Abstract
Thrombus removal by manual thrombectomy improves coronary flow and myocardial perfusion after percutaneous coronary intervention (PCI) in ST-segment elevation myocardial infarction (STEMI); growing interest is on mechanical devices for thrombectomy which may allow a larger thrombus removal as compared to manual devices. We aimed to perform the first direct and adjusted indirect meta-analysis of studies on manual and mechanical thrombectomy in PCI for STEMI. Methods: The literature was scanned for direct and indirect randomized comparisons between manual and/or mechanical thrombectomy and/or placebo by formal searches of electronic databases from November 1994 to June 2013. Clinical and procedural endpoints were selected. Results: Three studies directly comparing (2 RCTs and 1 non-randomized; N = 513) and 21 RCTs (N = 4514) indirectly comparing the two strategies were included in the meta-analysis. The direct meta-analysis showed comparable rates of survival (p = 0.88), re-infarction (MI) (p = 0.84) and procedural outcomes between the two strategies; direct evidence was however limited in number of enrolled patients. The indirect meta-analysis showed a superior reduction in mortality with manual thrombectomy compared to mechanical thrombectomy in the overall analysis (p = 0.01); by excluding trials with low percentage of patients with intracoronary thrombus (< 50%) at baseline, the two strategies were comparable in survival, but mechanical thrombectomy was associated with a significant reduction in re-MI (p < 0.001) and stroke (p = 0.04). Conclusions: This meta-analysis lends support to mechanical thrombectomy in the population with high thrombus burden only where, compared to manual thrombectomy, it is likely to provide higher benefits
Keywords: Mechanical thrombectomy, manual thrombectomy, meta-analysis
Introduction
Primary percutaneous coronary intervention (PCI) remains the most effective treatment strategy for patients presenting with STEMI [1]; however, despite the efficacy in achieving epicardial reperfusion in STEMI, this strategy is often limited by distal atherothrombotic embolization, leading to a suboptimal reperfusion and resulting in unfavorable short and long-term outcomes [2,3]. In the last years thrombectomy has emerged as a useful tool to further enhance the benefit of reperfusion during primary PCI by preventing distal embolization of infarct-related thrombus. Various adjunctive thrombectomy devices have been developed allowing manual or mechanical removal of intracoronary thrombus. To date the evidence from randomized controlled trials (RCTs) directly comparing these two types of thrombectomy is however limited; in this regard, a direct and indirect inference on the data coming from the studies comparing these two strategies could significantly increase the understanding of their comparative efficacy. The aim of this report was therefore to perform a comprehensive direct and adjusted indirect meta-analysis of studies on manual vs mechanical thrombectomy during PCI for STEMI.
Methods
A search covering the period from November 1994 to June 2013 was conducted by two independent investigators (EPN and GT) using MEDLINE/CENTRAL and Google Scholar databases, and conference proceedings from the American College of Cardiology, American Heart Association, European Society of Cardiology, Transcatheter Cardiovascular Therapeutics and EuroPCR scientific sessions. The following keywords were applied: randomized trial, myocardial infarction, reperfusion, primary angioplasty, rescue angioplasty, thrombectomy, thrombus aspiration, manual thrombectomy, mechanical thrombectomy, rheolytic thrombectomy, Diver catheter, Pronto catheter, Export catheter, Thrombus Vacuum Aspiration Catheter, Angiojet, Rescue, and X-sizer. Inclusion criteria were: 1) studies (RCTs or non-randomized studies) comparing directly manual vs mechanical thrombectomy AND/OR RCTs separately investigating manual vs mechanical thrombectomy in PCI for STEMI; 2) availability of complete clinical data, whereas exclusion criteria were i) follow-up data in less than 90% of patients and ii) ongoing studies or irretrievable data. References of retrieved studies were searched manually for additional trials. No language restrictions were applied. Data were abstracted on pre-specified forms by two independent investigators, neither involved in any of the studies retrieved; divergences were resolved by discussion with a third investigator.
Outcomes measures
Clinical endpoints were mortality, re-infarction (MI) and stroke at 30-day follow-up for the indirect meta-analysis. The same endpoints with the available follow-up were used for the direct meta-analysis.
Procedural endpoints were postprocedural epicardial flow, as evaluated by postprocedural Thrombolysis in Myocardial Infarction (TIMI-3), and myocardial perfusion, as evaluated by complete ST-segment resolution.
Data analysis
Data were analyzed according to the intention-to-treat principle. Odds ratio (OR) and 95% Confidence intervals (95% CI) were used as summary statistics. Heterogeneity was assessed by Cochran’s Q test. The statistical inconsistency test (I2) {((Q-df)/Q) × 100%, where Q is the chi-squared statistic and df its degrees of freedom} was also employed to overcome the low statistical power of Cochran’s Q test.
For the direct meta-analysis, pooled ORs were calculated using a fixed effect model with the Mantel-Haenszel method. The DerSimonian and Laird random effects model was used in case of significant heterogeneity and/or moderate or significant inconsistency (> 50%) across studies.
Adjusted indirect comparisons is a relatively novel methodology which allows to compare two different treatments not directly compared in the studies (treatment A = mechanical thrombectomy and C = manual thrombectomy) but which have a common comparator (treatment B = no thrombectomy); the use of statistical adjustment constructed from two trials that have a common comparator (comparison of manual versus mechanical thrombectomy using trials comparing A versus B and B versus C) has the advantage to maintain the randomization design of the comparison mechanical vs manual. We used the Bucher method [4] for indirect comparisons using a common comparator, which is a statistical method for estimating OR and corresponding uncertainty. Adjusted indirect comparisons of pooled estimates were then performed according to Song et al. [5]. The method is well validated and recommended as a preferred method for indirect comparison, superior to other methods, as it preserves the randomization and retains the methodological properties of the RCTs.
In detail, we generated from OR comparing manual or mechanical thrombectomy vs. placebo interaction OR for manual or mechanical thrombectomy, with pertinent 95% CI and Z scores for 2-tailed hypothesis testing (p significant if < 0.05); specifically, these interaction OR are calculated according to the following formula: ln (ORmanual vs. mechanical) = ln (ORmanual vs. no thrombectomy)-ln (ORmechanical vs. no thrombectomy), and var (ln (ORmanual vs. mechanical)) = var (ln (ORmanual vs. no thrombectomy))+var (ln (ORmechanical vs. no thrombectomy)), where ln is the natural logarithm, and var is the variance. Trial inconsistency was assessed with I2.
Separate indirect meta-analyses comparing the two strategies in the overall population and excluding trials enrolling < 50% patients with intracoronary thrombus at baseline angiography were planned and carried out.
Results
Eligible studies
Among the 436 potentially relevant publications, a total of 3 studies (two randomized and one non-randomized) directly comparing manual vs mechanical thrombectomy and 21 RCTs separately comparing these two strategies were initially identified (Figure 1). One trial was excluded because of comparison between two manual thrombectomy devices [6]. Therefore, 3 studies for the direct meta-analysis [7-9] with a total of 513 patients (249 allocated to manual vs 264 to mechanical thrombectomy) and 21 trials for the adjusted indirect meta-analysis [10-31] with a total of 4514 patients (2270 allocated to thrombectomy and a total number of 2244 patients without thrombectomy) were finally included. One trial was excluded because of comparison between two manual thrombectomy devices [28]. Trial characteristics are shown in Table 1.
Figure 1.
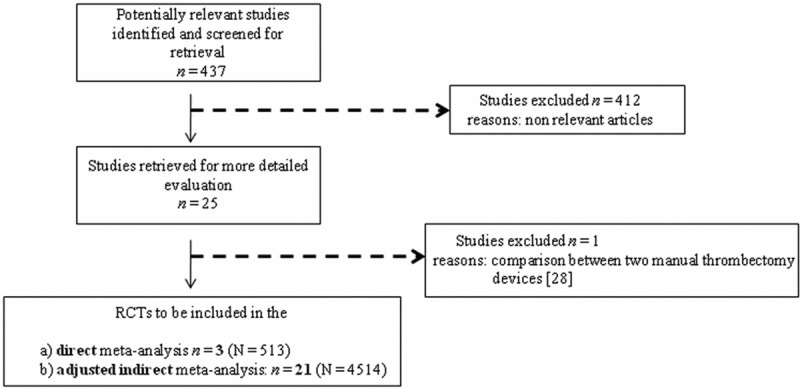
Flow chart of the systematic search for the direct and indirect meta-analysis
Table 1.
Main characteristics of the the studies included in the direct and indirect meta-analysis
| Study | Year of Publication | Design | Thrombectomy | Devices | Publication status | Patients | Thrombus T (%) | Thrombus C (%) | GP IIb/IIIa T (%) | GP IIb/IIIa C (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SMART [9] | abstract | Randomized | manual vs mechanical | NR | Abstract | 80 | 100 | 100 | NR | NR |
| Tarsia et al. [7] | 2010 | Retrospective | manual vs mechanical | Export/Diver-Angiojet | Full paper | 232 | 100 | 100 | 90.8 | 95.9 |
| TREAT-MI [8] | 2011 | Randomized | manual vs mechanical | Export-X-sizer | Full paper | 204 | 201 | 100 | 100 | 100 |
| REMEDIA [17] | 2005 | Randomized | Manual vs NO | Diver | Full paper | 96 | 58 | 55 | 68 | 63 |
| De Luca et al. [21] | 2006 | Randomized | Manual vs NO | Diver | Full paper | 76 | 100 | 100 | 100 | 100 |
| PIHRATE [25] | 2010 | Randomized | Manual vs NO | Export | Full paper | 196 | 70 | 70 | 62 | 63 |
| Noel et al. [18] | abstract | Randomized | Manual vs NO | Export | Abstract | 45 | NR | NR | NR | NR |
| Sardella et al. [27] | 2009 | Randomized | Manual vs NO | Export | Full paper | 175 | 100 | 100 | 100 | 100 |
| Chao et al. [26] | 2008 | Randomized | Manual vs NO | Export | Full paper | 74 | NR | NR | 19 | 32 |
| Chevalier et al. [30] | 2008 | Randomized | Manual vs NO | Export | Full paper | 249 | NR | NR | 66 | 70 |
| TAPAS [28] | 2008 | Randomized | Manual vs NO | Export | Full paper | 1060 | 49 | 44 | 93 | 90 |
| Lipiecki et al. [29] | 2009 | Randomized | Manual vs NO | Export | Full paper | 81 | NR | NR | 74 | 30 |
| Liistro et al. [23] | 2009 | Randomized | Manual vs NO | Export | Full paper | 111 | NR | NR | 100 | 100 |
| DEAR-MI [20] | 2006 | Randomized | Manual vs NO | Pronto | Full paper | 148 | NR | NR | 100 | 100 |
| Dudek et al. [12] | 2004 | Randomized | Mechanical vs NO | Rescue | Full paper | 41 | 100 | 100 | 0 | 0 |
| Kaltoft et al. [19] | 2006 | Randomized | Mechanical vs NO | Rescue | Full paper | 215 | 69 | 79 | 96 | 93 |
| VAMPIRE [24] | 2008 | Randomized | Mechanical vs NO | TVAC | Full paper | 349 | NR | NR | 0 | 0 |
| Beran et al. [10] | 2002 | Randomized | Mechanical vs NO | X-sizer | Full paper | 66 | 100 | 100 | 73 | 68 |
| Napodano et al. [11] | 2003 | Randomized | Mechanical vs NO | Rescue | Full paper | 92 | 100 | 100 | 43 | 41 |
| Lefevre et al. [15] | 2005 | Randomized | Mechanical vs NO | X-sizer | Full paper | 201 | 100 | 100 | 55 | 65 |
| Antoniucci et al. [13] | 2004 | Randomized | Mechanical vs NO | Angiojet | Full paper | 100 | NR | NR | 98 | 98 |
| AIMI [16] | 2006 | Randomized | Mechanical vs NO | Angiojet | Full paper | 480 | 49 | 44 | 95 | 94 |
| JETSTENT [22] | 2010 | Randomized | Mechanical vs NO | Angiojet | Full paper | 501 | 98.6 | 98.6 | 97 | 98 |
| Kuni et al. [14] | 2004 | Randomized | Mechanical vs NO | Rescue | Abstract | 258 | NR | NR | NR | NR |
T = treatment group, C = control group, no = no thrombectomy, NR = not reported.
Clinical endpoints
Mortality
Direct meta-analysis: no significant difference in mortality outcome was observed between the two strategies; 21 patients out of 212 and 23 patients out of 224 died in the manual and in the mechanical thrombectomy groups, respectively: (OR (95% CI) = 0.95 (0.51-1.78), p = 0.88) (Figure 2A).
Figure 2.
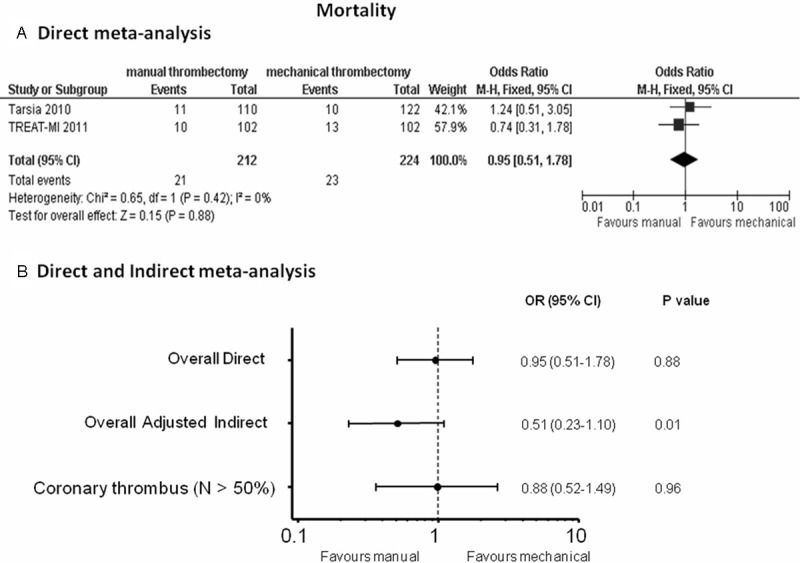
Odds ratios (ORs) with 95% confidence intervals (CIs) for mortality after manual vs mechanical thrombectomy in the direct meta-analysis (Panel A), overall direct and indirect meta-analysis and in the meta-analysis without TAPAS and AIMI trial, enrolling < 50% patients with intracoronary thrombus at baseline (Panel B). N = patients with visible coronary thrombus.
Indirect meta-analysis: Data on 30-day mortality were available in 4449 patients. Results of the adjusted indirect meta-analysis showed reduced mortality in the manual vs mechanical thrombectomy group (OR (95% CI) = 0.51 (0.23-1.10), p = 0.01) (Figure 2B); when discarding the TAPAS trial in the manual thrombectomy group and the AIMI trial in the mechanical thrombectomy group, both reporting an overall low percentage of patients with intracoronary thrombus at baseline, the effect on survival was comparable between the two strategies: OR (95% CI) = 0.88 (0.52-1.49), p = 0.96 (Figure 2B).
Re-infarction
Direct meta-analysis: re-MI rates were comparable between the two strategies: OR (95% CI) = 0.91 (0.36-2.31), p = 0.84 (Figure 3A).
Figure 3.
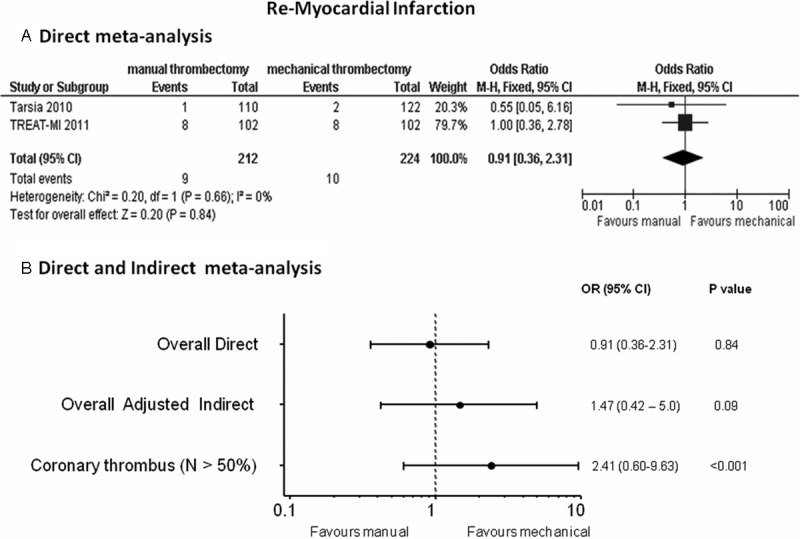
Odds ratios (ORs) with 95% confidence intervals (CIs) for MI after manual vs mechanical thrombectomy in the direct (Panel A), overall direct and indirect meta-analysis and in the meta-analysis without TAPAS and AIMI studies enrolling < 50% patients with intracoronary thrombus at baseline (Panel B). N = patients with visible coronary thrombus.
Indirect meta-analysis: data were available in 4056 (89.8%) patients. The overall indirect meta-analysis showed a non-significant trend in less re-MI rates in the mechanical thrombectomy group as compared to the manual thrombectomy arm: OR (95% CI)= 1.47 (0.42-5.0), p = 0.09 (Figure 3B); on the other hand, this trend in favor of mechanical thrombectomy turned out to be strongly significant when removing the TAPAS and AIMI trial with reported low percentage of patients with intracoronary thrombus at baseline: OR (95% CI) = 2.41 (0.60-9.63), p < 0.001 (Figure 3B).
Stroke
Data on stroke were consistently available for indirect meta-analysis only; they were available in 3859 (85.5%) patients. The overall indirect meta-analysis showed not significant increased rates of stroke in the manual vs mechanical thrombectomy arm: OR (95% CI) = 1.57 (0.23-10.35), p = 0.07 (Figure 4); when discarding TAPAS and AIMI studies, a higher risk of stroke was observed with manual thrombectomy: OR (95% CI) = 1.58 (0.21-11.80), p = 0.04 (Figure 4).
Figure 4.
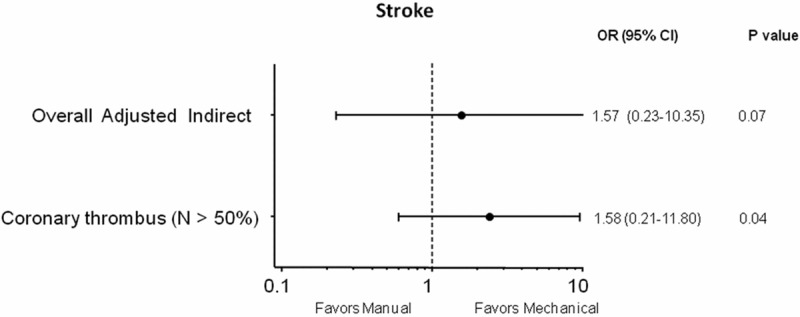
Odds ratios (ORs) with 95% confidence intervals (CIs) for stroke after manual vs mechanical thrombectomy in the overall indirect meta-analysis and in the meta-analysis without TAPAS and AIMI studies enrolling < 50% patients with intracoronary thrombus at baseline. N = patients with visible coronary thrombus.
Procedural endpoints
ST-segment resolution
Direct meta-analysis: no difference in the resolutions of ST-segment was found between manual and mechanical thrombectomy, although a significant heterogeneity and inconsistency was observed between the two RCTs: OR (95% CI) = 1.17 (0.69-1.99), p = 0.55 (Figure 5A).
Figure 5.
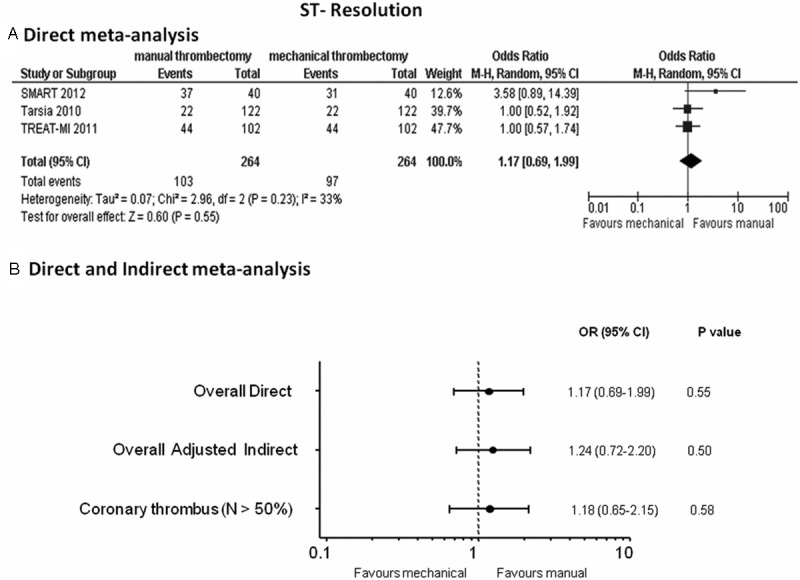
Odds ratios (ORs) with 95% confidence intervals (CIs) for ST-resolution after manual vs mechanical thrombectomy in the direct meta-analysis (Panel A), overall direct and indirect meta-analysis and in the meta-analysis without TAPAS and AIMI studies enrolling < 50% patients with intracoronary thrombus at baseline (Panel B). N = patients with visible coronary thrombus.
Indirect meta-analysis: data on complete ST-segment resolution were available in 3974 patients (89.3%). Comparable favorable results in normalizing ST-segment were observed in patients treated either with manual or mechanical thrombectomy in the overall adjusted indirect meta-analysis: OR (95% CI) = 1.24 (0.72-2.20), p = 0.50 (Figure 5B); when removing the TAPAS and AIMI studies, these findings persisted: OR (95% CI) = 1.18 (0.65-2.15), p = 0.58 (Figure 5B).
TIMI-3 coronary flow
Direct meta-analysis: manual and mechanical thrombectomy yielded similar TIMI-3 angiographic post-procedural results: OR (95% CI) = 0.92 (0.62-1.37), p = 0.69 (Figure 6A).
Figure 6.
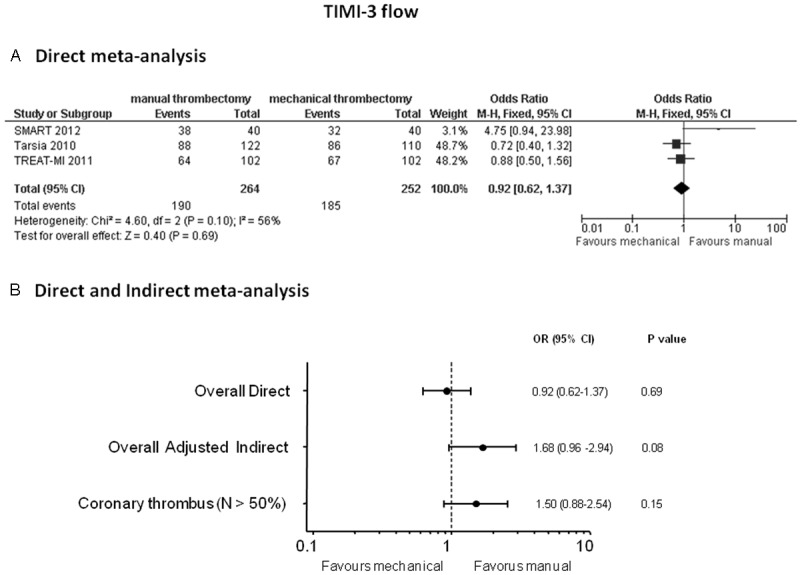
Odds ratios (ORs) with 95% confidence intervals (CIs) for TIMI-3 flow after manual vs mechanical thrombectomy in the direct meta-analysis (Panel A), overall indirect meta-analysis and in the meta-analysis without TAPAS and AIMI studies enrolling < 50 % patients with intracoronary thrombus at baseline (Panel B). N = patients with visible coronary thrombus.
Indirect meta-analysis: data on postprocedural TIMI-3 flow were available in 4192 (94.2%) patients. Non-significant higher rates of TIMI-3 flow restoration were noted in the manual thrombectomy group: OR (95% CI) 1.68 (0.96-2.94), p = 0.08 (Figure 6B); this trend however disappeared in the analysis by discarding TAPAS and AIMI trials where the two strategies provided comparable TIMI 3 flow rates: OR (95% CI) = 1.50 (0.88-2.54), p = 0.15 (Figure 6B).
Discussion
This is the first meta-analysis comparing to date manual vs mechanical thrombectomy in a systematic fashion; direct and indirect evidence were used to provide the most comprehensive source of evidence available to date; the main findings of this report are that 1) no clear clinical benefits emerged from mechanical thrombectomy in both the direct and overall indirect meta-analysis; in the meta-analysis without the inclusion of the TAPAS and AIMI trials, reporting low percentage (< 50%) of patients with intracoronary thrombus at baseline, 2) mechanical thrombectomy yielded a significant reduction of re-MI and stroke rates as compared to a manual strategy.
Several RCTs and a large meta-analysis have shown that primary PCI provides mortality benefits in comparison with thrombolysis, mainly due to better and sustained optimal epicardial perfusion; however, despite epicardial recanalization with angiographic TIMI-3 flow, suboptimal myocardial perfusion may occur in up to 20-40% of patients, affecting significantly the long-term survival.
Indeed, the patency of epicardial infarct related coronary artery after reperfusion is not a guarantee for adequate microvascular perfusion. Therefore, optimizing tissue-level reperfusion is a therapeutic goal in the setting of primary PCI. Distal embolization of material from coronary thrombus is a known predictor of coronary no-reflow and impaired tissue perfusion; the larger is the thrombus, the higher to chance to foster distal embolization.
In several studies thrombectomy has emerged as an important tool to reduce thrombus burden, counteracting distal embolization; In the Long-Term Clinical Efficacy of Thrombectomy Devices in Acute ST-Elevation Myocardial Infarction (ATTEMPT) meta-analysis [31], patient-level data were pooled, including 2686 patients from 11 trials. At a median of 1-year follow-up, all-cause mortality, death and MI, and major cardiovascular adverse events were significantly reduced with thrombectomy. As expected from results of individual trials, the survival benefit observed in this meta-analysis was confined to patients who were treated with manual thrombectomy, However, the investigators failed to obtain data from six eligible trials comprising approximately 1000 patients, which may have biased their results. Subsequently, updated meta-analyses have been performed incorporating more trials showing that thrombectomy was able to improve surrogate markers of reperfusion but not reinfarction and 30-day mortality.
Mechanical thrombectomy has emerged as a new promising tool which appears to be more effective in removing thrombus as compared to manual thrombectomy.
The recently published CompariSon of Manual Aspiration with Rheolytic Thrombectomy in Acute Myocardial Infarction: the (SMART) Primary PCI Trial [9], compared by optical coherence tomography the efficacy of mechanical thrombectomy vs manual thrombectomy in thrombus removal before infarct artery stenting in patients with STEMI; the study, while underpowered to assess clinical outcome, showed that mechanical thrombectomy as compared to manual thrombectomy, is more effective in thrombus removal and is associated with a better myocardial reperfusion. In the A Randomized Comparison of Manual Versus Mechanical Thrombus Removal in Primary Percutaneous Coronary Intervention in the Treatment of ST-Segment Elevation Myocardial Infarction (TREAT-MI) study [8], a single center study, mechanical thrombectomy failed to provide any further procedural or clinical improvement as compared to manual thrombectomy.
It must be noted that, however, the TREAT-MI trial was flawed by several biases: 1) paucity of clinical and angiographic data on the patients enrolled in the study; indeed, their hemodynamic condition, the size of the myocardial infarction, the extent of coronary disease in other major epicardial vessels, left ventricular function, presence of angiographic collaterals toward the infarct-related artery, all are not reported; 2) lack of adequate thrombus measurement precluded an adequate assessment of thrombus which was classified as absent in at least 16% of the patients receiving mechanical thrombectomy and 13% of the patients allocated to manual thrombectomy. Accordingly, a meta-analysis providing the results coming from a direct but also indirect evidence on manual and mechanical thrombectomy appears timely; despite the limited evidence available from direct meta-analysis, the adjusted indirect meta-analysis showed in a large population of 4514 patients that mechanical thrombectomy strongly reduced the incidence of re-MI when discarding from the analysis the two trials with lower thrombus burden.
High thrombus burden is in fact known to be associated with an increased incidence of distal embolization, a significant pathogenetic component of no-reflow, and may limit reperfusion at tissue level and is associated with a higher frequency of major adverse clinical events; moreover, stenting in the presence of high thrombus burden may hence increase the risk of late stent malposition when, days after, the thrombus is completely dissolved and which constitutes and important risk factor for stent thrombosis and re-MI.
Mechanical catheters, while more bulky, may provide more consistent advantages in removal of thrombus because of their intrinsic properties; for example within ANJOJET catheter there is a high-velocity saline jets which creates a strong negative pressure (Bernoulli effect) that entrains the thrombus to the catheter inflow windows where it is captured, fragmented, and evacuated through the outflow lumen; this may ultimately result in a larger thrombus removal when compared to manual aspiration.
This meta-analysis made clear the limited evidence of studies directly comparing manual vs mechanical thrombectomy; despite this, the findings coming from the large populations of indirect analysis have been useful to give new insights on this debated issue.
According to the present report, indeed the benefits from mechanical thrombectomy over manual thrombectomy are mainly related to the thrombus burden, which therefore should be assessed before starting the invasive procedure and might guide the choice of the proper thrombus removal device; the present study showed that patients presenting with visible thrombus experienced lower rates of re-MI after mechanical thrombectomy as compared to those treated with manual thrombectomy; conversely the benefits in the immediate procedural characteristics such as TIMI-3 flow and ST-segment resolutions were comparable between the two strategies; these findings reflect the efficacy of manual aspiration in improving coronary flow velocities and restoring coronary flow by removing part of the thrombus; the higher the thrombus however, the higher the possibility not to completely remove it with a manual passing of the device; conversely, the manual passage itself may favor in case of large thrombus burden microembolization and increase the possibility of re-MI.
Future powered head to head randomized comparisons in a population with large thrombus burden are certainly needed to definitively clarify the benefits of mechanical thrombectomy in reducing the rate of ischemic outcomes such as re-MI and stroke.
Limitations
The evidence from this meta-analysis is mainly derived by indirect comparisons; clearly, there are a number of constraints and limitations related to the method used for an indirect comparison analysis, but when performed according to established methods, it represents a reasonable and well validated statistical tool to qualify a comparison of effects especially when there is a limited direct evidence available. Therefore, indirect estimates obtained with adjusted methods which preserve randomization design appear informative to this purpose. Studies investigating mechanical thrombectomy used two essentially different catheters such as ANGIOJET and X-SIZER catheters; on the other hand, the stability of the results by performing separate analyses based on the mechanical catheter used provided similar results to those obtained in the overall analyses, suggesting the robustness of the overall estimates.
Conclusions
Mechanical thrombectomy, as compared to manual thrombectomy, provides higher benefits in reduction of re-MI and stroke in the population with high thrombus burden only. Large powered head-to-head comparisons in patients with high thrombus burden are warranted to definitively confirm the clinical benefits of mechanical thrombectomy.
Acknowledgments
The present contribution is a project of Systematic Investigation and Research on Interventions and Outcomes (SIRIO)-MEDICINE, a group of senior scientists and fellows collaborating worldwide to pursue research and innovation in medicine.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.van’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angio-graphic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction. Myocardial Blush Grade. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 3.Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van’t Hof AW, Hoorntje JC, Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23:1112–1117. doi: 10.1053/euhj.2001.3035. [DOI] [PubMed] [Google Scholar]
- 4.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 5.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analysis. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardella G, Mancone M, Nguyen BL, De Luca L, Di Roma A, Colantonio R, Petrolini A, Conti G, Fedele F. Aspiration by two thrombectomy devices in acute Myocardial Infarction (RETAMI) trial. Catheter Cardiovasc Interv. 2008;71:84–91. doi: 10.1002/ccd.21312. [DOI] [PubMed] [Google Scholar]
- 7.Tarsia G, De Michele M, Polosa D, Biondi-Zoccai G, Costantino F, Del Prete G, Osanna RA, Innelli P, Sisto F, Sheiban I, Lisanti P. Manual versus nonmanual thrombectomy in primary and rescue percutaneous coronary angioplasty. Heart Vessels. 2010;25:275–81. doi: 10.1007/s00380-009-1198-2. [DOI] [PubMed] [Google Scholar]
- 8.Vink MA, Patterson MS, van Etten J, Ijsselmuiden AJ, Dirksen MT, Amoroso G, Slagboom T, Laarman G, Kiemeneij F. A randomized comparison of manual versus mechanical thrombus removal in primary percutaneous coronary intervention in the treatment of ST-segment elevation myocardial infarction (TREAT-MI) Catheter Cardiovasc Interv. 2011;78:14–9. doi: 10.1002/ccd.22932. [DOI] [PubMed] [Google Scholar]
- 9.Parodi G, Valenti R, Migliorini A, Maehara A, Vergara R, Carrabba N, Mintz GS, Antoniucci D. Comparison of manual thrombus aspiration with rheolytic thrombectomy in acute myocardial infarction. Circ Cardiovasc Interv. 2013;6:224–30. doi: 10.1161/CIRCINTERVENTIONS.112.000172. [DOI] [PubMed] [Google Scholar]
- 10.Beran G, Lang I, Schreiber W, Denk S, Stefenelli T, Syeda B, Maurer G, Glogar D, Siostrzonek P. Intracoronary thrombectomy with the X-sizer catheter system improves epicardial flow and accelerates ST-segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation. 2002;105:2355–60. doi: 10.1161/01.cir.0000016350.02669.1d. [DOI] [PubMed] [Google Scholar]
- 11.Napodano M, Pasquetto G, Sacca S, Cernetti C, Scarabeo V, Pascotto P, Reimers B. Intraco-ronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2003;42:1395–402. doi: 10.1016/s0735-1097(03)01041-6. [DOI] [PubMed] [Google Scholar]
- 12.Dudek D, Mielecki W, Legutko J, Chyrchel M, Sorysz D, Bartuś S, Rzeszutko L, Dubiel JS. Percutaneous thrombectomy with the RESCUE system in acute myocardial infarction. Kardiol Pol. 2004;61:523–33. [PubMed] [Google Scholar]
- 13.Antoniucci D, Valenti R, Migliorini A, Parodi G, Memisha G, Santoro GM, Sciagrà R. Comp-arison of rheolytic thrombectomy before direct infarct artery stenting versus direct stenting alone in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2004;93:1033–5. doi: 10.1016/j.amjcard.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Kuni H, Kijima M, Araki T, Katoh A, Kubo T, Tomiyoshi S, Akira H, Hitoshi M. Lack of efficacy of intracoronary thrombus aspiration before coronary stenting in patients with Acute Myocardial Infarction: A Multicenter Rando-mized Trial. J Am Coll Cardiol. 2004;43(Suppl A):245A. [Google Scholar]
- 15.Lefevre T, Garcia E, Reimers B, Lang I, di Mario C, Colombo A, Neumann FJ, Chavarri MV, Brunel P, Grube E, Thomas M, Glatt B, Ludwig J X AMINE ST Investigators. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution: results of the X-sizer in AMI for negligible embolization and optimal ST resolution (XAMINE ST) trial. J Am Coll Cardiol. 2005;46:246–52. doi: 10.1016/j.jacc.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, Browne K, Iwaoka R, Azrin M, Stapleton D, Setum C, Popma J AIMI Investigators. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol. 2006;48:244–52. doi: 10.1016/j.jacc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, Garramone B, Giannico F, Niccoli G, Biondi-Zoccai GG, Schiavoni G, Mongiardo R, Crea F. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371–6. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Noel B, Morice MC, Lefevre T. Thromboaspiration in acute ST-elevation MI improves myocardial reperfusion. Circulation. 2005;112(Suppl II):519. [Google Scholar]
- 19.Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, Kristensen J, Thuesen L, Krusell LR, Kristensen SD, Andersen HR, Lassen JF, Rasmussen K, Rehling M, Nielsen TT, Bøtker HE. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. 2006;114:40–7. doi: 10.1161/CIRCULATIONAHA.105.595660. [DOI] [PubMed] [Google Scholar]
- 20.Silva-Orrego P, Colombo P, Bigi R, Gregori D, Delgado A, Salvade P, Oreglia J, Orrico P, de Biase A, Piccalò G, Bossi I, Klugmann S. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J Am Coll Cardiol. 2006;48:1552–9. doi: 10.1016/j.jacc.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 21.De Luca L, Sardella G, Davidson CJ, De Persio G, Beraldi M, Tommasone T, Mancone M, Nguyen BL, Agati L, Gheorghiade M, Fedele F. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST-elevation myocardial infarction. Heart. 2006;92:951–7. doi: 10.1136/hrt.2005.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliorini A, Stabile A, Rodriguez AE, Gandolfo C, Rodriguez Granillo AM, Valenti R, Parodi G, Neumann FJ, Colombo A, Antoniucci D JETSTENT Trial Investigators. Comparisonof AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. The JETSTENT trial. J Am Coll Cardiol. 2010;56:1298–306. doi: 10.1016/j.jacc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Liistro F, Grotti S, Angioli P, Falsini G, Ducci K, Baldassarre S, Sabini A, Brandini R, Capati E, Bolognese L. Impact of thrombus aspiration on myocardial tissue reperfusion and left ventricular functional recovery and remodeling after primary angioplasty. Circ Cardiovasc Interv. 2009;2:376–83. doi: 10.1161/CIRCINTERVENTIONS.109.852665. [DOI] [PubMed] [Google Scholar]
- 24.Ikari Y, Sakurada M, Kozuma K, Kawano S, Katsuki T, Kimura K, Suzuki T, Yamashita T, Takizawa A, Misumi K, Hashimoto H, Isshiki T VAMPIRE Investigators. Upfront thrombus aspiration in primary coronary intervention for patients with ST-segment elevation acute myocardial infarction: report of the VAMPIRE (VAcuuM aspiration thrombus REmoval) trial. JACC Cardiovasc Interv. 2008;1:424–31. doi: 10.1016/j.jcin.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Dudek D, Mielecki W, Burzotta F, Gasior M, Witkowski A, Horvath IG, Legutko J, Ochala A, Rubartelli P, Wojdyla RM, Siudak Z, Buchta P, Pregowski J, Aradi D, Machnik A, Hawranek M, Rakowski T, Dziewierz A, Zmudka K. Thrombus aspiration followed by direct stenting: a novel strategy of primary percutaneous coronary intervention in ST- segment elevation myocardial infarction. Results of the Polish-Italian-Hungarian R Andomized ThrombEctomy Trial (PIHRATE Trial) Am Heart J. 2010;160:966–72. doi: 10.1016/j.ahj.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Chao CL, Hung CS, Lin YH, Lin MS, Lin LC, Ho YL, Liu CP, Chiang CH, Kao HL. Time-dependent benefit of initial thrombosuction on myocardial reperfusion in primary percutaneous coronary intervention. Int J Clin Pract. 2008;62:555–61. doi: 10.1111/j.1742-1241.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 27.Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, Francone M, Di Roma A, Benedetti G, Conti G, Fedele F. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–15. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Svilaas T, Vlaar PJ, van der Horst IC, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primarypercutaneous coronary intervention. N Engl J Med. 2008;358:557–67. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 29.Lipiecki J, Monzy S, Durel N, Cachin F, Chabrot P, Muliez A, Morand D, Maublant J, Ponsonnaille J. Effect of thrombus aspiration on infarct size and left ventricular function in high-risk patients with acute myocardial infarction treated by percutaneous coronary intervention: results of a prospective controlled pilot study. Am Heart J. 2009;157:583, e1–e7. doi: 10.1016/j.ahj.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Chevalier B, Gilard M, Lang I, Commeau P, Roosen J, Hanssen M, Lefevre T, Carrié D, Bartorelli A, Montalescot G, Parikh K. Systematic primary aspiration in acute myocardial percutaneous intervention: a multicentre randomised controlled trial of the export aspiration catheter. EuroIntervention. 2008;4:222–8. doi: 10.4244/eijv4i2a40. [DOI] [PubMed] [Google Scholar]
- 31.Burzotta F, De Vita M, Gu YL, Isshiki T, Lefèvre T, Kaltoft A, Dudek D, Sardella G, Orrego PS, Antoniucci D, De Luca L, Biondi-Zoccai GG, Crea F, Zijlstra F. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–203. doi: 10.1093/eurheartj/ehp348. [DOI] [PubMed] [Google Scholar]


