Abstract
Actinobacillus actinomycetemcomitans is a major periodontopathic bacterium with multiple virulence factors, including lipopolysaccharide (LPS). Previous reports have demonstrated that LPS induced apoptosis in a murine macrophage-like cell line, J744.1, as well as in peritoneal macrophages from C3H/HeN mice in the presence of cycloheximide (CHX). However, the detailed molecular mechanisms involved in the apoptosis of macrophages induced by LPS and CHX are not well known. To clarify the possible role of LPS in the induction of macrophage apoptosis, we investigated cell death induced by LPS from A. actinomycetemcomitans and CHX in human macrophage-like U937 cells, which were differentiated by 12-O-tetradecanoylphorbol 13-acetate (TPA), and also assessed the molecular mechanisms involved in the process. We found that TPA-differentiated U937 cells usually showed resistance to LPS-induced apoptosis. However, in the presence of CHX, LPS induced release of cytochrome c without modifying steady-state levels of Bcl-2, Bcl-xL, Bax, and Bak. Treatment with LPS in the presence of CHX also led to activation of caspase-3 and apoptosis via, in part, the CD14/toll-like receptor 4 (TLR4). The induction of cytochrome c release may have been due to dephosphorylation of Akt and Bad, which were cooperatively induced by CHX and LPS. However, endogenous tumor necrosis factor alpha- and Fas-induced signals, extracellular signal-regulated kinase kinase/mitogen-activated protein kinases and I-κBα/nuclear factor-κB (NF-κB) were not required for caspase-3-dependent apoptosis. These results emphasize the possible important role of the mitochondrial apoptotic pathway leading to caspase-3 activation in LPS-induced apoptosis of human macrophages in the presence of CHX.
Actinobacillus actinomycetemcomitans is a gram-negative, capnophilic, and fermentative coccobacillus that has been implicated in the pathogenesis of various infectious diseases, such as endocarditis, pericarditis, meningitis, osteomyelitis, empyema, and subcutaneous abscess (15) as well as several types of periodontal disease (6, 47, 48). A. actinomycetemcomitans possesses or produces multiple virulence factors, including lipopolysaccharide (LPS). LPS from A. actinomycetemcomitans is recognized as an important pathogenic component in the initiation and progression of periodontal disease, since it stimulates host cells to produce inflammatory cytokines and induces bone resorption (12).
In inflamed tissue, apoptosis is a cellular event that underlies a wide variety of physiological phenomena, including the clonal selection of lymphocytes (49) and the removal of tissue inflammatory cells (1). Previous studies have shown apoptotic cell death in macrophages infected with A. actinomycetemcomitans (21) and the possible involvement of CD14 (37) and protein kinase C (42) in apoptosis. Infection with A. actinomycetemcomitans also induces apoptotic cell death of oral epithelial cells (20). Further, it has been demonstrated that a purified toxin from A. actinomycetemcomitans, which shows two major bands corresponding to molecular masses of approximately 80 and 85 kDa, causes cell cycle arrest in the G2/M phase and apoptosis in B lymphocyte HS-72 cells (43). These findings suggest that the ability of A. actinomycetemcomitans to promote apoptosis in various cell types may be important in the development of periodontal disease. However, there are few reports concerning the regulatory effect of A. actinomycetemcomitans LPS on apoptosis in various host cells.
The effects of LPS on apoptotic cell death differ among cell types and cell lines, and multiple molecular mechanisms are involved in its regulation. It has been reported that LPS inhibited apoptosis of human neutrophils through extracellular signal-regulated kinase (ERK) activation (28, 45), while activation of p38 mitogen-activated protein (MAP) kinase down regulated the LPS-induced inhibition of neutrophil apoptosis (45). LPS also prevented apoptotic cell death in human peripheral blood monocytes (34). Conversely, LPS administration has been shown to cause apoptosis in B cells (60), CD4+ 8+ thymocytes, and lymphoid organs (22). Further, LPS induced apoptotic cell death in endothelial cells via recruitment of the adaptor Fas-associated death domain (5). The LPS-induced apoptosis of human vascular endothelial cells has been shown to be mediated by tumor suppressor gene p53, proapoptotic Bax, caspase-1, and caspase-3 (36). In contrast, another report demonstrated that LPS did not cause apoptosis in a human dermal microvascular endothelial cell line, HMEC-1, though it did in the presence of cycloheximide (CHX) (11). LPS-induced apoptosis in the presence of CHX was also observed in a murine macrophage-like cell line, J744.1 (16-18), as well as in peritoneal macrophages from C3H/HeN mice (19). In contrast, caspase-3-like protease was the key enzyme in promoting apoptosis of J744.1 cells treated with LPS and CHX, and its activation was dependent on early LPS-induced signals such as LPS binding, protein tyrosine phosphorylation, and serine protease activity but not on later LPS signals such as MAP kinase kinase or MAP kinase (17). However, the detailed molecular mechanisms of LPS-induced apoptosis of macrophages in the presence of CHX are not well known.
In the present study, we confirmed apoptotic cell death induced by LPS from A. actinomycetemcomitans (strain Y4) and CHX in human macrophage-like U937 leukemic cells, which were differentiated by treatment with 12-O-tetradecanoylphorbol 13-acetate (TPA), and also assessed the molecular mechanisms involved with that process. Our results demonstrated that A. actinomycetemcomitans LPS, in the presence of CHX, induced cytochrome c release as well as subsequent caspase-3 activation and apoptosis in the cells and that the induction of cytochrome c release may have been due to dephosphorylation of Akt and Bad. We also found that the caspase-3-dependent apoptosis induced by LPS and CHX was mediated, in part, by CD14/toll-like receptor 4 (TLR4). However, endogenous tumor necrosis factor alpha (TNF-α)- and Fas-induced signals as well as ERK kinase (MEK)/MAP kinases and I-κBα/nuclear factor-κB (NF-κB) were not required. These results suggest that the mitochondrial apoptotic cascade leading to caspase-3 activation plays a crucial role in LPS-induced apoptosis of human macrophages in the presence of CHX.
MATERIALS AND METHODS
Materials.
Phosphate-buffered saline (PBS), RPMI 1640, penicillin-streptomycin, and fetal bovine serum (FBS) were obtained from Gibco BRL (Rockville, Md.). Recombinant human TNF-α (rhTNF-α) and anti-human TNF-α antibody (anti-hTNF-α) were obtained from Genzyme/Techne (Minneapolis, Minn.). SB203580 and gliotoxin were purchased from Calbiochem (Hornby, Canada). U0126 was obtained from Promega (Madison, Wis.). Benzyloxycarbonyl-Asp-CH2OC(O)-2,6,-dichlorobenzene (Z-asp-CH2-DCB) was purchased from the Peptide Institute, Inc. (Osaka, Japan). The antibody against mouse cytochrome c was obtained from Pharmingen (San Diego, Calif.). Mouse anti-human TLR4 antibody (HTA125) was donated by S. Akashi (Department of Microbiology and Immunology, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan). Polyclonal antibodies against Bcl-2, Bcl-xL, Bax, Bak, Bad, and Akt; mouse anti-human CD14 antibody (MY4), mouse immunoglobulin G2b (IgG2b); and mouse IgG2a were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Polyclonal antibodies against phosphorylated Akt (Thr308), phosphorylated Bad (Ser136 and Ser112), phosphorylated ERK1/2, ERK1/2, phosphorylated c-Jun N-terminal kinase 1/2 (JNK1/2), JNK1/2, phosphorylated p38, p38, and phosphorylated I-κBα and a Phototope-HRP Western Blot Detection System were obtained from Cell Signaling Technology, Inc. (Beverly, Mass.). TPA, CHX, Hoechst 33258, monoclonal anti-Fas ligand antibody (anti-FasL), PD098059, N-acetyl-l-cysteine (NAC), pyrrolidine dithiocarbamate (PDTC), LPS from Escherichia coli 055:B5 and the other reagents were obtained from Sigma (St. Louis, Mo.). LPS from A. actinomycetemcomitans Y4 and Porphyromonas gingivalis 381 were provided by T. Nishihara (Department of Oral Microbiology, Kyushu Dental College, Fukuoka, Japan). LPS was isolated from whole cells by the phenol-water procedure previously described by Westphal and Jann (54). The crude extracts were treated with nuclease, washed extensively with pyrogen-free water by ultracentrifugation, and lyophilized (39).
Cell line.
Human leukemia U937 cells were obtained from Japanese Cell Research Resources Bank (Tokyo, Japan). The cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (10 μg/ml) at 37°C in a 5% CO2 saturated atmosphere. Prior to each experiment, cells were treated with TPA (10 ng/ml) for 12 h to differentiate them into adherent macrophage-like cells (TPA-differentiated U937 cells).
Morphological assessment of apoptotic cells.
After treating TPA-differentiated U937 cells (105 cells), apoptosis was evaluated by morphological assessment using Hoechst 33258 staining. Briefly, following each treatment, cells were stained with Hoechst 33258 and evaluated under a fluorescence microscope for treatment-induced apoptosis. Apoptotic cells were identified by classical morphological features (i.e., condensed and fragmented nuclei, cell shrinkage, and formation of apoptotic bodies). Five randomly selected fields were evaluated to determine the percentage of apoptotic cells for each treatment condition.
Analysis of DNA fragmentation.
TPA-differentiated U937 cells (107 cells) were treated with LPS (1 μg/ml) from A. actinomycetemcomitans, P. gingivalis, or E. coli in the presence or absence of CHX (10 μg/ml) for 3 h. After each treatment, cellular DNA was extracted as reported previously (53). Briefly, cells were collected by centrifugation and washed with PBS and then lysed in a solution of 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, 0.5% (wt/vol) sodium dodecyl sulfate (SDS), and 0.1% (wt/vol) RNase A, with incubation for 60 min at 50°C. The lysates were incubated for an additional 60 min at 50°C with proteinase K (1 mg/ml) and then subjected to electrophoresis in a 1% (wt/vol) agarose gel in 40 mM Tris-acetate buffer (pH 7.4) at 50 V. After electrophoresis, DNA was visualized by staining with ethidium bromide.
Detection of cytochrome c release.
TPA-differentiated U937 cells (107 cells) were treated with A. actinomycetemcomitans LPS (1 μg/ml) and/or CHX (10 μg/ml) for 3 h. After each treatment, cells were harvested by centrifugation at 600 × g for 10 min at 4°C. The cell pellets were washed once with ice-cold PBS and resuspended with 5 volumes of buffer A (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 250 mM sucrose) and then were homogenized with 10 strokes of a Teflon homogenizer, after which the homogenates were centrifuged twice at 100,000 × g for 10 min at 4°C. The resulting supernatants (30 μg of protein for each treatment) were used for Western blot analysis with an anti-cytochrome c antibody.
Assay for caspase activity.
Following each treatment of TPA-differentiated U937 cells (107 cells), caspase activities were measured using colorimetric assay kits according to the manufacturer's instructions (Medical and Biological Laboratories Co., Ltd. Nagoya, Japan). Briefly, caspase activity in cytosolic extracts was measured by spectrophotometric detection of the chromophore p-nitroanilide (p-NA) after its cleavage from the labeled substrates DEVD (Asp-Glu-Val-Asp)-pNA for caspase-3, YVAD (Tyr-Val-Ala-Asp)-pNA for caspase-1, and IETD (Ile-Glu-Thr-Asp)-pNA for caspase-8. p-NA light emission was quantified using a microtiter plate reader at 400 nm (molar absorption coefficient, ɛ = 2.0 ×104 μmol/min).
Western blot analysis.
TPA-differentiated U937 cells (106 cells) were treated with A. actinomycetemcomitans LPS (1 μg/ml) and/or CHX (10 μg/ml) for 30 min or 3 h. After each treatment, the cells were washed with cold PBS and lysed by adding 200 μl of 1× SDS sample buffer (0.05 M Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 6% β-mercaptoethanol, 10% glycerol). The lysates were immediately scraped, collected into microcentrifuge tubes, and sonicated for 10 to 15 s on ice. The sonicated samples were then centrifuged at 17,360 × g for 15 min at 4°C. Protein amounts were determined using a protein assay kit (Bio-Rad Laboratories Inc., Hercules, Calif.). Each protein sample (20 or 30 μg/line) was run on an SDS-10% or -15% polyacrylamide gel for electrophoresis at 40 mA. The separated proteins were then electroblotted onto a polyvinylidene difluoride transfer membrane (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) using a semidry blotter (MilliBlot-SDE system; Millipore Co., Bedford, Mass.), according to the manufacturer's instructions. The membranes were washed once with 10 mM Tris-HCl (pH 7.2) containing 150 mM NaCl and 0.1% Tween 20 (TBS-T) and then were blocked for 1 h in TBS-T containing 5% (wt/vol) skim milk. After washing the membranes with TBS-T, each polyclonal antibody against ERK1/2, phosphorylated ERK1/2, JNK1/2, phosphorylated JNK1/2, p38, phosphorylated p38, phosphorylated I-κBα, Bcl-2, Bcl-xL, Bax, Bak, Bad, phosphorylated Bad (Ser136, Ser112), Akt, phosphorylated Akt (Thr308), and cytochrome c was added separately at a dilution of 1:500 or 1:1,000 in TBS-T containing 5% (wt/vol) skim milk or 5% bovine serum albumin and then incubated for 1 or 8 h at 4°C. After washing three times with TBS-T, the immunoreactive bands were visualized using a Phototope-HRP Western Blot Detection System. Briefly, the membrane was treated for 1 h with horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000) in TBS-T containing 5% (wt/vol) skim milk at room temperature. After washing three times with TBS-T, the membrane was incubated with LumiGLO reagent for 1 min at room temperature.
Statistical analysis.
All experiments were performed three times. For morphological assessment of apoptotic cells and an assay of caspase activity, the experiments were performed three times, with each conducted in triplicate. The means and standard deviations (SD) were then calculated, and the statistical significance of differences among the groups was examined by one-way analysis of variance and a post hoc t test. A post hoc t test was performed when analysis of variance test results indicated significance, which was determined at P < 0.01.
RESULTS
Periodontopathic bacterial LPS induce apoptotic cell death of TPA-differentiated U937 cells in the presence of CHX.
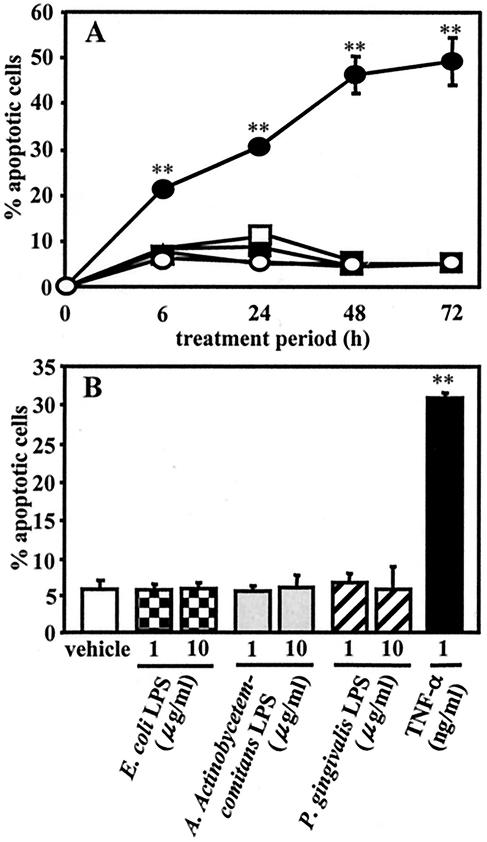
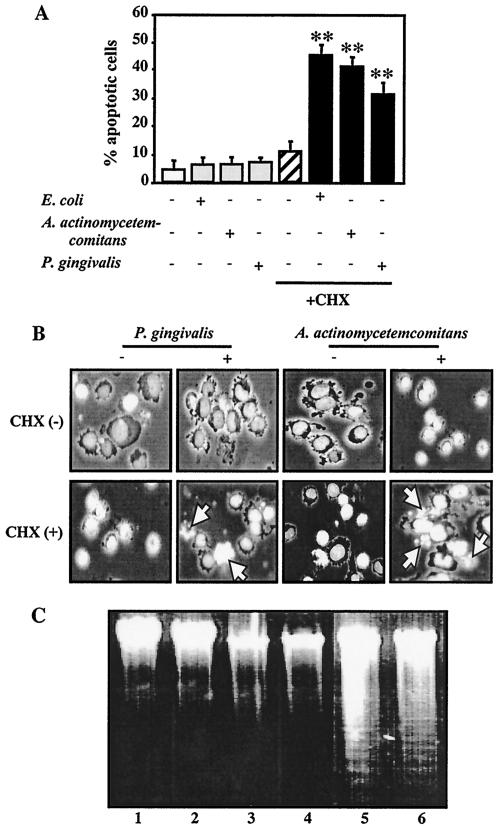
Human leukemia U937 cells were exposed to TPA for 12 h, and more than 90% of the cultured cells were found to be differentiated into adherent macrophage-like cells, which were designated TPA-differentiated U937 cells for this study. As shown in Fig. 1, rhTNF-α at 1 ng/ml induced apoptotic cell death in TPA-differentiated cells as early as 6 h, as detected by morphological assessment of Hoechst 33258-stained cells, and the percentage of apoptotic cells increased until 72 h. However, the induction of apoptotic cell death could not be observed when the cells were treated with a 1- or 10-μg/ml concentration of LPS from enterobacteria (E. coli) or periodontopathic bacteria (A. actinomycetemcomitans and P. gingivalis) for any of the indicated treatment times (Fig. 1). The morphological findings also demonstrated that induction of apoptotic cell death in cells treated for 3 h with CHX (10 μg/ml) alone could not be detected, whereas LPS from A. actinomycetemcomitans and P. gingivalis as well as E. coli at 1 μg/ml induced apoptotic cell death in the presence of CHX (10 μg/ml). After treatment, the ratio of apoptotic cells was 41% of cells treated with A. actinomycetemcomitans LPS and CHX, 32% of those treated with P. gingivalis LPS and CHX, and 47% of those treated with E. coli LPS and CHX (Fig. 2A and B).
FIG. 1.
Effects of LPS and rhTNF-α on apoptotic cell death in TPA-differentiated U937 cells. U937 cells were pretreated with TPA (10 ng/ml) for 12 h. (A) The TPA-differentiated U937 cells (105 cells) were further treated with E. coli LPS (1 μg/ml) (▴), A. actinomycetemcomitans LPS (□), P. gingivalis LPS (▪), rhTNF-α (1 ng/ml) (•), or nothing (○) for 6 to 72 h. (B) Other TPA-differentiated U937 cells (105 cells) were treated with a 1- or 10-μg/ml of LPS from E. coli, A. actinomycetemcomitans, or P. gingivalis; rhTNF-α (1 ng/ml); or nothing for 24 h. After each treatment, apoptosis induced in the cells was evaluated by morphological assessment using Hoechst 33258 dye, as described in Materials and Methods. Values shown (percent apoptotic cells) are the means ± SD (error bars) of three separate experiments, each conducted in triplicate. Differences from the value for untreated cells (vehicle) were considered significant (**) at a P of <0.01.
FIG. 2.
LPS-induced apoptosis of TPA-differentiated U937 cells in the presence of CHX. TPA-differentiated U937 cells (105 cells [A and B] or 107 cells [C]) were treated with a 1-μg/ml concentration of E. coli LPS, A. actinomycetemcomitans LPS, or P. gingivalis LPS in the presence or absence of CHX (10 μg/ml) for 3 h. Following each treatment, apoptosis in the cells was evaluated by morphological assessment using Hoechst 33258 dye (A and B) or analysis of DNA fragmentation (C), as described in Materials and Methods. (A) Values shown (percent apoptotic cells) are the means + SD (error bars) of three separate experiments, each conducted in triplicate. Differences from the value for untreated cells were considered significant (**) at a P of <0.01. (B) Morphological features of apoptotic cells (i.e., condensed and fragmented nuclei, cell shrinkage, and formation of apoptotic bodies). White arrow indicates apoptotic cells. (C) Agarose gel detection of DNA fragmentation. Lanes: 1 vehicle; 2, CHX alone; 3, A. actinomycetemcomitans LPS alone; 4, P. gingivalis LPS alone; 5, A. actinomycetemcomitans LPS plus CHX; 6, P. gingivalis LPS plus CHX.
Apoptosis induced by periodontopathic bacterial LPS in the presence of CHX was also confirmed in the DNA fragmentation analysis. Briefly, when the TPA-differentiated U937 cells were treated for 3 h with LPS from A. actinomycetemcomitans or P. gingivalis (1 μg/ml) and/or a 10-μg/ml concentration of CHX, LPS, or CHX alone did not cause fragmentation of chromosomal DNA. However, cotreatment with LPS and CHX induced DNA fragmentation in the cells (Fig. 2C).
CHX and A. actinomycetemcomitans LPS cooperatively induce dephosphorylation of Akt and Bad as well as subsequent cytochrome c release.
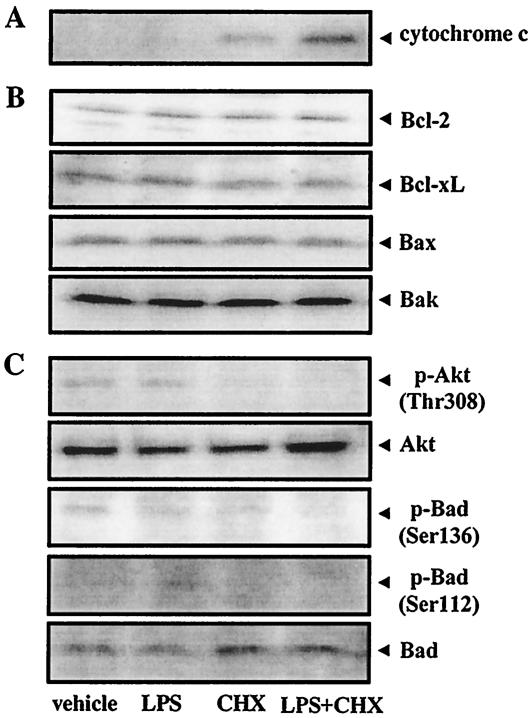
Cytochrome c release is considered to be an early key event in apoptosis (10, 30) that is prevented by antiapoptotic Bcl-2 and Bcl-xL (29, 51, 59) and induced by proapoptotic Bax and Bak (13, 38, 46). Accordingly, we confirmed cytochrome c release and the expression of Bcl-2 family proteins in TPA-differentiated U937 cells treated with CHX and/or A. actinomycetemcomitans LPS. Cytochrome c release was first detected when the cells were simultaneously treated with CHX (10 μg/ml) and LPS (1 μg/ml) for 1 h; however, treatment with CHX or LPS alone did not induce its release (data not shown). When the cells were treated for 3 h, CHX, but not LPS, slightly induced cytochrome c release, which was enhanced by cotreatment with LPS (Fig. 3A). Treatment with CHX or LPS alone or in combination did not change any steady-state Bcl-2, Bcl-xL, Bax, or Bak levels within 3 h of each treatment (Fig. 3B).
FIG. 3.
Effects of A. actinomycetemcomitans LPS and/or CHX on cytochrome c release, expression of Bcl-2 family proteins and phosphorylation of Akt and Bad. TPA-differentiated U937 cells (107 cells [A] or 106 cells [B and C]) were treated with A. actinomycetemcomitans LPS (1 μg/ml) in the presence or absence of CHX (10 μg/ml) for 15 min (C) or 3 h (A and B). Following each treatment, cytochrome c release (A); expression of Bcl-2, Bcl-xL, Bax, and Bak proteins (B); and phosphorylation of Bad (Ser136, Ser112) and Akt (Thr308) (C) were evaluated by Western blot analysis, as described in Materials and Methods.
Activated Akt is known to phosphorylate Bad in the serine 136 residue; however, the mechanism of the second phosphorylation at serine 112 is more controversial, as it allows binding to the 14-3-3 protein instead of Bcl-2 or Bcl-xL (33, 52, 35), resulting in the liberation of antiapoptotic proteins and promotion of cell survival. Conversely, following apoptotic stimuli, Bad is dephosphorylated, which is released from the 14-3-3 protein, subsequently dimerizes with antiapoptotic Bcl-xL (58, 61), and inactivates the antiapoptotic protein in the outer mitochondrial membrane, thereby promoting cell death. We examined changes in the levels of phosphorylated Akt (Thr308) and phosphorylated Bad (Ser136 and Ser112) in TPA-differentiated U937 cells treated with CHX (10 μg/ml) and/or A. actinomycetemcomitans LPS (1 μg/ml). The cells showed a constitutive expression of the phosphorylated forms of Akt and Bad (in both Ser136 and Ser112 sites). Further, steady-state phosphorylated Akt and phosphorylated Bad levels decreased in cells treated with CHX for 15 min, and this CHX-induced dephosphorylation of Akt and Bad was slightly enhanced by cotreatment with LPS (Fig. 3C).
Apoptosis induced by cotreatment with A. actinomycetemcomitans LPS and CHX is dependent on caspase-3 activation.
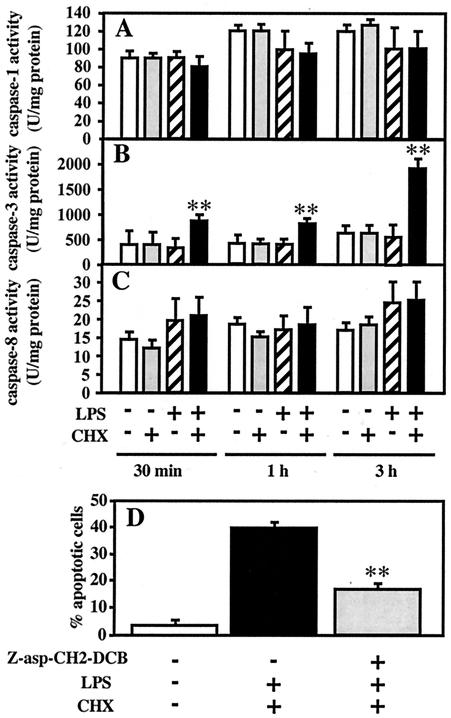
As noted above, cotreatment with A. actinomycetemcomitans LPS and CHX decreased steady-state phosphorylated Akt and Bad levels and subsequently induced cytochrome c release in TPA-differentiated U937 cells. Cytochrome c release activates caspase-9 and subsequently activates caspase-3 and the proteolytic cascade that leads to apoptosis (31). We investigated the participation of various caspases in apoptosis induced by cotreatment with A. actinomycetemcomitans LPS and CHX in TPA-differentiated U937 cells. When the cells were treated with LPS (1 μg/ml) and/or CHX (10 μg/ml) for 30 min, 1 h, and 3 h, caspase-1 and caspase-8 activities did not significantly change under any of the treatment conditions (Fig. 4A and 4C). Treatment with LPS or CHX alone also had no effect on caspase-3 activity. In contrast, significant caspase-3 activation in cells cotreated with LPS and CHX was first observed at 30 min and was remarkably enhanced at 3 h (Fig. 4B). Furthermore, apoptosis induced by simultaneous treatment with LPS and CHX was significantly inhibited by pretreatment for 1 h with 100 μM Z-asp-CH2-DCB, a caspase inhibitor (Fig. 4D).
FIG. 4.
Involvement of caspase-3 activation in apoptosis induced by A. actinomycetemcomitans LPS in the presence of CHX. TPA-differentiated U937 cells (107 cells) were treated with A. actinomycetemcomitans LPS (1 μg/ml) in the presence or absence of CHX (10 μg/ml) for 30 min, 1 h, and 3 h (A to C). Following each treatment, caspase-1 (A), caspase-3 (B), and caspase-8 (C) activities were measured using colorimetric assay kits according to the manufacturer's instructions. (D) TPA-differentiated U937 cells (105 cells) were also pretreated for 1 h with a 100 μM concentration of a caspase inhibitor, Z-asp-CH2-DCB, or nothing, and were subsequently cotreated with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml) for 3 h. Following each treatment, the effect of Z-asp-CH2-DCB on apoptosis induced by LPS and CHX (D) was evaluated by morphological assessment using Hoechst 33258-dye, as described in Materials and Methods. Values shown (units per milligram of protein [A to C] or percent apoptotic cells [D]) are the means + SD (error bars) of three separate experiments, each conducted in triplicate. Differences from the value for untreated cells (B) or cells cotreated with LPS and CHX (D) were considered significant (**) at a P of <0.01.
A. actinomycetemcomitans LPS induces caspase-3-dependent apoptosis in the presence of CHX via, in part, CD14/TLR4.
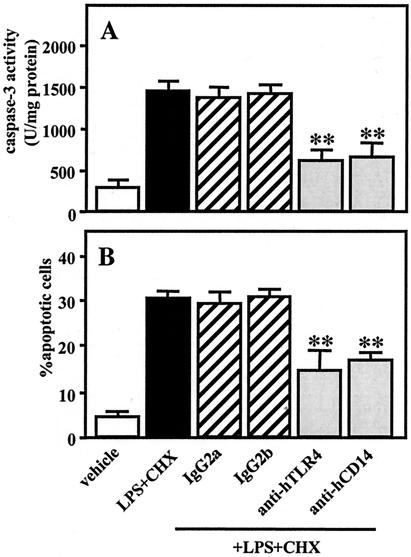
CD14 acts as a receptor for LPS combined with LPS-binding protein (56), and TLR4 has been well-documented to act as a LPS-signaling receptor (55). We examined whether CD14 and TLR4 mediate caspase-3 activation and apoptosis induced by A. actinomycetemcomitans LPS (1 μg/ml) in combination with CHX (10 μg/ml) in TPA-differentiated U937 cells. Pretreatment with MY4 (5 μg/ml), an anti-human CD14, or HTA125 (20 μg/ml), an anti-human TLR4 antibody, for 1 h significantly, though not completely, inhibited caspase-3 activity (Fig. 5A) and apoptosis (Fig. 5B) in cells subsequently cotreated with LPS and CHX for 3 h. In contrast, pretreatment with isotype-matched mouse IgG2b (5 μg/ml) or mouse IgG2a (20 μg/ml), control antibodies against MY4 and HTA125, respectively, had no effect on the caspase-3 activation or apoptosis induced by LPS and CHX (Fig. 5).
FIG. 5.
Participation of CD14/TLR4 in caspase-3 activity and apoptotic cell death induced by A. actinomycetemcomitans LPS in the presence of CHX. TPA-differentiated U937 cells (107 cells [A] or 105 cells [B]) were pretreated for 1 h with MY4 (5 μg/ml), control isotype-matched mouse IgG2b (5 μg/ml), HTA125 (20 μg/ml), control isotype-matched mouse IgG2a (20 μg/ml), or nothing and were subsequently cotreated with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml) for 3 h. Following each treatment, caspase-3 activity (A) was evaluated using colorimetric assay kits, and apoptosis (B) was evaluated by morphological assessment using Hoechst 33258 dye, as described in Materials and Methods. Values shown (units per milligram of protein [A] or percent apoptotic cells [B]) are the means + SD (error bars) of three separate experiments, each conducted in triplicate. Differences from the value for cells cotreated with LPS and CHX were considered significant (**) at a P of <0.01.
Endogenous TNF-α- and Fas-induced signals are not required for caspase-3-dependent apoptosis induced by cotreatment with A. actinomycetemcomitans LPS and CHX.
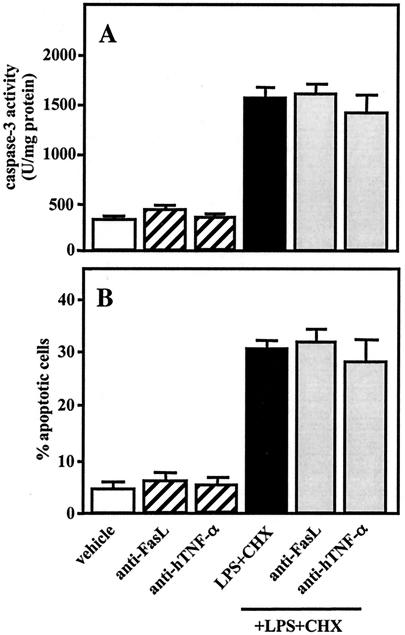
TNF-α- and Fas-induced signals cause apoptosis of U937 cells in the presence of CHX (9, 40, 41). LPS from E. coli and P. gingivalis stimulates production of TNF-α in phorbol myristic acid (PMA)-treated macrophage-like U937 cells (44). PMA-treated THP-1 cells, which also differentiate into macrophage-like cells, express high levels of FasL (however, not Fas), while LPS induces Fas expression in those cells (4). We investigated the participation of endogenous TNF-α- and Fas-induced signals in the caspase-3 activation and apoptosis induced by cotreatment with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml) in TPA-differentiated U937 cells. Stimulation with LPS, but not CHX, produced TNF-α in the cells, whereas TNF-α was not detected in the culture medium when the cells were simultaneously treated with LPS and CHX (data not shown). Further, pretreatment with anti-FasL (10 μg/ml) or anti-hTNF-α (5 μg/ml) for 1 h had no effect on caspase-3 activity (Fig. 6A) or apoptosis (Fig. 6B) in cells subsequently cotreated with LPS and CHX for 3 h.
FIG. 6.
Effects of anti-FasL and anti-hTNF-α on caspase-3 activity and apoptosis induced by A. actinomycetemcomitans LPS and CHX. TPA-differentiated U937 cells (107 cells [A] or 105 cells [B]) were pretreated for 1 h with anti-FasL (10 μg/ml), anti-hTNF-α (5 μg/ml), or nothing and were subsequently cotreated with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml) for 3 h. Following each treatment, caspase-3 activity (A) was determined using colorimetric assay kits and apoptosis (B) by morphological assessment using Hoechst 33258 dye, as described in Materials and Methods. Values shown (units per milligram of protein [A] or percent apoptotic cells [B]) are the means + SD (error bars) of three separate experiments, each conducted in triplicate.
MEK/MAP kinases and I-κBα/NF-κB are not involved in A. actinomycetemcomitans LPS-induced apoptosis in the presence of CHX.
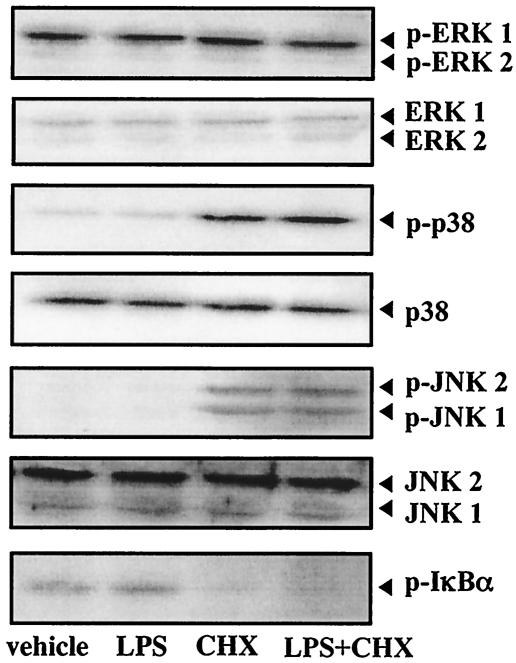
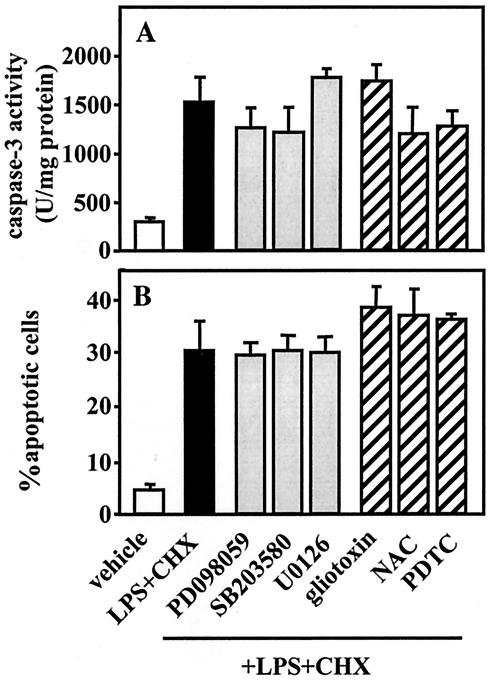
The roles of MAP kinases and I-κB/NF-κB in the regulation of apoptosis have been evaluated using many cell types and stimuli; however, they remain largely undefined. To clarify the participation of MEK/MAP kinases and I-κBα-NF-κB pathways in caspase-3-dependent apoptosis induced by simultaneous treatment with A. actinomycetemcomitans LPS and CHX in TPA-differentiated U937 cells, we first investigated the effects of LPS (1 μg/ml) and/or CHX (10 μg/ml) on the activation of MAP kinases, such as ERK1/2, p38, and JNK1/2, as well as I-κBα. As shown in Fig. 7, when the cells were treated with LPS or CHX alone or in combination for 30 min, the steady-state phosphorylated ERK1/2 level was not remarkably changed under any of the treatment conditions. CHX, but not LPS, stimulated the phosphorylation of p38 and JNK1/2, though cotreatment with LPS had no effect on the CHX-stimulated activation. Conversely, CHX, but not LPS, suppressed the phosphorylation of I-κBα, and the level of phosphorylated I-κBα decreased by CHX was not altered by cotreatment with LPS (Fig. 7). We next examined the effects of inhibitors of MAP kinases and NF-κB activation on caspase-3 activity and apoptosis in cells subsequently cotreated with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml). When the cells were pretreated with each inhibitor for 1 h, the MAP kinase inhibitors—PD098059 [100 μM; MAP kinase/MEK1/2 inhibitor], U0126 [5 μM; MEK1/2 inhibitor], and SB203580 [10 μM; p38 inhibitor]) and the inhibitors of NF-κB activation (gliotoxin [250 ng/ml], NAC [5 mM], and PDTC [100 μM]) had no significant effect on caspase-3 activation (Fig. 8A) or apoptosis (Fig. 8B) induced by cotreatment with LPS and CHX for 3 h.
FIG. 7.
Activation of MAP kinases and I-κBα in TPA-differentiated U937 cells treated with A. actinomycetemcomitans LPS and/or CHX. TPA-differentiated U937 cells (106 cells) were treated with A. actinomycetemcomitans LPS (1 μg/ml) and/or CHX (10 μg/ml) for 30 min. Following each treatment, the expressions of ERK1/2, phosphorylated ERK1/2 (p-ERK1/2), p38, phosphorylated p38 (p-p38), JNK1/2, phosphorylated JNK1/2 (p-JNK1/2), and phosphorylated I-κBα (p-I-κBα) were evaluated by Western blot analysis, as described in Materials and Methods.
FIG. 8.
Effects of inhibitors of MEK/MAP kinases or NF-κB activation on caspase-3 activity and apoptosis induced by A. actinomycetemcomitans LPS and CHX. TPA-differentiated U937 cells (107 cells [A] or 105 cells [B]) were pretreated for 1 h with PD098059 (100 μM; MEK1/2/MAP kinase inhibitor), U0126 (5 μM; MEK1/2 inhibitor), SB203580 (10 μM; p38 inhibitor), inhibitors of NF-κB activation (gliotoxin [250 ng/ml], NAC [5 mM], or PDTC [100 μM]), or nothing and were subsequently cotreated with A. actinomycetemcomitans LPS (1 μg/ml) and CHX (10 μg/ml) for 3 h. Following each treatment, caspase-3 activity (A) was determined using the colorimetric assay kits and apoptosis (B) was evaluated by morphological assessment using Hoechst 33258 dye, as described in Materials and Methods. Values shown (units per milligram of protein [A] or percent apoptotic cells [B]) are the means + SD (error bars) of three separate experiments, each conducted in triplicate.
DISCUSSION
We examined the possible role of LPS from A. actinomycetemcomitans in the induction of apoptosis in human macrophage-like TPA-differentiated U937 cells. Our findings demonstrated that the LPS from A. actinomycetemcomitans and P. gingivalis, both periodontopathic bacteria, as well as from E. coli, an enterobacterium, alone did not induce apoptosis of the cells; however, each induced apoptotic cell death in the presence of CHX. Apoptosis induced by LPS in the presence of CHX has also been shown in a murine macrophage-like cell line, J744.1 (16-18), as well as in peritoneal macrophages from C3H/HeN mice (19). Those findings suggested that macrophages usually show resistance to LPS-induced apoptosis and led us to speculate that CHX inhibits the actions of antiapoptotic molecules in macrophages, which are spontaneously expressed. However LPS-induced apoptosis of macrophages in the presence of CHX is not an actual physiological phenomenon, and this model cannot be directly applied to in vivo conditions encountered with infectious diseases, including periodontal disease. Nevertheless, to clarify the possible role of LPS in the induction of macrophage apoptosis, it is important to understand the mechanisms of apoptosis induced by LPS and CHX.
Recent evidence has shown that mitochondria play a crucial role in many forms of apoptosis by releasing apoptogenic factors such as cytochrome c (10, 30) from the intermembrane space into the cytoplasm, which activates the downstream execution phase of apoptosis. Antiapoptotic Bcl-2 and Bcl-xL prevent cytochrome c release (59, 29, 51), whereas proapoptotic Bax and Bak induce it (13, 38, 46). A previous study (50) showed that treatment of TPA-differentiated U937 cells with CHX for 24 h triggered apoptosis and also induced cytochrome c release and decreased Bcl-xL expression without modifying Bcl-2, Mcl-1, or Bax protein levels. In the present study, short term exposure (within 3 h) of the cells to CHX also induced cytochrome c release, which was enhanced by A. actinomycetemcomitans LPS, whereas exposure to LPS alone had no effect. However, treatment with CHX and/or LPS did not change the steady-state protein levels of Bcl-2, Bax, and Bak as well as Bcl-xL. Previous reports (8, 23, 24) have established Akt as a major determinant of cell survival. Akt mediates cell survival induced by various growth factors and cytokines in a variety of cell types and blocks apoptosis induced by multiple apoptotic stimuli (7, 14). Further, in the presence of survival factors, activated Akt phosphorylates Bad in the Ser136 residue and sometimes in Ser112, allowing it to bind to 14-3-3 protein instead of Bcl-2 or Bcl-xL (33, 52, 35), resulting in the liberation of antiapoptotic proteins and terminal promotion of cell survival. It has also been shown that Akt inhibits cytochrome c release, thereby preventing initiation of the apoptotic cascade leading to activation of caspases (25). Conversely, in the absence of survival signals or following apoptotic stimuli, Bad is dephosphorylated. Dephosphorylated Bad is released from 14-3-3 protein, dimerizes with antiapoptotic Bcl-xL (58, 61), and displaces and releases Bax from Bcl-xL, which resides in a constitutive form bound to Bax (58). Thereafter, Bax translocates to mitochondria in which it promotes the release of cytochrome c and activation of the caspase cascade (31, 3). The present results demonstrated that steady-state phosphorylated Akt and phosphorylated Bad levels in TPA-differentiated U937 cells were decreased by treatment with CHX for 15 min, however, not by A. actinomycetemcomitans LPS, while the CHX-induced dephosphorylation of Akt and Bad was slightly enhanced by cotreatment with LPS. These findings suggest that CHX induces the dephosphorylation of Akt and Bad, resulting in cytochrome c release, without modifying steady-state antiapoptotic or proapoptotic protein levels, while LPS weakly enhances this CHX activity.
It has been proposed that the release of cytochrome c activates caspase-9, and subsequently caspase-3 and the proteolytic cascade leading to apoptosis (31). A previous study (50) reported that exposure of TPA-differentiated U937 cells to CHX for 24 h activated procaspase-2L, −3, and −8. However, in this study, the activities of caspase-1, -3, and -8 in the cells were not changed by CHX or A. actinomycetemcomitans LPS up to 3 h after treatment. In contrast, caspase-3 activity, but not caspase-1 and -8 activities, was significantly increased by cotreatment with LPS and CHX during the treatment time periods. We also found that apoptosis induced by simultaneous treatment with LPS and CHX was significantly inhibited by pretreatment with Z-asp-CH2-DCB. A previous report demonstrated that Z-asp-CH2-DCB inhibited caspase-3- and -6-like activities, whereas it was less effective on the inhibition of caspase-1- and -8-like activities (17). Therefore, together with the present results, it is suggested that caspase-3 is the key enzyme involved in the progress of apoptosis of TPA-differentiated U937 cells treated with LPS and CHX. The predominant role of caspase-3-like protease in the promotion of macrophage apoptosis has also been shown in previous reports using J774.1 cells (17, 18); however, the possibility of involvement of other caspases in apoptosis of TPA-differentiated U937 cells induced by LPS and CHX is undeniable at present. In the present study, cytochrome c release was first detected when TPA-differentiated U937 cells were treated with CHX and LPS for 1 h; however, significant caspase-3 activation in the treated cells was first observed at 30 min. Therefore, we considered that there may be a feedback amplification of cytochrome c release by caspase-3 activation induced by LPS and CHX in the cells, because a recent study (57) demonstrated that caspase-3 induces cytochrome c release by inducing permeability transition pore opening which is associated with changes in mitochondrial respiration and redox potential.
CD14/TLR4 has been well-documented to mediate LPS-signaling (55, 56). In the present study, pretreatment with MY4 or HTA125 significantly, but not completely, inhibited caspase-3 activity and apoptosis in TPA-differentiated U937 cells subsequently cotreated with A. actinomycetemcomitans LPS and CHX. This result indicates that in the presence of CHX, A. actinomycetemcomitans LPS induces caspase-3 activity and apoptosis in cells via, in part, CD14/TLR4.
TNF-α- and Fas-induced signals are known to cause apoptosis of undifferentiated U937 cells in the presence of CHX (9, 41, 40), whereas TPA-differentiated U937 cells have demonstrated resistance to Fas- and TNF-α-induced apoptosis (50). Vitamin D3- and retinoic acid-treated U937 cells, both of which differentiate into macrophage-like cells, also acquire resistance against Fas- or TNF receptor-mediated apoptosis (27). On the other hand, LPS from E. coli and P. gingivalis stimulate macrophage-like PMA-treated U937 cells to produce TNF-α (44). Further, macrophage-like PMA-treated THP-1 cells express high levels of FasL but do not express Fas, whereas LPS induces Fas expression in those cells (4). These findings led us to hypothesize that endogenous TNF-α- and Fas-mediated signals are involved in the caspase-3 activation and apoptosis induced by LPS and CHX in TPA-differentiated U937 cells. However, the results reported here also suggest that endogenous TNF-α- and Fas-mediated signals are not required for caspase-3-dependent apoptosis induced by A. actinomycetemcomitans LPS and CHX, because TNF-α production could not be detected in cells cotreated with LPS and CHX (data not shown), and pretreatment with anti-FasL or anti-hTNF-α had no effect on caspase-3 activity or apoptosis subsequently induced by LPS and CHX. These findings support those of previous reports showing that endogenous TNF-α does not mediate the cytotoxicity of mouse peritoneal macrophages (19) and J774.1 cells (2) induced by LPS and CHX. Therefore, macrophage apoptosis induced by LPS in the presence of CHX is suggested to be due to some direct effect of LPS.
The roles of MEK/MAP kinases and I-κB/NF-κB in the regulation of apoptosis have been evaluated using many cell types and stimuli; however, they have not been completely elucidated. Previous studies have shown that LPS from some gram-negative bacterial species activate MAP kinase/AP-1 and/or IKK/I-κB/NF-κB in various macrophage cell lines as well as macrophages isolated from different species. Previously, we also observed that A. actinomycetemcomitans LPS weakly stimulated phosphorylation of MAP kinases and I-κBα in TPA-differentiated THP-1 cells (unpublished data). However, in the present study, treatment with A. actinomycetemcomitans LPS did not cause significant changes in the levels of the phosphorylated forms of MAP kinases or I-κBα in TPA-differentiated U937 cells, while E. coli LPS only weakly activated those (data not shown). Therefore, the magnitude of the effect of LPS on the phosphorylation of MAP kinases and I-κBα may be different among bacterial species or macrophage cell lines. We also found that CHX stimulated the phosphorylation of p38 and JNK1/2, while LPS showed no effect on the CHX-stimulated activation of p38 and JNK1/2. The activation of MAP kinases by CHX using murine RAW 264.7 macrophages has also recently been presented in another study (32). Conversely, CHX suppressed the phosphorylation of I-κBα and the CHX-decreased phosphorylated I-κBα level was not changed by cotreatment with LPS. Akt activation can promote the phosphorylation of I-κBα and subsequent NF-κB activation (26), while CHX induced the dephosphorylation of Akt in TPA-differentiated U937 cells in the present study. Therefore, CHX-induced dephosphorylation of Akt may participate with subsequent dephosphorylation of I-κBα in those cells. However, neither the MEK/MAP kinase inhibitors nor the inhibitors of NF-κB activation had a significant effect on caspase-3 activation or apoptosis induced by cotreatment with LPS and CHX. This suggests that MEK/MAP kinases and I-κBα/NF-κB are not involved in LPS-induced caspase-3-dependent apoptosis in the presence of CHX. The present results agree with previously provided evidence (17) that MAP kinase kinase and MAP kinase were not involved in the activation of caspase-3-like protease in J744.1 cells treated with LPS and CHX.
Our present results clarified the precise mechanisms by which LPS from A. actinomycetemcomitans induces apoptosis in human macrophages cultured with CHX. Further, they suggest that TPA-differentiated U937 cells usually show resistance to LPS-induced apoptosis, and CHX has a potential to cancel that resistance, because CHX, but not LPS, induced dephosphorylation of Akt and Bad as well as the subsequent cytochrome c release without modifying the steady-state protein levels of Bcl-2, Bcl-xL, Bax, and Bak, while LPS weakly enhanced the CHX actions. Accordingly, LPS can subsequently induce caspase-3 activation and apoptosis in the presence of CHX via, in part, CD14/TLR4. However, endogenous TNF-α- and Fas-induced signals, MEK/MAP kinases, and I-κBα/NF-κB are not required for caspase-3-dependent apoptosis. We considered that these findings emphasize the important role of the mitochondrial apoptotic pathway leading to caspase-3 activation in LPS-induced apoptosis of human macrophages in the presence of CHX.
Acknowledgments
This work was supported in part by a grant from the Japanese Ministry of Education, Science, and Culture (11470463).
Editor: V. J. DiRita
REFERENCES
- 1.Afford, S. C., J. Pongracz, R. A. Stockley, J. Crocker, and D. Burnett. 1992. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J. Biol. Chem. 267:21612-21616. [PubMed] [Google Scholar]
- 2.Amano, F., and H. Karahashi. 1996. A cytotoxic effect of lipopolysaccharide on a macrophage-like cell line, J774.1, in the presence of cycloheximide. J. Endotoxin Res. 3:415-423. [DOI] [PubMed] [Google Scholar]
- 3.Bratton, S. B., G. Walker, S. M. Srinivasula, X. M. Sun, M. Butterworth, E. S. Alnemri, and G. M. Cohen. 2001. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 20:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner, T. A., D. Chatterjee, Z. Han, M. F. Tsan, and J. H. Wyche. 1999. THP-1 monocytic leukemia cells express Fas ligand constitutively and kill Fas-positive Jurkat cells. Leuk. Res. 23:865-870. [DOI] [PubMed] [Google Scholar]
- 5.Choi, K. B., F. Wong, J. M. Harlan, P. M. Chaudhary, L. Hood, and A. Karsan. 1998. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J. Biol. Chem. 273:20185-20188. [DOI] [PubMed] [Google Scholar]
- 6.Christersson, L. A., U. M. Wikesjo, B. Albini, J. J. Zambon, and R. J. Genco. 1987. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J. Periodontol. 58:540-545. [DOI] [PubMed] [Google Scholar]
- 7.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 8.Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665. [DOI] [PubMed] [Google Scholar]
- 9.Galea-Lauri, J., A. J. Richardson, D. S. Latchman, and D. R. Katz. 1996. Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-alpha and cycloheximide: a possible role in immunopathology. J. Immunol. 157:4109-4118. [PubMed] [Google Scholar]
- 10.Goldstein, J. C., N. J. Waterhouse, P. Juin, G. I. Evan, and D. R. Green. 2000. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2:156-162. [DOI] [PubMed] [Google Scholar]
- 11.Hu, X., E. Yee, J. M. Harlan, F. Wong, and A. Karsan. 1998. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood 92:2759-2765. [PubMed] [Google Scholar]
- 12.Ishihara, Y., T. Nishihara, E. Maki, T. Noguchi, and T. Koga. 1991. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J. Periodont. Res. 26:155-160. [DOI] [PubMed] [Google Scholar]
- 13.Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, A. H., D. J. Weber, E. Z. Oddone, and J. R. Perfect. 1989. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev. Infect. Dis. 11:46-63. [DOI] [PubMed] [Google Scholar]
- 16.Karahashi, H., and F. Amano. 1998. Apoptotic changes preceding necrosis in lipopolysaccharide-treated macrophages in the presence of cycloheximide. Exp. Cell Res. 241:373-383. [DOI] [PubMed] [Google Scholar]
- 17.Karahashi, H., and F. Amano. 1999. LPS-induced signals in activation of caspase-3-like protease, a key enzyme regulating apoptotic cell damage into a macrophage-like cell line, J774.1, in the presence of cycloheximide. J. Leukoc. Biol. 66:689-696. [DOI] [PubMed] [Google Scholar]
- 18.Karahashi, H., and F. Amano. 2000. Changes of caspase activities involved in apoptosis of a macrophage-like cell line J774.1/JA-4 treated with lipopolysaccharide (LPS) and cycloheximide. Biol. Pharm. Bull. 23:140-144. [DOI] [PubMed] [Google Scholar]
- 19.Karahashi, H., and F. Amano. 2000. Lipopolysaccharide (LPS)-induced cell death of C3H mouse peritoneal macrophages in the presence of cycloheximide: different susceptibilities of C3H/HeN and C3H/HeJ mice macrophages. J. Endotoxin Res. 6:33-39. [DOI] [PubMed] [Google Scholar]
- 20.Kato, S., K. Nakashima, M. Inoue, J. Tomioka, K. Nonaka, T. Nishihara, and Y. Kowashi. 2000. Hum. epithelial cell death caused by Actinobacillus actinomycetemcomitans infection. J. Med. Microbiol. 49:739-745. [DOI] [PubMed] [Google Scholar]
- 21.Kato, S., M. Muro, S. Akifusa, N. Hanada, I. Semba, T. Fujii, Y. Kowashi, and T. Nishihara. 1995. Evidence for apoptosis of murine macrophages by Actinobacillus actinomycetemcomitans infection. Infect. Immun. 63:3914-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato, Y., A. Morikawa, T. Sugiyama, N. Koide, G. Z. Jiang, T. Lwin, T. Yoshida, and T. Yokochi. 1997. Augmentation of lipopolysaccharide-induced thymocyte apoptosis by interferon-gamma. Cell. Immunol. 177:103-108. [DOI] [PubMed] [Google Scholar]
- 23.Kauffmann-Zeh, A., P. Rodriguez-Viciana, E. Ulrich, C. Gilbert, P. Coffer, J. Downward, and G. Evan. 1997. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385:544-548. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701-713. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khwaja, A. 1999. Akt is more than just a Bad kinase. Nature 401:33-34. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi, H., R. Iizuka, S. Sugiyama, G. Gon, H. Mori, M. Arai, K. Mizumoto, and S. Imajoh-Ohmi. 1996. Monocytic differentiation modulates apoptotic response to cytotoxic anti-Fas antibody and tumor necrosis factor alpha in human monoblast U937 cells. J. Leukoc. Biol. 60:778-783. [DOI] [PubMed] [Google Scholar]
- 28.Klein, J. B., A. Buridi, P. Y. Coxon, M. J. Rane, T. Manning, R. Kettritz, and K. R. McLeish. 2001. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell. Signal. 13:335-343. [DOI] [PubMed] [Google Scholar]
- 29.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 30.Li, K., Y. Li, J. M. Shelton, J. A. Richardson, E. Spencer, Z. J. Chen, X. Wang, and R. S. Williams. 2000. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101:389-399. [DOI] [PubMed] [Google Scholar]
- 31.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 32.Lin, W. W., and Y. M. Hsu. 2000. Cycloheximide-induced cPLA(2) activation is via the MKP-1 down-regulation and ERK activation. Cell. Signal. 12:457-461. [DOI] [PubMed] [Google Scholar]
- 33.Lizcano, J. M., N. Morrice, and P. Cohen. 2000. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 349:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Mangan, D. F., G. R. Welch, and S. M. Wahl. 1991. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J. Immunol. 146:1541-1546. [PubMed] [Google Scholar]
- 35.Masters, S. C., H. Yang, S. R. Datta, M. E. Greenberg, and H. Fu. 2001. 14-3-3 inhibits Bad-induced cell death through interaction with serine-136. Mol. Pharmacol. 60:1325-1331. [DOI] [PubMed] [Google Scholar]
- 36.Munshi, N., A. Z. Fernandis, R. P. Cherla, I. W. Park, and R. K. Ganju. 2002. Lipopolysaccharide-induced apoptosis of endothelial cells and its inhibition by vascular endothelial growth factor. J. Immunol. 168:5860-5866. [DOI] [PubMed] [Google Scholar]
- 37.Muro, M., T. Koseki, S. Akifusa, S. Kato, Y. Kowashi, Y. Ohsaki, K. Yamato, M. Nishijima, and T. Nishihara. 1997. Role of CD14 molecules in internalization of Actinobacillus actinomycetemcomitans by macrophages and subsequent induction of apoptosis. Infect. Immun. 65:1147-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita, M., S. Shimizu, T. Ito, T. Chittenden, R. J. Lutz, H. Matsuda, and Y. Tsujimoto. 1998. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:14681-14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishihara, T., T. Fujiwara, T. Koga, and S. Hamada. 1986. Chemical composition and immunobiological properties of lipopolysaccharide and lipid-associated proteoglycan from Actinobacillus actinomycetemcomitans. J. Periodont. Res. 21:521-530. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi, K., M. Naito, S. Kataoka, S. Yonehara, and T. Tsuruo. 1995. A recessive mutant of the U937 cell line acquired resistance to anti-Fas and anti-p55 tumor necrosis factor receptor antibody-induced apoptosis. Cell Growth Differ. 6:1271-1277. [PubMed] [Google Scholar]
- 41.Noguchi, K., M. Naito, M. Oshimura, T. Mashima, N. Fujita, S. Yonehara, and T. Tsuruo. 1996. Chromosome 22 complements apoptosis in Fas-and TNF-resistant mutant UK110 cells. Oncogene 13:39-46. [PubMed] [Google Scholar]
- 42.Nonaka, K., A. Ishisaki, M., Muro, S. Kato, M. Oido, K. Nakashima, Y. Kowashi, and T. Nishihara. 1998. Possible involvement of protein kinase C in apoptotic cell death of macrophages infected with Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 159:247-254. [DOI] [PubMed] [Google Scholar]
- 43.Ohguchi, M., A. Ishisaki, N. Okahashi, M. Koide, T. Koseki, K. Yamato, T. Noguchi, and T. Nishihara. 1998. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect. Immun. 66:5980-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts, F. A., G. J. Richardson, and S. M. Michalek. 1997. Effects of Porphyromonas gingivalis and Escherichia coli lipopolysaccharides on mononuclear phagocytes. Infect. Immun. 65:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth, K., J. Friel, B. Nolan, and P. Bankey. 2001. Inhibition of p38 mitogen activated protein kinase increases lipopolysaccharide induced inhibition of apoptosis in neutrophils by activating extracellular signal-regulated kinase. Surgery 130:242-248. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu, S., Y. Eguchi, W. Kamiike, Y. Funahashi, A. Mignon, V. Lacronique, H. Matsuda, and Y. Tsujimoto. 1998. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. USA 95:1455-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slots, J., and M. A. Listgarten. 1988. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J. Clin. Periodontol. 15:85-93. [DOI] [PubMed] [Google Scholar]
- 48.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, C. A., G. T. Williams, R. Kingston, E. J. Jenkinson, and J. J. Owen. 1989. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature 337:181-184. [DOI] [PubMed] [Google Scholar]
- 50.Sordet, O., A. Bettaieb, JM. Bruey, B. Eymin, N. Droin, M. Ivarsson, C. Garrido, and E. Solary. 1999. Selective inhibition of apoptosis by TPA-induced differentiation of U937 leukemic cells. Cell Death Differ. 6:351-361. [DOI] [PubMed] [Google Scholar]
- 51.Susin, S. A., N. Zamzami, M. Castedo, T. Hirsch, P. Marchetti, A. Macho, E. Daugas, M. Geuskens, and G. Kroemer. 1996. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 184:1331-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan, Y., M. R. Demeter, H. Ruan, and M. J. Comb. 2000. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J. Biol. Chem. 275:25865-25869. [DOI] [PubMed] [Google Scholar]
- 53.Watabe, M., Y. Masuda, S. Nakajo, T. Yoshida, Y. Kuroiwa, and K. Nakaya. 1996. The cooperative interaction of two different signaling pathways in response to bufalin induces apoptosis in human leukemia U937 cells. J. Biol. Chem. 271:14067-14072. [DOI] [PubMed] [Google Scholar]
- 54.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 55.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 57.Xia, T., C. Jiang, L. Li, C. Wu, Q. Chen, and S. S. Liu. 2002. A study on permeability transition pore opening and cytochrome c release from mitochondria, induced by caspase-3 in vitro. FEBS Lett. 510:62-66. [DOI] [PubMed] [Google Scholar]
- 58.Yang, E., J. Zha, J. Jockel, L. H. Boise, C. B. Thompson, and S. J. Korsmeyer. 1995. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285-291. [DOI] [PubMed] [Google Scholar]
- 59.Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. I. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129-1132. [DOI] [PubMed] [Google Scholar]
- 60.Yokochi, T., Y. Kato, T. Sugiyama, N. Koide, A. Morikawa, G. Z. Jiang, M. Kawai, T. Yoshida, M. Fukada, and K. Takahashi. 1996. Lipopolysaccharide induces apoptotic cell death of B memory cells and regulates B cell memory in antigen-nonspecific manner. FEMS Immunol. Med. Microbiol. 15:1-8. [DOI] [PubMed] [Google Scholar]
- 61.Zha, J., H. Harada, E. Yang, J. Jockel, and S. J. Korsmeyer. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619-628. [DOI] [PubMed] [Google Scholar]