Abstract
Background
Arcobacter spp. have in recent years received increasing interest as potential emerging enteropathogens and zoonotic agents. They are associated with various animals including poultry and can be isolated from meat products. The possibilities of persistence and cross-contamination in slaughterhouses during meat processing are not well established. We have evaluated the occurrence and persistence of Arcobacter spp. in a Danish slaughterhouse and determined the sensitivity of isolates to sodium hypochlorite, a commonly used biocide.
Results
Arcobacter contamination was examined in a broiler slaughterhouse by selective enrichment of 235 swabs from the processing line during two production days and after sanitizing in between. In total 13.6% of samples were positive for A. butzleri with the majority (29 of 32 isolates) originating from the evisceration machine. No Arcobacter spp. was isolated after cleaning. A. butzleri isolates confirmed by PCR were typed by multilocus sequence typing (MLST) resulting in 10 new sequence types (STs). Two sequence types were isolated on both processing days. Minimum inhibitory concentration (MIC) to sodium hypochlorite was determined to 0.5% hypochlorite biocide (500 ppm chlorine) for most isolates, which allows growth of A. butzleri within the working concentration of the biocide (0.2 - 0.5%).
Conclusions
A. butzleri was readily isolated from a Danish broiler slaughterhouse, primarily in the evisceration machine. Typing by MLST showed high strain variability but the recurrence of two STs indicate that some persistence or cross-contamination takes place. Importantly, the isolates tolerated sodium hypochlorite, a biocide commonly employed in slaughterhouse sanitizing, at levels close to the disinfection concentration, and thus, A. butzleri may survive the disinfection process although this was not observed in our study.
Keywords: Arcobacter butzleri, MLST, Chicken slaughterhouse, MIC, Sodium hypochlorite
Background
Arcobacter is member of the family Campylobacteraceae[1] and was first isolated in 1977 from aborted bovine and porcine fetuses [2,3]. The genus includes several species of which A. butzleri, A. cryaerophilus and to some extent A. skirrowii have been considered as animal pathogens implicated in abortions, mastitis and gastrointestinal disorders [4] and as human pathogens causing gastroenteritis and occasionally bacteraemia [5-8]. The genome sequence of A. butzleri revealed the presence of homologues of several virulence genes known to contribute to the pathogenesis of Campylobacter jejuni but also close resemblance to species of the Helicobacteraceae[9]. Arcobacter spp. have also been isolated from healthy humans and animals [8,10-12] but in general the prevalence of this emerging pathogen in human disease is likely to be underestimated due to lack of a standard detection procedure [13,14]. Methods for detection and isolation of Arcobacter spp. have been suggested and are continuously developing [15-18].
Arcobacter spp. have been isolated from a variety of meat products such as beef, pork, poultry and lamb, with notably high prevalence in poultry meat [19-22]. Consistently, poultry seem to be a major reservoir with flock prevalence between 4.3% and 100%, determined with various isolation techniques [23-28]. Some controversies have occurred about the location of A. butzleri in broilers, but the intestines and the cloacae seem to be colonized [18,23,29,30]. In poultry slaughterhouses, Arcobacter spp. have been isolated from the processing line, process water and carcasses and an increasing prevalence through a slaughter week has been indicated [27,28]. In a Danish study, all of 30 chicken carcasses sampled at a processing plant were positive for A. butzleri and six of these were concurrently positive for A. cryaerophilus[25]. A. butzleri has the ability to attach to surfaces of stainless steel and plastic and is capable of growing at 10°C and forming biofilm [31,32]. These findings suggest that A. butzleri may be able to persist in the food processing environment for extended periods of time and contaminate processing equipment despite of sanitizing [28]. In light of this information, we have addressed if A. butzleri chicken slaughterhouse isolates tolerate the commonly used biocide sodium hypochlorite in disinfection concentration causing the risk of isolates persisting in this environment. Further, multilocus sequence typing (MLST) of A. butzleri isolates was used to evaluate the heterogeneity of the isolates obtained on two consecutive production days and after the sanitizing in between.
Methods
Bacterial strains
Reference strains used in this study are listed in Table 1. Bacteria were routinely grown in Brain-Heart-Infusion broth (BHI; OXOID, Greve, Denmark) or Arcobacter broth (OXOID) and on Arcobacter agar (OXOID; Agar No. 3 incl. Arcobacter broth and 50 mL L-1 defibrinated calf blood) at 28°C in microaerobic atmosphere generated using CampyGen (OXOID) in sealed jars. For dilution and washing of bacteria peptone-saline water was used (NaCl 9.0 g L-1 and peptone 1.0 g L-1).
Table 1.
Arcobacterstrains used in this study
| Species | ID | Obtained from |
|---|---|---|
|
A. butzleri |
LMG 10828 |
BCCM/LMG (Ghent, Belgium) |
|
A. cryaerophilus |
LMG 7536 |
CCUG (Göteborg, Sweden) |
| LMG 10829 |
BCCM/LMG (Ghent, Belgium) |
|
|
A. skirrowii |
LMG 6621 |
CCUG (Göteborg, Sweden) |
|
A. trophiarum |
LMG 25534 |
BCCM/LMG (Ghent, Belgium) |
|
A. cibarius |
LMG 21996 |
BCCM/LMG (Ghent, Belgium) |
| A. thereius | LMG 24486 | BCCM/LMG (Ghent, Belgium) |
Specifications of the broiler slaughterhouse
A Danish slaughterhouse was selected for an investigation of the occurrence of Arcobacter during two processing days. The slaughter capacity was 180,000 chickens per day and the products ranged from whole chickens, breast fillets, wings and chicken legs. After slaughter, scalding, defeathering, evisceration and washing, chickens were conveyed through a 3-hour air chilling step before cutting and packaging. In general, the temperature in the processing areas was 10–12°C. The temperature of the scalding water was 53?±?2°C. The cleaning following directly after each processing day was performed with flusher and foam followed by disinfection. A biocide containing sodium hypochlorite was used for disinfection six days of the week and once per week a quaternary ammonium compound was used. The disinfection biocide containing sodium hypochlorite (Divosan Hypo Plus, JohnsonDiversey, Nivaa, Denmark) was used in a 0.2 – 0.5% working solution, corresponding to 200–500 ppm active chlorine and with a contact time of ten minutes as determined by the manufacturer.
Sample collection
Samples were collected three times at four sampling points during two consecutive production days (see Table 2 for details). Sterile cotton swabs moistened with Arcobacter broth sampled surfaces directly or indirectly in contact with parts of the carcasses. A total of 235 swabs were taken with 55 samples collected from the scalding tank (scalding tank water and overflow), 60 samples from the evisceration machine, 60 samples from the area “cutting of breast fillet” and 60 samples from the area “boning of chicken legs”. A. butzleri LMG 10828, A. cryaerophilus LMG 7536 and A. skirrowii LMG 6621 on agar were used as positive controls and sterile broth samples were used as negative controls. After sampling, swabs were stored individually in 5 mL Arcobacter broth in transport tubes under cooled conditions and processed within 30 h.
Table 2.
Prevalence ofArcobacterspp. in the processing line of a Danish broiler slaughterhouse determined by cultural enrichment and isolation or by direct PCR on enrichment cultures
| Processing day 1 | After cleaning | Processing day 2 | ||||
|---|---|---|---|---|---|---|
|
Place |
Enrichment |
PCR |
Enrichment |
PCR |
Enrichment |
PCR |
|
Scalding tank |
0/15 |
0/15 |
0/20 |
0/20 |
3/20 |
2/20 |
|
Evisceration machine |
10/20 |
13/20 |
0/20 |
2/20 |
19/20 |
17/20 |
|
Area “cutting of breast fillet” |
0/20 |
0/20 |
0/20 |
0/20 |
0/20 |
0/20 |
|
Area “boning of chicken leg” |
0/20 |
2/20 |
0/20 |
0/20 |
0/20 |
0/20 |
|
Prevalence |
10/75 |
15/75 |
0/80 |
2/80 |
22/80 |
19/80 |
| In percent | 13.3% | 20% | 0% | 2.5% | 27.5% | 23.8% |
Selective isolation
For selective enrichment favoring Arcobacter spp. the samples were added 50 ml L-1 defibrinated calf blood and selective supplement comprising of 5-flourouacil (100 mg L-1), amphotericin B (10 mg L-1), cefoperazone (16 mg L-1), novobiocin (32 mg L-1) and trimethoprim (64 mg L-1) [33] and incubated in 48 h in microaerobic atmosphere at 28°C. The samples were then stored in 15% glycerol at – 80°C until further processing. The samples were thawed in refrigerator at 5°C and aliquots of 250 μL were subcultured individually in 7 mL Arcobacter broth, 5% defibrinated calf blood and the selective supplement before incubation for 48 h in microaerobic atmosphere at 28°C. Subsequent 100 μL was inoculated on Arcobacter agar with selective supplement and incubated in 48 to 96 h in microaerobic atmosphere at 28°C. Furthermore, the frozen enrichments were subjected to direct PCR identification of Arcobacter spp. on crude DNA lysates. After thawing, 300 mL enrichment culture was centrifuged (8.000?×?g for 5 min), the supernatant was removed and the pellet was added 300 mL miliQ water. Bacteria were lysed by heating to 99°C for 15 min, cooled down and centrifuged (10.000?×?g for 2 min). The supernatants were used as DNA templates in a genus specific PCR amplifying a 181-bp DNA fragment of the 16S rRNA gene from Arcobacter spp. [34].
Species identification by multiplex-PCR
Presumptive Arcobacter colonies, determined by colony morphology (<1.5 mm greyish/white colonies) and cell morphology (motile, small, curved rods by microscopy), were subcultured on Arcobacter agar and incubated in microaerobic atmosphere at 28°C for 48 h. BHI cultures, grown for 20 h at 28°C, were centrifuged at 11,000?×?g for 3 min. Pellets were suspended in 0.5 mL peptone-saline water and centrifuged for 3 min at 14.000 × g. DNA was extracted using QIAGEN DNeasy® Blood & Tissue kit following the manufacturer’s instructions with pretreatment for Gram-negative bacteria. All PCR assays were carried out using DreamTaq™ Green PCR Master Mix (2x) (Fermentas Life Sciences) and primers were obtained from TAG Copenhagen (Copenhagen, Denmark). Multiplex-PCR (mPCR) was used for confirmation and identification at species level [35] using a range of Arcobacter species as controls (Table 1). Isolates not positive in the mPCR were furthermore evaluated by PCR using primers specific for A. trophiarum[36].
Multilocus sequence typing (MLST)
MLST, a typing method based on partial sequence information of seven housekeeping loci (aspA, atpA, glnA, gltA, glyA, pgm and tkt), was used for characterization of A. butzleri isolates below species level [37]. The MLST gene products were sequenced by Macrogen Inc. (Seoul, Korea) and the sequencing results were processed with CLC Main Work Bench version 5.7.1. To compare with known A. butzleri MLST profiles the profiles from the isolates were subjected to the Arcobacter specific MLST scheme: [http://pubmlst.org/arcobacter/] [38].
Phylogenetic analysis
Concatenated A. butzleri alleles were aligned using ClustalX [39]. Phylogenetic analyses were conducted using MEGA (Version 5.1; [40]). Un-rooted neighbor-joining phylogenetic trees were constructed with bootstrap analysis (1000 replicates).
Determination of minimal inhibitory concentration (MIC)
The tolerance towards a slaughterhouse biocide containing sodium hypochlorite (NaClO; Divosan Hypo Plus) was assessed using a standard dilution assay for determination of MIC. The MICs were determined by preparing dilutions starting from the maximal recommended working solution (0.5% and two-fold dilutions from 0.4 to 0.05%) of the biocide in 96-well microtiterplates [41,42]. BHI cultures of the slaughterhouse isolates and A. butzleri LMG 10828, grown for 20 h at 28°C, were diluted in BHI broth to give absorbance at 600 nm (A600) of 0.01 and were inoculated to biocide broth dilutions to a total volume of 200 μL per well. Positive (no NaClO) and negative (no inoculum) growth controls were included and the experiment was performed with quadruple determinations. The lowest NaClO concentration to inhibit bacterial growth after 20 h of incubation at 28°C in microaerobic atmosphere was considered as the MIC. To verify that inhibition from the slaughterhouse biocide was caused by the active ingredient, NaClO, the experiment was repeated with NaClO from Sigma-Aldrich (Broendby, Denmark; active chlorine; 4.00 - 4.99%).
Results and discussion
Occurrence of Arcobacter in a Danish slaughterhouse
A Danish chicken slaughterhouse, with a capacity of 180,000 chickens per day, was subjected to swab sampling on selected sites over two consecutive processing days during production, and after the sanitizing in between, to evaluate the Arcobacter contamination. In total, 32 of 235 (13.6%) samples were confirmed positive for A. butzleri by multiplex PCR after selective enrichment [33,43] but none of the samples taken on clean surfaces after sanitizing were positive. The majority of the isolates (29 of 32) were recovered from the evisceration machine; hence 48.3% of the samples from this site were positive. The distribution in the processing line is shown in Table 2. Using a direct PCR-technique, 36 of 235 (15.3%) samples were found positive for Arcobacter spp. and by this method, two samples from the meat processing area (cutting and boning) were positive as well as two samples from the evisceration machine after cleaning, which were not found in the selective enrichment. Otherwise, Arcobacter positive samples were identical using the two methods, except for three additional positives in the evisceration machine (Day 1) and two ‘false negative’ samples from the evisceration machine (Day 2) that were disqualified due to unspecific bands on agarose gels. The high prevalence of A. butzleri in the evisceration area supports earlier reports of Arcobacter positive chicken gut samples [23,26,28] and suggests that contamination is likely to originate from the chicken gut.
A. butzleri was the only species isolated. This may be due to the enrichment procedure, as controls of A. cryaerophilus and A. skirrowii failed to grow under the enrichment conditions. Enrichment culturing has previously been shown to favor outgrowth of A. butzleri[33], but variations in the enrichments culturing used in this study (prolonged cooled storage before enrichment and inclusion of a freeze storage stage of enriched samples) have likely had a negative effect on the survival of Arcobacter isolates, especially non-A. butzleri species [29]. This is supported by the difference in prevalence of Arcobacter on the first and second sampling day, as the prevalence was twice as high in samples from the second day, which had a shorter storage time. The A. butzleri, A. cryaerophilus and A. skirrowii controls were all detected using direct PCR. Interestingly, we detected Arcobacter in two out of 20 swabs from the evisceration machine after cleaning, but these were not isolated using selective plating. Arcobacter spp. have previously been recovered from cleaned processing machines in poultry slaughterhouses, even after a three-day break in the production, as well as from slaughtered broilers before evisceration [27]. Furthermore, A. butzleri is able to multiply at the temperature present in slaughterhouses (10°C) and to form biofilm on stainless steel surfaces [31], which suggest that A. butzleri may be able to establish itself in the slaughterhouse environment. In consequence, we investigated the heterogeneity of A. butzleri isolated from chicken carcasses and slaughterhouse equipment by MLST and the ability of the obtained isolates to tolerate disinfection with the routinely used biocide (sodium hypochlorite).
Characterization of A. butzleri isolates by multilocus sequence typing (MLST) and phylogeny
Typing of Arcobacter by MLST was demonstrated to be useful for discrimination below species level of isolates of A. butzleri, A. cryaerophilus, A. skirrowii and A. cibarius and a database for Arcobacter MLST profiles are available online [http://pubmlst.org/arcobacter/] [37,38]. We performed MLST analysis of 32 A. butzleri isolates obtained in the chicken slaughterhouse, resulting in identification of 10 new sequence types (STs) comprising both known alleles and 13 new alleles (Table 3). No known STs were identified. The relatively high numbers of new alleles and STs show the heterogeneity of the A. butzleri isolates, but also reflects the limited number of profiles in the Arcobacter MLST database. With three isolates on the first sampling day and 12 on the second sampling day, ST 367 was found to be the most prevailing (47% of isolates, see Table 4). Furthermore, ST 367 (15 isolates) and ST 373 (five isolates) were isolated during both production days in the evisceration machine. The two STs 367 and 373 have no identical alleles and it seems more likely that the reoccurrence of these STs are due to cross-contamination rather than identical sequence types being present at different broiler flock farms.
Table 3.
Overview of MLST results ofA. butzleriLMG 10828 and slaughterhouse isolates with allelic profiles according to theArcobacterMLST database[37]
|
Isolation area |
Allelic profile |
ST |
||||||
|---|---|---|---|---|---|---|---|---|
| aspA | atpA | glnA | gltA | glyA | pgm | tkt | ||
|
Evisceration machine |
153 |
4 |
11 |
11 |
177 |
102 |
11 |
367 |
|
Evisceration machine |
5 |
12 |
122* |
15 |
36 |
86 |
2 |
368 |
|
Evisceration machine |
30 |
34 |
9 |
30 |
144 |
35 |
175* |
369 |
|
Scalding tank |
206* |
143* |
2 |
37 |
166 |
53 |
162 |
370 |
|
Evisceration machine |
14 |
49 |
26 |
55 |
449* |
102 |
176* |
371 |
|
Evisceration machine |
23 |
44 |
24 |
14 |
450* |
101 |
20 |
372 |
|
Evisceration machine |
205* |
144* |
123* |
55 |
447* |
233* |
177* |
373 |
|
Evisceration machine |
5 |
5 |
5 |
19 |
120 |
11 |
65 |
374 |
|
Evisceration machine |
20 |
25 |
7 |
2 |
354 |
32 |
2 |
375 |
| Evisceration machine | 175 | 25 | 41 | 19 | 88 | 101 | 14 | 376 |
* New allele.
Table 4.
Occurrence and isolation site of 32Arcobacter butzleriisolates assigned ten different MLST sequence types (ST)
| |
Day 1 |
Day 2 |
||
|---|---|---|---|---|
| ST | No. of isolates | Area | No. of isolates | Area |
| 367 |
3 |
Evisceration |
12 |
Evisceration |
| 368 |
1 |
Evisceration |
- |
- |
| 369 |
1 |
Evisceration |
- |
- |
| 370 |
- |
- |
3 |
Scalding tank |
| 371 |
- |
- |
2 |
Evisceration |
| 372 |
- |
- |
1 |
Evisceration |
| 373 |
4 |
Evisceration |
1 |
Evisceration |
| 374 |
1 |
Evisceration |
- |
- |
| 375 |
- |
- |
1 |
Evisceration |
| 376 | - | - | 2 | Evisceration |
Isolation of A. butzleri from the scalding water during production on the second sampling day suggests a risk of cross-contamination within a production period. However, Arcobacter spp. are unlikely to survive more than few minutes in the scalding water (53?±?2°C) [44] and the ST found in scalding water was not found elsewhere in the production. Phylogenetic analysis of the concatenated A. butzleri alleles was conducted to evaluate possible clustering within the isolates. As established with the MLST, the strain diversity is high and in conjunction with the relatively low number of isolates this results in a phylogenetic tree (Figure 1) that is not very robust (low bootstrap values) and shows no clustering of isolates. Arcobacters have previously been characterized by molecular typing using ERIC-PCR and RAPD-PCR [45,46], by MLST [9] and by AFLP and PFGE profiling [47]. In general, these molecular fingerprinting techniques show a high discrimination of strains due to considerable heterogeneity of Arcobacter[15]. A great advantage of MLST genotyping is the online available database of Arcobacter spp. isolates collected worldwide [38] and thereby the possibility to compare results. Yet, so far it has not been possible to relate specific STs with hosts or geographical areas [37].
Figure 1.
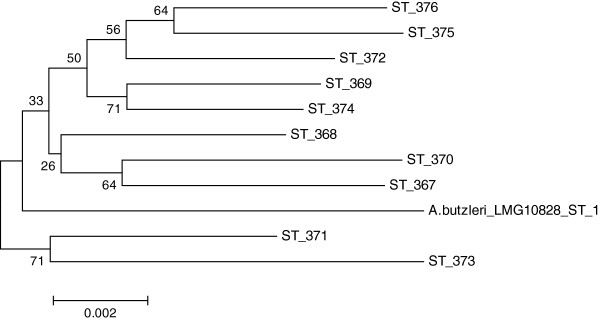
Neighbour-joining phylogenetic tree of concatenated A. butzleri alleles from ST 367 to ST 376 (and including A. butzleri LMG 10828 ST 1) isolated at a Danish chicken slaughterhouse. Bootstrap analysis with 1000 replications was performed, based on 3,341 bp, and bootstrap values are shown at the nodes.
Biocide tolerance
In the slaughterhouse, cleaning is performed on a daily basis by foaming all surfaces with a detergent and high pressure cleaning with hot water. Following cleaning, disinfection is performed with a biocide containing sodium hypochlorite (NaClO/Chlorine) at concentrations of 5–15%. The working solution of the biocide is 0.2 – 0.5% corresponding to 200–500 ppm active chlorine and the contact time is approximately 10 min. To address the susceptibility of A. butzleri to sodium hypochlorite we determined the minimum inhibitory concentration (MIC) by a standard broth dilution method used in similar studies [41,42]. We found that the majority of arcobacters were only inhibited by the sodium hypochlorite containing biocide in the maximum recommended working solution; 0.5% corresponding to 500 ppm active chlorine. A. butzleri LMG 10828 had a MIC of 0.5% sodium hypochlorite, as did 31 of the 32 slaughterhouse isolates (97%) (data not shown). The last slaughterhouse isolate and the remaining Arcobacter type strains (Table 1) had a MIC of 0.2% corresponding to 200 ppm active chlorine. This tolerance was confirmed using reagent grade sodium hypochlorite (Sigma-Aldrich). During regular cleaning operations the biocides are allowed a contact time of 10 min when applied for disinfection. Here we determine that the lower working concentration (0.2%) of sodium hypochlorite does not have a lethal effect on A. butzleri even after 20 h of exposure. Despite the biocide tolerance of Arcobacter isolates we did not obtain any isolates in the slaughterhouse after cleaning and disinfection with chlorine containing detergent, although two of a total of 80 swabs taken after sanitizing were Arcobacter positive by direct PCR. This suggests that the combined cleaning and disinfection procedures are generally working satisfactorily, but in highly contaminated areas it might not be sufficient.
We isolated A. butzleri almost exclusively from the evisceration area in the chicken slaughter house. Previous studies report findings of arcobacters in the intestines of chickens [23,48] and increased prevalence of A. butzleri on neck skin after evisceration with identical genotypes in the intestines and on the carcasses within a flock [28]. These findings suggest that arcobacter contamination occurs at the evisceration step and that contamination from the intestinal content to the carcass surface takes place. We determined a high biocide tolerance of the A. butzleri isolates obtained from the slaughterhouse, illustrating that A. butzleri can grow at concentrations close to the working solution of NaClO and we confirmed the presence of Arcobacter in two swab samples taken after sanitizing. Considering the disinfection contact time of 10 min, it is likely that A. butzleri may survive sanitizing. This assumption is supported by the isolation of two cases of identical STs of A. butzleri, determined by MLST, reoccurring on two consecutive production days, suggesting either persistence or continuous re-contamination of A. butzleri.
Conclusions
The MLST genotyping of A. butzleri, isolated on two consecutive production days in a chicken slaughterhouse, showed high heterogeneity of the isolates but nonetheless reoccurrence of two sequence types on both production days. We further show that A. butzleri can survive and multiply in prolonged exposure (20 h) to working concentrations of a commonly used disinfecting biocide, sodium hypochlorite. Thus, A. butzleri may be able to persist in the slaughterhouse after disinfection.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LHR conceived the research idea and performed the laboratory work, analyzed the data and wrote the manuscript. JK helped in sample collection, analyzing and interpreting data and preparation of the manuscript. JPC and HI helped in designing the study and supervision of the work and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Louise Hesselbjerg Rasmussen, Email: lohra@regionsjaelland.dk.
Jette Kjeldgaard, Email: jekje@sund.ku.dk.
Jens Peter Christensen, Email: jpch@sund.ku.dk.
Hanne Ingmer, Email: hi@sund.ku.dk.
Acknowledgements
The work was supported by the Danish Research Council for Strategic Research.
References
- Vandamme P, Deley J. Proposal for a new family, Campylobacteraceae. Int J Syst Bacteriol. 1991;41:451–455. doi: 10.1099/00207713-41-3-451. [DOI] [Google Scholar]
- Ellis WA, Neill SD, O'Brien JJ, Ferguson HW, Hanna J. Isolation of Spirillum/Vibrio-like organisms from bovine fetuses. Vet Rec. 1977;100:451–452. doi: 10.1136/vr.100.21.451. [DOI] [PubMed] [Google Scholar]
- Ellis WA, Neill SD, O'Brien JJ, Hanna J. Isolation of Spirillum-like organisms from pig fetuses. Vet Rec. 1978;102:106. doi: 10.1136/vr.102.5.106-a. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Vancanneyt M, Pot B, Mels L, Hoste B, Dewettinck D. et al. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb-nov and Arcobacter skirrowii sp-nov, an aerotolerant bacterium isolated from veterinary specimens. Int J Syst Bacteriol. 1992;42:344–356. doi: 10.1099/00207713-42-3-344. [DOI] [PubMed] [Google Scholar]
- Vandamme P, Pugina P, Benzi G, Van Etterijck R, Vlaes L, Kersters K. et al. Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J Clin Microbiol. 1992;30:2335–2337. doi: 10.1128/jcm.30.9.2335-2337.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- On SLW, Stacey A, Smyth J. Isolation of Arcobacter butzleri from a neonate with bacteremia. J Infect. 1995;31:225–227. doi: 10.1016/s0163-4453(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Yan JJ, Ko WC, Huang AH, Chen HM, Jin YT, Wu JJ. Arcobacter butzleri bacteremia in a patient with liver cirrhosis. J Formos Med Assoc. 2000;99:166–169. [PubMed] [Google Scholar]
- Vandenberg O, Dediste A, Houf K, Ibekwem S, Souayah H, Cadranel S. et al. Arcobacter species in humans. Emerg Infect Dis. 2004;10:1863–1867. doi: 10.3201/eid1010.040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WG, Parker CT, Rubenfield M, Mendz GL, Wösten MM, Ussery DW. et al. The complete genome sequence and analysis of the Epsilonproteobacterium Arcobacter butzleri. PLoS One. 2007;2:e1358. doi: 10.1371/journal.pone.0001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley IV, Wells SJ, Harmon KM, Green A, Schroeder-Tucker L, Glover M. et al. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl Environ Microbiol. 2000;66:1994–2000. doi: 10.1128/AEM.66.5.1994-2000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Óngör H, Cetinkaya B, Acik MN, Atabay HI. Investigation of arcobacters in meat and faecal samples of clinically healthy cattle in Turkey. Lett Appl Microbiol. 2004;38:339–344. doi: 10.1111/j.1472-765X.2004.01494.x. [DOI] [PubMed] [Google Scholar]
- Houf K, Stephan R. Isolation and characterization of the emerging foodborn pathogen Arcobacter from human stool. J Microbiol Methods. 2007;68:408–413. doi: 10.1016/j.mimet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Prouzet-Mauleon V, Labadi L, Bouges N, Menard A, Megraud F. Arcobacter butzleri: Underestimated enteropathogen. Emerg Inf Dis. 2006;12:307–309. doi: 10.3201/eid1202.050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J, On SLW, Harrington CS, Gerner-Smidt P. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. J Clin Microbiol. 2000;38:286–291. doi: 10.1128/jcm.38.1.286-291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I, Garcia T, Fernandez S, Martin R. Current status on Arcobacter research: An update on DNA-based identification and typing methodologies. Food Anal Method. 2012;5:956–968. doi: 10.1007/s12161-011-9343-9. [DOI] [Google Scholar]
- Lehner A, Tasara T, Stephan R. Relevant aspects of Arcobacter spp. as potential foodborne pathogen. Int J Food Microbiol. 2005;102:127–135. doi: 10.1016/j.ijfoodmicro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Van Driessche E, Houf K, Van Hoof J, De Zutter L, Vandamme P. Isolation of Arcobacter species from animal feces. FEMS Microbiol Lett. 2003;229:243–248. doi: 10.1016/S0378-1097(03)00840-1. [DOI] [PubMed] [Google Scholar]
- Eifert JD, Castle RM, Pierson FW, Larsen CT, Hackney CR. Comparison of sampling techniques for detection of Arcobacter butzleri from chickens. Poult Sci. 2003;82:1898–1902. doi: 10.1093/ps/82.12.1898. [DOI] [PubMed] [Google Scholar]
- Kabeya H, Maruyama S, Morita Y, Ohsuga T, Ozawa S, Kobayashi Y. et al. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int J Food Microbiol. 2004;90:303–308. doi: 10.1016/S0168-1605(03)00322-2. [DOI] [PubMed] [Google Scholar]
- Rivas L, Fegan N, Vanderlinde P. Isolation and characterisation of Arcobacter butzleri from meat. Int J Food Microbiol. 2004;91:31–41. doi: 10.1016/S0168-1605(03)00328-3. [DOI] [PubMed] [Google Scholar]
- Scullion R, Harrington CS, Madden RH. A comparison of three methods for the isolation of Arcobacter spp. from retail raw poultry in Northern Ireland. J Food Prot. 2004;67:799–804. doi: 10.4315/0362-028x-67.4.799. [DOI] [PubMed] [Google Scholar]
- Lee MH, Cheon DS, Choi S, Lee BH, Jung JY, Choi C. Prevalence of Arcobacter species isolated from retail meats in Korea. J Food Prot. 2010;73:1313–1316. doi: 10.4315/0362-028x-73.7.1313. [DOI] [PubMed] [Google Scholar]
- Ho HTK, Lipman LJA, Gaastra W. The introduction of Arcobacter spp. in poultry slaughterhouses. Int J Food Microbiol. 2008;125:223–229. doi: 10.1016/j.ijfoodmicro.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Atanassova V, Kessen V, Reich F, Klein G. Incidence of Arcobacter spp. in poultry: quantitative and qualitative analysis and PCR differentiation. J Food Prot. 2008;71:2536. doi: 10.4315/0362-028x-71.12.2533. [DOI] [PubMed] [Google Scholar]
- Atabay HI, Waino M, Madsen M. Detection and diversity of various Arcobacter species in Danish poultry. Int J Food Microbiol. 2006;109:139–145. doi: 10.1016/j.ijfoodmicro.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Kabeya H, Maruyama S, Morita Y, Kubo M, Yamamoto K, Arai S. et al. Distribution of Arcobacter species among livestock in Japan. Vet Microbiol. 2003;93:153–158. doi: 10.1016/S0378-1135(02)00312-7. [DOI] [PubMed] [Google Scholar]
- Houf K, De Zutter L, Van Hoof J, Vandamme P. Occurrence and distribution of Arcobacter species in poultry processing. J Food Prot. 2002;65:1233–1239. doi: 10.4315/0362-028x-65.8.1233. [DOI] [PubMed] [Google Scholar]
- Houf K, De Zutter L, Verbeke B, Van Hoof J, Vandamme P. Molecular characterization of Arcobacter isolates collected in a poultry slaughterhouse. J Food Prot. 2003;66:364–369. doi: 10.4315/0362-028x-66.3.364. [DOI] [PubMed] [Google Scholar]
- Van Driessche E, Houf K. Discrepancy between the occurrence of Arcobacter in chickens and broiler carcass contamination. Poult Sci. 2007;86:744–751. doi: 10.1093/ps/86.4.744. [DOI] [PubMed] [Google Scholar]
- Ho H, Lipman L, Gaastra W. The presence of Arcobacter species in breeding hens and eggs from these hens. Poult Sci. 2008;87:2404–2407. doi: 10.3382/ps.2008-00092. [DOI] [PubMed] [Google Scholar]
- Kjeldgaard J, Jørgensen K, Ingmer H. Growth and survival at chiller temperatures of Arcobacter butzleri. Int J Food Microbiol. 2009;131:256–259. doi: 10.1016/j.ijfoodmicro.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Assanta MA, Roy D, Lemay MJ, Montpetit D. Attachment of Arcobacter butzleri, a new waterborne pathogen, to water distribution pipe surfaces. J Food Prot. 2002;65:1240–1247. doi: 10.4315/0362-028x-65.8.1240. [DOI] [PubMed] [Google Scholar]
- Houf K, Devriese LA, De Zutter L, Van Hoof J, Vandamme P. Development of a new protocol for the isolation and quantification of Arcobacter species from poultry products. Int J Food Microbiol. 2001;71:189–196. doi: 10.1016/S0168-1605(01)00605-5. [DOI] [PubMed] [Google Scholar]
- González I, García T, Antolín A, Hernández PE, Martín R. Development of a combined PCR-culture technique for the rapid detection of Arcobacter spp. in chicken meat. Lett Appl Microbiol. 2000;30:207–212. doi: 10.1046/j.1472-765x.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- Douidah L, De Zutter L, Vandamme P, Houf K. Identification of five human and mammal associated Arcobacter species by a novel multiplex-PCR assay. J Microbiol Methods. 2010;80:281–286. doi: 10.1016/j.mimet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- De Smet S, Vandamme P, De Zutter L, On SLW, Douidah L, Houf K. Arcobacter trophiarum sp. nov., isolated from fattening pigs. Int J Syst Evol Microbiol. 2011;61:356–361. doi: 10.1099/ijs.0.022665-0. [DOI] [PubMed] [Google Scholar]
- Miller WG, Wesley IV, On SLW, Houf K, Megraud F, Wang GL. et al. First multi-locus sequence typing scheme for Arcobacter spp. BMC Microbiol. 2009;9:196. doi: 10.1186/1471-2180-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WG. Arcobacter MLST Database. 2012. http://pubmlst.org/arcobacter/
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H. et al. Clustal W and Clustal X version 2.0. Bioinform. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavri A, Kurincic M, Mozina SS. The prevalence of antibiotic and biocide resistance among Campylobacter coli and Campylobacter jejuni from different sources. Food Technol Biotechnol. 2012;50:371–376. [Google Scholar]
- Sheridan À, Lenahan M, Duffy G, Fanning S, Burgess C. The potential for biocide tolerance in Escherichia coli and its impact on the response to food processing stresses. Food Control. 2012;26:98–106. doi: 10.1016/j.foodcont.2012.01.018. [DOI] [Google Scholar]
- Houf K, Tutenel A, De Zutter L, Van Hoof J, Vandamme P. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol Lett. 2000;193:89–94. doi: 10.1111/j.1574-6968.2000.tb09407.x. [DOI] [PubMed] [Google Scholar]
- Van Driessche E, Houf K. Survival capacity in water of Arcobacter species under different temperature conditions. J Appl Microbiol. 2008;105:443–451. doi: 10.1111/j.1365-2672.2008.03762.x. [DOI] [PubMed] [Google Scholar]
- Houf K, De Zutter L, Van Hoof J, Vandamme P. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl Environ Microbiol. 2002;68:2172–2178. doi: 10.1128/AEM.68.5.2172-2178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabay HI, Bang DD, Aydin F, Erdogan HM, Madsen M. Discrimination of Arcobacter butzleri isolates by polymerase chain reaction-mediated DNA fingerprinting. Lett Appl Microbiol. 2002;35:141–145. doi: 10.1046/j.1472-765X.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Ferrus MA, Gonzalez R, Hernandez J. Molecular fingerprinting of Campylobacter and Arcobacter isolated from chicken and water. Int Microbiol. 2007;10:85–90. [PubMed] [Google Scholar]
- Atabay HI, Corry JEL. The prevalence of Campylobacters and Arcobacters in broiler chickens. J Appl Microbiol. 1997;83:619–626. doi: 10.1046/j.1365-2672.1997.00277.x. [DOI] [PubMed] [Google Scholar]


