Abstract
Fusobacterium nucleatum is a gram-negative anaerobe ubiquitous to the oral cavity. It is associated with periodontal disease. It is also associated with preterm birth and has been isolated from the amniotic fluid, placenta, and chorioamnionic membranes of women delivering prematurely. Periodontal disease is a newly recognized risk factor for preterm birth. This study examined the possible mechanism underlying the link between these two diseases. F. nucleatum strains isolated from amniotic fluids and placentas along with those isolated from orally related sources invaded both epithelial and endothelial cells. The invasive ability may enable F. nucleatum to colonize and infect the pregnant uterus. Transient bacteremia caused by periodontal infection may facilitate bacterial transmission from the oral cavity to the uterus. To test this hypothesis, we intravenously injected F. nucleatum into pregnant CF-1 mice. The injection resulted in premature delivery, stillbirths, and nonsustained live births. The bacterial infection was restricted inside the uterus, without spreading systemically. F. nucleatum was first detected in the blood vessels in murine placentas. Invasion of the endothelial cells lining the blood vessels was observed. The bacteria then crossed the endothelium, proliferated in surrounding tissues, and finally spread to the amniotic fluid. The pattern of infection paralleled that in humans. This study represents the first evidence that F. nucleatum may be transmitted hematogenously to the placenta and cause adverse pregnancy outcomes. The results strengthen the link between periodontal disease and preterm birth. Our study also indicates that invasion may be an important virulence mechanism for F. nucleatum to infect the placenta.
Periodontal disease is a highly prevalent infectious disease affecting the majority of the world's population to various degrees. It is caused by heavy colonization with various gram-negative, anaerobic bacteria in subgingival plaque. Preterm birth is the number one cause of infant mortality and morbidity. It is also highly prevalent and occurs in 7 to 11% of all births in the United States alone. These two seemingly unrelated health problems have recently been linked, with periodontal disease being recognized as a potential risk factor for preterm birth, based on epidemiologic studies (18). The specific mechanism by which these two diseases are correlated is not fully understood.
Studies have shown that intrauterine infections are highly prevalent among women who give birth prematurely (2, 7, 21). Four possible mechanisms exist for microbes to spread to the uterus, an otherwise sterile environment: (i) the organisms from the vagina and the cervix ascend to the uterus; (ii) the organisms originate from elsewhere and are transmitted hematogenously; (iii) the organisms from the peritoneal cavity seed retrogradely through the fallopian tube; and (iv) they are inoculated accidentally inside the uterus during invasive procedures, such as amniocentesis and chorionic villous sampling (21). Ascending infection is considered to be by far the most common route of infection. Hematogenous spread of organisms from other body sites to the uterus is a second route. A variety of microorganisms have been isolated from the placenta, amniotic fluid and chorioamnion cultures, with Fusobacterium nucleatum, Ureaplasma urealyticum, Mycoplasma hominis, and Bacteroides urealyticus being the most prevalent species (2, 10, 21, 22). Among these, U. urealyticum, M. hominis, and B. urealyticus are opportunistic pathogens indigenous to the female lower genital tract and often associated with bacterial vaginosis, which is recognized as a risk factor for preterm birth (3, 10, 23).
It is believed that U. urealyticum, M. hominis, B. urealyticus, and certain other species commonly found in the lower genital tract are transmitted to the uterus through the ascending route to infect the fetoplacental unit. F. nucleatum, a filamentous gram-negative anaerobe, however, is an oropulmonary pathogen that is infrequently found in the vaginal tract (2, 9, 10). Although associated with bacterial vaginosis, the organism is relatively uncommon compared to the frequencies of other species linked with the disease (12, 13). F. nucleatum is ubiquitous in the oral cavity and is one of the most abundant species in subgingival plaque. During periodontal infections, the cell mass of F. nucleatum increases more than 10,000-fold (17). The frequency of F. nucleatum infection in amniotic fluid is approximately 10 to 30% in women in preterm labor with intact membranes and 10% in women with preterm premature rupture of membranes, in considerable excess compared to most other single species (2, 10). The species of Fusobacterium most frequently isolated from the lower genital tract, F. naviforme and F. gonidiaformans, are rarely isolated from amniotic fluid cultures (9, 10). These data suggest that F. nucleatum in the oral cavity may spread hematogenously to infect the pregnant uterus. Consistent with this hypothesis, Lin et al. recently reported that when the oral bacterium Porphyromonas gingivalis was disseminated systemically in pregnant mice, bacterial DNA was detected in the murine placentas associated with intrauterine growth restriction (15).
In a previous study, we showed that F. nucleatum invaded oral epithelial cells (8). A spontaneous mutant, F. nucleatum 12230 lam, was less invasive (8). Invasion is a mechanism for pathogenic bacteria to colonize different tissue sites. In the present study, F. nucleatum strains isolated from the amniotic fluid and placentas of women who gave birth prematurely were tested for their ability to invade epithelial and endothelial cells. Furthermore, although F. nucleatum has been cultured frequently from infections associated with preterm birth, often isolated as a sole infectious agent from amniotic fluid among women in preterm labor with intact membranes, a causal relationship with preterm delivery has not been demonstrated experimentally. In particular, infection by a hematogenous route has not been investigated. Therefore, we tested the ability of F. nucleatum to induce preterm birth or other adverse pregnancy outcomes when introduced into the bloodstream of pregnant mice.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
F. nucleatum 12230 is a transtracheal isolate and has been used in previous studies (8). F. nucleatum 12230 lam is a spontaneous mutant defective in autoaggregation and attachment and invasion of epithelial cells (8). F. nucleatum DUMC 2929 and 1356 were isolated from amniotic fluid by transabdominal amniocentesis from two women who gave birth prematurely prior to rupture of the fetal membranes. F. nucleatum DUMC 3349 and 2079 were isolated from the placentas of women who gave birth prematurely immediately following the birth. F. gonidiaformans strains were isolated from the vaginal tract of women with bacterial vaginosis. All fusobacterial strains were maintained on either Trypticase soy agar (BBL) plates supplemented with 5 μg of hemin (Sigma, St. Louis, Mo.) per ml, 1 μg of menadione (Sigma) per ml, and 5% defimbriated sheep blood (Cleveland Scientific) or in Trypticase soy broth (BBL) supplemented with 5 μg of hemin per ml and 1 μg of menadione per ml. The cultures were incubated at 37°C in an anaerobic chamber with an atmosphere of 90% N2, 5% H2, and 5% CO2. Escherichia coli DH5α was used as a control strain and was maintained in LB broth (Difco) or on LB agar (Difco) and incubated at 37°C in air.
Endothelial and epithelial cell attachment and invasion assay.
Human umbilical vein endothelial cells (HUVEC; ATCC CRL-1730; American Type Culture Collection, Manassas, Va.) were maintained in F12K medium (Mediatech, Herndon, Va.) supplemented with 0.1 mg of heparin (Sigma) per ml, 0.03 to 0.05 mg of endothelial cell growth supplement (Sigma) per ml, and 10% fetal bovine serum (Mediatech). The human oral mucosal epithelial cell line KB (ATCC CCL-17) was maintained in minimal essential medium (Gibco-BRL, Rockville, Md.) supplemented with 10% fetal bovine serum. The cultures were grown at 37°C under 5% CO2.
The attachment and invasion assays were carried out as previously described (8). Cells were seeded into 24-well trays and allowed to grow to near confluence. The bacteria were then added to the monolayers at a multiplicity of infection of 50:1 to 100:1 and incubated at 37°C under 5% CO2. For invasion assays, the bacteria were incubated with the monolayers for 4 h, followed by two washes with phosphate-buffered saline (PBS; Sigma). Fresh medium containing 300 μg of gentamicin per ml and 200 μg of metronidazole per ml was then added to the monolayers and incubated for an additional hour to kill the extracellular bacteria. At these antibiotic concentrations, both KB and HUVEC cells were unaffected, as determined by trypan blue staining, while >99.9% of the original bacterial inoculum was killed. Following the incubation, the monolayers were washed again with PBS and lysed with sterile water, and the bacteria released from the monolayers were enumerated on blood agar plates. For attachment assays, the bacteria were incubated with the monolayers for 1 h before being washed with PBS, lysed with water, and then enumerated on blood agar plates. Control experiments showed that the viability of the bacteria was not affected by the water treatment. Bacterial proliferation in the tissue culture media during the assay was minimal. Therefore, the levels of attachment and invasion were expressed as the percentage of bacteria recovered following cell lysis relative to the total number of bacteria added initially. All experiments were performed in duplicate or triplicate and repeated at least three times.
Mating, intravenous injection of pregnant mice, and LD50 of fetal death.
The animal protocol was approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Ten-week-old outbred CF-1 mice (Charles River Laboratories, Wilmington, Mass.) were mated at a female-to-male ratio of 2:1. Positive mating was indicated by the presence of a white vaginal plug, and the day that it appeared was termed day 1 of gestation. The normal length of gestation of CF-1 mice is 20 to 21 days. Bacteria were injected on day 16 or 17 of gestation. Fresh bacterial cultures of F. nucleatum 12230, 12230 lam, and DUMC 2929 were washed with PBS, and their optical density at 600 nm was determined to estimate the CFU of the cultures before 100 μl of each culture was injected into the tail vein of each mouse. Serial dilutions of the cultures were plated on blood agar plates to obtain an actual number of bacteria injected.
To determine the 50% lethal dose (LD50), 3 to 12 pregnant mice were injected at each dose. The doses ranged from 105 to 108 CFU per mouse. For each dose, the fetal death rate was calculated as the percentage of dead pups out of the total number of pups delivered at that particular dose. The LD50 was defined as the dose that resulted in a 50% fetal death rate. The following formula, modified from a previous one (20), was used for the estimation of LD50: ln(LD50) = (50 − X)/(Y − X) + ln(dose X), where X is the nearest death rate below 50% and Y is the nearest death rate above 50%. The results were confirmed with logistic regression models. As a control, pregnant mice were injected with either 100 μl of PBS or 100 μl of E. coli DH5α.
Kinetics of F. nucleatum infection of pregnant mice.
To determine the kinetics of infection, pregnant mice were challenged with F. nucleatum 12230 by tail vein injection at a dose of 0.5 to 1 LD50. At 6, 18, 24, 48, and 72 h postinjection, groups of three to four mice were sacrificed. The liver, spleen, placentas, amniotic fluid, and fetuses were harvested from each pregnant mouse. Placentas, amniotic fluid, and fetuses from the same mouse were pooled regardless of whether each individual fetoplacental unit was infected. Serial dilutions of amniotic fluid were plated onto blood agar plates to enumerate the bacteria. The liver, spleen, placentas, and fetuses were weighed and homogenized in sterile PBS, followed by serial dilutions and plating. The identities of the organisms recovered on blood agar plates were verified by Gram stain. No contaminating bacteria were detected. The bacterial concentration was expressed as CFU per milliliter in amniotic fluid and as CFU per gram of tissue for the liver, spleen, placentas, and fetuses. As a control, nonpregnant mice were also challenged with F. nucleatum 12230 by tail vein injection at a dose of 3 × 106 to 4 × 106 or 3 × 107 to 4 × 107 CFU. At 6, 18, 24, 48, and 72 h postinjection, groups of two to four mice were sacrificed. The liver and spleen were harvested from each nonpregnant mouse, and the bacterial titers were determined as described above. The standard deviation at each time point was defined as the square root of the variance of the measure, that is,
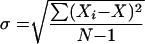 |
Immunohistologic and ultrastructural studies of mouse placentas.
Pregnant mice were injected with F. nucleatum 12230 at a dose of about 1 LD50 per mouse as described above. Mouse placentas were harvested at 24, 48, and 72 h postinjection. The specimens were fixed in formalin (10% neutral formaldehyde), dehydrated with increasing concentrations of ethanol, and embedded in paraffin. For immunohistochemical staining, 3-μm tissue sections were placed onto electrostatically charged slides, heated in a 60°C oven for 1 h, deparaffinized in xylene, and rehydrated sequentially in 100, 95, and 80% ethanol and water. This was followed by incubation in 3% H2O2 to quench endogenous peroxidase. After being rinsed with water, the slides were incubated with avidin and then biotin, each for 10 min, to block endogenous biotin activity. The slides were incubated with serum-free protein block (Dako, Carpinteria, Calif.) for 20 min, followed by incubation in rabbit antiserum raised against whole F. nucleatum 12230 or normal rabbit serum (1:2,000 dilution each) for 18 h at room temperature. Subsequently, the slides were incubated with biotinylated goat anti-rabbit immunoglobulin G (Dako) for 60 min. The assay was then continued on an autoimmunostainer (Dako Autostainer Universal Staining System). Streptavidin-horseradish peroxidase conjugate and 3′,3-diaminobenzidine were used to detect the bacteria (stained brown). Slides were counterstained with Gills no. 3 hematoxylin (Fisher Scientific, Pittsburgh, Pa.). For transmission electron microscopy (TEM), tissues from paraffin-embedded blocks were deparaffinized and rehydrated as above. The specimens were postfixed with 1% osmium tetraoxide for 1 h at room temperature, dehydrated, and embedded in Epon 812. The thin sections were double stained in uranyl acetate and lead citrate and examined with a JEM-1200EX transmission electron microscope.
RESULTS
F. nucleatum attaches to and invades human epithelial and endothelial cells.
We previously reported invasion of oral mucosal epithelial KB cells by transtracheal and oral isolates of F. nucleatum (8). In the present study, invasion of HUVEC and KB cells by intrauterine F. nucleatum isolates was determined (Table 1). E. coli DH5α is noninvasive and was used as a negative control. F. nucleatum strains, regardless of the source of isolation, all invaded both the KB and HUVEC cells to different extents. F. nucleatum 12230 lam was less invasive than its wild-type strain. F. gonidiaformans DUMC CF65-1 and CF63-1, two isolates from the vaginal tracts of women with bacterial vaginosis, were noninvasive in both cell lines. F. nucleatum also adhered to HUVECs, whereas F. gonidiaformans did not (Table 1).
TABLE 1.
Invasion of KB and HUVEC cells and attachment to HUVEC cells by different fusobacteria
| Strain | Invasiona (%)
|
Attachment to HUVEC cells (%) | |
|---|---|---|---|
| KB cells | HUVEC cells | ||
| F. nucleatum 12230 | 1.5 ± 0.0 | 0.5 ± 0.1 | 1.5 ± 0.6 |
| F. nucleatum 12230 lam | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| F. nucleatum DUMC 2929 | 1.5 ± 0.1 | 0.2 ± 0.0 | 0.5 ± 0.0 |
| F. nucleatum DUMC 3349 | 2.2 ± 0.5 | 2.0 ± 0.3 | ND |
| F. nucleatum DUMC 1356 | 2.3 ± 0.2 | 0.6 ± 0.1 | ND |
| F. nucleatum DUMC 2079 | 1.3 ± 0.1 | 0.4 ± 0.1 | 1.5 ± 0.6 |
| F. gonidiaformans DUMC CF65-1 | <0.01 | <0.01 | <0.01 |
| F. gonidiaformans DUMC CF63-1 | <0.01 | <0.01 | <0.01 |
| E. coli DH5α | <0.01 | <0.01 | ND |
Invasion and attachment were calculated as (no. of bacteria recovered after cell lysis) × 100/(total bacteria initially added). The values here are results from one representative experiment, shown as the average of duplicates ± standard deviation. Each experiment was repeated at least three times. The limit of detection was <0.01%. ND, not determined.
Intravenous injection of F. nucleatum induces stillbirth in pregnant mice.
Pregnant CF-1 mice were challenged at day 16 to 17 of gestation with either orally related strain F. nucleatum 12230 or 12230 lam or uterine-related strain DUMC 2929 at various doses by tail vein injection. The resultant infection by all three strains caused similar adverse pregnancy outcomes, manifested mainly as preterm births or term stillbirths, with occasional nonsustained live births (all died within a day). The fetal death rate increased with dose (Table 2). The LD50s of all three strains were comparable, as determined by statistical analysis with logistic regression models (modeling fetal death rate as a function of dose). As a control, four pregnant CF-1 mice were each injected with 100 μl of PBS. Together, they delivered 50 pups, all alive and well. In addition, three pregnant CF-1 mice were injected with E. coli DH5α, each at a dose of 7 × 106 CFU. These three mice delivered a total of 32 healthy pups, with no deaths.
TABLE 2.
Effect of intravenous injection of fusobacteria on pregnancy outcome in mice
| F. nucleatum strain | Dose (CFU) | No. of mice infected | No. of pups delivereda | No. of dead pupsa,b | Death ratec (%) | LD50 (CFU)d |
|---|---|---|---|---|---|---|
| 12230 | 4 × 105 | 3 | 49 | 3 | 6 | 4 × 107 |
| 2 × 107 | 8 | 87 | 20 | 23 | ||
| 4 × 107 | 12 | 126 | 77 | 61 | ||
| 7 × 107 | 6 | 63 | 59 | 93 | ||
| 12230 lam | 4 × 106 | 3 | 30 | 0 | 0 | 6 × 107 |
| 2 × 107 | 5 | 33 | 3 | 9 | ||
| 4 × 107 | 4 | 35 | 7 | 20 | ||
| 1 × 108 | 4 | 36 | 36 | 100 | ||
| 2929 | 8 × 106 | 8 | 91 | 18 | 20 | 2 × 107 |
| 1 × 107 | 4 | 47 | 32 | 68 | ||
| 3 × 107 | 4 | 44 | 27 | 61 |
Values shown were combined for all mice infected with the same dose.
Includes premature births and term stillbirths.
The death rate was calculated as (number of dead pups)/(number of pups delivered) × 100. The dead pup values include all pups delivered dead, either at term or prematurely, and those that died after delivery.
The LD50 was determined as the dose that resulted in a fetal death rate of 50% and was calculated as described in Materials and Methods.
F. nucleatum colonizes and proliferates specifically inside the mouse uterus.
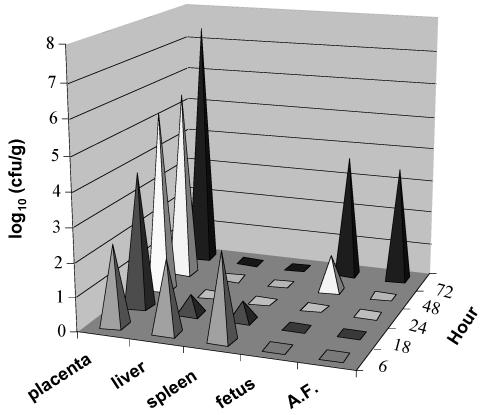
Does F. nucleatum cause fetal death by general, systemic infection or by localized infection, as occurs with human intrauterine infection? To address this question, a kinetic study was performed by intravenous injection of F. nucleatum 12230 into pregnant CF-1 mice. At 6 h postinjection, live F. nucleatum was isolated from the liver, spleen, and placentas at approximately equal quantities per g of tissue (Fig. 1). This generalized spread could be attributed to the intravenous route of infection, with bacteria reaching all parts of the body via the circulation. However, the organism did not persist in the liver or spleen in this model. Counts of viable F. nucleatum in the liver and spleen decreased with time, and the organisms had been completely eliminated from both organs by 24 h postinjection (Fig. 1). In contrast, the organism established stable colonization in the placentas and proliferated rapidly over time (Fig. 1). By 48 to 72 h postinjection, F. nucleatum had spread to the amniotic fluid and fetuses (Fig. 1). As this was also the time that fetal death was observed, it is likely that F. nucleatum caused fetal death by localized infection inside the uterus. When mice were injected with lower doses of F. nucleatum (105 or 106 CFU/mouse), fewer placentas were infected, resulting in fewer dead fetuses. However, the placentas that were infected showed the same rapid kinetics of infection as seen in mice injected with higher doses of the organism (data not shown). When injected intravenously into nonpregnant mice, only a few bacteria were isolated from the liver and spleen at 6 h but none at 18, 24, 48, or 72 h postinjection.
FIG. 1.
Kinetic study of intravenous injection of F. nucleatum into pregnant mice. Mice were infected with a dose of 1 × 107 to 2.5 × 107 CFU of F. nucleatum 12230. At 6, 18, 24, 48, and 72 h postinfection, three to four mice were dissected. The bacterial counts in the placentas, liver, spleen, fetuses, and amniotic fluid (A.F.) of each mouse were expressed as log10 CFU per gram of tissue or per milliliter of fluid. The results shown are the averages for all mice dissected at each time point. The standard deviations were <20% for values above 1 log10 (CFU/g) but >50% for those less than 1 log10 (CFU/g).
F. nucleatum infection of the mouse placenta originates in the decidua basalis.
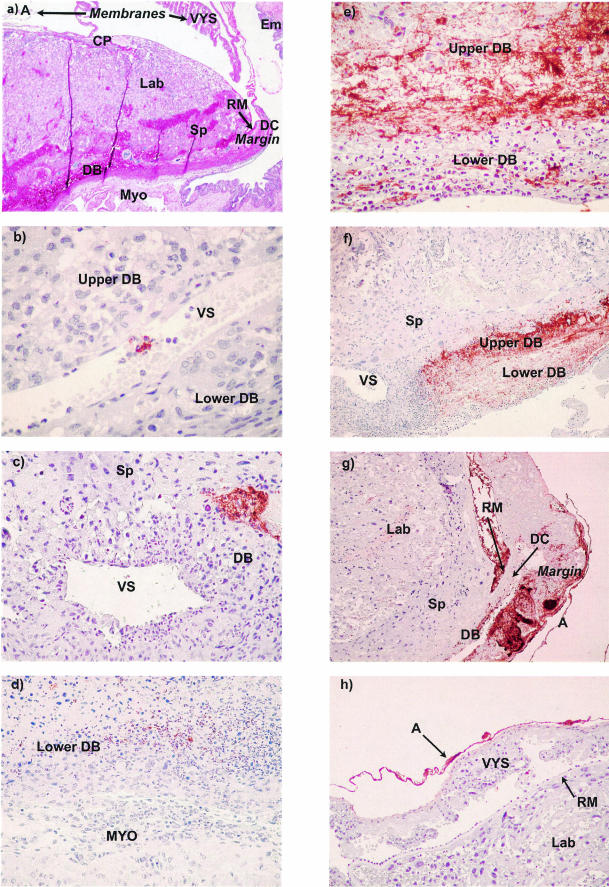
In order to precisely determine the pattern of spread of F. nucleatum and its relationship to the inflammatory response, immunohistochemical analyses were performed on placentas either uninfected or infected with F. nucleatum 12230. Antisera raised against whole F. nucleatum 12230 were used to detect the presence of the organism (Fig. 2). Murine placental anatomy in the second half of pregnancy closely parallels that of the human placenta (Fig. 2a). Maternal blood enters the gas-exchanging region via modified arteries in the central decidua basalis and exits via more laterally oriented large decidual veins (sinuses). Since the gas-exchanging regions are generally not involved in either human or murine fusobacterial infection, they will not be described further. The decidua basalis extends to the margin of the placental disk (marginal zone), where it continues out into the placental membranes as the decidua capsularis. Membrane structure is similar to that of the human placenta with the exception of an additional layer interposed between the choriodecidual layer (decidua capsularis and mural trophoblast) and the amnion. This additional layer is the yolk sac with its thickened basement membrane (Reichert's membrane).
FIG. 2.
Histologic and immunohistochemical analysis of the sequence of infection and host response to F. nucleatum at 24 h (b and c), 48 h (d), and 72 h (e, f, g, and h) postinfection. (a) Basic anatomy of the placenta and membranes with the surrounding uterus at day 16 of gestation. The placenta includes the chorioallantoic plate, labyrinth (where vascularized fetal tissue and trophoblast-lined maternal blood spaces interdigitate for gas exchange), spongiotrophoblast, margin zone (junction of the decidua basalis, decidua capsularis, and Reichert's membrane of the parietal yolk sac), and Reichert's membrane. The membranes include the visceral yolk sac and amnion. The uterus includes the decidua capsularis, decidua basalis, myometrium, and endometrium. Magnification, 20×. (b) Immunoreactive F. nucleatum within a large venous sinus in the decidua basalis (DB). Venous sinuses separate the lateral decidua basalis into the upper portion of the decidua basalis, adjacent to the spongiotrophoblast, and the lower portion of the decidua basalis, adjacent to the myometrium. Magnification, 400×. (c) Single focus of F. nucleatum above a venous sinus in the upper decidua basalis. Magnification, 200×. (d) Neutrophilic response to F. nucleatum in the lower portion of the decidua basalis. Magnification, 100×. (e) Massive proliferation of F. nucleatum in the upper portion of decidua basalis. Degenerating neutrophilic exudate is seen in the lower portion of the decidua basalis. Magnification, 200×. (f) Lateral spread of proliferating F. nucleatum in the upper portion of the decidua basalis. Magnification, 40×. (g) Placenta and membranes (lateral at right, medial at left), showing spread of F. nucleatum to the margin. F. nucleatum has penetrated Reichert's membrane to colonize the visceral yolk sac and amnion. Magnification, 20×. (h) Infection of the amnion. Magnification, 200×.. A, amnion; CP, chorioallantoic plate; DB, decidua basalis; DC, decidua capsularis; Em, endometrium; Lab, labyrinth; margin, margin zone; MYO, myometrium; RM, Reichert's membrane of parietal yolk sac; SP, spongiotrophoblast; VS, venous sinus; VYS, visceral yolk sac.
At 24 h postinjection, the organisms and inflammation were restricted to the maternal decidua basalis, either in the venous sinuses (Fig. 2b) or in isolated foci adjacent to the venous sinuses (Fig. 2c). At 48 h, organisms, inflammation, and necrosis all became more apparent (Fig. 2d). By 72 h, the organisms had spread beyond the decidua basalis and were frequently detected in the placental membranes and fetal vessels of the placenta (Fig. 2e to h). Of note, the maternal neutrophilic inflammatory response was largely limited to maternal tissues (lower decidua basalis) below the venous sinuses (Fig. 2e and f). The organisms spread contiguously in the decidua basalis, proliferated to high levels in the marginal zone, and eventually crossed Reichert's membrane to infect the placental membranes (the visceral yolk sac and amnion in the mouse) (Fig. 2g and h). This sequence parallels the development of chorioamnionitis in humans. The progression of F. nucleatum infection in various regions of the placenta is summarized further in Table 3. As the infection progressed, the occurrence of fetal death increased, the infection changed from a focal to a diffuse pattern, and the organisms spread laterally from the decidua basalis to the marginal zone, the fetal membranes, and the labyrinth (Table 3).
TABLE 3.
Progression of infection of fetoplacental units infected by F. nucleatum
| Time of infection (h) | Ni/Nta | Fetal statusb (no. of fetuses)
|
Extent of infectionc (no. of fetuses)
|
Localization of F. nucleatumc (no. of fetal units)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Alive | Dead | Focal | Diffuse | DB | Margin | Membranes | Labyrinth | ||
| 0 (uninfected) | 0/10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 2/14 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | 0 |
| 48 | 9/11 | 4 | 5 | 8 | 1 | 5 | 5 | 1 | 0 |
| 72 | 7/9 | 0 | 7 | 1 | 6 | 6 | 5 | 5 | 5 |
Ni, number of fetal units infected with F. nucleatum; Nt, total number sampled. Fetal units were sampled from two mice at each time point.
Fetal status of F. nucleatum-infected fetaplacental units was determined by the color of fetuses at the time of dissection. Alive, number of fetuses that remained pink; dead, number of fetuses that had already turned purple.
Determined by immunohistochemical staining of infected fetaplacental units with anti-F. nucleatum antibodies. Shown in each column are the numbers of fetaplacental units with each particular infectious pattern (focal or diffuse) or with infections observed in each particular anatomic area (decidua basalis [DB], margin, membranes, or labyrinth). Focal, isolated symmetric area(s) of bacterial colonization; diffuse, continuous zone of colonization encompassing one third or more of the placental structure affected; DB, maternal decidua basalis; margin, lateral point of intersection between the maternal decidua basalis, maternal decidua capsularis, fetal parietal yolk sac, and placental spongiotrophoblast; membrane, visceral yolk sac and/or amnion; Labyrinth, (zone where vascularized fetal tissue and trophoblast-lined maternal blood spaces interdigitate for gas exchange). Also see Fig. 2a for placental anatomy.
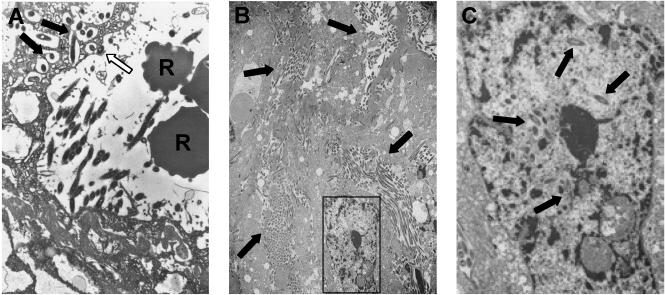
Consistent with the immunohistochemical analysis, F. nucleatum 12230 was detected by TEM in venous sinuses at 24 h postinjection (Fig. 3a). Organisms were found to be either attached to or internalized in the venous endothelial cells. This sequence of attachment and invasion was consistent with what had been observed in our previous TEM study of in vitro invasion of epithelial cells by F. nucleatum (8). That is, only a few organisms were internalized in a single cell, and they were located in the cytoplasm. At 48 and 72 h postinjection, no bacteria were detected in blood vessels or endothelial cells (data not shown). Instead, F. nucleatum 12230 had massively invaded the decidua basalis by 72 h (Fig. 3b). Large quantities of extracellular and intracellular bacteria were observed, and they formed clusters, much like microcolonies. The intracellular bacteria were detected not only in the cytoplasm but also in the nucleus (Fig. 3c). This localization differed significantly from that seen at 24 h and in our previous TEM studies (8).
FIG. 3.
TEM analysis of murine placentas infected with F. nucleatum 12230. (A) At 24 h postinfection. R, red blood cells in the blood vessel. The solid arrows point to bacteria internalized in the endothelial cells lining the veins. The open arrow points to an organism attached to the endothelial cells. Magnification, 6,000×. (B) At 72 h postinfection. Arrows indicate bacteria that have proliferated in the cytoplasm or outside the cells. Magnification, 1,500×. (C) Enlarged view of the area boxed in panel B. Arrows indicate bacteria in the nucleus.
DISCUSSION
Studies have shown that intrauterine infections in humans cause preterm birth. F. nucleatum is one of the most prevalent species found in infections of amniotic fluid and placenta and notably is often implicated as a sole infectious agent in preterm labor with intact fetal membranes. It is also associated with various forms of periodontal disease. Because of its association with both periodontal disease and preterm birth, F. nucleatum is an ideal model organism for investigating the correlation between these two diseases. During periodontal infection, when the oral mucosa is injured and inflamed and the quantities of periodontal pathogens increase dramatically, transient bacteremia may occur (1, 4, 5). This can lead to selective colonization of undesired sites. In the current study, we bypassed initial transmission of organisms from the oral cavity into the bloodstream and addressed the question of what effects F. nucleatum has on pregnancy if it enters the circulation.
We chose to intravenously inject pregnant mice on day 16 to 17 of gestation, a relatively late stage in the pregnancy, for several reasons. Stress or infection prior to day 14 tends to lead to resorption of the fetuses. Preterm birth in humans also occurs during late stages in gestation, and the murine infection was initiated at a gestational age proportional to 28 to 32 weeks in humans. Also, the structure of the mouse placenta at late stages of gestation is remarkably similar to that of human placenta (6).
Our study showed that intravenous injection of pregnant mice with F. nucleatum isolated from either amniotic fluid infection or an oropulmonary source resulted in fetal death. This is the first experimental demonstration that hematogenous injection with an orally associated species can cause adverse pregnancy outcomes. This murine model closely paralleled human intrauterine infection by F. nucleatum in several important aspects. First, infection was located inside the uterus, and live organisms could be isolated from the placenta, amniotic fluid, and fetuses. Second, fetal death was due to local infection of the fetoplacental unit rather than systemic effects of maternal bloodstream infection. Third, immunohistochemical analysis indicated that the placental infection eventually spread to the amnion, mimicking chorioamnionitis in humans.
The abnormal pregnancy outcomes in our murine model were manifested as premature births and term stillbirths, with occasional nonsustained live births. This differs somewhat from the outcome of intrauterine F. nucleatum infection in humans, where delivery and medical intervention can often prevent fetal death. In mice, preterm delivery only occurred if the entire litter was dead; mixtures of live and dead fetuses were all delivered at full term. Since the birth of one abnormal fetus cannot occur in the mouse without delivery of the other unaffected mice, preterm birth is not a successful adaptive strategy in animals with multiple gestational sacs.
Immunohistochemical analysis revealed that F. nucleatum infection, accompanied by inflammation, was initiated in the decidua basalis of the placenta, an area characterized by large venous sinuses. A similar localization of organisms at this site has previously been reported in murine fetoplacental listeriosis (19). We speculate that the slow blood flow rate and consequent low shear forces in the venous sinuses provide an opportunity for F. nucleatum to adhere to and invade the endothelial cells. The bacteria may then cross the endothelium and establish infection in the decidua basalis. Indeed, the majority of F. nucleatum infections detected by immunohistochemical analysis at earlier time points were located either within or directly adjacent to the venous sinuses. The infection intensified with time, indicating proliferation of the bacteria, consistent with the kinetic study. Also consistent were electron microscopic studies, which confirmed the tendency of F. nucleatum to migrate from within the venous sinuses and proliferate in the decidua basalis.
TEM studies also provided the first in vivo evidence of F. nucleatum attachment and invasion of tissue cells, supporting our in vitro findings of attachment and invasion of epithelial and endothelial cells by F. nucleatum. The major discrepancy between our in vivo and in vitro studies was the observation by TEM of massive invasion of the decidua basalis, with bacteria present in the nucleus at 72 h postinjection. This discrepancy could be due either to the difference in infection time or to local factors promoting bacterial proliferation. During the in vitro studies, F. nucleatum was incubated with host cells for no more than a few hours. It is possible that, with prolonged incubation, similar massive proliferation of intracellular F. nucleatum might be observed in the in vitro assays.
The vaginal isolates of F. gonidiaformans, which is rarely found to be associated with preterm birth, did not invade either endothelial or epithelial cells, nor did it adhere to the endothelial cells. The inability of F. gonidiaformans to invade was probably due to its inability to attach to the host cells. This observation is consistent with the suggestion that attachment and invasion may be important virulence mechanisms of F. nucleatum. F. nucleatum possesses a unique adhesion molecule which is absent in F. gonidiaformans but present in F. nucleatum 12230 lam (Han et al., unpublished data). This may partially explain why the lam mutant, although defective, is still capable of attaching to and invading tissue culture cells, which may then account for its unaffected virulence in mice. It is likely that F. nucleatum encodes additional virulence factors, such as phospholipase, lipopolysaccharides, and the ability to induce proinflammatory cytokines (8, 14, 16, 24), which may also be critical to its role in the pathogenesis of preterm birth.
Our study suggests that systemic injection of F. nucleatum into mice prior to pregnancy would be unlikely to contribute to an adverse pregnancy outcome. Although F. nucleatum has been demonstrated to cause intrahepatic and intra-abdominal abscesses by intraperitoneal challenge (11), in our model the bacteria were eliminated rather quickly from the liver and spleen. It appeared that colonization would not occur unless the placenta was present. The ability of F. nucleatum to proliferate in the placenta and eventually spread to the amniotic fluid and fetuses may be attributed in part to local immunosuppression in the reproductive organs during pregnancy. Venous sinusoids also exist in the liver, but the presence of abundant immunocompetent cells may explain the rapid clearance of F. nucleatum that we observed at this site. An alternative but not mutually exclusive explanation is that F. nucleatum may preferentially infect the cell types found in the decidua basalis, decidual stromal cells and interstitial trophoblastic cells (glycogen cells).
In summary, we found that intravenous inoculation of mice with F. nucleatum, an orally related species, induced adverse pregnancy outcomes. This indicates that F. nucleatum is capable of colonizing the uterus via a hematogenous route in addition to the commonly acknowledged ascending route. The sequence of infection in murine placentas mimicked that in humans leading to preterm birth. Live organisms were isolated from the infected murine placentas. Therefore, we have fulfilled Koch's postulates identifying F. nucleatum as a causative agent of adverse fetal outcomes in mice analogous to those seen with human preterm birth. The current model will be useful for studies of the mechanism of preterm birth caused by intrauterine infection. Our results indicate that invasion of the maternal venous endothelium in the decidualized endometrium of pregnancy may be an important virulence mechanism for F. nucleatum and perhaps other oral bacteria to colonize the placenta and cause stillbirth or preterm birth. This study also sheds light on the mechanisms underlying the link between periodontal infection and preterm birth and may be useful for investigating the impact of oral health on other diseases.
Acknowledgments
This study was supported by NIH grants DE 14447 and DE 14924 to Y.W.H., Pathology Associates of University Hospitals grant 20.2 to R.W.R., and NIH grant AR 39750 to T.S.M.
Editor: A. D. O'Brien
REFERENCES
- 1.Carroll, G. C., and R. J. Sebor. 1980. Dental flossing and its relationship to transient bacteremia. J. Periodontol. 51:691-692. [DOI] [PubMed] [Google Scholar]
- 2.Chaim, W., and M. Mazor. 1992. Intraamniotic infection with fusobacteria. Arch. Gynecol. Obstet. 251:1-7. [DOI] [PubMed] [Google Scholar]
- 3.Chaim, W., M. Mazor, and J. R. Leiberman. 1997. The relationship between bacterial vaginosis and preterm birth. A review. Arch. Gynecol. Obstet. 259:51-58. [DOI] [PubMed] [Google Scholar]
- 4.Daly, C., D. Mitchell, D. Grossberg, J. Highfield, and D. Stewart. 1997. Bacteraemia caused by periodontal probing. Aust. Dent. J. 42:77-80. [DOI] [PubMed] [Google Scholar]
- 5.Daly, C. G., D. H. Mitchell, J. E. Highfield, D. E. Grossberg, and D. Stewart. 2001. Bacteremia due to periodontal probing: a clinical and microbiological investigation. J. Periodontol. 72:210-214. [DOI] [PubMed] [Google Scholar]
- 6.Georgiades, P., A. C. Ferguson-Smith, and G. J. Burton. 2002. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23:3-19. [DOI] [PubMed] [Google Scholar]
- 7.Goncalves, L. F., T. Chaiworapongsa, and R. Romeo. 2002. Intrauterine infection and prematurity. Ment. Retard Dev. Disabil. Res. Rev. 8:3-13. [DOI] [PubMed] [Google Scholar]
- 8.Han, Y. W., W. Shi, G. T. Huang, S. Kinder Haake, N. H. Park, H. Kuramitsu, and R. J. Genco. 2000. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill, G. B. 1993. Investigating the source of amniotic fluid isolates of fusobacteria. Clin. Infect. Dis. 16(Suppl. 4):S423-S424. [DOI] [PubMed] [Google Scholar]
- 10.Hill, G. B. 1998. Preterm birth: associations with genital and possibly oral microflora. Ann. Periodontol. 3:222-232. [DOI] [PubMed] [Google Scholar]
- 11.Hill, G. B., S. Osterhout, and P. C. Pratt. 1974. Liver abscess production by non-spore-forming anaerobic bacteria in a mouse model. Infect. Immun. 9:599-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillier, S. L., M. A. Krohn, L. K. Rabe, S. J. Klebanoff, and D. A. Eschenbach. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16(Suppl. 4):S273-S281. [DOI] [PubMed] [Google Scholar]
- 13.Hitti, J., S. L. Hillier, K. J. Agnew, M. A. Krohn, D. P. Reisner, and D. A. Eschenbach. 2001. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet. Gynecol. 97:211-219. [DOI] [PubMed] [Google Scholar]
- 14.Hofstad, T., and K. Sveen. 1979. Endotoxins of anaerobic gram-negative rods. Scand. J. Infect. Dis. Suppl. 19:42-45. [PubMed] [Google Scholar]
- 15.Lin, D., M. A. Smith, J. Elter, C. Champagne, C. L. Downey, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 71:5163-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikamo, H., K. Kawazoe, Y. Sato, A. Imai, and T. Tamaya. 1998. Preterm labor and bacterial intraamniotic infection: arachidonic acid liberation by phospholipase A2 of Fusobacterium nucleatum. Am. J. Obstet. Gynecol. 179:1579-1582. [DOI] [PubMed] [Google Scholar]
- 17.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontology 2000. 5:66-77. [DOI] [PubMed] [Google Scholar]
- 18.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 19.Redline, R. W., and C. Y. Lu. 1987. Role of local immunosuppression in murine fetoplacental listeriosis. J. Clin. Investig. 79:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 21.Romero, R., C. Avila, C. A. Brekus, and R. Morotti. 1991. The role of systemic and intrauterine infection in preterm parturition. Ann. N. Y. Acad. Sci. 622:355-375. [DOI] [PubMed] [Google Scholar]
- 22.Watts, D. H., M. A. Krohn, S. L. Hillier, and D. A. Eschenbach. 1992. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet. Gynecol. 79:351-357. [DOI] [PubMed] [Google Scholar]
- 23.Yoon, B. H., R. Romero, M. Kim, E. C. Kim, T. Kim, J. S. Park, and J. K. Jun. 2000. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am. J. Obstet. Gynecol. 183:1130-1137. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura, A., Y. Hara, T. Kaneko, and I. Kato. 1997. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J. Periodont. Res. 32:279-286. [DOI] [PubMed] [Google Scholar]