Abstract
Purpose
The purpose of the present pilot study was to provide a preliminary estimate of the minimum detectable difference (MDD) and minimum clinically important difference (MCID) of the six-minute walk test (6MWT) and daily activity in outpatients with chronic heart failure (CHF).
Methods
A convenience sample of 22 adults with stable New York Heart Association Functional Class II and III CHF performed two baseline 6MWTs separated by 30 minutes of rest. Subjects then wore a triaxial accelerometer for 7 days to monitor daily activity. After 7 weeks of usual care, subjects again wore the accelerometer for 7 days and then returned to the clinic to complete the Global Rating of Change Scale (GRS) with regard to their heart disease and perform another set of 6MWTs. For the 6MWT, the MDD was calculated using the two baseline 6MWT distances. For daily activity, the MDD was calculated using two methods: (1) day-to-day test-retest reliability during baseline monitoring, and (2) baseline to follow-up test-retest reliability in those who reported no change on the GRS. The MCID for the 6MWT and daily activity was calculated using the mean and 95% confidence interval (CI95%) for those subjects who reported ‘improvement’ on the GRS.
Results
The MDD at the CI95% for the 6MWT was 32.4 meters. The MCID for the 6MWT was 30.1 (CI95% 20.8, 39.4) meters. The MDD for daily activity was 5,909 vector magnitude units (VMU·hr.−1) The MCID for daily activity was 1,337 VMU·hr.−1 There was good alignment of the MDD and MCID for the 6MWT, suggesting that clinically meaningful change is approximately 32 meters. However, the calculated MCID was substantially less than measurement error as represented by the MDD, indicating that the MCID was underestimated in this sample or that daily activity may be robust to change in overall disease status.
Key Words: six-minute walk test, daily activity, heart failure, clinically meaningful change
INTRODUCTION
Exercise intolerance is the most frequent and debilitating symptom in patients with chronic heart failure (CHF),1,2 and is both a cause and result of the cycle of physical inactivity and subsequent deconditioning.3,4,5,6,7,8,9,10,11 Rehabilitation interventions are directed at disrupting the perpetuation of this cycle. The effect of these interventions on deconditioning has been widely studied;12 however, only a limited number of studies have examined the effect of rehabilitation interventions on physical (daily) inactivity.9,13,14,15,16 Unfortunately, few studies have investigated how measures of deconditioning and daily activity change in relationship to one another, and even fewer have investigated what amount of change in these measures is considered to be clinically meaningful.
Measurement of daily activity can use a variety of instrumentation strategies, including questionnaires, measurement of energy expenditure, and activity monitors.17 Self-report questionnaires have repeatedly been shown to overestimate daily activity,18,19,20,21,22 including individuals with cardiac diseases.23 Measurement of energy expenditure with doubly-labeled water or metabolic gas exchange analysis, while most accurate, is not feasible for routine field testing.17 Activity monitors, however, provide reasonable estimates of daily activity and are easy to incorporate into study protocols. Activity monitors include pedometers, heart rate monitors, accelerometers, and integrated multi-sensors. Of these methods used for measuring daily activity, triaxial accelerometers appear to provide sufficient accuracy, feasibility, and subject adherence for measuring daily activity in patients with cardiac disease.18 However, there is also growing interest in the use of activity data generated by the single axis accelerometers contained in implanted cardiac defibrillators.24,25,26 These devices overcome difficulties of monitor wearing non-adherence and short monitoring periods to better capture changes in daily activity over greater periods of time. Despite the increasing interest in daily activity, its measurement by accelerometry, and 5 prior studies examining the impact of exercise on daily activity, no study to date has determined what amount of change in daily activity is considered to be meaningful or sufficient to interrupt the cycle of inactivity and deconditioning.
The six-minute walk test (6MWT) is a commonly used outcome measure in both the clinical and research settings for patients with CHF.27 It is somewhat unclear if the construct represented by the 6MWT is a submaximal proxy for maximal, graded exercise tests that measure peak aerobic capacity or whether it represents a different construct such as functional exercise tolerance and capacity for completing activities of daily living (ADLs).27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 Positive correlations between 6MWT distance and peak oxygen consumption (0.57 to 0.88) have been demonstrated across a wide range of patients with CHF and chronic obstructive pulmonary disease (COPD),28,29,30,31,32,33,34,35 though some have proposed that the 6MWT might best be described as a measure of functional exercise tolerance that is more closely associated with ability to perform ADLs.36,37,38,39,40,41 The 6MWT has demonstrated good reliability [intraclass correlation coefficients (ICCs) of 0.82 to 0.99] in patients with CHF,29,35,42,43,44,45,46,47,48,49 and is likely to be highest when following a standardized procedure.27,50 Despite its widespread use as an outcome measure, only 3 studies45,46,48 have investigated and reported what amount of change is considered to be clinically meaningful among patients with CHF, and none of these studies included measures of daily activity.
The effect of rehabilitation on daily activity has been investigated in 5 studies. van den Berg-Emons et al9 studied the effect of 3 months of aerobic exercise training on daily activity measured by accelerometry. Despite improvements in factors known to be associated with daily activity, including strength,51 aerobic capacity,11 and 6MWT distance,11 no changes in daily activity were observed in the intervention group. Mueller et al13 reported on a 6-year period following cardiac rehabilitation, and found no difference in a daily activity questionnaire between the exercise and control groups. Gottlieb et al14 found no change in daily activity measured by a uniaxial accelerometer following a 6-month exercise program despite improvements in exercise tolerance measured using cardiopulmonary exercise testing and the 6MWT. Willenheimer et al15 noted an insignificant trend of decreased daily activity despite a 4-month exercise program, though the decrease was not of the same magnitude as that of the control group. Only Witham et al16 found significant improvements in daily activity measured by accelerometry following a 3-month seated exercise program. Interestingly, they did not find any improvement in 6MWT distance. The 3 studies9,14,16 that measured changes in both the 6MWT and daily activity in the same sample demonstrated conflicting results with regard to how each measure changed in relationship to the other, and no study commented on whether the observed changes were clinically meaningful. It is, therefore, not only uncertain as to how both the 6MWT and daily activity change in relationship to each other in response to intervention and/or disease progression, it is also unclear what amount of change in these measures is clinically meaningful.
Foundational to the planning and interpretation of clinical trials is the knowledge of what amount of change in an outcome measure is clinically meaningful. Two common methods for determining clinically meaningful change are the minimum detectable difference (MDD) and the minimum clinically important difference (MCID). Each represents a different construct in that the MDD reflects measurement error and the MCID reflects perceptible change. The MDD represents the amount of change that must be achieved to reflect a ‘true’ change in clinical status that exceeds the measurement error inherent in a given instrument.52 Although the MDD is a statistical calculation based on the reproducibility or test-retest reliability of an instrument, it assumes that exceeding this threshold should reflect a change that is clinically meaningful. Alternately, the MCID is the smallest change that a patient would perceive as beneficial.53,54 Two broad methods for calculating perceptible change include distribution-based and anchor-based methods.54 Distribution-based methods include calculation of an effect size that provides a ratio of change in an outcome to the baseline standard deviation of that measure for interpreting whether the effect observed in a given trial was statistically significant, while anchor-based methods determine the mean amount of change associated with subjects’ perceived level of change using the Global Rating of Change Scale (GRS).
Meaningful change in daily activity in individuals with CHF has only been investigated by Shoemaker et al,55 who calculated an MCID of 0.5 and 1.0 hours per day for deterioration and improvement, respectively, in patients with implanted cardiac defibrillators. Daily activity was measured using the single axis accelerometer contained within the implanted device, and the results were only generalizable to similar individuals with similar devices. With regard to the 6MWT, only Opasich et al46and Pinna et al48 have calculated and reported the MDD for the 6MWT in a single sample of patients with CHF, which ranged from 39 m to 55 m depending on the confidence level and number of trials used (eg, 45 m using a 95% confidence interval and two trials). Using a 7-point, patient-rated GRS, O'Keeffe et al45 estimated the 6MWT anchor-based MCID to be 24 m and 43 m, respectively, for those who deteriorated or improved. O'Keefe et al45 also estimated the 6MWT distribution-based MCID to reflect an effect size of 2.13 and .85, respectively, for those who deteriorated or improved. The anchor-based MCID reported by Spertus et al56 used a 15-point, clinician-rated GRS collapsed into 7 categories: no change or large, moderate, and small improvement or decline. They found that mean 6MWT distances for moderate decline or improvement were 90 m and 55 m, respectively. More recently, a systematic review by Shoemaker et al57 calculated the MDD based on reported ICCs across 13 studies. The mean MDD was 43 m with a wide range of 20 m to 151 m.
In summary, no study has examined the MDD and MCID for the 6MWT in the same sample; and therefore, the degree of agreement between these two measurement strategies for determining clinically meaningful change is not known. Additionally, only one study has provided an estimate of meaningful change in daily activity, and was unique to measurement of individuals with implanted cardiac defibrillators. Therefore, the purpose of the present pilot study was to provide a preliminary estimate of the MDD and MCID for the 6MWT and daily activity in outpatients with CHF to assist in planning a randomized clinical trial to improve daily activity in this population.
METHODS
Design
This 8-week observational study measured the 6MWT and daily activity at baseline and 8 weeks later. Subjects were instructed to maintain their usual routine. At the beginning of the final clinic visit following completion of the 8-week observation period, subjects were interviewed about significant health events in the preceding 8 weeks, including hospitalizations and physician office visits, which were checked against the subject's medical record. Subjects also completed the GRS46,49 (Figure 1) which asked them to rate the amount of perceived change in their condition since enrolling in the study. The GRS and the medical record review of patient status served as the anchor for determining change when calculating the MCID (perceptible change).
Figure 1.
Global Rating of Change Scale.
Subjects
A convenience sample (n=22) was recruited from a tertiary care outpatient heart failure clinic. To be included in the study, subjects must: (1) have been >40 years old, (2) have had echocardiographic evidence of left ventricular systolic dysfunction [left ventricular ejection fraction (LVEF) < 40%], (3) have been clinically stable on optimized medical therapy, (4) have rated themselves as New York Heart Association-Functional Class (NYHA-FC) II or III, and (5) have had sufficient mental capacity to be able to accurately report any changes in symptoms or functional status. Subjects were excluded if their functional limitations were primarily due to noncardiovascular causes. The study was approved by the human subjects review committees at Western Michigan University and Spectrum Health.
Measurements Six-minute walk test
The 6MWT was administered on a 100-foot hallway course, with standardized instructions, encouragement, and monitoring as recommended in the American Thoracic Society guidelines.42 Following a 30 minute rest period,49 the test was repeated. The test-retest distances at baseline formed the basis of the MDD for the 6MWT, and the longest distance walked at baseline and follow-up served as the basis for the anchor-based MCID to reflect the subject's best effort. The investigator conducting the 6MWT at follow-up was blinded to the baseline 6MWT distance.
Accelerometer-based measures of daily activity
Triaxial accelerometers, such as the RT3 (Stayhealthy, Inc., Monrovia, CA) are small devices the size of a pager that are worn on the hip to detect movement/vibration in 3 orthogonal planes (X, Y, and Z). This movement/vibration is recorded and averaged in one minute epochs with date and time of day labels. ‘Activity counts’ are recorded by the RT3 as a summed vector magnitude unit (VMU, calculated by √(X2+Y2+Z2)), and are the measurement proxy for daily activity. Triaxial accelerometers have a higher correlation than uniaxial accelerometers (such as a pedometer) to activity intensity as measured by oxygen consumption and heart rate in children,58,59 and have demonstrated high intraclass correlation coefficients (.97) in elderly nursing home residents.60 The RT3 has demonstrated good intra- and intermonitor reliability using a vibration table with an ICC of .99,60 as well as good intermonitor reliability measured using laboratory-based daily activity with coefficients of variation ranging from 6% to 14%.61
Each subject wore the same RT3 accelerometer on the dominant hip during waking hours for 7 days in the first and seventh weeks. The 7-day monitoring period was chosen because it has been shown to more accurately depict weekly routines and reduce the likelihood of a significant ‘Hawthorne Effect.’18,62,63 The same monitor was used for baseline and follow-up for each subject to minimize error that might result from minor differences in individual monitors.62
Prior to statistical analysis, the RT3 data were ‘cleaned’63 by detecting and eliminating non-wearing periods and data generated while in a moving vehicle,63,64 which may overestimate daily activity by up to 16%.63,65 Subjects were asked to keep a log to record significant events, periods of not wearing the monitor, as well as days/times of traveling in a motor vehicle. Daily activity data from the RT3 (mean VMU·hr−1) were used to calculate the MDD using two different approaches: (1) day-to-day test-retest reliability during the baseline period for all subjects, and (2) baseline to follow-up test-retest reliability in those subjects who reported “about the same” on the GRS. Daily activity data from baseline and follow-up in those who reported “improvement” was used to calculate the anchor-based MCID. The investigator preparing the follow-up activity data for analysis was blinded to the baseline activity data values for each subject. Although there are potential confounding variables with longer test-retest intervals, previous studies investigating the MCID for the 6MWT have included test-retest intervals of 4 weeks46 up to one year47 in those subjects who reported no change from baseline.
Global Rating Scale
The GRS was administered at the beginning of the follow-up visit. It asked subjects to rate the amount of change in their heart disease over the two month study period (see Figure 1).46,66 Due to the small sample size, the GRS was dichotomized into ‘improved’ and ‘not improved’ using the dichotomization strategy portrayed in Figure 1.
Statistical Analysis
Descriptive statistics for subjects were calculated for the overall group, as well as for those reporting “improvement” and “no improvement” in heart disease over the study period. The two groups were compared using independent t-tests for baseline characteristics.
The MDD at the 95% confidence interval (MDD95%) was calculated as follows67: MDD95%= z * SEM * √2 where z = 1.96 and SEM (standard error of measurement) = σbaseline √(1-ICC). The repeated measures two-way, random effects, single measure agreement definition ICC (2,1) was calculated using SPSS 16.0 for the two baseline 6MWT distances and for daily activity according to the aforementioned day-to-day and baseline to follow-up strategies.
The MCID for the 6MWT was calculated using the mean and 95% confidence interval of the difference between the longest baseline and follow-up 6MWT distances for those who reported “improvement” or “no improvement” using the aforementioned GRS dichotomization strategy. The MCID for daily activity as measured by accelerometry was determined by calculating the mean and 95% confidence interval of the change in VMU·hr−1 between baseline and follow-up for those who reported “improvement” and “no improvement” on the GRS.
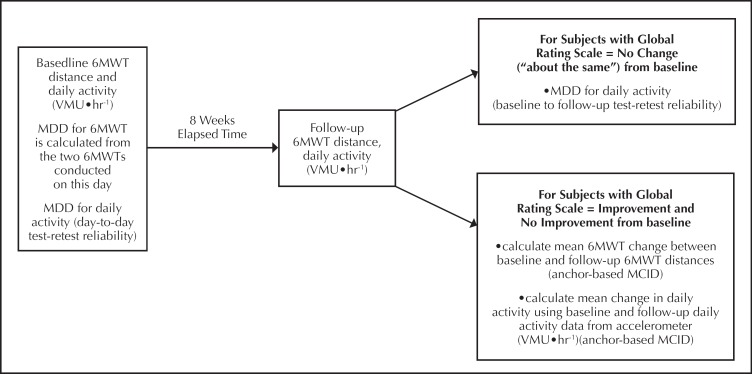
The relationship between the 6MWT and daily activity baseline and change values was assessed using the Pearson correlation coefficient. Figure 2 provides a graphic depiction of the timing of measurements and the statistical analysis.
Figure 2.
Overview of study design. bbreviations: 6MWT, six-minute walk test; MDD, minumum detectable difference; MCID, minimum clinically important difference
RESULTS
A total of 22 subjects were enrolled in the study. Two subjects failed to complete the study protocol; one due to voluntary withdrawal because of problems with transportation to the clinic, and the other due to death unrelated to participation in the study protocol. Another two subjects were hospitalized during the 8-week interim study period; one for acute renal failure and the other for pneumonia. Subject compliance with wearing the RT3 activity monitor was excellent with a mean (standard deviation) of 6.64 (.95) days and 12.8 (2.5) hours per day. Table 1 provides a summary of overall subject characteristics as well as characteristics stratified by the dichotomized GRS responses. On average, 6MWT distances increased by 5 m to 8 m on the second trial. Anecdotally, all subjects reported that 30 minutes was a sufficient rest period between trials. There were no statistically significant differences in baseline characteristics between those reporting “improvement” and “no improvement” except for left ventricular ejection fraction (LVEF) and NYHA-FC, with those reporting “improvement” being younger (p=.034) and having a lower NYHA-FC (p = .044).
Table 1.
Descriptive Statistics
| All Subjects (N=22) | Subjects Reporting “No Improvement” (N=8) | Subjects Reporting “Improvement” (N=12) | |
|---|---|---|---|
| Age | 62.5 (12.6) | 70.6 (14.0) | 58.3 (10.3) |
| LVEF | 25.5 (9.1) | 25.8 (12.7) | 25.4 (8.0) |
| NYHA-FC | 2.4(.45)
|
2.6 (.5) | 2.2 (.3) |
| All Medications (#) | 11.7 (4.6) | 12.8 (2.3) | 9.9 (5.1) |
| 6MWT (m) Trial #1 | 347.1 (95.4) | 342.5 (94.2) | 375.8 (75.6) |
| Trial #2 | 351.3 (98.3) | 350.0 (88.7) | 380.7 (83.7) |
| Daily Activity (VMU·hr−1) | 8,527.3 (4,468.9) | 9, 115.3 (5,309.3) | 9,128.8 (3,615.8) |
| Global Rating Scale | 12 reported “improvement” | ||
8 reported “no improvement”
|
Data are presented as mean (standard deviation)
Abbreviations: LVEF, left ventricular ejection fraction; NYHA-FC, New York Heart Association Functional Classification; 6MWT, six-minute walk test; VMU, vector magnitude units
The ICC (2,1) for the 6MWT was 0.99 (95% confidence interval = 0.97, 0.99) demonstrating excellent test-retest reliability. The MDD95% for the 6MWT was 32.4 m. Regarding the anchor-based MCID for the 6MWT, the mean change in 6MWT from baseline to follow-up for those reporting “improvement” in heart disease was 30.1 (95% confidence interval 20.8 to 39.4) m and was statistically significantly different than the −9.4 (95% confidence interval −31.8 to 13.1) m for those reporting “no improvement” (p = .004).
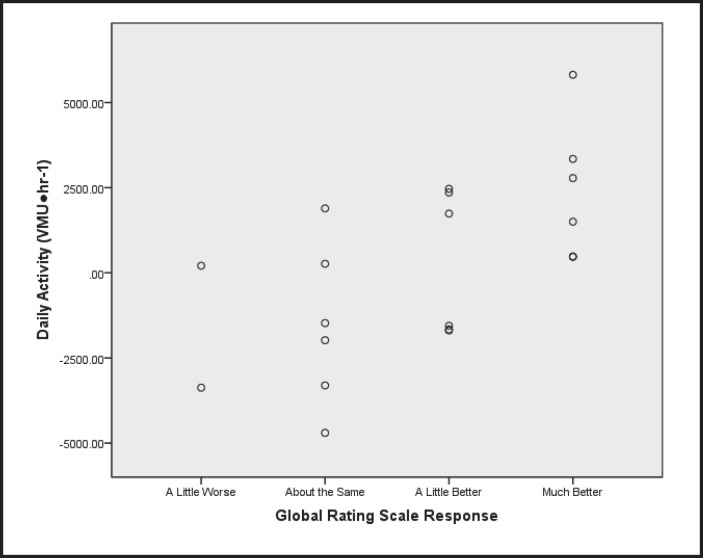
The ICC (2,1) for the RT3 accelerometer-based measure of daily activity for day-to-day test-retest reliability at baseline across all subjects was .68 (95% confidence interval = 0.51, 0.84) with a resulting MDD95% of 5,908.8 VMU·hr.−1 The ICC (2,1) for baseline to follow-up test-retest reliability in those subjects reporting “about the same” on the GRS was 0.86 (95% confidence interval = 0.36, 0.98), with a resulting MDD95% of 5,819.9 VMU·hr.−1 Regarding the anchor-based MCID for the RT3-based daily activity, the mean change in daily activity from baseline to follow-up for those reporting “improvement” was 1,337.4 (95% confidence interval −105.1 to 2,779.9) VMU·hr−1 and was statistically significantly different than the −1,558.0 (95% confidence interval −3,420.2 to 304.2) VMU·hr-1 for those reporting “no improvement.” Figure 3 depicts the scatterplot of daily activity across all levels of the GRS.
Figure 3.
Association between change in daily activity and Global Rating Scale response.
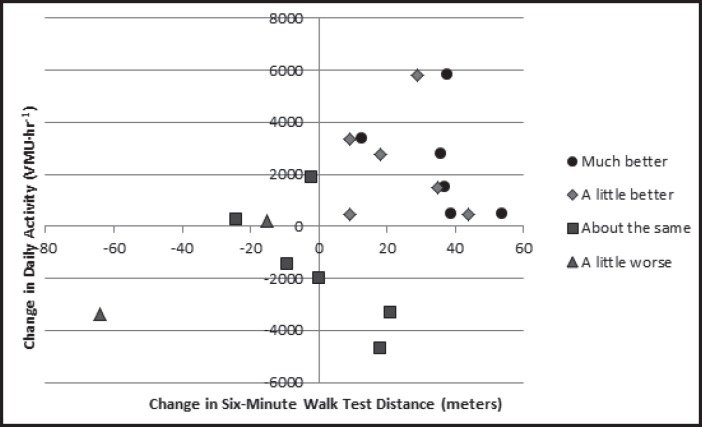
The correlation between the baseline 6MWT and RT3-based daily activity values was moderate (0.59, p = .004); however, there was a lower correlation of 0.44 (p=.05) for the change values in these variables. When examining the results graphically, those reporting no change in heart disease from baseline to follow-up appear to have a negative linear association between changes in 6MWT and daily activity (Figure 4).
Figure 4.
Association between changes in six-minute walk test distance and daily activity based on Global Rating Scale responses.
DISCUSSION
The present study served as a pilot project to investigate the alignment of two methods for estimating clinically meaningful change in the 6MWT and an accelerometer-based measure of daily activity to assist in planning a randomized clinical trial to improve daily activity in individuals with CHF. The results from the present study also provide insight into the relationship between changes in 6MWT performance and daily activity over time. For the 6MWT, there was good alignment of the MDD95% and MCID, suggesting that a change of approximately 30 m to 32 m can be considered to be clinically meaningful. The results for daily activity, however, were not as clear. The mean change in daily activity for those subjects with a GRS response indicating improvement in their heart disease over the two-month study period was substantially less than the MDD95%. Finally, change in 6MWT performance did not appear to be strongly related to change in daily activity.
First, with regard to the 6MWT, the MDD95% calculated on this small sample was 32 m. This is lower than the MDD95% of 45 m calculated by Pinna et al48 and slightly lower than the 39 m calculated by Opasich et al46 using an average of trials when calculating the ICC. Four of the 13 MDD estimates calculated from reported ICCs by Shoemaker et al57 were under 35 m. Therefore, the MDD estimate in the present study is consistent with the existing literature. The MCID calculated based on a dichotomization of the GRS into “improvement” and “no improvement” in the present study was 30 m. This threshold is between the 24 m and 43 m for those subjects who had deteriorated or improved, respectively, in the study by O'Keeffe et al.45 However, Spertus et al56 reported substantially larger MCID estimates of 55 m and 90 m for those who improved or declined, respectively. They used a clinician-based anchor using a 15-level GRS collapsed to 7 levels for data analysis and found that the mean change in 6MWT for small improvement or decline was not significantly different than the mean change for those for whom the clinicians believed did not change. Unfortunately, they did not report their schema for collapsing the 15 levels, and it is, therefore, not clear whether their attempt at a finer discrimination of change resulted in mis-categorizing those with a small amount of change as being unchanged. This would result in a higher MCID threshold. Therefore, excluding Spertus et al,56there not only appears to be excellent agreement between the results of the present study to two prior studies46,47 and 4 other estimates calculated in a systematic review57 but there also appears to be excellent alignment of a threshold for meaningful change in the 6MWT using two different constructs-the MDD95% and the anchor-based MCID. This suggests that changes of greater 32 m likely represent change that exceeds both the measurement error of the 6MWT and that which is perceptible by the individual.
With regard to daily activity, the results of this study are less clear. The MDD95% for the RT3-based measure of daily activity was calculated to be approximately 5,900 VMU·hr-1 using two different methods. This value is over two times greater than the mean change of 1,337 VMU·hr-1 in those subjects reporting improvement in heart disease on the GRS. This is problematic because it implies that perceptible change in the instrument (MCID) is far less than the instrument's measurement error (MDD). It does appear that both the measurement error calculated in this study is accurate and that subjects accurately responded to the GRS. The MDD95% determined by the present study is very close to the 5,829 VMU·hr-1 calculated based on data from patients with COPD reported by Hecht et al.63 Furthermore, based on the results of the 6MWT in the present study, subjects appeared to be able to accurately respond to the GRS. Visual analysis of the scatterplot of change in daily activity based on GRS response reveals that those reporting “much better” on the GRS appeared to consistently have positive changes in daily activity; however, 3 of the subjects reporting feeling “a bit better” had declines in daily activity that lowers the MCID threshold. This is a limitation of the anchor-based MCID and is the reason that an external objective criterion against which the threshold is compared is recommended.66 In the present study, the objective external criterion of the MDD suggests that the present study considerably underestimated the MCID. It is also possible that daily activity may be robust to change in overall disease status; significant changes in daily activity may only be apparent in subjects with major changes in CHF disease status.
Unfortunately, no prior study has used the GRS to determine the MCID for triaxial accelerometer-based daily activity in subjects with CHF to allow for direct comparison to the results of the present study. However, Witham et al16 demonstrated statistically significant improvement in RT3-based measures of daily activity of 18% in an exercise intervention group compared to a 13% decline in the control group. Applying those percentages to the subjects in the present study results in an expected change of approximately 1,535 VMU·hr-1 for the mean baseline daily activity for all subjects, which is substantially lower than the MDD, but is comparable to the mean amount of change for the subjects reporting improvement in the present study. This would suggest that the statistically significant changes in daily activity found by Witham et al16 may not have been clinically meaningful in light of the MDD determined by the present study.
Finally, with regard to how the 6MWT and daily activity change in relationship to each other, the results of the present study suggest a complex interaction. Correlation coefficients for accelerometer-based measures of daily activity and baseline 6MWT performance have previously been shown to be between 0.43 and 0.68.16,19,25,68 This is highly consistent with the present study. However, despite the correlation between 6MWT and daily activity measured at a given point in time, the association between changes in both variables is much weaker. As seen in Figure 4, there appears to be a weak positive linear association between the two variables in those subjects reporting a change from baseline and a negative linear association for those reporting “about the same.” It is important to note that the 6MWT is a measure of performance, and daily activity is a measure of behavior, which may explain the discrepant results noted by previous authors9,14,16 with regard to changes in these variables in response to exercise intervention. Daily activity, as a behavior, is multifactorial. Unfortunately, the present study did not attempt to capture potential confounding factors for change in daily activity such as self-efficacy, depression, anxiety, and feeling disabled.3,51
Limitations
There are several limitations to the present study. First, the MDD and MCID for daily activity were calculated with somewhat overlapping methods. For example, one of the methods for determining the MDD was based on baseline and follow-up data in those subjects who reported being “about the same” as opposed to being calculated based on two separate, closely approximated baseline observation periods. The authors felt that including a second baseline monitoring period would have detracted from subject recruitment. Therefore, the MDD calculated based on day-to-day test-retest reliability was used and was in excellent agreement with the other method.
The second potential limitation is that of seasonal changes confounding daily activity changes from baseline to follow-up,69 with activity levels being approximately 10% lower during the winter months. Three subjects were recruited mid-fall where the follow-up might be influenced by the onset of the winter season. However, all 3 of these subjects demonstrated an increase in activity upon follow-up. It is not known whether the increase in activity was of a lower magnitude than if the follow-up was not during a different season.
The third potential limitation is that confounding variables such as depression that might explain discrepancies between changes in 6MWT and daily activity were not measured. The fourth limitation is that the GRS was dichotomized into “improvement” or “no improvement” and did not allow for determination of the anchor-based MCID for decline; however, the purpose of determining an MCID for this pilot study was to prepare for a randomized trial that hypothesizes improvement as a result of intervention.
Finally, although the subjects included in this study are representative of the majority of individuals with moderate-to-severe CHF based on age, LVEF, NYHA-FC, and 6MWT distances compared to those reported in the systematic review by Shoemaker et al,57 the small sample size of this pilot study limits generalizability of these results.
CONCLUSIONS
There was good alignment of the MDD95% and MCID for the 6MWT in individuals with CHF, suggesting that a change of 30 m to 32 m is required to be considered clinically meaningful. However, there was not good alignment of the MDD95% and MCID for accelerometer-based daily activity. The calculated MCID was substantially less than measurement error as represented by the MDD, indicating that the MCID was underestimated in this sample or that daily activity may be robust to change in overall disease status. Additionally, it remains unclear how the 6MWT and daily activity change in relationship to each other.
ACKNOWLEDGEMENTS
This paper was completed in partial fulfillment of the PhD in Interdisciplinary Health Sciences at Western Michigan University. This study was funded in part by the Blue Cross Blue Shield of Michigan Foundation.
REFERENCES
- 1.Gordan A, Tyni-Lenne R, Persson H, Kauser L, Hultman E, Sylven C. Markedly improved skeletal muscle function with local muscle training in patients with chronic heart failure. Clin. Cardiol. 1996;19:568–574. doi: 10.1002/clc.4960190709. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 3.Oka RK, Gortner SR, Stotts NA, Haskell WL. Predictors of physical activity with chronic heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:159–163. doi: 10.1016/s0002-9149(96)90588-3. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg-Emons H, Bussmann J, Balk A, et al. Level of activities associated with mobility during everyday life in patients with chronic congestive heart failure as measured with an “Activity Monitor.”. Phys Ther. 2001;81:1502–1511. [PubMed] [Google Scholar]
- 5.Davies SW, Jordan SL, Lipkin DP. Use of limb movement sensors as indicators of the level of everyday physical activity in chronic congestive heart failure. Am J Cardiol. 1992;69:1581–1586. doi: 10.1016/0002-9149(92)90707-6. [DOI] [PubMed] [Google Scholar]
- 6.Hoodless DJ, Stainer K, Savic N, et al. Reduced customary activity in chronic heart failure: assessment with a new shoe-mounted pedometer. Int J Cardiol. 1994;43:39–42. doi: 10.1016/0167-5273(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JT, Andrews R, Evans A, Cowley AJ. Failure of “effective” treatment for heart failure to improve customary activity. Br Heart J. 1995;70:373–376. doi: 10.1136/hrt.74.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toth MJ, Gottlieb SS, Goran MI, et al. Daily energy expenditure in free-living heart failure patients. Am J Physiol. 1997;272:E469–E475. doi: 10.1152/ajpendo.1997.272.3.E469. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg-Emons R, Balk A, Bussmann H, Stam H. Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur J Heart Fail. 2004;6:95–100. doi: 10.1016/j.ejheart.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Oka RK, Stotts NA, Dae MW, et al. Daily physical activity levels in congestive heart failure. Am J Cardiol. 1993;71:921–925. doi: 10.1016/0002-9149(93)90907-t. [DOI] [PubMed] [Google Scholar]
- 11.Witham MD, Argo IS, Johnston DW, Struthers AD, McMurdo MET. Predictors of exercise capacity and everyday activity in older heart failure patients. Eur Heart J Failure. 2006;8:203–207. doi: 10.1016/j.ejheart.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Davies EJ, Moxham T, Rees K, Singh S, Coats AJS, Ebrahim S, Lough F, Taylor RS. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2010;4:CD003331. doi: 10.1002/14651858.CD003331.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil. 2007;21:923–931. doi: 10.1177/0269215507079097. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb SS, Fisher ML, Freudenberger R, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Cardiac Failure. 1999;5:188–194. doi: 10.1016/s1071-9164(99)90002-7. [DOI] [PubMed] [Google Scholar]
- 15.Willenheimer Rydberg E, Cline C, et al. Effects on quality of life symptoms, and daily activity 6 onths after termination of san exercise programme in heart failure patients. Int J Cardiol. 2001;77:25–31. doi: 10.1016/s0167-5273(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 16.Witham MD, Gray JM, Argo IS. Effect of a seated exercise program to improve physical function and health status in frail patients > 70 years of age with heart failure. Am J Cardiol. 2005;95:1120–1124. doi: 10.1016/j.amjcard.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Casburi R. Activity monitoring in assessing activities of daily living. COPD. 2007;4:251–255. doi: 10.1080/15412550701480158. [DOI] [PubMed] [Google Scholar]
- 18.Steele BG, Holt L, Belza B, et al. Quantitating physical activity in COPD using a triaxial acceleromter. Chest. 2000;117:1359–1367. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]
- 19.Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2005;86:1979–1985. doi: 10.1016/j.apmr.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Cust AE, Armstrong BK, Smith BJ, et al. Self-reported confidence in recall as a predictor of validity and repeatability of physical activity questionnaire data. Epidemiology. 2009;20:433–441. doi: 10.1097/EDE.0b013e3181931539. [DOI] [PubMed] [Google Scholar]
- 21.Taber DR, Stevens J, Murray DM, et al. The effect of a physical activity intervention on bias in self-reported activity. Ann Epidemiol. 2009;19:316–322. doi: 10.1016/j.annepidem.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slootmaker SM, Schuit AJ, Chinapaw MJM, Seidell JC, van Mechelen W. Disagreement in physical activity assessed by accelerometer and self-report in subgroups of age, gender, education, and weight status. Int J Behavioral Nutr Phys Activity. 2009;6:17. doi: 10.1186/1479-5868-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orrell A, Doherty P, Coulton S, et al. Failure to validate the Health Survey for England physical activity module in a cardiac population. Health Policy. 2007;84:262–268. doi: 10.1016/j.healthpol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Gulati SK, McKenzie J, Crossley G, Papp MA, Sims J, Andrulli J. Device measured physical activity: is it the new 6-minute hall walk? J Cardiac Failure. 2009;15(6):S119–120. [Google Scholar]
- 25.Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–2394. doi: 10.1161/01.CIR.0000139841.42454.78. [DOI] [PubMed] [Google Scholar]
- 26.Small RS, Wickemeyer W, Germaqny R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Cardiac Failure. 2009;15:475–481. doi: 10.1016/j.cardfail.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society, Board of Directors ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 28.Opasich C, Pinna GD, Mazza A, et al. Six-minute walking performance in patients with moderate to severe heart failure: is it a useful indicator? Eur Heart J. 2001;22:488–496. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 29.Cahalin L, Pappagianopolous P, Prevost S, Wain J, Ginns L. The relationship of the six-minute walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108:452–459. doi: 10.1378/chest.108.2.452. [DOI] [PubMed] [Google Scholar]
- 30.Rostagno C, Galanti G, Comeglio M, et al. Comparison of different methods of functional evaluation in patients with chronic heart failure. Eur J Heart Fail. 2000;2:273–280. doi: 10.1016/s1388-9842(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-min walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 32.Riley M, McParland J, Stanford CF, et al. Oxygen consumption during corridor walk testing in chronic cardiac failure. Eur Heart J. 1992;13:789–793. doi: 10.1093/oxfordjournals.eurheartj.a060258. [DOI] [PubMed] [Google Scholar]
- 33.Lucas C, Stevenson LW, Johnson W, et al. The six-minute walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J. 1999;138:618–624. doi: 10.1016/s0002-8703(99)70174-2. [DOI] [PubMed] [Google Scholar]
- 34.Cahalin LP, Mathier MA, Semigran MJ, Dec W, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 35.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136:449–457. doi: 10.1016/s0002-8703(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 36.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J. 1986;292:653–655. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guazzi M, Opasich C. Functional evaluation of patients with chronic pulmonary hypertension. Ital Heart J. 2005;6:789–794. [PubMed] [Google Scholar]
- 38.Guyatt GH, Thompson PJ, Berman LB, et al. How should we measure function in patients with chronic heart and lung disease? J Chron Dis. 1985;38:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 39.Guyatt GH, Sullivan MJJ, Fallen EL, et al. A controlled trial of digoxin in congestive heart failure. Am J Cardiol. 1988;61:371–375. doi: 10.1016/0002-9149(88)90947-2. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf F, Tsuyuki T. Using endpoints in heart failure trials: design considerations. Eur Heart J. 1996;17:4–6. doi: 10.1093/oxfordjournals.eurheartj.a014690. [DOI] [PubMed] [Google Scholar]
- 41.Bittner V, Weiner DH, Yusuf S, for the SOLVD Investigators et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- 42.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J. 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demers C, McKelvie RS, Negassa A, Yusuf S. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142(4):698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 44.Ingle L, Wilkinson M, Carroll S, et al. Cardiorespiratory requirements of the 6-min walk test in older patients with left ventricular systolic dysfunction and no major structural heart disease. Int J Sports Med. 2007;28:678–684. doi: 10.1055/s-2007-964886. [DOI] [PubMed] [Google Scholar]
- 45.O'Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–382. doi: 10.1136/hrt.80.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opasich C, Pinna GD, Mazza A, et al. Reproducibility of the six-minute walking test in patients with chronic congestive heart failure: practical implications. Am J Cardiol. 1998;81:1497–1500. doi: 10.1016/s0002-9149(98)00218-5. [DOI] [PubMed] [Google Scholar]
- 47.Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JGF. The reproducibility and sensitivity of the 6-minute walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26:1742–1751. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 48.Pinna GD, Opasich C, Mazza A, Tangenti A, Maestri R, Sanarico M. Reproducibility of the six-minute walking test in chronic heart failure patients. Statist Med. 2000;19:3087–3094. doi: 10.1002/1097-0258(20001130)19:22<3087::aid-sim628>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 49.Guimaraes GV, Carvalho VO, Bocchi EA. Reproducibility of the self-controlled six-minute walking test in heart failure patients. Clinics. 2008;63:201–206. doi: 10.1590/s1807-59322008000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guyatt GH, Pugsley SO, Sullivan MJ, Thompson PJ, Berman L, Jones NL Fallen EL, Taylor DW. Effect of encouragement on walking test performance. Thorax. 1984;39:818–822. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Berg-Emons H, Bussmann J, Balk A, Stam HJ. Factors associated with the level of movement-related everyday activity and quality of life in people with chronic heart failure. Phys Ther. 2005;85:1340–1348. [PubMed] [Google Scholar]
- 52.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed. Upper Saddle, NJ: Prentice Hall; 2008. [Google Scholar]
- 53.Jaeschke R, Singer J, Guyatt GH. Measure of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 54.Lydlick E, Epstein RS. Interpretation of quality of life measures. Qual Life Res. 1993;2:221–226. doi: 10.1007/BF00435226. [DOI] [PubMed] [Google Scholar]
- 55.Shoemaker M, Curtis A, Vangsnes E, Dickinson M, Paul R. Analysis of daily activity from implanted cardiac defibrillators: the minimum clinically important difference and relationship to mortality/life expectancy. World J Cardiovasc Dis. 2012;2(3):129–135. [Google Scholar]
- 56.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heat failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Shoemaker M, Curtis A, Vansgnes E, Dickinson M. Triangulating clinically meaningful change in the six-minute walk test in individuals with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2012;23(3):5–15. [PMC free article] [PubMed] [Google Scholar]
- 58.Eston RG, Rowlands AV, Ingledew DK. Validity of heart rate, pedometry, and accelerometry for predicting the energy cost of children's activities. J Appl Physiol. 1998;84:362–371. doi: 10.1152/jappl.1998.84.1.362. [DOI] [PubMed] [Google Scholar]
- 59.Ott AE, Pate RR, Trost SG, Ward DS, Saunder R. The use of uniaxial and triaxial accelerometers to measure children's free play physical activity. Pediatr Exerc Sci. 2000;71:36–43. [Google Scholar]
- 60.Powell SM, Jones DI, Rowlands AV. Technical variability of the RT3 accelerometer. Med Sci Sport Exerc. 2003;35:1773–1778. doi: 10.1249/01.MSS.0000089341.68754.BA. [DOI] [PubMed] [Google Scholar]
- 61.Powell SM, Rowlands AV. Intermonitor variability of the RT3 accelerometer during typical physical activities. Med Sci Sport Exerc. 2004;36:324–330. doi: 10.1249/01.MSS.0000113743.68789.36. [DOI] [PubMed] [Google Scholar]
- 62.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Hecht A, Ma S, Porszaz J, Casaburi R. Methodology for using long-term accelerometry monitoring to describe daily activity patterns in COPD. COPD. 2009;6:121–129. doi: 10.1080/15412550902755044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumukadas D, Laidlaw S, Witham MD. Using the RT3 accelerometer to measure everyday activity in functionally impaired older people. Aging Clin Exp Res. 2007;20:15–18. doi: 10.1007/BF03324742. [DOI] [PubMed] [Google Scholar]
- 65.Bouten CV, Westerterp KR, Verduin M, Janssen JD. Assessment of energy expenditure for physical activity using a triaxial accelerometer. Med Sci Sports Exerc. 1994;26(12):1516–1523. [PubMed] [Google Scholar]
- 66.Quintana JM, Escobar A, Bilbao A, et al. Responsiveness and clinically important differences for the WOMAC and SF-36 after hip joint replacement. Osteoarthritis Cartilage. 2005;13:1076–1083. doi: 10.1016/j.joca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Beaton DE, Bombardier C, Katz JN, et al. Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome Measures in Rheumatology. Minimal Clinically Important Differences. J Rheumatol. 2001;28:400–405. [PubMed] [Google Scholar]
- 67.Gatchel RJ, Mayer TG. Testing minimal clinically important difference: consensus or conundrum? Spine J. 2010;10:321–327. doi: 10.1016/j.spinee.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Jehn M, Schmidt-Trucksaess A, Schuster T, et al. Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: applicability in telemedicine. J Cardiac Failure. 2009;15(4):334–340. doi: 10.1016/j.cardfail.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Buchowski MS, Choi L, Majchrzak KM, et al. Seasonal changes in amount and patterns of physical activity in women. J Phys Act Health. 2009;6:252–261. doi: 10.1123/jpah.6.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]