, the angiocath was placed at the bottom of the tube, and the tube was kept in an upright position. In tubes containing no SIgA, 0.5 mg of bovine serum albumin/ml was added to maintain the same protein concentration in all tubes. During the next 2.5 days, the medium was changed four times at 4- to 18-h intervals. (Shorter time intervals were used as the biofilms became thicker and used nutrients at a faster rate.) Tubes containing no SIgA were changed at the same time intervals as tubes continuing SIgA. For each change, tubes were gently washed three times with PBS by slowly removing and adding solution by using a 16-gauge, 5.25-in. angiocath. This washing method was effective for removing almost all of the nonadherent bacteria.
The incorporation of 14C by bacteria was used as a measure of the amount of bacteria and was taken to be proportional to the number of bacteria. For this measurement, tubes were washed three times as described above, and some bacteria were removed from the tubes by vortexing at maximum speed for 30 s in 1 ml of PBS. The suspended bacteria were removed, and the remaining adherent bacteria were removed by vortexing them at maximum speed for 30 s with 100 mg of washed, sterilized sand in 1 ml of PBS. The suspended bacterial cells were washed three times with PBS, resuspended in 50 μl of deionized water, dissolved in 500 μl of Solvable, and diluted into 10 ml of scintillation fluid (Packard Hionic-Fouor). The amount of 14C was then quantified by using a Wallac 1409 liquid scintillation counter. Using a serial dilution of 14C-labeled bacteria, we found the results of this method to be linear (r2 > 0.99) over greater than a thousandfold range (from 85,000 disintegrations per minute [dpm] to 40 dpm). The presence of CFU was not used as a measure of activity since we could not separate completely the bacteria within biofilms with confidence without using conditions that might kill some of the bacteria.
Binding of SIgA to immobilized E. coli.
The binding of SIgA to immobilized E. coli was measured by enzyme-linked immunosorbent assay (ELISA) as follows. E. coli samples were washed with PBS, and 109 E. coli organisms in 60 μl of PBS with 0.2% NaN3 were added to each well of a Costar 96-well assay plate (flat bottom, polystyrene high binding). After an overnight incubation at 4°C, wells were washed four times with 200 μl of PBS/well and blocked with 0.5% human serum albumin in PBS for 1 h at room temperature. After this incubation, the blocking agent was discarded, and the wells were washed three times with 200 μl of PBS/well. SIgA (0.5 mg/ml; 50 μl/well) diluted in PBS was added, followed by incubation for 1.5 h at 4°C. The wells were then washed three times with PBS, alkaline phosphatase-conjugated goat antibodies specific for human α-chain were added, and the wells were incubated for 1 h. The wells were then washed three times with PBS, and 100 μl of a developing solution consisting of 1.0 mg of p-nitrophenyl phosphate/ml in 100 mM diethanolamine-0.5 mM MgCl2-0.2% NaN3 (pH 9.5) was added. The A405 was determined by using an EL 340 BioKinetics reader (Bio-Tek Instruments, Winooski, Vt.). The absorbance in wells containing no E. coli was taken to be background and was subtracted from the total.
Binding of SIgA to suspended (nonimmobilized) E. coli.
To determine the amount of SIgA bound by suspended (nonimmobilized) E. coli, the E. coli samples were incubated with SIgA and washed, and the bound SIgA was quantified. For this purpose, ca. 2.7 × 109 E. coli was added to a final volume of 1.5 ml of PBS containing 1.0 mg of SIgA. The bacteria were incubated at room temperature for 2 h and then washed three times with PBS. Bacteria were solubilized by boiling for 12 min in 180 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 44% (vol/vol) β-mercaptoethanol. Then, 20 μl of this preparation was loaded per lane onto a 4 to 20% acrylamide gradient gel, separated electrophoretically, and transferred to a polyvinylidene difluoride membrane. Membranes were blocked overnight with 1% bovine serum albumin in PBS and then incubated for 1 h at room temperature with alkaline-phosphatase conjugated, affinity-purified goat antibody specific for human α-chain in blocking buffer. Blots were washed and developed as described above. Bands corresponding to SIgA were digitized and quantified by fitting peak intensities to Gaussian distributions by using the program GRAMS/32 (version 5.10; Galactic Industries Corp.). To determine absolute amounts of SIgA in a given lane, the areas under the peaks obtained from unknown samples were compared to a standard curve made with purified SIgA of known concentration. All standard curves obtained by using this method had a correlation coefficient (r2) greater than 0.99.
Binding of SIgA to bacterial antigens as determined by immunoblotting.
Approximately 3 × 1010 bacteria were solubilized in 200 μl of SDS sample buffer containing 25% (vol/vol) β-mercaptoethanol. Then, 20 μl of a one-to-five dilution of this preparation in SDS sample buffer was loaded per lane onto a 4 to 20% acrylamide gradient gel, separated electrophoretically, and transferred to a polyvinylidene difluoride membrane. Membranes were blocked overnight with 0.5% human serum albumin and 0.1% Tween 20 in PBS, followed by incubation for 2 h at room temperature with 100 μg of human SIgA/ml. Blots were washed three times with PBS and then incubated for 1 h with alkaline phosphatase-conjugated, affinity-purified goat antibody specific for human α-chain in blocking buffer. Finally, blots were washed three times with PBS and then developed with 0.33 mg of nitroblue tetrazolium and 0.165 mg of BCIP (5-bromo-4-chloro-3-indolylphosphate)/ml in 100 mM Tris-HCl-100 mM NaCl2-5.0 mM MgCl2 at pH 9.5.
RESULTS
SIgA-mediated agglutination of various E. coli strains in the presence or absence of 25 mM α-CH3Man.
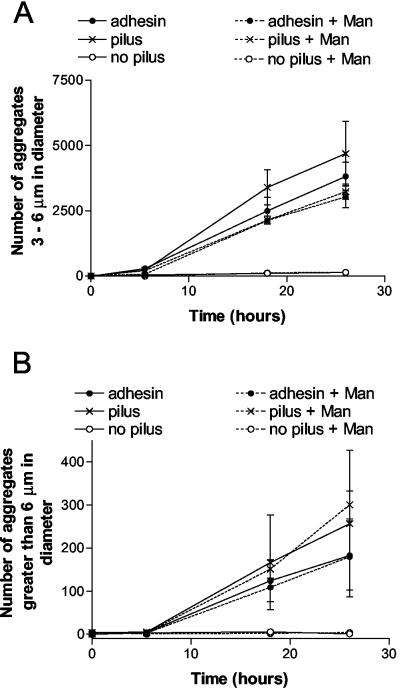
The extent to which SIgA-mediated agglutination of E. coli was dependent on the αMan was evaluated. To this end, the agglutination of various E. coli strains by SIgA in the presence or absence of mannose-specific adhesin was measured by using a Coulter counter as described in Materials and Methods. Controls with no SIgA failed to agglutinate, indicating that agglutination was indeed specific for SIgA. As shown in Fig. 1, E. coli expressing pili with adhesin was agglutinated by SIgA. Similarly, E. coli expressing pili without the adhesin was also agglutinated by SIgA. In contrast, the E. coli expressing no pili and no adhesin was not agglutinated by SIgA, indicating that the presence of the pili in the absence of the adhesin can facilitate SIgA-mediated agglutination. Consistent with this idea, the presence of 25 mM α-CH3Man did not prevent the SIgA-mediated agglutination of either E. coli expressing pili plus adhesin or E. coli expressing pilus only (Fig. 1).
FIG. 1.
Agglutination of various strains of E. coli in the presence of SIgA. The numbers and sizes of particles after the addition of 0.5 mg of SIgA/ml to various strains of E. coli (ORN225, ORN226, and ORN227; see Table 1) were measured as a function of time with a Coulter counter. For presentation purposes, smaller aggregates (3 to 6 μm) (A) were plotted separately than larger aggregates (>6 μm) (B), which formed slower than the smaller aggregates. Experiments were performed in duplicate, and the standard errors are shown.
In addition to experiments with 25 mM α-CH3Man to inhibit binding of the adhesin to E. coli, some experiments were conducted in which 100 mM α-CH3Man was utilized. Consistent with previous results (61), the presence of 100 mM α-CH3Man inhibited 96% of the SIgA-mediated agglutination (determined at 17.5 h with the number of aggregates greater than 3 μm as a measure of aggregation) of E. coli expressing pili with adhesin. However, 100 mM α-CH3Man also inhibited 90% of the SIgA-mediated agglutination of E. coli expressing pili without the mannose-specific adhesin. This latter finding indicated that at least some of the inhibition of SIgA-mediated aggregation observed in the presence of 100 mM α-CH3Man was not specific for the mannose-specific adhesin.
Secretory IgA-mediated biofilm formation by various strains of E. coli.
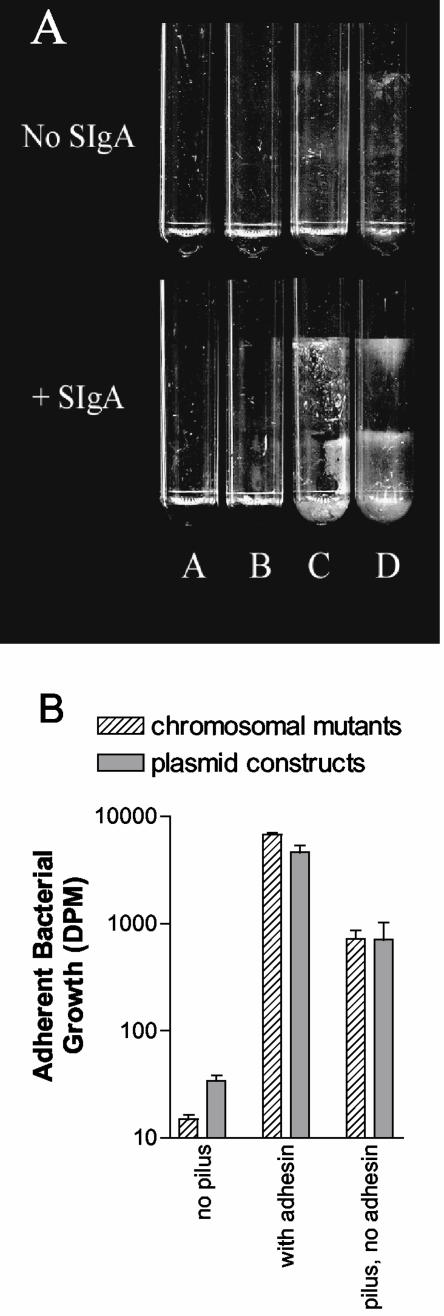
Under conditions in which free floating or free-swimming (planktonic) bacteria are repeatedly washed away, E. coli and other microorganisms form adherent plaques, or biofilms, on various surfaces (11). Biofilm formation may be facilitated by factors produced by the bacteria themselves or may be facilitated by the addition of factors such as SIgA that mediate interbacterial interactions. Thus, SIgA-mediated biofilm formation by E. coli can be used as an independent marker for the interaction between E. coli and SIgA. E. coli expressing pili and adhesin formed biofilms in the presence of SIgA but not in the absence of SIgA (Fig. 2). Similarly, E. coli expressing pili with no adhesin formed biofilms in the presence of SIgA but not in the absence of SIgA (Fig. 2A). Thus, biofilm formation by these bacteria was mediated by SIgA. In contrast, E. coli expressing no pili did not form SIgA-mediated biofilms (Fig. 2A), indicating that the pili, even without the presence of adhesin, are able to mediate biofilm formation by SIgA. Quantitative evaluation of the biofilms formed revealed that, in the absence of SIgA, the amount of biofilm formation decreased by 99% in E. coli expressing pili plus adhesin and by 97% in E. coli expressing pili only. However, the SIgA-mediated biofilm formed by E. coli expressing pili with adhesin contained ∼10-fold more radiolabeled material than the SIgA-mediated biofilm formed by E. coli expressing pili without adhesin (Fig. 2B). This observation suggests that the presence of the adhesin plays an important role but is not absolutely required in the interaction between SIgA and E. coli.
FIG. 2.
SIgA-mediated biofilm formation of E. coli as a function of pilus and adhesin expression (A) Experiments with chromosomal mutants. Tubes A contain no bacteria, tubes B contain E. coli (chromosomal mutants) without pilus or adhesin (ORN225; Table 1), tubes C contain E. coli with pilus but no adhesin (ORN226; Table 1), and tubes D contain E. coli with pilus and adhesin (ORN227; Table 1). (B) Quantification of the SIgA-mediated biofilm growth by various E. coli expressing pili with adhesin, pili without adhesin, or neither pili nor adhesin. Chromosomal mutants were the same used in the experiment described in Fig. 2A. Plasmid constructs expressing pili with adhesin (ORN201 containing pSH2; Table 1), pili without adhesin (ORN201 containing pORN307; Table 1), or neither pili nor adhesin (ORN201 containing pACY184; Table 1) were also used. Biofilm growth did not occur in the absence of immunoglobulin (see Fig. 3), indicating that the biofilm growth was mediated by SIgA. The growth is shown on a log scale. Growth was quantified by measuring the amount of 14C-glucose incorporated into the biofilm as described in Materials and Methods. Experiments were performed in duplicate, and the standard errors are shown.
The study required to remove biofilms from the polystyrene tubes provided further evidence that the interaction between SIgA and the adhesin was important in biofilm formation. A total of 91% of the SIgA-mediated biofilms formed by E. coli expressing pili without adhesin could be removed by vortexing without the use of sand as an abrasive. In contrast, only 35% of the SIgA-mediated biofilm formed by E. coli expressing pili with adhesin could be removed by vortexing without sand, the rest requiring sand to facilitate removal. This observation provides further evidence that the presence of the adhesin plays an important role but is not absolutely required in the interaction between SIgA and E. coli.
The experiments described above (Fig. 2A, B) were conducted with chromosomal mutants of E. coli in which the expression of the adhesin (FimH) was eliminated by deletion of the terminal 22 codons of the fimH gene. Deletion of the terminal 22 codons eliminated functional expression of the adhesin as measured by the mannose-dependent agglutination of guinea pig erythrocytes and by the failure of anti-adhesin antibodies to bind (21). However, to ensure that the results shown in Fig. 2 were not due to sIgA binding by a hypothetical truncated adhesin (FimH), we conducted additional experiments in which E. coli containing a recombinant plasmid carrying the genes for just the pilus fiber (lacking the fimH gene entirely) was compared to an E. coli carrying the genes for complete piliation (pilus plus adhesin). E. coli containing just the cloning vector was used and expressed no pili (see Table 1). The results (Fig. 2B) revealed that there was no discernible difference between the plasmid containing mutant lacking the entire fimH gene and the chromosomal mutant with a deletion of part of the fimH gene. The chromosomal mutant set was used exclusively for the remainder or the experiments.
Role of pili and adhesin in the interaction between IgM and E. coli.
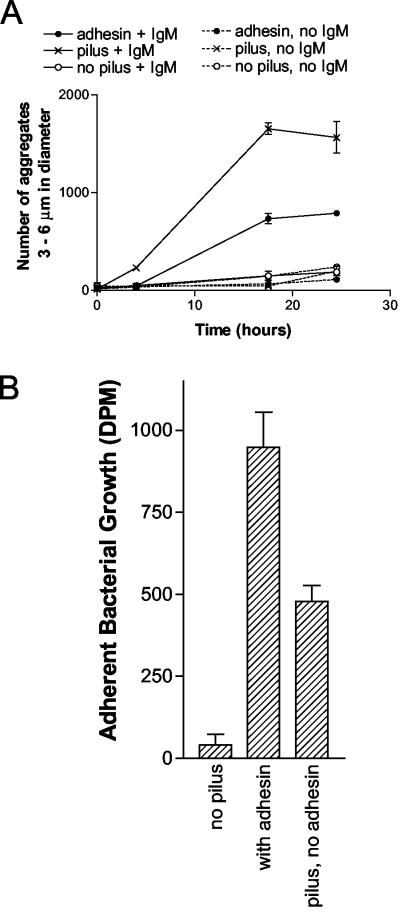
To determine whether pili without adhesin might also facilitate interactions between E. coli and a polyvalent immunoglobulin other than SIgA, the role of pili in the agglutination of E. coli by human plasma IgM was evaluated. As shown in Fig. 3A, IgM agglutinated E. coli expressing pili without adhesin better than E. coli expressing pili with adhesin. This finding was not expected, since IgM contains a large quantity and variety of terminal mannose structures (27). Similar to results obtained with SIgA, IgM failed to agglutinate E. coli expressing no pili or adhesin. Thus, pili in the absence of adhesin were required for interactions between IgM and E. coli.
FIG. 3.
IgM-mediated aggregation and biofilm formation of E. coli. (A) Agglutination of various E. coli (chromosomal mutants ORN225, ORN226, and ORN227; Table 1) in the presence or absence of human IgM. In contrast to results obtained with SIgA, aggregates greater than 6 μm in diameter were not formed by any of the E. coli in the presence of the IgM. (B) IgM-mediated biofilm formation by various E. coli (chromosomal mutants). All experiments were performed in duplicate, and the standard errors are shown.
Both E. coli expressing pili and adhesin and E. coli expressing pili only formed an IgM-mediated biofilm (Fig. 3B). In the absence of IgM, there were 87 and 92% reductions of the biofilm formation by E. coli expressing pili and adhesin and E. coli expressing pili only, respectively. Thus, the biofilms formed by these bacteria were indeed mediated by IgM. In contrast, E. coli expressing neither pili nor adhesin formed no IgM-mediated biofilm. These findings confirmed that pili without adhesin were required for mediate interactions between IgM and E. coli.
Binding of SIgA to various strains of E. coli during agglutination.
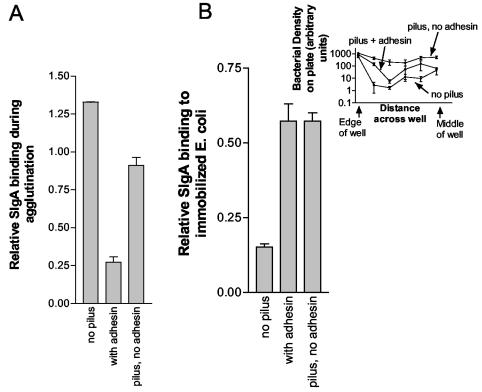
Agglutination and biofilm formation are indirect measures of the interaction between SIgA and E. coli. Depending on the efficiency of the agglutination reaction, agglutination may not be directly proportional to the amount of immunoglobulin binding to the bacteria. To determine to what extent pili with or without adhesin might facilitate the binding of SIgA to E. coli, we measured the binding of SIgA to E. coli expressing pili with or without adhesin and binding to E. coli expressing no pili. Direct binding of SIgA to E. coli was measured after a 2-h incubation in which E. coli expressing pili with or without adhesin was agglutinated. The measurement revealed that more SIgA bound to E. coli expressing no pili than to E. coli expressing pili without adhesin (Fig. 4A). Further, SIgA bound better to E. coli expressing pili without adhesin than to E. coli expressing pili with adhesin (Fig. 4A). This finding might seem unexpected since it suggests an inverse relationship between agglutination or biofilm formation (refer to Fig. 1 and 2) and SIgA binding. However, the E. coli bearing pilus with adhesin were, based on visual observation of aggregate size, strongly agglutinated during immunoprecipitation under the conditions used. On the other hand, under the same conditions, E. coli bearing pilus without adhesin were weakly agglutinated and E. coli without pilus or adhesin were not visibly agglutinated. Thus, under the conditions described here, agglutination of E. coli by SIgA may have sterically inhibited binding of SIgA except that required for agglutination. Regardless of any potential inhibition of binding by agglutination, these results demonstrate that the pilus does not facilitate an increase in the magnitude of binding of SIgA to E. coli.
FIG. 4.
SIgA binding to E. coli as a function of pilus and adhesin expression. (A) Immunoprecipitation of SIgA (1.0 mg/ml) was carried out for 2 h at 22.5°C with 7.2 × 107 E. coli/ml. The unbound SIgA was washed away, bacteria and bound SIgA were solubilized in SDS, and the amount of SIgA was quantified by immunoblotting and densitometric evaluation of the blots. Chromosomal mutants (ORN225, ORN226, and ORN227; Table 1) were utilized in the study. The experiment was conducted in duplicate, and the standard errors are shown. (B) Binding of human SIgA to immobilized E. coli (ORN225, ORN226, and ORN227; Table 1) expressing pilus with or without adhesin and to E. coli expressing no pilus. Binding of SIgA to E. coli was determined by ELISA with 0.5 mg of SIgA/ml to probe the E. coli. The number of E. coli bound to the plate is shown in the inset (note log scale). The number of E. coli bound was determined at various positions in the well by inserting a grid in each well (94-μm cell screen filter) and counting the number of E. coli in a given section manually. The ELISA was run in quadruplicate, and the standard errors are shown.
The number of SIgA molecules bound per E. coli cell is of interest. An approximation of the number of SIgA molecules bound to a given E. coli was obtained by quantitative evaluation of SIgA bound to E. coli, separated by SDS-PAGE, and stained by immunoblotting. The densitometric intensity of SIgA bound to E. coli was compared to the intensity of a purified SIgA preparation of a known concentration. Based on that comparison, under the conditions used for binding, ca. 625 SIgA molecules bound per E. coli cell bearing no pili or adhesin. Similarly, 430 SIgA molecules bound to each E. coli bearing pilus without adhesin and ca. 130 SIgA molecules bound to each E. coli bearing pilus plus adhesin.
Binding of SIgA to immobilized E. coli.
To further determine to what extent the pilus and the adhesin might affect binding of SIgA, the binding of antibody to E. coli was evaluated under conditions in which the E. coli could not be agglutinated. For this purpose, the E. coli was immobilized on an assay plate and the binding of SIgA to the E. coli was determined by ELISA. The results were complicated by the fact that E. coli expressing pili without adhesin bound much better to the assay plate than did E. coli expressing pili with adhesin (Fig. 4B, inset). Similarly, E. coli expressing pili with adhesin bound to the plate better than did E. coli expressing no pili (Fig. 4B, inset). The fact that pili with adhesin facilitate the binding of E. coli to various surfaces in a mannose-dependent manner (inhibited by 10 mM mannose) has been well established (24, 39, 40). However, the finding of increased adherence of E. coli expressing pili without adhesin was unexpected. The uneven distribution of E. coli on the plate (Fig. 4B, inset) made quantification of the number of bound bacteria difficult to accurately assess. However, some conclusions were still forthcoming from the data; The binding of SIgA to E. coli expressing pilus plus adhesin was similar to the binding of SIgA to E. coli expressing pilus only (Fig. 4B), despite the fact that more E. coli expressing pilus only bound to the plates. Thus, regardless of the role of pilus or adhesin in binding of E. coli to the assay plates, SIgA bound better to immobilized E. coli expressing pilus plus adhesin than to E. coli expressing pilus with no adhesin.
In addition to these experiments, the binding of SIgA to whole-cell extracts of E. coli was evaluated. Neither the presence of the pilus nor the adhesin had an impact on the amount of binding to whole-cell extracts of E. coli as judged by separation of the extracts by using SDS-PAGE, Western blotting, and immunostaining of the blots with purified SIgA (data not shown). Immunoblots of the E. coli extracts did reveal binding of SIgA to numerous bands, indicating that SIgA recognize a wide variety of antigens expressed by the E. coli. Given that most of the bacterial proteins were probably denatured, it is likely that the binding observed on immunoblots was due to the specificity of the antibody rather than to the specificity of bacterial adhesins.
DISCUSSION
We present several lines of evidence indicating that, in E. coli K-12, the type 1 pilus fiber was required for SIgA-mediated agglutination and biofilm formation and that a functional adhesin was not required to carry out the reactions. First, at concentrations of α-CH3Man that do not inhibit agglutination of E. coli expressing pilus without adhesin, there was little or no inhibition of SIgA-mediated aggregation of E. coli expressing adhesin. Thus, at least some component of the SIgA-dependent aggregation of E. coli appears to be independent of mannose binding. Second, the presence of pilus, even in the absence of adhesin, results in a dramatic increase in SIgA-mediated aggregation and SIgA mediated biofilm formation of E. coli. Lastly, in experiments with an immunoglobulin other than SIgA, aggregation and biofilm formation by IgM was also facilitated by the presence of pilus without adhesin. Importantly, under conditions in which E. coli was aggregated, the binding of SIgA to E. coli was not dependent on the presence of the pilus, with or without adhesin. This suggests that enhanced agglutination in the presence of pilus likely reflects the fact that the agglutination process is dependent on several parameters that are separate from binding of agglutinin (10, 54). For example, cell surface rigidity and electrostatic repulsion are two parameters that affect agglutination and that may be dramatically affected by the presence of pilus. In other words, pili may, for example, act as “entangling” surface fibrils that lack any activity in promoting mannose sensitive adhesion on their own. Consistent with this idea, other investigators have concluded that bacterial surface components such as pili, flagella, or exopolysaccharides can reduce the effects of electrostatic repulsive forces or improve the effective radius for binding during biofilm formation (non-SIgA mediated) (38, 58). On the other hand, the idea that SIgA bind directly to pili, thus facilitating agglutination, has not been ruled out.
There are additional findings which suggest that lack of antibody binding by SIgA or IgM does not account for failure of these antibodies to agglutinate nonpiliated bacteria: the results presented herein indicate that very few SIgA molecules (<130 per E. coli) are required to mediate agglutination of E. coli expressing pilus with or without adhesin. The view that few immunoglobulin molecules are needed to agglutinate target cells is not new, since Greenbury et al. found that about 25 IgM molecules per cell were required to agglutinate human red blood cells (19). This observation, in addition to the idea that natural antibodies recognize a wide variety of bacterial antigens, suggests that binding of sufficient immunoglobulin to facilitate agglutination of E. coli should readily occur. This may not be the case, however, if the amount of immunoglobulin required for agglutination is high. Thus, it may be argued that a failure of antibody to agglutinate some bacteria is a result of a relatively large amount of binding required to facilitate agglutination rather than a lack of actual binding.
These studies do not diminish the importance of the mannose-specific adhesin in the interaction between SIgA and E. coli. The studies described here were conducted in the presence of relatively high concentrations (0.5 to 1.0 mg/ml) of SIgA, which could obfuscate any interactions that might occur only under conditions in which the adhesin is expressed. Consistent with this idea, Wold et al. found that E. coli was agglutinated at high IgA concentrations regardless of the presence of α-CH3Man (61). The tendency of biofilms formed by E. coli expressing both pili and adhesin to be composed of more “strongly adherent” (based on the requirement of a physical abrasive to disrupt the biofilm) bacteria than biofilms formed by bacteria expressing pili without adhesin is consistent with an important role of the adhesin in the SIgA-mediated agglutination reaction. Also, our direct binding assays conducted on immobilized cells (i.e., under conditions in which physical aggregation could not influence immunoglobulin binding) indicated a role for the adhesin in contributing to binding. On the other hand, both aggregation and biofilm formation were completely eliminated when the pilus fiber was eliminated, but not when the adhesin was eliminated. Consequently, whereas the adhesin likely contributes to aggregation and certainly contributes to biofilm formation, the pilus fiber itself plays a crucial role in both of these processes. Although the presence of an intact adhesin improved SIgA-mediated biofilm formation, the adhesin may have slowed the kinetics of SIgA-mediated bacterial aggregation and clearly slowed the kinetics of IgM-mediated bacterial aggregation. Although these results might seem contradictory, at least one explanation is evident: rapid association of bacteria, reflecting a low free-energy barrier to intercellular association, may give rise to relatively heterogeneous intercellular interactions, which could, in turn, result in a weak aggregate or biofilm. On the other hand, a slower aggregate formation, associated with a stronger free-energy barrier to association, may be associated with a greater degree of cooperativity for binding and may result in a more uniform, stronger, aggregate. In other words, the presence of the adhesin may alter the tendency for nucleation versus propagation of an aggregate in a manner that strengthens biofilm formation (for which propagation may be more important) but weakens aggregation (for which nucleation may be more important).
Although incidental to the primary focus of the present study, our observation that mannose can become a nonspecific inhibitor at high concentrations has important technical implications. In particular, inhibition of bacterial interactions by relatively high concentrations of α-CH3Man is not an indication that the mannose-specific adhesin is important in those interactions. This observation is not without precedent; agglutination of Saccharomyces cerevisiae (baker's yeast) by E. coli is inhibited by 50 mM α-CH3Man. However, Eshdat et al. found that agglutination of yeast by E. coli, which occurs in the absence of type 1 pili, can be inhibited by 50 mM α-CH3Man (14). The reason for inhibition due to high concentrations of mannose or mannose derivatives found by Eshdat et al. and by us could be a nonspecific effect of viscosity.
These studies provide new insights into the role of the type 1 pilus in the SIgA-mediated agglutination reaction and thus into its potential role in colonization of the gut. For example, the pilus fiber itself may be particularly important in the interactions between microbes and SIgA1, an immunoglobulin that apparently lacks receptors for the mannose specific adhesin. In addition, the finding that agglutination by SIgA does not necessarily correspond to binding of SIgA is probably of more than technical importance; biologically important processes mediated by SIgA may depend on factors other than the binding of SIgA, including the expression of cell surface molecules, that may have a dramatic impact on intercellular interactions.
Acknowledgments
This study was supported in part by a Howard Hughes fellowship to A.D., by the Fannie E. Rippel Foundation, and by grants RO1 AI22223 and P30 DK34987 from the National Institutes of Health.
We thank Ann Wilson for technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Baenziger, J., and S. Kornfeld. 1974. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J. Biol. Chem. 249:7260-7269. [PubMed] [Google Scholar]
- 2.Baenziger, J., and S. Kornfeld. 1974. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J. Biol. Chem. 249:7270-7281. [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Bloch, C. A., B. A. Stocker, and P. E. Orndorff. 1992. A key role for type 1 pili in enterobacterial communicability. Mol. Microbiol. 6:697-701. [DOI] [PubMed] [Google Scholar]
- 5.Bollinger, R. R., M. L. Everett, D. Palestrant, S. D. Love, S. S. Lin, and W. Parker. 2003. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology 109:580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen, G. D., and A. D. Rovira. 1976. Microbial colonization of plant roots. Annu. Rev. Phytopathol. 14:121-144. [Google Scholar]
- 7.Brandtzaeg, P. 1998. Development and basic mechanisms of human gut immunity. Nutr. Rev. 56:S5-S18. [DOI] [PubMed] [Google Scholar]
- 8.Casali, P., and E. W. Schettino. 1996. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 210:167-179. [DOI] [PubMed] [Google Scholar]
- 9.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien, S., L. A. Sung, S. Simchon, M. M. Lee, K. M. Jan, and R. Skalak. 1983. Energy balance in red cell interactions. Ann. N. Y. Acad. Sci. 416:190-206. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 12.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 13.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshdat, Y., V. Speth, and K. Jann. 1981. Participation of pili and cell wall adhesion in the yeast agglutination activity of Escherichia coli. Infect. Immun. 34:980-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraysse, N., F. Couderc, and V. Poinsot. 2003. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 270:1365-1380. [DOI] [PubMed] [Google Scholar]
- 16.Friman, V., I. Adlerberth, H. Connell, C. Svanborg, L. A. Hanson, and A. E. Wold. 1996. Decreased expression of mannose-specific adhesins by Escherichia coli in the colonic microflora of immunoglobulin A-deficient individuals. Infect. Immun. 64:2794-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gbarah, A., C. G. Gahmberg, I. Ofek, U. Jacobi, and N. Sharon. 1991. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect. Immun. 59:4524-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giampapa, C. S., S. N. Abraham, T. M. Chiang, and E. H. Beachey. 1988. Isolation and characterization of a receptor for type 1 fimbriae of Escherichia coli from guinea pig erythrocytes. J. Biol. Chem. 263:5362-5367. [PubMed] [Google Scholar]
- 19.Greenbury, C. L., D. H. Moore, and L. A. C. Nunn. 1963. Reaction of 7S and 19S components of immune rabbit antisera with human group A and AB red cells. Immunology 6:421-433. [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, A., M. Gopal, and K. V. Tilak. 2000. Mechanism of plant growth promotion by rhizobacteria. Ind. J. Exp. Biol. 38:856-862. [PubMed] [Google Scholar]
- 21.Hamrick, T. S., S. L. Harris, P. A. Spears, E. A. Havell, J. R. Horton, P. W. Russell, and P. E. Orndorff. 2000. Genetic characterization of Escherichia coli type 1 pilus adhesin mutants and identification of a novel binding phenotype. J. Bacteriol. 182:4012-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamrick, T. S., E. A. Havell, J. R. Horton, and P. E. Orndorff. 2000. Host and bacterial factors involved in the innate ability of mouse macrophages to eliminate internalized unopsonized Escherichia coli. Infect. Immun. 68:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson, M. S., J. Hempel, and C. C. Brinton, Jr. 1988. Purification of the Escherichia coli type 1 pilin and minor pilus proteins and partial characterization of the adhesin protein. J. Bacteriol. 170:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris, S. L., D. A. Elliott, M. C. Blake, L. M. Must, M. Messenger, and P. E. Orndorff. 1990. Isolation and characterization of mutants with lesions affecting pellicle formation and erythrocyte agglutination by type 1 piliated Escherichia coli. J. Bacteriol. 172:6411-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 27.Hickman, S., R. Kornfeld, C. K. Osterland, and S. Kornfeld. 1972. The structure of the glycopeptides of a human M-immunoglobulin. J. Biol. Chem. 247:2156-2163. [PubMed] [Google Scholar]
- 28.Jones, C. H., J. S. Pinkner, R. Roth, J. Heuser, A. V. Nicholes, S. N. Abraham, and S. J. Hultgren. 1995. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 92:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keith, B. R., S. L. Harris, P. W. Russell, and P. E. Orndorff. 1990. Effect of type 1 piliation on in vitro killing of Escherichia coli by mouse peritoneal macrophages. Infect. Immun. 58:3448-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keith, B. R., L. Maurer, P. A. Spears, and P. E. Orndorff. 1986. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 53:693-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 32.Krogfelt, K. A., H. Bergmans, and P. Klemm. 1990. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 58:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lederberg, E. M., and S. N. Cohen. 1974. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J. Bacteriol. 119:1072-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markovitz, A. 1977. Genetics and regulation of bacterial capsular polysaccharide synthesis and radiation sensitivity, p. 415-462. In I. W. Sutherland (ed.), Surface carbohydrates of the prokaryotic cell, vol. I. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 35.Maurer, L., and P. E. Orndorff. 1987. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J. Bacteriol. 169:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Moshier, A., M. S. Reddy, and F. A. Scannapieco. 1996. Role of type 1 fimbriae in the adhesion of Escherichia coli to salivary mucin and secretory immunoglobulin A. Curr. Microbiol. 33:200-208. [DOI] [PubMed] [Google Scholar]
- 38.Neu, T. R., and K. C. Marshall. 1990. Bacterial polymers: physicochemical aspects of their interactions at interfaces. J. Biomaterials Appl. 5:107-133. [DOI] [PubMed] [Google Scholar]
- 39.Old, D. C., I. Corneil, L. F. Gibson, A. D. Thomson, and J. P. Duguid. 1968. Fimbriation, pellicle formation and the amount of growth of salmonellas in broth. J. Gen. Microbiol. 51:1-16. [DOI] [PubMed] [Google Scholar]
- 40.Old, D. C., and J. P. Duguid. 1970. Selective outgrowth of fimbriate bacteria in static liquid medium. J. Bacteriol. 103:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen, A., A. Arnqvist, M. Hammar, S. Sukupolvi, and S. Normark. 1993. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol. Microbiol. 7:523-536. [DOI] [PubMed] [Google Scholar]
- 42.Olsen, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338:652-655. [DOI] [PubMed] [Google Scholar]
- 43.Orndorff, P. E. 1987. Genetic study of piliation in Escherichia coli: implications for understanding microbe-host interactions at the molecular level. Pathol. Immunopathol. Res. 6:82-92. [DOI] [PubMed] [Google Scholar]
- 44.Orndorff, P. E., and C. A. Bloch. 1990. The role of type 1 pili in the pathogenesis of Escherichia coli infections: a short review and some new ideas. Microb. Pathog. 9:75-79. [DOI] [PubMed] [Google Scholar]
- 45.Orndorff, P. E., and S. Falkow. 1984. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J. Bacteriol. 159:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orndorff, P. E., P. A. Spears, D. Schauer, and S. Falkow. 1985. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J. Bacteriol. 164:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponniah, S., R. O. Endres, D. L. Hasty, and S. N. Abraham. 1991. Fragmentation of Escherichia coli type 1 fimbriae exposes cryptic d-mannose-binding sites. J. Bacteriol. 173:4195-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouttu, R., T. Puustinen, R. Virkola, J. Hacker, P. Klemm, and T. K. Korhonen. 1999. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia coli to collagens. Mol. Microbiol. 31:1747-1757. [DOI] [PubMed] [Google Scholar]
- 49.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli, and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 51.Russell, P. W., and P. E. Orndorff. 1992. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J. Bacteriol. 174:5923-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated autoaggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 53.Schiffrin, E. J., and S. Blum. 2002. Interactions between the microbiota and the intestinal mucosa. Eur. J. Clin. Nutr. 56:S60-S64. [DOI] [PubMed] [Google Scholar]
- 54.Skalak, R. 1984. Aggregation and disaggregation of red blood cells. Biorheology 21:463-476. [DOI] [PubMed] [Google Scholar]
- 55.Sokurenko, E. V., H. S. Courtney, D. E. Ohman, P. Klemm, and D. L. Hasty. 1994. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J. Bacteriol. 176:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonnenwirth, A. C. 1979. Antibody response to anaerobic bacteria. Rev. Infect. Dis. 1:337-341. [DOI] [PubMed] [Google Scholar]
- 57.Spears, P. A., D. Schauer, and P. E. Orndorff. 1986. Metastable regulation of type 1 piliation in Escherichia coli and isolation and characterization of a phenotypically stable mutant. J. Bacteriol. 168:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Loosdrecht, M. C., J. Lyklema, W. Norde, and A. J. Zehnder. 1990. Influence of interfaces on microbial activity. Microbiol. Rev. 54:75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams, R. C., and R. J. Gibbons. 1972. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science 177:697-699. [DOI] [PubMed] [Google Scholar]
- 60.Wold, A. E., and I. Adlerberth. 2000. Breast feeding and the intestinal microflora of the infant-implications for protection against infectious diseases. Adv. Exp. Med. Biol. 478:77-93. [DOI] [PubMed] [Google Scholar]
- 61.Wold, A. E., J. Mestecky, M. Tomana, A. Kobata, H. Ohbayashi, T. Endo, and C. S. Eden. 1990. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect. Immun. 58:3073-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodall, L. D., P. W. Russell, S. L. Harris, and P. E. Orndorff. 1993. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J. Bacteriol. 175:2770-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]