Abstract
The anti-infectious activity of probiotic Bifidobacteria against Shiga toxin-producing Escherichia coli (STEC) O157:H7 was examined in a fatal mouse STEC infection model. Stable colonization of the murine intestines was achieved by the oral administration of Bifidobacterium breve strain Yakult (naturally resistant to streptomycin sulfate) as long as the mice were treated with streptomycin in their drinking water (5 mg/ml). The pathogenicity of STEC infection, characterized by marked body weight loss and subsequent death, observed in the infected controls was dramatically inhibited in the B. breve-colonized group. Moreover, Stx production by STEC cells in the intestine was almost completely inhibited in the B. breve-colonized group. A comparison of anti-STEC activity among several Bifidobacterium strains with natural resistance to streptomycin revealed that strains such as Bifidobacterium bifidum ATCC 15696 and Bifidobacterium catenulatum ATCC 27539T did not confer an anti-infectious activity, despite achieving high population levels similar to those of effective strains, such as B. breve strain Yakult and Bifidobacterium pseudocatenulatum DSM 20439. The effective strains produced a high concentration of acetic acid (56 mM) and lowered the pH of the intestine (to pH 6.75) compared to the infected control group (acetic acid concentration, 28 mM; pH, 7.15); these effects were thought to be related to the anti-infectious activity of these strains because the combination of a high concentration of acetic acid and a low pH was found to inhibit Stx production during STEC growth in vitro.
A complex intestinal microflora provides protection against colonization by many pathogenic infectious agents (for reviews, see references 8 and 40), and the term colonization resistance was first used by van der Waaij et al. in 1971 to indicate a resistance to colonization by exogenous, potentially pathogenic microorganisms (PPMOs) (39). Vollaard and Clasener concluded that the flora providing colonization resistance to exogenous microorganisms are identical to the flora limiting the concentration of indigenous PPMOs (40).
Shiga toxin-producing Escherichia coli (STEC) infection models in streptomycin (SM)-treated mice have been the most popular (15, 20, 22, 41). An increased susceptibility to STEC infection by treating mice with antibiotics can be explained by the disruption of colonization resistance. However, high dosages of inoculum (more than 106 CFU/body) are usually required to establish an STEC infection. These effects were not precisely examined (determination of viable STEC counts or quantification of Shiga-like toxins) in previous studies. We have developed an SM-treated murine STEC infection model in which 100% lethality was achieved after inoculation with only ∼5 × 103 CFU of STEC, followed by multiple mitomycin C (MMC) treatments (29). Moreover, a periodic quantitative analysis of Stx production in the intestines showed that there was a transient but dramatic increase of Stxs (especially Stx2) in the lower intestines after multiple MMC treatments during the early stationary phase of STEC growth in the lower intestines.
Probiotics are viable cell preparations or foods containing viable bacterial cultures or components of bacterial cells that have beneficial effects on the health of the host (19). Many of these probiotics are lactic acid bacteria, and anaerobic bifidobacteria have been reported to be useful in the treatment of disturbed intestinal microflora and diarrheal diseases (for a review, see reference 18). Feeding probiotic bifidobacteria to experimental animals has been reported to prevent gram-negative bacterial infections (23, 30, 32). Some probiotic bifidobacterial strains have been reported to lessen the severity of oral STEC infection in murine experimental infection models. Most of these reports, however, utilized gnotobiotic animal models (2, 31, 37, 38), and definite data have not been obtained in studies with conventional animals. Moreover, the precise mechanism of protection has not yet been clarified. The main purpose of the present study was to test the hypothesis that intestinal colonization by probiotic bifidobacteria prevents antibiotic-induced disruptions in the intestinal environment and reduces the lethal toxicity of STEC by using our previously reported lethal murine STEC infection model (29).
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 6-week-old male BALB/c mice were purchased from Charles River Japan, Inc. (Kanagawa, Japan). Groups of 7 or 8 mice were housed in polypropylene cages (CLEA Japan, Tokyo, Japan) with sterilized bedding under controlled lighting (12 h light, 12 h dark), temperature (24°C), and relative humidity (55%) conditions. The mice were maintained on an MF diet (Oriental Yeast, Tokyo, Japan) and sterilized water (126°C for 30 min) containing Cl2 at a final concentration of 1.5 ppm (μg/ml), ad libitum. SM sulfate (Sigma Chemical, St. Louis, Mo.) was dissolved in the drinking water at a concentration of 5 mg/ml. The water bottles were exchanged with freshly prepared bottles every 3 days. All experimental procedures were performed according to the standards set forth in the Guide for the Care and Use of Laboratory Animals (24).
Bifidobacteria.
Bifidobacterium breve strain Yakult, Bifidobacterium pseudocatenulatum DSM 20439, Bifidobacterium bifidum ATCC 15696, and Bifidobacterium catenulatum ATCC 27539T were used after the selection of the strains had been confirmed by growth in PY broth (16) containing SM at a dose of 4 mg/ml. All bifidobacterial strains were identified by PCR assay with the corresponding species-specific primers for 16S rRNA (21). Each bifidobacterial strain was cultivated separately in GAM broth (Nissui Pharmaceutical, Tokyo, Japan) for 24 h at 37°C, washed with saline twice, and then suspended in saline at a concentration of 109 CFU/ml. Colonization by bifidobacteria was established by three consecutive daily administrations of the bacteria to separate groups of mice receiving SM in their drinking water. Periodic examinations of viable counts of B. breve in stools were performed in subsets of 6 mice from each group. Briefly, fresh stool specimens (1 to 2 pellets) were weighed and placed in an Eppendorf tube containing 1 ml of sterilized anaerobic buffer solution [KH2PO4, 0.0225% wt/vol; K2HPO4, 0.0225% wt/vol; NaCl, 0.045% wt/vol; (NH4)2SO4, 0.0225% wt/vol; CaCl2, 0.00225% wt/vol; MgSO4, 0.00225% wt/vol; Na2C03, 0.3% wt/vol; l-cysteine hydrochloride, 0.05% wt/vol; resazurin, 0.0001% wt/vol] and homogenized with a pestle. TOS agar (33) supplemented with 0.625 g of SM/ml and 1 μg of carbenicillin disodium salt (Sigma)/ml (T-CBPC agar) was used for the quantitation of the B. breve strain Yakult, and CPLX agar (42) supplemented with 0.625 g of SM/ml was used for the selective isolation of other Bifidobacterium strains. The media were cultured anaerobically in an atmosphere of 7% H2 and 5% CO2 in N2 at 37°C for 72 h, and the colonies on the plates were counted.
STEC O157:H7 infection.
A clinically isolated STEC O157:H7 strain 89020087, which produces both Stx1 and Stx2, was used throughout the study. Cells were grown overnight in Casamino Acids-yeast extract broth (14) at 37°C. A murine gastrointestinal infection model (29) was developed based on the methods of Wadolkowski et al. (41). Briefly, STEC cells were suspended at a concentration of 5 × 104 CFU/ml in saline, and a 100-μl portion of the suspension was administered orally to mice. MMC (0.25 mg/kg; Kyowa Hakko Kogyo, Tokyo, Japan) was administered intraperitoneally a total of three times, once each at 18, 21, and 24 h postinoculation, when the fecal excretion levels of STEC reached as much as 109 CFU/g of feces. To assess the viable STEC counts in the feces, intestinal contents, livers, and mesenteric lymph nodes, samples were removed aseptically from the mice and homogenized in 1 ml (5 ml for liver) of sterile saline solution by using a Teflon grinder. The number of viable STEC cells was determined by their growth on sorbitol-MacConkey agar (Nissui Seiyaku, Tokyo, Japan) supplemented with cefixime (2.5 mg/ml; Sigma) and potassium tellurite (0.05 mg/ml; Oxoid, Bashingstoke, Hampshire, United Kingdom) at 37°C for 24 h.
Stx assay.
Stxs (Stx1 and Stx2) in the intestinal contents (both free and bacterium associated) were extracted as follows. Briefly, sections of the gastrointestinal tracts were prepared as described above. After homogenization, samples were sonicated at 28 kHz for 60 min in ice-cold water to completely disrupt the bacteria and then centrifuged at 30,000 × g for 10 min to remove undisrupted debris. The supernatants were then filtered through a 0.45-μm-pore-size membrane filter and then ultrafiltrated (molecular weight cutoff, 20,000; 5,000 × g for 60 min) to remove low-molecular-weight substances, such as SM sulfate, which can affect Stx quantification when the reversed passive latex agglutination (RPLA) test (Denka Seiken, Tokyo, Japan) is used. After centrifugation, the resulting fraction on the membrane in the tube was reconstituted in the original volume of phosphate-buffered saline and then serial twofold diluted with phosphate-buffered saline supplemented with 0.5% bovine serum albumin and 0.1% NaN3. Both Stx1 and Stx2 were then quantified by the RPLA test. The Stx concentrations in the intestinal contents were then calculated relative to a standard curve of purified Stx1 or Stx2 and expressed as micrograms per tissue weight.
Histopathology.
Mice were dissected on day 2 or 7 after STEC infection. The mesenteric lymph nodes, femur, thymus, lungs, bronchus, heart, small intestine, cecum, colon, liver, spleen, kidneys, suprarenal gland, and brain were divided longitudinally and fixed overnight in 10% neutral buffered formalin. Paraffin-embedded sections stained with hematoxylin and eosin were then examined by light microscopy.
Detection of organic acids in cecal contents.
The cecal contents were homogenized in 1 ml of distilled water, and the homogenate was centrifuged at 13,000 × g at 4°C for 10 min. A mixture of 0.9 ml of the resulting supernatant and 0.1 ml of 1.5 mM perchloric acid was mixed well in a glass tube and allowed to stand at 4°C for 12 h. The suspension was then passed through a filter with a pore size of 0.45 μm (Millipore Japan, Tokyo, Japan). The organic acid content of the sample was analyzed by high-performance liquid chromatography as described in a previous report (18). The high-performance liquid chromatography was performed with a Waters system (Waters 432 Conductivity Detector; Waters, Milford, Mass.) equipped with two columns (Shodex Rspack KC-811; Showa Denko, Tokyo, Japan). The concentrations of organic acids were calculated by using external standards.
Combined effect of pH and AA on STEC growth and Stx production.
The pH and concentration of acetic acid (AA) were adjusted in tryptic soy broth so that the conditions were the same as those found in the cecal contents of the STEC-infected control group (pH 7.15; AA concentration, 28 mM) or in the B. breve-colonized cecum (pH 6.75; AA concentration, 56 mM). Then, STEC in media at a concentration of 105 CFU/ml was added and cultivated anaerobically in an atmosphere of 100% N2 at 37°C; MMC at a final concentration of 1 μg/ml was added after 8 h of cultivation. Viable bacterial counts were determined periodically after 0, 2, 4, 6, 8, 12, and 16 h of incubation. Stx concentrations were determined after 16 h of cultivation (8 h after the addition of MMC).
Statistical analysis.
The average number of bacteria was analyzed by using the Dunnet test to determine significant differences between the treatment and control groups. Differences in survival ratios were determined by using Fisher's exact probability test followed by correction with the Bonferroni inequality equation. A significant difference was defined as a P value of <0.05.
RESULTS
Inhibition of lethal STEC O157:H7 intestinal infection by B. breve colonization of the intestines in SM-treated mice.
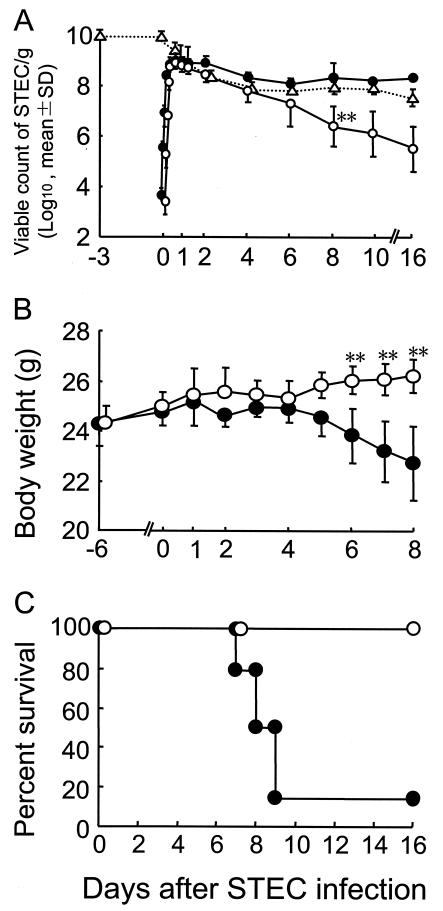
The excretion levels of STEC in feces after the oral administration of 5 × 103 CFU suggested that STEC proliferated dramatically in the intestines within 24 h of the infection (Fig. 1A). B. breve strain Yakult, when administered daily for three consecutive days (108 CFU/mouse/day), aggressively proliferated in the intestine, reaching a population level of 109 CFU/g of cecal contents; this level of proliferation was maintained at the time of the STEC infection on day 3, after the last administration of B. breve (Fig. 1A). The stable colonization of the intestines by B. breve did not inhibit the STEC cells from proliferating logarithmically during the initial phase of the infection, but once the proliferation had reached a plateau, further colonization was significantly inhibited for 16 days (Fig. 1A).
FIG. 1.
Inhibition of lethal intestinal STEC infection by B. breve colonization in SM-treated mice. SM sulfate at a concentration of 5 mg/ml in drinking water was given to 28 mice from day −6 until day 16. B. breve strain Yakult (1 × 108 to 3 × 108 CFU/mouse/day) in 0.1 ml of saline was administered to half of the mice once a day from day −5 to −3, and the other half of the mice were administered saline on the same schedule as that for the B. breve treatment. Mice were infected orally with STEC (5 × 103 CFU) on day 0 and then treated with MMC at an inoculum dose of 0.25 mg/kg of body weight three times at 18, 21, and 24 h after the STEC infection. (A) Feces for bacteriological analysis were obtained from 6 randomly selected mice in each group on days 0 (at 3, 6, 9, 12, 15, and 18 h), 1, 3, 4, 7, 10, and 16 after the STEC infection, with the exception of the control group on days 10 to 16 (n = 2). Viable counts of STEC and B. breve were examined as described in the text. Symbols: •, number of STEC organisms in the STEC-infected control mice; ○, number of STEC organisms in the B. breve-treated mice; ▵, number of B. breve organisms in B. breve-treated mice. (B) All 14 mice in each group were weighed every day until day 8. Symbols: •, STEC-infected control; ○, B. breve-treated mice. (C) The STEC-infected control mice (•) and B. breve-treated mice (○) were observed for survival for 14 days after the challenge infection. **, a significant difference was observed between the B. breve-treated and the untreated control groups (P < 0.01).
In the STEC-infected control group, a dramatic decrease in body weight and subsequent death was observed in 12 of 14 mice in the group within 10 days after MMC treatment (Fig. 1B and C). On the other hand, body weight was maintained and none of the mice died in the B. breve-treated group throughout the observation period. Extraintestinal STEC translocation was not observed in either group (data not shown), suggesting that sepsis was not the cause of death in the STEC-infected control group.
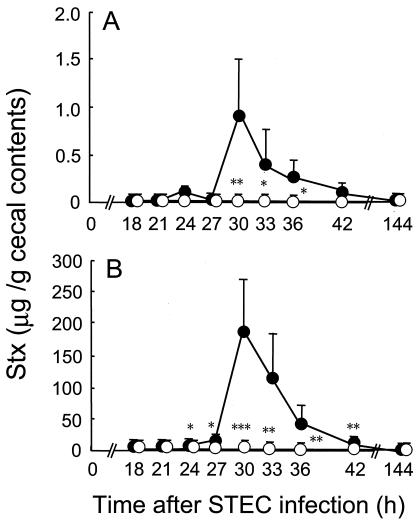
In the next series of experiments, mice were dissected at various intervals after MMC treatment, and the Stx levels in the cecal contents were analyzed by RPLA. Transient but dramatic increases in the concentrations of both types of Stx were observed 3 to 9 h after the last MMC treatment in the STEC-infected control group (Fig. 2). The Stx2 titers were relatively higher than those of Stx1 throughout the experimental period (Fig. 2). No significant increases in the Stx titers were detected in the B. breve-treated group after MMC treatment, and the titers were less than 1/50 (Stx1) and 1/500 (Stx2) of those in the controls, respectively (Fig. 2). The Shiga toxins were produced mainly in the lower parts of the intestine, whereas B. breve markedly inhibited production of both types of the toxin (Table 1).
FIG. 2.
Inhibition of MMC-induced production of Shiga toxins by B. breve colonization. Mice were infected with STEC and treated with MMC as described in the legend to Fig. 1 and then dissected at the indicated periods after STEC infection to examine Stx production. The concentrations of Stx1 (A) and Stx2 (B) in the intestinal contents were determined by RPLA test as described in the text. The results were expressed as the means and standard deviations of the results from 6 mice. Significant differences in Stx concentration were observed between the B. breve-treated and the untreated control groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
TABLE 1.
Inhibition of MMC-induced production of Shiga toxins by B. breve colonizationc
| Intestinal part | Stx1 (μg/g of intestinal contents) ina:
|
Stx2 (μg/g of intestinal contents) ina:
|
||
|---|---|---|---|---|
| Untreated control | B. breve-treated mice | Untreated control | B. breve-treated mice | |
| Small intestine | NDb | ND | 0.04 ± 0.02 | ND |
| Cecum | 0.9 ± 0.6 | 0.02 ± 0.03d | 181.5 ± 85.6 | 0.4 ± 0.2d |
| Large intestine | 0.2 ± 0.2 | 0.02 ± 0.01e | 33.2 ± 33.8 | 0.3 ± 0.4d |
The concentrations of Stx1 and Stx2 in intestinal contents were determined by the RPLA test as described in the text, and the results are expressed as the means and standard deviations of the results for 6 mice.
ND, not detected.
Mice were infected with STEC and treated with MMC as described in the legend to Fig. 1 and dissected at 30 h after STEC infection for examination of Stx production. To examine localization of Stx in the intestines, each part of the intestine was resected at 30 h after infection.
Significant difference between the B. breve-treated mice and the untreated control group (P < 0.01).
Significant difference between the B. breve-treated mice and the untreated control group (P < 0.001).
Histological analysis.
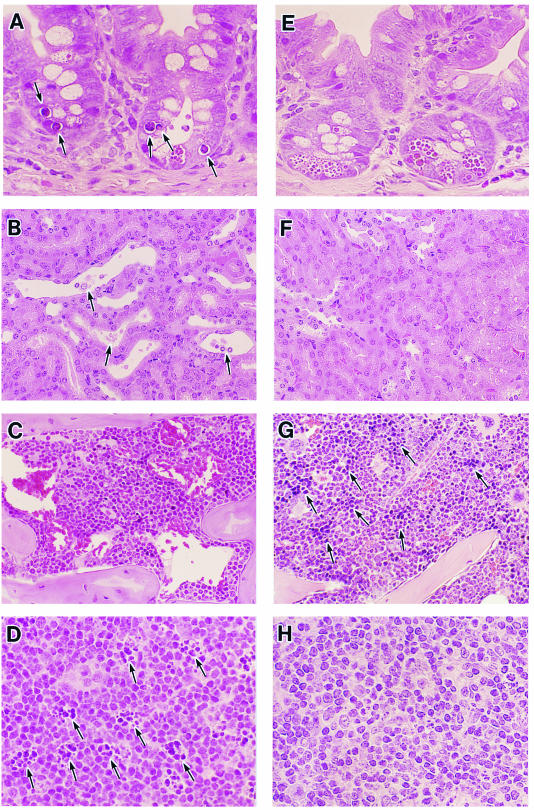
In the STEC-infected control mice, mild damages appearing to be apoptotic were observed in cryptic areas of the intestinal mucosa 30 h after STEC infection, when the concentrations of Stxs in the intestines peaked (Fig. 3A). Injuries in the hematopoietic organs, such as the bone marrow (erythroblastopenia) (Fig. 3C), mesenteric lymph nodes (apoptosis) (Fig. 3D), and toxic tubular necrosis accompanied by distention (Fig. 3B) were clearly observed on day 7 after STEC infection in the moribund STEC-infected control group. In the B. breve-treated group, however, no significant histopathologic disorders (Fig. 3E, F, G, and H) were observed, and erythroblast hematopoiesis was clearly observed in femur and spleen specimens. A hematological analysis showed no clear hemolytic-uremic syndrome signs, such as thrombocytopenia or hematolytic anemia, in either the STEC-infected control group or the B. breve-treated group. Significant increases in creatinine and blood urea nitrogen were detected in the infected control group but not in the B. breve-treated mice (creatinine, 0.6 ± 0.2 mg/dl for the control, 0.3 ± 0.0 mg/dl for the B. breve-treated mice, P < 0.01; blood urea nitrogen, 27.8 ± 2.2 mg/dl for the control, 23.4 ± 2.3 mg/dl for the B. breve-treated mice, P < 0.01). No distinct characteristics of lower leg paralysis or histopathological damage to the other organs, including the brain, were observed (data not shown). These results suggest that the intestinal mucosa injuries were caused by the transient but marked increase in Stx after MMC treatment.
FIG. 3.
Histopathological analysis. Hematoxylin and eosin staining of the ileum (A and E), kidney (B and F), bone marrow (C and G), and mesenteric lymph node (D and H) from a mouse in the STEC-infected control group (STEC inoculum, 3.8 × 103 CFU) (A to D) and a mouse in the B. breve-treated group (E to H). Organs were obtained on day 2 (A and E) or 7 (B to D and F to H) after STEC infection. Black arrows: panel A, changes suggestive of apoptosis; panel B, necrotic tubular endothelial cells with distention; panel D, changes suggestive of apoptotic bodies; panel G, erythroblasts. Magnifications in both groups: ileum, ×520; kidney, ×520; bone marrow, ×260; mesenteric lymph node, ×520.
Comparison of antitoxic activity among several strains of bifidobacteria with natural resistance to SM.
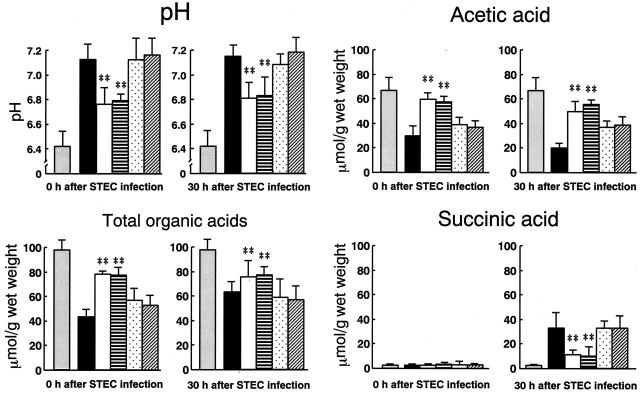
The anti-infectious activities of four bifidobacterial strains belonging to four species and confirmed to exhibit a natural resistance to SM in vitro were assessed for their antitoxic activity against STEC in vivo. Although all the strains were colonized in the intestine at similarly high population levels, marked differences in antitoxic activity were observed among the strains (Table 2). Two of four strains that were tested showed potent antitoxic activity, but B. bifidum ATCC 15696 and B. catenulatum ATCC 27539T did not exhibit an antitoxic activity. No correlations between antitoxic activity and colonization level were observed among the strains (Table 2). As shown in Fig. 4, the total organic acid concentration decreased and the pH increased in the cecum after SM treatment in the STEC-infected control group; these levels remained unchanged at 30 h after the STEC infection. The disruption in the balance of organic acid concentrations was characterized by a decrease in the acetate concentration and an increase in the succinate concentration when compared with the healthy controls (Fig. 4). Significantly lower pHs, higher concentrations of both total organic acids and AA, and lower concentrations of succinic acid were observed at 30 h after STEC infection in the B. breve- and B. pseudocatenulatum-treated groups than in the STEC-infected control group. No significant changes in these markers were observed in the groups treated with the ineffective strains, B. bifidum and B. catenulatum, when compared with the infected controls.
TABLE 2.
Comparison of antitoxic activity among several strains of bifidobacteria with natural resistance to SM sulfatea
| Treatment | STEC viable counts/g of feces 18 h after infection (log10, mean ± SD) | No. of deaths/total no. of mice (survival time [days, mean ± SD]) | Concn in cecal contents ofb:
|
|
|---|---|---|---|---|
| Stx1 | Stx2 | |||
| None (untreated control) | 9.2 ± 0.2 | 8/10 (8.0 ± 1.2) | 0.7 ± 0.5 | 200.0 ± 162.1 |
| B. breve strain Yakult | 9.0 ± 0.3 | 0/10c | 0.1 ± 0.1c | 1.9 ± 1.2d |
| B. pseudocatenulatum DSM 20439 | 9.1 ± 0.3 | 0/10c | 0.1 ± 0.1c | 1.4 ± 1.4d |
| B. bifidum ATCC 15696 | 9.1 ± 0.2 | 8/10 (8.6 ± 1.4) | 0.6 ± 0.6 | 141.3 ± 99.7 |
| B. catenulatum ATCC 27539T | 9.0 ± 0.3 | 7/10 (8.6 ± 1.4) | 0.8 ± 0.3 | 155.7 ± 128.6 |
SM sulfate at a concentration of 5 mg/ml in drinking water was given to mice from day −6 to day 16. Bifidobacterial strains (1 × 108 to 4 × 108 CFU/mouse/day) at an inoculum size of 0.1 ml/mouse were administered to separate groups of mice (10 mice/group) once a day from day −5 to day −3. Population levels of bifidobacteria at the time of STEC infection (log10; mean ± standard deviation) are as follows: B. breve strain Yakult, 9.7 ± 0.3; B. pseudocatenulatum DSM 20439, 9.7 ± 0.1; B. bifidum ATCC 15696, 9.6 ± 0.2; B. catenulatum ATCC 27539T, 9.8 ± 0.3. Mice were orally infected with STEC at a dose of 8.1 × 103 CFU on day 6 after starting SM treatment. MMC (0.25 mg/kg of body weight) was administered a total of three times, once each at 18, 21, and 24 h postinfection. Mice were observed for survival for 16 days after STEC infection. Mice were sacrificed 6 h after the last MMC shot, and Stx concentrations in the cecal contents were determined by the RPLA test as described in the text.
Results are expressed as mean Stx concentrations (micrograms per gram of cecal contents) ± standard deviations for the results from 6 mice.
Significant difference between the Bifidobacterium-treated mice and the untreated control mice (P < 0.05).
Significant difference between the Bifidobacterium-treated mice and the untreated control mice (P < 0.01).
FIG. 4.
Changes in intestinal pH and concentrations of organic acids after STEC infection in SM-treated mice. Mice were treated as shown in Table 2. Cecal contents were obtained from mice both at the time of STEC infection (0 h) and 30 h after STEC infection. pH and organic acid concentrations were determined as described in the text. Results are expressed as the means and standard deviations of the results from 6 mice. Columns: grey, nontreated healthy mice; black, SM-treated mice; white, B. breve strain Yakult-treated mice; hatched, B. pseudocatenulatum DSM20439-treated mice; slashed, B. bifidum ATCC 15696-treated mice; vertically lined, B. catenulatum ATCC 27539T-treated mice. **, significant differences are shown for the Bifidobacterium-treated groups versus the untreated control group (P < 0.01).
Effect of pH and acetate concentration on Stx production in vitro.
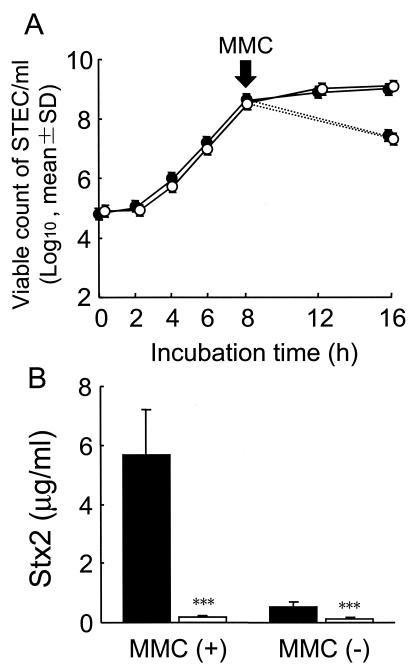
The differences in pH and acetate concentration observed between the lower intestines of the infected control group and the B. breve-treated group, when reproduced in vitro, produced no differences in the STEC growth patterns, with or without the addition of MMC (Fig. 5A). As shown in Fig. 5B, the addition of MMC to media simulating the conditions observed in the infected control group markedly enhanced the production of Stx2 by the STEC cells. Stx2 production at levels as low as 1/30 of that produced under the control conditions were detected when the conditions in the B. breve-treated group were reproduced, and the inhibitory activity of the combined lower pH and higher acetate concentration conditions was exerted almost equally in cultures with or without the addition of MMC. Superinduction of Stx2 by the other agents such as ciprofloxacin hydrochloride and UV under the STEC-infected control condition was detected in a similar fashion as that induced by MMC, which was also markedly inhibited under the conditions in the B. breve-treated group (data not shown).
FIG. 5.
Inhibition of Stx production but not STEC proliferation at higher AA concentrations and lower pH values in vitro. The pH and concentration of AA were adjusted in the growth medium so that the conditions were the same as those in the control cecum (•) (pH 7.15; AA concentration, 28 mM) or the B. breve-colonized cecum (○) (pH 6.75; AA concentration, 56 mM). STEC was added to each medium at a final concentration of 105 CFU/ml and cultivated at 37°C for 8 h. Cultures were then divided into two groups, and either 20 μl of fresh medium or MMC at a final concentration of 1 μg/ml in 20 μl of medium was added to each group, and the tubes were incubated for an additional 8 h. (A) Viable bacterial counts were determined at the indicated periods during incubation. The straight line and the dotted line show growth without (−) and with (+) MMC, respectively. (B) Stx2 concentrations were determined after incubation for 16 h. Columns: black, control, white, B. breve. Results are expressed as the means and standard deviations of the results from triplicate cultures. ***, significant differences are shown for growth under the B. breve colonization conditions versus growth under control conditions (P < 0.001).
DISCUSSION
It was previously demonstrated that the injection of a lethal dose of 5-fluorouracil (400 mg/kg) into mice induced an extraordinary increase in the levels of indigenous E. coli in the intestine and the systemic translocation of these bacteria to the liver; Bifidobacterium was the only species whose intestinal population markedly decreased after treatment with this chemotherapeutic agent (25, 26). Moreover, the daily administration of fermented milk containing probiotic bifidobacteria prevented both the drug-induced intestinal outgrowth and the extraintestinal translocation of indigenous E. coli (3). Analysis of the organic acid concentrations in the intestinal contents suggested that the administered species compensated for a decrease in the production of organic acids by the disrupted indigenous microflora. More recently, it was demonstrated that the antibiotic-induced intestinal overgrowth and extraintestinal translocation of Salmonella enterica serovar Typhimurium was markedly inhibited by precolonization of the intestine by specific strains, such as the probiotic B. breve strain Yakult, and suggested that the pH-lowering and acid-producing effects of this strain appeared to be important for enabling this anti-infectious activity (4). These observations suggested that compensation for chemotherapy-induced disruptions in colonization resistance throughout the use of probiotic bifidobacteria may be effective for preventing intestinal infections by PPMOs and that not only the population level but also the metabolic activity of the intestinal colonizer is important for this anti-infectious activity. In the present study, we clarified that the stable colonization of intestines by specific strains of bifidobacteria, such as B. breve strain Yakult and B. pseudocatenulatum DSM 20439, results in the protection of mice from lethal STEC infections, possibly by inhibiting the production of Stxs in the intestines. To our knowledge, this is the first clear evidence that specific strains of bifidobacteria, including certain probiotics, have such a potent anti-infectious activity against lethal STEC O157:H7 infection in a lethal mouse infection model. The important features of the mechanism responsible for the anti-infectious activity of bifidobacteria are as follows: (i) the inhibition of Stx production but not of STEC growth and (ii) the improvement of intestinal environmental factors, such as pH and acetate concentration.
Treatment of mice with SM depleted the facultative intestinal flora, which appeared to allow the explosive opportunistic proliferation of SM-resistant STEC cells (Fig. 1A), and the initial logarithmic phase of STEC proliferation in the intestines was not influenced by the B. breve cocolonization. Organic acids, such as AA, lactic acid, and citric acid, have been reported to possess a higher bactericidal activity than inorganic acids, such as hydrochloric acid; furthermore, the bactericidal activity of organic acids depends mainly on their undissociated form (6, 13). Undissociated organic acids can permeate the cell membrane by diffusion and release protons within the cell. The influx of protons is thought to induce cytoplasm acidification and dissipate the membrane proton potential (6, 10, 13). This leads to the disruption of the proton motive force and the inhibition of substrate transport mechanisms, energy-yielding processes, and macromolecule synthesis (7, 12). In addition, anion accumulation is assumed to exert a bacterial toxicity (28). In a previous study, it was reported that the cytotoxic properties of undissociated lactic acid on STEC strain 89020087 in vitro was divided into two phases: a bacteriostatic phase (between 3.2 to 62 mM) and a bactericidal phase (over 62 mM) (27). Several investigators have noted the ability of STEC O157:H7 to survive in acidic conditions; a possible explanation for this survival ability could be an acid tolerance response (5, 9, 12). We analyzed the cytotoxic properties of undissociated AA against the STEC strain and found that an undissociated AA concentration of more than 20 mM was needed to exert cytotoxic or growth-inhibitory activity against the STEC strain in vitro (data not shown), and the higher acetate concentration and lower pH in the B. breve-colonized cecum, when reproduced in vitro, was not found to inhibit STEC growth (Fig. 5A). Taken together, these results may explain the reason why B. breve colonization did not inhibit STEC growth in vivo.
On the other hand, the higher concentration of AA and the lower pH in the B. breve-colonized intestines appear to play a somewhat important role in the inhibition of toxin production because the inhibitory effect of the combination of pH and acetate on Stx production was confirmed by in vitro experiments (Fig. 5B). The mechanism by which the acetate concentration and the pH of the intestines inhibit Stx production is not clear. Quorum sensing is a mechanism through which gene expression in bacteria is regulated by cell density (11). Recently, quorum-sensing systems have been reported to be involved in the expression of several pathogenic genes such as LEE, which encodes a component of a type III secretion system in STEC (17, 34, 36); the expression levels of such genes vary with the bacterial growth phases (1). Little is known about the regulatory mechanisms of Stx production (35), and both the host- and bacterium-related factors affecting Stx production remain to be elucidated. The present results suggest that environmental regulation via molecules in the intestine, such as AA, is an important regulator of Stx gene expression in intestinal colonies of STEC. Studies to determine the mechanism of Stx production in STEC at the gene expression level are in progress.
Acknowledgments
We thank Kazumi Uchida and Kana Hashimoto for performing the histopathological analyses.
Editor: A. D. O'Brien
REFERENCES
- 1.Abe, H., I. Tatsuno, T. Tobe, A. Okutani, and C. Sasakawa. 2002. Bicarbonate ion stimulates the expression of locus of enterocyte effacement-encoded genes in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:3500-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba, Y., H. Ishikawa, K. Shimizu, S. Noda, Y. Kitada, M. Sasaki, and Y. Koga. 2002. Role of internalization in the pathogenicity of Shiga toxin-producing Escherichia coli infection in gnotobiotic murine model. Microbiol. Immunol. 46:723-731. [DOI] [PubMed] [Google Scholar]
- 3.Asahara, T., K. Shimizu, K. Nomoto, M. Watanuki, and R. Tanaka. 2001. Antibacterial effect of fermented milk containing Bifidobacterium breve, Bifidobacterium bifidum and Lactobacillus acidophilus against indigenous Escherichia coli infection in mice. Microb. Ecol. Health Dis. 13:16-24. [Google Scholar]
- 4.Asahara, T., K. Nomoto, K. Shimizu, M. Watanuki, and R. Tanaka. 2001. Increased resistance of mice to Salmonella enterica serovar Typhimurium infection by synbiotic administration of Bifidobacteria and transgalactosylated oligosaccharides. J. Appl. Microbiol. 91:985-996. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin, M. M., and A. R. Datta. 1995. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 61:1669-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocklehurst, T. F., and B. M. Lund. 1990. The influence of pH, temperature and organic acids on the initiation of growth of Yersinia enterocolitica. J. Appl. Bacteriol. 69:390-397. [DOI] [PubMed] [Google Scholar]
- 7.Cherrington, C. A., M. Hinton, and I. Chopra. 1990. Effect of short-chain organic acids on macromolecular synthesis in Escherichia coli. J. Appl. Bacteriol. 68:69-74. [DOI] [PubMed] [Google Scholar]
- 8.Clasener, H. A. L., E. J. Vollaard, and J. L. Whitby. 1987. Long-term prophylaxis of infection by selective decontamination in leukopenia and in mechanical ventilation. Rev. Infect. Dis. 9:295-328. [DOI] [PubMed] [Google Scholar]
- 9.Conner, D. E., and J. S. Kotrola. 1995. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl. Environ. Microbiol. 61:382-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer, J. A., and J. H. Prestegard. 1997. NMR studies of pH induced transport of carboxylic acids across phospholipid vesicle membranes. Biochem. Biophys. Res. Commun. 75:295-301. [DOI] [PubMed] [Google Scholar]
- 11.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diez-Gonzalez, F., and J. B. Russell. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology 143:1175-1180. [DOI] [PubMed] [Google Scholar]
- 13.Eklund, T. 1983. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 54:383-389. [DOI] [PubMed] [Google Scholar]
- 14.Evans, D. J., D. G. Evans, and S. L. Gorbach. 1973. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect. Immun. 8:725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii, J., T. Kita, S. Yoshida, T. Takeda, H. Kobayashi, N. Tanaka, K. Ohsato, and Y. Mizuguti. 1994. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H- in mitomycin-treated mice. Infect. Immun. 62:3447-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holdeman, L. V., E. P. Cato, and W. E. C. Moore (ed.). 1977. Anaerobe laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg.
- 17.Kanamaru, K., K. Kanamaru, I. Tatsuno, T. Tobe, and C. Sasakawa. 2000. SdiA, an Escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 38:805-816. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi, H., and T. Yajima. 1992. Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J. Sci. Food Agric. 60:139-146. [Google Scholar]
- 19.Lee, Y. K., K. Nomoto, S. Salminen, and S. L. Gorbach. 1999. Probiotics, p. 1-4. In Y. K. Lee, K. Nomoto, S. Salminen, and S. L. Gorbach (ed.), Handbook of probiotics. John Wiley & Sons, Inc., New York, N.Y.
- 20.Lindgren, S. W., A. R. Melton, and A. D. O'Brien. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nader de Macías, M. E., M. C. Apella, N. C. Romero, S. N. González, and G. Oliver. 1992. Inhibition of Shigella sonnei by Lactobacillus casei and Lact. acidophilus. J. Appl. Bacteriol. 73:407-411. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85-23. National Institutes of Health, Bethesda, Md.
- 25.Nomoto, K., T. Yokokura, Y. Yoshikai, M. Mitsuyama, and K. Nomoto. 1991. Induction of lethal infection with indigenous Escherichia coli in mice by fluorouracil. Can. J. Microbiol. 37:244-247. [DOI] [PubMed] [Google Scholar]
- 26.Nomoto, K., T. Yokokura, and N. Tomita. 1998. Induction of lethal endogenous Escherichia coli infection in mice by administration of 5-FU in combination with 1-β-D-arabinofuranosyluracil (Sorivudine). Biosci. Microflora 17:115-123. [Google Scholar]
- 27.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 28.Russell, J. B. 1991. Resistance of Streptococcus bovis to acetic acid at low pH: relationship between intracellular pH and anion accumulation. Appl. Environ. Microbiol. 57:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu, K., T. Asahara, K. Nomoto, R. Tanaka, T. Hamabata, A. Ozawa, and Y. Takeda. 2003. Development of a lethal Shiga toxin-producing Escherichia coli-infection mouse model using multiple mitomycin C treatment. Microb. Pathog. 35:1-9. [DOI] [PubMed] [Google Scholar]
- 30.Shu, Q., H. Lin, K. J. Rutherfurd, S. G. Fenwick, J. Prasad, P. K. Gopal, and H. S. Gill. 2000. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol. Immunol. 44:213-222. [DOI] [PubMed] [Google Scholar]
- 31.Shu, Q., and H. S. Gill. 2001. A dietary probiotic (Bifidobacterium lactis HN019) reduces the severity of Escherichia coli O157:H7 infection in mice. Med. Microbiol. Immunol. 189:147-152. [DOI] [PubMed] [Google Scholar]
- 32.Silva, A. M., E. A. Bambirra, A. L. Oliveira, P. P. Souza, D. A. Gomes, E. C. Vieira, and J. R. Nicoli. 1999. Protective effect of bifidus milk on the experimental infection with Salmonella enteritidis subsp. typhimurium in conventional and gnotobiotic mice. J. Appl. Microbiol. 86:331-336. [DOI] [PubMed] [Google Scholar]
- 33.Sonoike, K., M. Mada, and M. Mutai. 1986. Selective agar medium for counting viable cells of bifidobacteria in fermented milk. J. Food Hyg. Soc. Jpn. 27:238-244. [Google Scholar]
- 34.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 70:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi, H., M. Takahashi, H. Yamaguchi, T. Osaki, A. Komatsu, Y. Fujioka, and S. Kamiya. 2002. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. J. Med. Microbiol. 51:336-343. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi, M., H. Taguchi, H. Yamaguchi, T. Osaki, R. Sakazaki, and S. Kamiya. 1999. Antagonistic interaction between Clostridium butyricum and enterohemorrhagic Escherichia coli O157:H7. Kansenshogaku Zasshi 73:7-14. [DOI] [PubMed] [Google Scholar]
- 39.Van der Waaij, D., J. M. Berghuis-de Vries, and J. E. C. Lekkerkerk-van der Wees. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 69:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollaard, E. J., and H. A. L. Clasener. 1994. Colonization resistance. Antimicrob. Agents Chemother. 38:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wadolkowski, E. A., J. A. Burris, and A. D. O'Brien. 1990. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58:2438-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuki, N., K. Matsumoto, H. Takayama, M. Morotomi, and R. Tanaka. 1999. Isolation and identification of Bifidobacterium from feces of experimental rats: species identification and preparation of improved selective medium. J. Intest. Microbiol. 12:97-102. [Google Scholar]