Abstract
Oropharyngeal candidiasis, typically caused by Candida albicans, is the most common oral disease associated with human immunodeficiency virus type 1 (HIV-1) infection. Secretory leukocyte protease inhibitor (SLPI), a 12-kDa antiprotease, suppresses the growth of C. albicans in vitro. To determine whether the mucosal protein plays a role in protecting oral tissues against fungal infection, we conducted a cross-sectional study investigating the oral and systemic health and salivary SLPI levels in 91 dentate HIV-1-infected adults receiving medical care in the southeastern United States. Participants with a self-reported history of clinical oropharyngeal candidiasis during the previous 2 years constituted the test group (n = 52), while the comparison group (n = 39) had no oropharyngeal candidiasis during that period. Data collected from medical records, oral examination, and SLPI enzyme-linked immunosorbent assay quantitation of whole saliva were analyzed by t test, analysis of variance, linear regression, and unconditional logistic regression. The test group had a significantly higher mean salivary SLPI level than the comparison group (1.9 μg/ml versus 1.1 μg/ml, P < 0.05). Linear regression modeling identified CD4 cell count and history of oropharyngeal candidiasis as key predictors of salivary SLPI and revealed a significant interaction (P < 0.05) between immunosuppression (CD4 cell count below 200 cells/μl) and positive history of oropharyngeal candidiasis in predicting salivary SLPI level. By logistic regression modeling, a salivary SLPI level exceeding 2.1 μg/ml, low CD4 count, antiretroviral monotherapy, and smoking were key predictors of oropharyngeal candidiasis. These data support a key role for SLPI in the oral mucosal defense against C. albicans. The antimicrobial mucosal protein may serve as an indicator of previous oropharyngeal candidiasis infection among immunosuppressed persons.
Oropharyngeal candidiasis, typically caused by Candida albicans, is the most frequent opportunistic oral disease in persons infected with human immunodeficiency virus (HIV) and other immunosuppressive conditions (12, 33). Although the microorganism is a harmless commensal in the oral cavity of healthy individuals, C. albicans can rapidly proliferate, invade tissues, and cause symptomatic mucosal lesions in immunocompromised persons (12). Oropharyngeal candidiasis is a sentinel indicator of immunodeficiency and HIV disease progression (20, 34). The asymptomatic presence of C. albicans in the oral cavity increases the likelihood of developing clinical disease, especially following immune suppression (4, 11, 13). In a cross-sectional study conducted by Campisi and colleagues (4), 62% of HIV-1-positive persons harbored Candida species in their oral cavity, compared to 29% of HIV-1-negative persons. In a recent longitudinal study, Ohmit and coworkers (32) found the acquisition and prevalence rates of oral Candida colonization and candidiasis to be eightfold higher among HIV-1-seropositive women compared to at-risk uninfected women.
Multiple host defense mechanisms control the proliferation and invasion of C. albicans in the oral cavity (28). Systemic cell-mediated immunity is considered the key host defense mechanism against this microorganism, as evidenced by the high incidence of oropharyngeal candidiasis in patients with reduced CD4+ T lymphocyte counts (CD4 counts) (12). A role for local cell-mediated immunity is suggested by a shift from a Th1- to Th2-type cytokine profile in the saliva of HIV-infected persons with oral candidal lesions (24). Humoral immunity is not considered a critical protective defense, as persons with and without oropharyngeal candidiasis exhibit similar Candida-specific antibody responses in saliva (44). Much less is known about the importance of soluble endogenous antimicrobial peptides and proteins such as salivary histatins, lactoferrin, and defensins in the natural resistance of oral tissues against C. albicans (25).
Antifungal activity has been recently described for the antimicrobial mucosal protein secretory leukocyte protease inhibitor (SLPI; also known as antileukoprotease and mucus protease inhibitor) (40). The 12-kDa single-chained polypeptide is secreted by epithelial cells lining the mucosal surfaces of the oral, nasal, respiratory, and reproductive tracts (16). Tomee et al. (42) reported that recombinant SLPI inhibited the in vitro growth of C. albicans and Aspergillus fumigatus. Recombinant SLPI also killed the yeast form of C. albicans in a dose-dependent manner at concentrations ranging from 2 to 15 μM, with over 80% fungicidal activity at 15 μM. The antifungal activity of recombinant SLPI was localized to its amino-terminal domain, distinct from the antiprotease and antibacterial functional sites residing in its carboxyl-terminal domain (10). Because these experiments were conducted under low-salt conditions (10 mM sodium phosphate buffer), it is not clear whether SLPI has antifungal activity under physiological conditions.
Although SLPI was initially characterized from parotid gland secretions (31), little is known of the role that the cationic protein plays in oral mucosal disease. Most studies of SLPI function have been conducted in the respiratory tract (17), reproductive system (8), and skin (1). As the major serine protease inhibitor in upper airway cells (17) and neutrophils (37), SLPI protects mucosal membranes from attack by neutrophil elastase during inflammation (35). The mucosal protein also blocks the in vitro replication of HIV-1 (19, 29, 38-41, 43), non-HIV-1 viruses (influenza A and Sendai viruses) (2), and bacteria (Escherichia coli and Staphylococcus aureus) (18), and promotes cutaneous wound healing in mice (1, 45). Thus, salivary SLPI may play a role in the innate oral mucosal defense against pathogenic microorganisms such as C. albicans.
To date, no published studies have investigated either the predictors of salivary SLPI or the relationship between salivary SLPI and Candida infection in vivo. The current investigation examined the level of salivary SLPI in relation to CD4 cell count and history of oropharyngeal candidiasis among HIV-1-infected individuals while controlling for antifungal medications, smoking, and other factors. The study also evaluated whether salivary SLPI may serve as an indicator of previous oral candidal infection in this immunosuppressed population.
MATERIALS AND METHODS
Study population.
The cross-sectional analysis included 91 dentate adult persons and was nested within a cohort study of 631 HIV-positive persons followed for 2 years at the University of North Carolina Hospitals, Chapel Hill, N.C. The participants in the current study included all subjects of the larger cohort study who had at least four teeth in all quadrants of the mouth and provided saliva samples. For the current study, the test group consisted of 52 participants who had a history of oropharyngeal candidiasis at any time during the 2 years preceding and including the study visit, while the comparison group was comprised of 39 participants who had no history of oropharyngeal candidiasis during that period. Written informed consent was obtained from each participant prior to enrollment, according to the University of North Carolina (UNC) Institutional Review Board.
Questionnaire, clinical examination, and medical record review.
Before the clinical examination, a trained social researcher administered a detailed questionnaire to collect data describing sociodemographics and smoking history. A single calibrated dental examiner trained in oral medicine and blinded to the participant's questionnaire responses performed a comprehensive oral examination for HIV-associated oral diseases, following standard clinical criteria (9). White and red areas caused by trauma or friction were ignored. A diagnosis of oropharyngeal candidiasis was based solely on clinical appearance and included the pseudomembraneous, erythematous, and angular cheilitis forms; no staining, cytology, or culture was performed. After the clinical examination, the medical record for each participant was reviewed to obtain data describing CD4 count, viral load in blood (typically within 2 months of the study visit), and current antiretroviral and antifungal medication use.
Saliva collection and salivary SLPI quantitation.
Participants refrained from eating and toothbrushing for at least 30 min before saliva collection. Whole (mixed) unstimulated human saliva was collected by expectoration into chilled sterile containers as we have described (40) and stored at −70°C within 2 h of collection. Samples were later thawed, mixed briefly, and analyzed for SLPI content with a commercial human SLPI enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, Minn.).
Data management.
This study analyzed variables from the interview questionnaire, medical record review, examination findings, and salivary SLPI quantitation. Data were double entered, checked for consistency, crosschecked, and synchronized with the medical records and interview questionnaire.
Due to the small number of participants with clinically detected oropharyngeal candidiasis at the study visit, the intermittent nature of oropharyngeal candidiasis (11, 12), and the high prevalence of Candida carriage reported among HIV-1-positive individuals (4, 32), we used a history of oropharyngeal candidiasis as a surrogate indicator of oropharyngeal candidiasis. In support of this decision, a recent study by the Centers for Disease Control and Prevention found a self-reported history of oropharyngeal candidiasis to be a good predictor of clinical oropharyngeal candidiasis, as evaluated by repeat-measure analysis (6). To directly test this reasoning in our study population, we created a composite three-level candidiasis variable: history of oropharyngeal candidiasis only, current oropharyngeal candidiasis plus history of oropharyngeal candidiasis, and neither current oropharyngeal candidiasis nor history of oropharyngeal candidiasis. No participant had current oropharyngeal candidiasis without a history of oropharyngeal candidiasis, so this level was not needed.
Based on the previously reported mean salivary SLPI level of 1.2 μg/ml in an HIV-1-positive cohort (40), we calculated that this study had 80% power for a minimum detectable difference of 0.455 μg/ml in salivary SLPI levels between participants with and without a positive history of oropharyngeal candidiasis, with a two-tailed test at an alpha of 0.05. For some analyses, salivary SLPI levels were dichotomized, with a cutoff value of 2.1 μg/ml. This value was the minimal concentration that reduced Candida growth in vitro (42) and was equal to the mean level plus three times the standard deviation for mean salivary SLPI concentration in a cohort of HIV-1-positive persons (40).
Statistical analyses.
Statistical analyses were performed with SAS (version 8.2; SAS, Cary, N.C.). Differences in mean salivary SLPI levels between test and comparison groups were evaluated based on CD4 count groups and viral load groups with the t test, one-way analysis of variance, and chi-square tests.
The goals of the multivariable analyses were to determine whether salivary SLPI levels were different between those with and without a positive history of oropharyngeal candidiasis after adjusting for important variables and to develop an explanatory model for salivary SLPI level by finding the best-fitting and most parsimonious yet biologically reasonable model to describe the relationship between the outcome and explanatory variables. Linear regression models to predict salivary SLPI were generated with continuous salivary SLPI as the outcome and candidal status, smoking status, and categorized CD4 levels and viral load as the explanatory variables. Interaction terms were tested with history of oropharyngeal candidiasis status as the main explanatory variable. The main-effects model and the interaction terms were assessed with a chunkwise test. Variables that were statistically not significant were removed to arrive at a parsimonious final model. If any statistical interaction became evident, analysis for the involved variables was limited to simple effects within their categories. Predicted salivary SLPI values were examined against observed values. Another model similar to the final model was evaluated by replacing the categorical CD4 count variable with CD4 count as a continuous variable. Predicted salivary SLPI levels were plotted and, in case of interactions, mean salivary SLPI levels in the CD4 cell groups were tested between levels of the stratifying variables. Unconditional logistic regression models with Proc Logistic were analyzed with dichotomized salivary SLPI as the outcome variable to evaluate predictors of high salivary SLPI levels.
For all multivariable analyses, the hierarchically well-formulated model principle (21) was used; for any given variable in the model, all lower-order components of the variables must also be contained in the model. To arrive at a final model, we tested hierarchical sets of models with the likelihood ratio test. Where present, interactions were assessed through the interaction contrast ratio (ICR). With Rothman and Greenland's notations (36), ICR = RR11 − RR01 − RR10 + RR00; where ICR is the relative excess risk for interaction, whereas RR01 and R10 are the odds ratios for exposure to any one of the exposures, RR11 is the odds ratio for doubly exposed, and R00 is the unitary value for the referent (doubly unexposed group). Regression diagnostics were performed, and the utility of the models was evaluated by outputting predicted scores and residuals.
A secondary goal of the analyses was to evaluate the usefulness of a dichotomous high/low salivary SLPI variable as an explanatory variable for history of oropharyngeal candidiasis as an outcome and to control for confounding. Unconditional logistic regression was performed as described above to model the probability of positive history of oropharyngeal candidiasis.
RESULTS
Study population characteristics.
Table 1 summarizes the characteristics of the study population. Most participants were black, male, and between 30 and 39 years of age and were not taking an antifungal medication. Approximately half were current tobacco smokers, while 24.4% were former smokers and 21.1% had never smoked. The majority (83.3%) had CD4 counts below 500 cells/ml, the threshold below which oral manifestations typically appear (33). Approximately half of the population currently used combination antiretroviral therapy and had plasma viral loads below 2.0 × 104 copies/ml.
TABLE 1.
Study population characteristics
| Characteristic | No. of subjects (n = 91) | % of subjects |
|---|---|---|
| Age (yr) | ||
| 18-29 | 17 | 18.7 |
| 30-39 | 40 | 43.9 |
| 40+ | 34 | 37.4 |
| Race/ethnicity | ||
| White | 34 | 37.4 |
| Black | 57 | 62.6 |
| Sex | ||
| Male | 74 | 81.3 |
| Female | 17 | 18.7 |
| Smoking | ||
| Current | 49 | 54.4 |
| Former | 22 | 24.4 |
| Never | 19 | 21.1 |
| CD4+-T-cell count (cells/μl) | ||
| <200 | 36 | 40.0 |
| 200-499 | 39 | 43.3 |
| >499 | 15 | 16.7 |
| Viral load in plasma (HIV RNA copies/ml) | ||
| <2.0 × 104 | 50 | 60.2 |
| 2.0 × 104-5.0 × 104 | 12 | 14.5 |
| >5.0 × 104 | 21 | 25.3 |
| Anti-HIV medication | ||
| Combination therapy | 42 | 46.7 |
| Monotherapy | 21 | 23.3 |
| None | 27 | 30.0 |
| Antifungal medication | ||
| Yes | 14 | 15.6 |
| No | 76 | 84.4 |
| Current OCa | ||
| Yes | 13 | 14.3 |
| No | 78 | 85.7 |
| History of OC | ||
| Yes (test group) | 52 | 57.1 |
| No (comparison group) | 39 | 42.9 |
| Current OC and history of OC | ||
| Both positive | 13 | 14.2 |
| Both negative | 39 | 42.9 |
| Positive history of OC and negative OC | 39 | 42.9 |
| Hairy leukoplakia | ||
| Yes | 13 | 14.4 |
| No | 77 | 85.6 |
OC, oral candidiasis.
A majority (52 of 91, or 57.1%) of the participants had had at least one episode of oropharyngeal candidiasis during the previous 2 years. Current oropharyngeal candidiasis, in its pseudomembraneous, erythematous, or angular cheilitis form, was detected at the examination in 13 (14.3%) participants. Oral hairy leukoplakia (13 participants) and linear gingival erythema (4 participants) were the only other HIV-associated oral lesions detected in the study population.
Salivary SLPI levels and oral candidal disease.
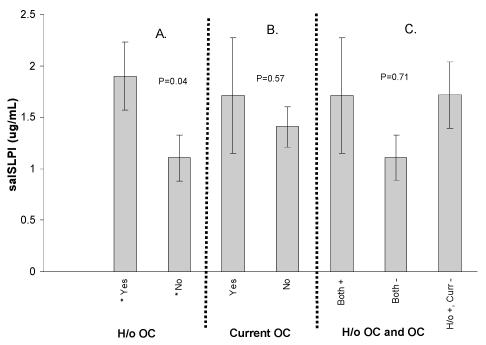
The mean (± standard error) salivary SLPI concentration for the study population was 1.45 ± 0.19 μg/ml (median, 0.79 μg/ml), which is comparable to the mean salivary SLPI level that we previously described in a different HIV-1-infected cohort (40). Salivary SLPI levels for study participants according to candidal status are shown in Fig. 1. The mean salivary SLPI level was significantly higher among participants with a history of oropharyngeal candidiasis (1.99 ± 0.80 μg/ml) compared to participants with no history of oropharyngeal candidiasis (1.11 ± 0.16 μg/ml, t test, P = 0.04, Fig. 1A). At the time of the study visit, the mean salivary SLPI level was comparable between participants with and without current oropharyngeal candidiasis (1.71 ± 0.56 μg/ml versus 1.41 ± 0.20 μg/ml, P > 0.05, Fig. 1B).
FIG. 1.
Salivary SLPI (salSLPI) levels and oral candidiasis (OC) in an HIV-1-infected population. The SLPI content of saliva from each participant was measured by ELISA and compared with their history of oropharyngeal candidiasis (H/o OC, panel A), current oropharyngeal candidiasis (panel B), and both history of and current oropharyngeal candidiasis (panel C). P values were determined by t test or analysis of variance (*, significantly different).
Using analysis of variance, we found no significant difference between salivary SLPI levels based on the composite candidiasis variable (model P = 0.71, Fig. 1C). Therefore, we were confident in using a history of oropharyngeal candidiasis as our test definition for additional analyses. With this definition, participants with a previous oropharyngeal candidiasis episode had a slightly lower mean CD4 count (211 ± 61 cells/μl) and somewhat higher mean viral load in plasma (1.2 × 105 ± 5.0 × 105 copies/ml) compared to participants without an oropharyngeal candidiasis episode (395 ± 31 cells/μl and 5.7 × 104 ± 4.1 × 104 copies/ml, respectively); however, the differences failed to reach statistical significance. The collective data suggest that salivary SLPI levels are elevated in immunosuppressed individuals who have experienced a previous episode of oropharyngeal candidiasis.
Impact of CD4 cell count and history of oropharyngeal candidiasis on salivary SLPI levels.
We assessed the relationship of systemic immunosuppression (CD4 counts) and salivary SLPI level with oropharyngeal candidiasis experience. In bivariable analyses, participants with low CD4 counts (below 200 cells/μl) were five times as likely as those with higher CD4 counts (200 cells/μl or greater) to have a positive history of oropharyngeal candidiasis (crude odds ratio = 5.1; 95% confidence interval = 1.9, 13.9). Those with higher salivary SLPI levels were 2.5 times as likely as those with low salivary SLPI levels to have a positive history of oropharyngeal candidiasis [odds ratio = 2.5 (0.6, 7.3)]. When stratified by salivary SLPI levels, the odds ratios for CD4 and history of oropharyngeal candidiasis varied between low salivary SLPI (odds ratio = 9.5) and high salivary SLPI (odds ratio = 1.0) levels, the ratio of the two stratum-specific odds ratios being 9.5. The Breslow-Day chi-square statistic (3.9/1 df, P = 0.04, general association P = 0.0006) suggested heterogeneity of odds ratios between the salivary SLPI strata, indicating modification of the effect of CD4 on candidal status by salivary SLPI levels.
To determine whether mean salivary SLPI levels differed between participants based on oropharyngeal candidiasis experience after adjusting for key variables, linear regression models were developed with salivary SLPI as a continuous-outcome variable and history of oropharyngeal candidiasis, smoking status, and categorized CD4 level and viral load as explanatory variables. Table 2 shows the full and final linear regression models for predicting salivary SLPI as a continuous outcome. Viral load in plasma, antifungal medications, and smoking were not significant factors in the full model and were removed to develop the final model. Explanatory variables that significantly contributed to both models were low CD4 count (below 200 μl) and a positive history of oropharyngeal candidiasis. The final model with categorical CD4 levels was: salivary SLPI = 0.946 + 0.780 μg/ml (if CD4 count is <200 cells/μl, 0 otherwise) + 1.298 μg/ml (if history of oropharyngeal candidiasis is positive, 0 otherwise) − 1.743 μg/ml (if CD4 count is <200 and if history of oropharyngeal candidiasis is positive, 0 otherwise).
TABLE 2.
Linear regression source table, parameter estimates, and model equations for outcomes of continuous salivary SLPI and continuous CD4 cell counta
| Variable | Full model with continuous salivary SLPI
|
Final model with continuous salivary SLPI
|
Final model with continuous CD4 count
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Est | SE | P | Est | SE | P | Est | SE | P | |
| Intercept | 1.534 | 0.542 | 0.006 | 0.946 | 0.311 | 0.003 | 1.520 | 0.487 | 0.002 |
| CD4 cell count (continuous), cells/μl | −0.001 | 0.001 | |||||||
| CD4 count <200 cell/μl* | 0.446 | 0.887 | 0.780 | 0.686 | |||||
| CD4 count 200-500 cells/μl* | −0.358 | 0.545 | 0.514 | ||||||
| Viral load >50,000 copies/ml* | 0.175 | 0.506 | 0.730 | ||||||
| Viral load 20,000-50,000 copies/ml* | −0.317 | 0.592 | 0.594 | ||||||
| Current smoker (yes = 1) | −0.523 | 0.410 | 0.206 | ||||||
| History of candidiasis (yes = 1) | 1.527 | 0.515 | 1.298 | 0.476 | −0.401 | 0.590 | |||
| CD4 count <200 and history of candidiasis | −2.000 | 0.935 | 0.036 | −1.743 | 0.841 | 0.004 | |||
| CD4 cell count (continuous) and history of candidiasis | 0.004 | 0.002 | 0.012 | ||||||
Est, estimate; *, indicator variable.
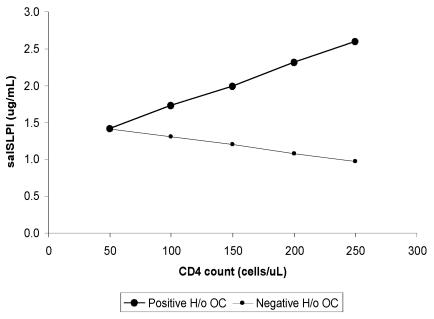
A significant interaction between low CD4 count and oropharyngeal candidiasis experience was detected in the linear regression model predicting the salivary SLPI value as a continuous variable (P = 0.004, Table 2). The graph for the interaction is shown in Fig. 2. According to this linear regression model, salivary SLPI levels among participants with a positive history of oropharyngeal candidiasis are predicted to increase with increasing CD4 counts. The opposite trend of lower salivary SLPI level with increasing CD4 count, however, is predicted among those without a history of oropharyngeal candidiasis. These results support the odds ratio heterogeneity between salivary SLPI strata identified in the bivariate analyses described above and suggest that salivary SLPI levels are modified by immune status and candidal experience.
FIG. 2.
Salivary SLPI (salSLPI) levels from the linear regression model were plotted for participants with and without a positive oropharyngeal candidiasis history, with CD4 count as a continuous variable, to demonstrate the interaction between CD4 cell count and history of oropharyngeal candidiasis (H/o OC) as related to salivary SLPI level.
Logistic regression models were also used to predict salivary SLPI as a dichotomous outcome (high versus low, with 2.1 μg/ml as the cutoff). As shown in Table 3, viral load in plasma, use of antifungal medication, and smoking were not significant factors and were removed to develop the final model. The interaction term P value just reached the alpha value of 0.05. We retained the term in the final model because interaction tests are known to have low power (36), and the P value came to the statistical threshold of 0.05.
TABLE 3.
Logistic regression model for predicting dichotomous salivary SLPI outcome with cutoff at 2.1 μg/mla
| Parameter | Full model
|
Final model
|
||||
|---|---|---|---|---|---|---|
| Est | SE | P | Est | SE | P | |
| Intercept | −1.82 | 0.83 | 0.02 | −1.87 | 0.54 | 0.0005 |
| CD4 count (cells/ml) | ||||||
| <200 | 1.27 | 1.09 | 0.96 | 0.99 | ||
| ≥200 | Refb | Ref | ||||
| History of candidiasis | ||||||
| Yes | 2.02 | 0.75 | 1.78 | 0.69 | ||
| No | Ref | Ref | ||||
| CD4 count and history of candidiasis | ||||||
| <200/ml, yes | −2.61 | 1.30 | 0.04 | −2.25 | 1.19 | 0.05 |
| ≥200/ml, no | Ref | Ref | ||||
| Antifungal medicine | ||||||
| Yes | 0.79 | 0.13, 4.96 | 0.80 | |||
| No | Ref | |||||
| Viral load (copies/ml) | ||||||
| <20,000 | 1.94 | 0.57, 6.53 | 0.28 | |||
| ≥20,000 | Ref | |||||
| Smoking | ||||||
| Former | 0.56 | 0.12, 2.63 | 0.46 | |||
| Current | 0.41 | 0.11, 1.57 | 0.19 | |||
| Never | Ref | |||||
Values are estimates (Est) and standard error for the first four parameters and odds ratios and 95% confidence intervals for the last three parameters. In the evaluation of the interaction between oral candidiasis experience and CD4 cell count, ICR = RR11 − RR10 − RR01 + 1; therefore, ICR = 1.64 − 2.62 − 5.94 + 1 = −5.92. The odds ratios for the final model (adjusted estimates) were: positive history and CD4 count of <200/μl, 1.64; positive history and CD4 count of ≥200/μl, 5.94; negative history and CD4 count of <200/μl, 2.62; and negative history and CD4 count of ≥200/μl, 1.00 (Ref).
Ref, reference.
Further examination of the interaction between candidal experience and CD4 count (Table 3) revealed that participants with a positive history of oropharyngeal candidiasis and higher CD4 counts were six times as likely to have a high salivary SLPI level as those with no history of oropharyngeal candidiasis and high CD4 count (odds ratio = 5.94), supporting the model shown in Fig. 2. With a positive history of oropharyngeal candidiasis and low CD4 cell count as the two exposure groups, the interaction of candidal status with CD4 cell count was significant: ICR = 1.64 − 2.62 − 5.94 + 1.00 = −5.92 (i.e., subadditive). Therefore, the risks for individual exposures do not add up mathematically to the risk for the doubly exposed. In biological terms, this finding suggests that other mechanisms might be involved in completely explaining relationships among CD4 cell count, history of oropharyngeal candidiasis, and salivary SLPI.
Effects of salivary SLPI on history of oropharyngeal candidiasis.
Although our primary outcome variable of interest was salivary SLPI, we also wanted to determine the usefulness of a dichotomous high/low salivary SLPI categorization as an explanatory variable for positive history of oropharyngeal candidiasis as an outcome and control for confounding. Thus, unconditional logistic regression models were developed, modeling the probability of having a positive history of oropharyngeal candidiasis. As shown in Table 4, high salivary SLPI level, low CD4 count, anti-HIV monotherapy, and smoking were significant contributors to an oropharyngeal candidiasis episode. Here, too, we found the interaction explained earlier involving salivary SLPI, oropharyngeal candidiasis experience, and CD4 count, in that the relationship between CD4 count and history of oropharyngeal candidiasis was significantly modified by the salivary SLPI level. The ICR of −21.84 suggests subadditivity and supports the earlier finding that other factors likely influence the relationship among salivary SLPI, CD4 count, and history of oropharyngeal candidiasis.
TABLE 4.
Logistic regression model for predicting the probability of positive history of oral candidiasisa
| Parameter | Estimate | SE | Odds ratio | 95% confidence interval | P |
|---|---|---|---|---|---|
| Intercept | −2.31 | 0.91 | 0.01 | ||
| CD4 (<200 cells/μl = 1; >200 cells/μl = 0) | 2.34 | 0.70 | |||
| Anti-HIV medication (monotherapy = 1; none = 0) | −2.01 | 0.86 | 0.13 | 0.03, 0.72 | 0.01 |
| Anti-HIV medication (combination therapy = 1; none = 0) | 0.93 | 0.65 | 2.53 | 0.71, 9.03 | 0.15 |
| Smoking (ever = 1; never = 0) | 1.53 | 0.76 | 4.62 | 1.05, 20.37 | 0.04 |
| Salivary SLPI (<2.1 μg/ml = 0; ≥2.1 μg/ml = 1) | 3.00 | 0.96 | |||
| Salivary SLPI and CD4 cell count | −3.31 | 1.47 | 0.02 |
In the evaluation of the interaction between salivary SLPI level and CD4 cell count group, ICR = RR11 − RR10 − RR01 + 1; therefore, ICR = 7.61 − 10.37 − 20.08 + 1 = −21.84. The odds ratios for the final model (adjusted estimates) were salivary SLPI >2.1 μg/ml and CD4 count <200/μl, 7.61; salivary SLPI >2.1 μg/ml and CD4 count >200/μl, 20.08; salivary SLPI <2.1 μg/ml and CD4 count <200/μl, 10.37; and salivary SLPI <2.1 μg/ml and CD4 count >200/μl, 1.00 (reference).
DISCUSSION
In this first investigation of potential antifungal activity by SLPI in vivo, we examined the associations between salivary SLPI and viral, immunologic, and oral health parameters within an HIV-1-infected population. High Candida carriage has been demonstrated among HIV-1-positive persons (4, 11, 12), and oropharyngeal candidiasis is found in 95% of AIDS patients (27). Although our study did not determine carriage rates, we used a history of oropharyngeal candidiasis as a surrogate measure for high candidal carriage. Given the low prevalence of clinically apparent oropharyngeal candidiasis lesions among study participants, the strategy allowed reasonable power to detect meaningful associations between salivary SLPI and history of oropharyngeal candidiasis. In our study, participants with a positive history of oropharyngeal candidiasis had significantly higher levels of salivary SLPI than participants without an oropharyngeal candidiasis episode (P < 0.05). Positive history of oropharyngeal candidiasis and high CD4 count were predictive of salivary SLPI production regardless of whether salivary SLPI was evaluated as a continuous or dichotomous outcome. In additional analyses, salivary SLPI was a key explanatory variable for history of oropharyngeal candidiasis, suggesting that the mucosal protein may serve as an indicator of candidal status in immunocompromised individuals. Together with the reported in vitro antifungal activity of SLPI (42), the data support a role for salivary SLPI in the local oral defense against C. albicans.
It is intuitively appealing to expect higher salivary SLPI levels among participants without an oropharyngeal candidiasis history based on SLPI's candidicidal activity in vitro (42). Due to our study design, we could not identify the temporal association between salivary SLPI elevation and development of oropharyngeal candidiasis. In our study, it is likely that salivary SLPI production was upregulated in response to oropharyngeal candidiasis infection in an attempt to inactivate the pathogenic microorganisms and resolve the clinical disease. Despite elevated salivary SLPI levels, which may be limited physiologically to an individualized threshold, the oral defenses were overwhelmed by the fungal infection, and clinical disease was recognized by participants who reported a positive history of oropharyngeal candidiasis. Furthermore, participants in the test group had already been exposed to Candida organisms at the time of saliva collection. Therefore, it is likely that their salivary SLPI values represent levels close to their individual thresholds. In such a situation, an increase in salivary SLPI is expected to be associated with greater odds of being a participant with previous or current oropharyngeal candidiasis.
The overall candidal burden and species of candidal microorganisms colonizing oral tissues may have altered the oral salivary SLPI levels. Because tests to confirm the presence and identity of microbes were not performed on the saliva specimens, we could not evaluate this hypothesis. This potentially important issue should be addressed in future studies.
Systemic and local cell-mediated immunity responses likely influence local salivary SLPI production and oral disease, underscoring the interplay between the innate and cell-mediated immune responses against C. albicans. Subclinical candidal infection may promote the maintenance of high salivary SLPI levels due to immune dysfunction in the oral cavity that alters salivary SLPI regulation such that a high level is maintained even after the infection is resolved. In support of this idea, immunohistochemical analyses have localized CD8+ T cells to the interface of the lamina propria and submucosa in oropharyngeal candidiasis lesions in HIV-infected individuals, a location that is distant from the outer layers of the epithelium where active Candida infection occurs (30). In an immune dysfunctional state, especially when CD4+ cell numbers are sharply reduced, CD8+ T cells may be important for host defense against oropharyngeal candidiasis (30). Also, the systemic immunosuppression characteristic of HIV-1 infection modifies the normal immune responses in the oral cavity, as evidenced by altered antibody and cytokine responses in saliva (3, 7, 23) and reduced numbers of Langerhans dendritic cells (26) and CD4+ T cells (30) in the oral mucosa of HIV-1-infected persons.
The relationship between salivary SLPI and history of oropharyngeal candidiasis persisted after adjustment for other potential confounding factors. The associations between oropharyngeal candidiasis, immunosuppression, and viremia are well documented (14, 15, 22, 34). In our study, participants with high salivary SLPI levels were more likely to have had an oropharyngeal candidiasis episode than those with low salivary SLPI levels after adjusting for CD4 count, antiretroviral medication, and smoking (Tables 2 and 3). These findings suggest that salivary SLPI is a previously unidentified independent indicator of oropharyngeal candidiasis history.
Linear regression modeling showed a significant interaction between immunosuppression and positive history of oropharyngeal candidiasis in predicting high salivary SLPI content. Among participants with a positive history of oropharyngeal candidiasis, the odds of having a high salivary SLPI level when mildly to moderately immunosuppressed was 3.6-fold higher than in those with severe immunosuppression (odds ratios, 5.94 versus 1.64, Table 3). In contrast, among participants without an oropharyngeal candidiasis experience, the odds ratio for having a high salivary SLPI level was twofold higher among those with CD4 counts below 200 cells/μl compared to those with counts above this threshold (odds ratios, 2.62 versus 1.00, Table 3). These results suggest that an intact immune system is needed to generate a strong salivary SLPI response among individuals with oral candidal disease but not in disease-free individuals. With history of oropharyngeal candidiasis as the outcome variable (Table 4), logistic regression modeling confirmed the interaction of salivary SLPI, candidal history, and CD4 count.
In an attempt to explain candidal experience in terms of CD4 count, salivary SLPI levels, and other important variables, we considered cigarette smoking as a potential factor in the models predicting salivary SLPI and history of oropharyngeal candidiasis. In a recent study of pulmonary oxidative stress, mice exposed to cigarette smoke exhibited a 50% reduction in SLPI antiprotease activity compared to unexposed mice (5). Although we found lower salivary SLPI levels among current smokers, the difference was not statistically significant, and smoking did not significantly contribute to the model for prediction of salivary SLPI as either a continuous or dichotomous outcome variable (Tables 2 and 3). However, smoking was associated with a fourfold increase in the odds of having a positive history of oropharyngeal candidiasis [odds ratio, 4.62 (1.05, 20.37), P = 0.04] even after adjusting for covariates, including salivary SLPI (Table 4). From these observations, we speculate that while cigarette smoke suppresses salivary SLPI levels (as among smokers who report no oropharyngeal candidiasis episode), exposure to Candida species may lead to an upregulation of salivary SLPI (as among smokers who report a positive history of oropharyngeal candidiasis) that may overcome the suppressive effects of smoking.
Our study suggests that upregulation of the anti-inflammatory protein contributes to the mucosal defense of oral tissues against Candida infection. It is possible that other inflammatory conditions or microbial infections coexisting with oropharyngeal candidiasis, such as periodontal or HIV-associated oral pathogens, could also trigger an increase in salivary SLPI. Therefore, future studies of SLPI function in the oral cavity should take into account possible confounding influences by competing disease groups and local inflammatory conditions that may influence salivary SLPI production.
Several salivary peptides and proteins, including the cationic peptides, histatins, defensins and the antimicrobial protein lactoferrin, inhibit C. albicans growth in vitro (25). Based on our current findings, SLPI joins the growing list of endogenous antifungal factors that may serve as potential therapeutic agents for preventing and/or treating candidal infections. More study is needed to determine the relative antifungal activities of these factors in oral secretions. Longitudinal investigation of salivary SLPI gene expression and protein production in patients with and without clinical oropharyngeal candidiasis will give further insight into the antimicrobial role of this protein in the oral cavity.
In conclusion, this study demonstrated that a history of oropharyngeal candidiasis is a strong independent predictor of salivary SLPI level in an HIV-1-positive population and that the salivary SLPI-oropharyngeal candidiasis relationship is modified by overall immune status. Elevated salivary SLPI production may be a defensive oral response to Candida attack in HIV-1-infected persons. Upregulation of the antimicrobial mucosal protein may serve as a biomarker for oropharyngeal candidiasis, particularly in immunosuppressed persons, and warrants further investigation.
Acknowledgments
We thank Stephanie Freel and Patrick Garrison for technical assistance, Dawn R. Porter for participant interviews, the UNC Core Retrovirology lab (Susan Fiscus, director) for blood processing and viral load testing, and Robert W. Ryder for guidance.
Funding support was provided by NIH/NIDCR grants (R01DE12162,R29DE11369, and 5T32DE07191) and the UNC Center for AIDS Research (NICHD/NIAID 9P30-AI50410).
Editor: T. R. Kozel
REFERENCES
- 1.Ashcroft, G. S., K. Lei, W. Jin, G. Longenecker, A. B. Kulkarni, T. Greenwell-Wild, H. Hale-Donze, G. McGrady, X. Y. Song, and S. M. Wahl. 2000. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat. Med. 6:1147-1153. [DOI] [PubMed] [Google Scholar]
- 2.Beppu, Y., Y. Imamura, M. Tashiro, T. Twoatari, H. Ariga, and H. Kido. 1997. Human mucus protease inhibitor in airway fluids is a potential defensive compound against infection with influenza A and Sendai viruses. J. Biochem. 121:309-316. [DOI] [PubMed] [Google Scholar]
- 3.Bradney, C. P., G. D. Sempowski, H. X. Liao, B. F. Haynes, and H. F. Staats. 2002. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J. Virol. 76:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campisi, G., G. Pizzo, M. E. Milici, and S. Mancuso. 2002. Candidal carriage in the oral cavity of human immunodeficiency virus-infected participants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 93:281-286. [DOI] [PubMed] [Google Scholar]
- 5.Cavarra, E., M. Lucatelli, F. Gambelli, B. Bartalesi, S. Fineschi, A. Szarka, F. Giannerini, P. A. Martorana, and G. Lungarella. 2001. Human SLPI inactivation after cigarette smoke exposure in a new in vivo model of pulmonary oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 281:L412-L417. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1998. Report of the NIH panel to define principles of therapy of HIV infection and guidelines for the use of antiretroviral agents in HIV infected adults and adolescents. Morbid. Mortal. Wkly. Rep. 47:1-82. [Google Scholar]
- 7.Challacombe, S. J., and S. P. Sweet. 2002. Oral mucosal immunity and HIV infection: current status. Oral Dis. 8(Suppl. 2):55-62. [DOI] [PubMed] [Google Scholar]
- 8.Draper, D., W. Donohoe, L. Mortimer, and R. P. Heine. 1998. Cysteine proteases of Trichomonas vaginalis degrade secretory leukocyte protease inhibitor. J. Infect. Dis. 178:815-819. [DOI] [PubMed] [Google Scholar]
- 9.EC Clearinghouse on Oral Problems Related to HIV Infection and World Health Organization Collaborating Center on Oral Manifestations of the Immunodeficiency Virus. 1993. Classification and diagnostic criteria for oral lesions in HIV infection. J. Oral Pathol. Med. 22:289-291. [PubMed] [Google Scholar]
- 10.Eisenberg, S. P., K. K. Hale, P. Heimdal, and R. C. Thompson. 1990. Location of the protease inhibitory region of secretory leukocyte protease inhibitor. J. Biol. Chem. 256:7976-7981. [PubMed] [Google Scholar]
- 11.Fetter, A., M. Partisani, H. Koenig, M. Kremer, and J. M. Lang. 1993. Asymptomatic oral Candida albicans carriage in HIV infection: frequency and predisposing factors. J. Oral Pathol. Med. 22:57-59. [DOI] [PubMed] [Google Scholar]
- 12.Fidel, P. L. Jr., and J. D. Sobel. 2002. Host defense against oral, esophageal, and gastrointestinal candidiasis, p. 179-192. In R. A. Calderone (ed.) Candida and candidiasis. American Society for Microbiology, Washington, D.C.
- 13.Glick, M., and M. A. Siegel. 1999. Viral and fungal infections of the oral cavity in immunocompetent patients. Infect. Dis. Clin. N. Am. 13:817-831. [DOI] [PubMed] [Google Scholar]
- 14.Gottfredsson, M., O. S. Indridason, G. M. D. de Almeida, A. E. Heald, and J. R. Perfect. 1999. Association of plasma levels of human immunodeficiency virus type 1 RNA and oropharyngeal Candida colonization. J. Infect. Dis. 180:534-587. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan, D., E. Komaroff, M. Redford, J. A. Phelan, M. Navazesh, M. E. Alves, H. Kamrath, R. Mulligan, C. E. Barr, and J. S. Greenspan. 2000. Oral mucosal lesions and HIV viral load in the Women’s Interagency HIV Study (WIHS). J. Acquir. Immune Defic. Syndr. 25:44-50. [DOI] [PubMed] [Google Scholar]
- 16.Grütter, M. G., G. Fendrich, R. Huber, and W. Bode. 1988. The 2.5 Å X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 7:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemstra, P. S. 2002. Novel roles of protease inhibitors in infection and inflammation. Biochem. Soc. Trans. 30:116-120. [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra, P. S., R. J. Maassen, J. Stolk, R. Heinzel-Wieland, G. J. Steffens, and J. H. Dijkman. 1996. Antibacterial activity of antileukoprotease. Infect. Immun. 64:4520-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hocini, H., P. Becquart, H. Bouhlal, H. Adle-Biassette, M. D. Kazatchine, and L. Belec. 2000. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin. Diagn. Lab. Immunol. 7:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-358. [DOI] [PubMed] [Google Scholar]
- 21.Klienbaum, D. J. 1994. Logistic regression—a self learning text. Springer-Verlag, New York, N.Y.
- 22.Koloktronis, A., A. Kioses, D. Antoniades, K. Mandraveli, I. Doutos, and P. Papanayotou. 1994. Immunologic status in individuals infected with HIV oral candidiasis and hairy leukoplakia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 78:41-46. [DOI] [PubMed] [Google Scholar]
- 23.Leigh, J. E., C. Steele, F. Wormley, and P. L. Fidel, Jr. 2002. Salivary cytokine profiles in the immunocompetent individual with Candida-associated denture stomatitis. Oral Microbiol. Immunol. 17:311-314. [DOI] [PubMed] [Google Scholar]
- 24.Leigh, J. E., C. Steele, F. L. Wormley, Jr., W. Luo, R. A. Clark, W. R. Gallaher, and P. L. Fidel, Jr. 1998. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373-380. [DOI] [PubMed] [Google Scholar]
- 25.Lupetti, A., R. Danesi, J. W. van't Wout, J. T. van Dissel, S. Senesi, and P. H. Nibbering. 2002. Antimicrobial peptides: therapeutic potential for the treatment of Candida infections. Expert Opin. Investig. Drugs 11:309-318. [DOI] [PubMed] [Google Scholar]
- 26.Maurer, D., and G. Stingl. 2001. Langerhans cells, p 35-50. M. In Lotze and A. W. Thomson (ed.), Dendritic cells, 2nd ed. Academic Press, London, England.
- 27.McCarthy, G. M. 1992. Host factors associated with HIV-related oral candidiasis: a review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 73:181-186. [DOI] [PubMed] [Google Scholar]
- 28.McGhee, J. R., M. E. Lamm, and W. Strober. 1994. Mucosal immune response - an overview, p. 485-506. In P. L. Ogra et al. (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 29.McNeely, T. B., M. Dealy, D. J. Dripps, J. M. Orenstein, S. P. Eisenberg, and S. M. Wahl. 1995. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 96:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers, T. A., J. E. Leigh, A. R. Arribas, S. Hager, R. Clark, E. Lilly, and P. L. Fidel, Jr. 2003. Immunohistochemical evaluation of T cells in oral lesions from human immunodeficiency virus-positive persons with oropharyngeal candidiasis. Infect Immun. 71:956-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlsson, M., U. Fryksmark, A. Polling, H. Tegner, and K. Ohlsson. 1984. Localization of antileukoprotease in the parotid and the submandibular salivary glands. Acta Otolaryngol. 98:147-151. [DOI] [PubMed] [Google Scholar]
- 32.Ohmit, S. E., J. D. Sobel, P. Schuman, A. Duerr, K. Mayer, A. Rompalo, R. S. Klein, and the HIV Epidemiology Research Study Group. 2003. Longitudinal study of mucosal Candida species colonization and candidiasis among human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect. Dis. 188:118-127. [DOI] [PubMed] [Google Scholar]
- 33.Patton, L. L. 2003. HIV disease. Dent. Clin. North Am. 47:467-492. [DOI] [PubMed] [Google Scholar]
- 34.Patton, L. L., R. G. McKaig, J. J. Eron, Jr., H. P. Lawrence, and R. P. Strauss. 1999. Oral hairy leukoplakia and oral candidiasis as predictors of HIV viral load. AIDS 13:2174-2176. [DOI] [PubMed] [Google Scholar]
- 35.Rice, W. G., and S. J. Weiss. 1990. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukocyte protease inhibitor. Science 249:178-181. [DOI] [PubMed] [Google Scholar]
- 36.Rothman, K. J., and S. Greenland. 1998. Modern epidemiology, 2nd ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 37.Sallenave, J. M., M. Si-Ta har, G. Cox, M. Chignard, and J. Gauldie. 1997. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J. Leukoc. Biol. 61:695-702. [DOI] [PubMed] [Google Scholar]
- 38.Shine, N., S. C. Wang, K. Konopka, E. A. Burks, N Düzgünes, and C. P. Whitman. 2002. Secretory leukocyte protease inhibitor: inhibition of human immunodeficiency virus-1 infection of monocytic THP-1 cells by a newly cloned protein. Bioorg. Chem. 30:249-263. [DOI] [PubMed] [Google Scholar]
- 39.Shugars, D. C., D. L. Sauls, and J. B. Weinberg. 1997. Secretory leukocyte protease inhibitor blocks infectivity of primary monocytes and mononuclear cells with both monocytotropic and lymphocytotropic strains of human immunodeficiency virus type I. Oral Dis. 3(Suppl. 1):S70-S72. [DOI] [PubMed] [Google Scholar]
- 40.Shugars, D. C., A. L. Alexander, K. Fu, and S. A. Freel. 1999. Endogenous salivary inhibitors of human immunodeficiency virus. Arch. Oral Biol. 44:445-453. [DOI] [PubMed] [Google Scholar]
- 41.Skott, P., E. Lucht, M. Ehnlund, and E. Björling. 2002. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral Dis. 8:160-167. [DOI] [PubMed] [Google Scholar]
- 42.Tomee, J. F. C., P. S. Heimstra, R. Heinzel-Wieland, and H. F. Kauffman. 1997. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J. Infect. Dis. 176:740-747. [DOI] [PubMed] [Google Scholar]
- 43.Wahl, S. M., T. B. McNeely, E. N. Janoff, D. Shugars, P. Worley, C. Tucker, and J. M. Orenstein. 1997. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 3(Suppl. 1):S64-S69. [DOI] [PubMed] [Google Scholar]
- 44.Wozniak, K. L., J. E. Leigh, S. Hager, R. K. Swoboda, and P. L. Fidel, Jr. 2002. A comprehensive study of Candida-specific antibodies in saliva of HIV-infected persons with oropharyngeal candidiasis. J. Infect. Dis. 185:1269-1276. [DOI] [PubMed] [Google Scholar]
- 45.Zhu, J., C. Nathan, W. Jin, G. S. Ashcroft, S. M. Wahl, L. Lacomis, H. Erdjument-Bromage, P. Tempst, C. D. Wright, and A. Ding. 2002. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111:867-878. [DOI] [PubMed] [Google Scholar]