Abstract
Isogenic Enterococcus faecalis strains that differ in their expression of aggregation substance (AS) and its cognate receptor, enterococcal binding substance (EBS), were compared for urovirulence in mice. Strain OG1SSp/pCF500 (inducible AS+, constitutive EBS+) failed to outcompete isogenic derivative INY3000 (AS− EBS−) in the urine, bladders, or kidneys of mice harvested at 48 h postinoculation. Neither mouse nor human urine induced AS expression by OG1SSp/pCF500. Recombinant strain OG1SSp/pINY1801 (constitutive AS+, EBS+) exhibited plasmid segregation that was as extensive in vivo as in vitro. These data suggest that AS and EBS do not contribute to upper or lower urinary tract colonization by E. faecalis and that growth in urine does not induce AS expression by strains carrying plasmids in the pCF10 family.
Enterococcus, a leading cause of hospital-acquired infections, is associated specifically with urinary tract infection (UTI), device-related infections, bacteremia, and endocarditis (16). Putative virulence factors include gelatinase, cytolysin, capsule, hyaluronidase, the fsr quorum-sensing system, enterococcal surface protein (Esp), and aggregation substance (AS) (2, 3, 6, 8, 12, 14, 17-20, 24, 25, 27, 28, 30-32). A role in urovirulence has been directly demonstrated only for Esp (24), but AS also may conceivably have a role in UTI pathogenesis. AS is a plasmid-encoded enterococcal surface protein that interacts with enterococcal binding substance (EBS), its cognate receptor on other enterococci, to form mating complexes. Enterococci containing an AS-encoding sex plasmid express AS when stimulated by a peptide pheromone secreted from other enterococci, whereas most enterococci constitutively express EBS (7, 15). AS is epidemiologically associated with UTI (6), increases enterococcal adherence to cultured renal tubular cells (14), and protects Enterococcus faecalis from killing by polymorphonuclear leukocytes (19), an important urinary tract defense element. However, AS has not been directly assessed as a urovirulence factor. We therefore sought to determine whether the AS-EBS combination enhances urinary tract colonization by E. faecalis.
Strains.
Four derivatives of OG1SSp, a streptomycin-spectinomycin-resistant mutant of wild-type E. faecalis strain OG1 (4), were studied (Table 1). These included two transformants of OG1SSp, one with pCF500 (a derivative of wild-type pheromone plasmid pCF10 that, like pCF10, confers pheromone-inducible AS expression and, in addition, has a selectable erythromycin resistance marker) and the other with pINY1801, which contains a fragment of pCF10 cloned into shuttle vector pWM401 and constitutively expresses AS (4, 23). Also studied were INY3000, an EBS-deficient Tn916 mutant of OG1SSp (1, 29) and a transformant of INY3000 containing shuttle vector pWM401 (23, 29).
TABLE 1.
E. faecalis strains and plasmids used
| Strain | Phenotypic markers | Reference(s) |
|---|---|---|
| OG1SSp/pINY1801 | AS+ EBS+ Cmr | 4, 23 |
| INY3000/pWM401 | AS− EBS− Tcr Cmr | 23, 29 |
| OG1SSp/pCF500 | AS+ EBS+ Emr Tcr | 5 |
| INY3000 | AS− EBS− Tcr | 29 |
Mouse model.
The mouse model of ascending unobstructed UTI has been described previously (13, 21). Both dual-strain and single-strain bacterial challenges were used. Female CBA/J or Swiss Webster mice were inoculated perurethrally by an infusion pump with broth-grown bacteria. The absence of vesico-ureteral reflux was confirmed by immediate postinoculation kidney cultures. For colonization experiments, urine, bladder, and kidneys were harvested sterilely at 24 h, 48 h, or 5 days postinoculation and cultured quantitatively with appropriate media (13, 21).
The bacteria for inoculation were grown at 37°C in tryptic soy broth or Todd-Hewitt broth (THB) either to mid-log phase or overnight and then pelleted. For competition experiments, test strains were grown separately and then combined. Selective cultures were used to enumerate each strain from the inoculum (input ratio) and from mouse cultures (output ratio). The output ratio divided by the input ratio gave the competitive index, of which the log10 was analyzed (22).
In vitro induction of AS expression.
Overnight cultures of OG1/pCF500 and INY3000 in THB were inoculated 1/1,000 in 1 ml of THB. After 4 h of growth at 37°C, the cultures were pelleted, washed with phosphate-buffered saline (PBS) (pH 7.4), and resuspended in 50 μl of PBS, THB with 50 ng of cCF10 (the cognate pheromone for pCF500), or mouse or human urine. At intervals, 9 μl from each sample was mixed with 1 μl of 1% sodium dodecyl sulfate, boiled for 10 min, and stored on ice. These preparations were then spotted onto nitrocellulose and blocked overnight in 0.01 M PBS with 10% milk at 4°C. Immunoblotting was performed with an antibody against an N-terminal domain of Asc10, the AS variant expressed by OG1/pCF500 (20), at a 1/2,500 dilution with purified Asc10 protein as a control and chemiluminescent detection.
Comparative urovirulence: OG1SSp/pCF500 and INY3000.
Approximately equal amounts of strains OG1SSp/pCF500 and INY3000 were administered as a mixed inoculum to 20 CBA/J mice in a competition model of ascending UTI. For urine, bladders, and kidneys harvested 48 h after inoculation and cultured quantitatively, replica plating confirmed the absence of plasmid segregation, i.e., all colonies from nonselective primary plates were resistant either to both tetracycline and erythromycin (putative OG1SSp/pCF500) or to tetracycline alone (putative INY3000). Macroscopic growth characteristics, indicative of the AS-EBS phenotype (i.e., +/+ or −/−), corresponded precisely with the inferred strain identities.
At each site cultured, pure growth or a predominance of INY3000 occurred as frequently as did pure growth or a predominance of OG1SSp/pCF500 (Table 2). Results within each outcome category (i.e., columns in Table 2) were combined to increase the number of observations per comparison, but still no difference was evident between OG1SSp/pCF500 and INY3000 (Table 2, bottom row). Even merging the “pure growth” and “predominance” categories across all sample types yielded no between-strain difference, i.e., 20 cultures favored OG1SSp/pCF500 and 22 favored INY3000 (P > 0.10, McNemar's test). Comparisons of relative bacterial concentrations likewise revealed no significant differences between the two strains for any individual site or all sites combined, with the median log10(competitive index) (for OG1SSp/pCF500 to INY3000) being 0.20 for urine, −1.29 for bladders, −0.07 for right kidneys, 0.01 for left kidneys, and −0.08 overall (P > 0.10 for each comparison, Wilcoxon rank sum test).
TABLE 2.
Competition of E. faecalis strains OG1SSp/pCF500 and INY3000 in the CBA/J mouse model of ascending urinary tract infection
| Origin of specimen | Total no. of specimens | No. (%) with the following resulta:
|
||||
|---|---|---|---|---|---|---|
| No growth | OG1SSp/pCF500 only | OG1SSp/pCF500 > INY3000 | INY3000 > OG1SSp/pCF500 | INY3000 only | ||
| Urine | 10 | 1 (10) | 2 (20) | 3 (30) | 2 (20) | 2 (20) |
| Bladder | 18 | 7 (39) | 3 (17) | 1 (6) | 3 (17) | 4 (22) |
| Right kidney | 18 | 8 (44) | 0 | 5 (28) | 3 (17) | 2 (11) |
| Left kidney | 18 | 6 (33) | 2 (11) | 4 (22) | 4 (22) | 2 (11) |
| Total | 64 | 22 (34) | 7 (11) | 13 (20) | 12 (19) | 10 (16) |
P values of >0.10 (McNemar's test) for all comparisons of results from “OG1SSp/pCF500 only” and “INY3000 only” and for all comparisons of results from “OG1SSp/pCF500 > INY3000” and “INY3000 > OG1SSp/pCF500.”
Expression of AS in mouse urine.
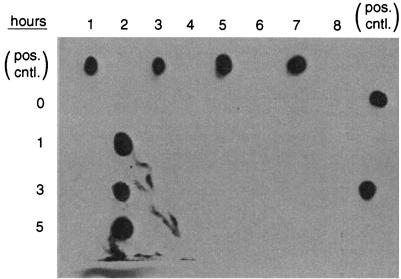
Since bacterial recovery from infected mice was insufficient for the direct detection of AS expression in vivo, expression was instead assessed ex vivo. No expression of AS by either OG1SSp/pCF500 or INY300 was detected by immunoblotting after bacterial incubation for up to 5 h in PBS alone or in human or mouse urine (Fig. 1). In contrast, under the same conditions, OG1/pCF500 vigorously expressed AS after stimulation with cCF10.
FIG. 1.
Induction of expression of AS by E. faecalis. Lane 1, OG1/pCF500 in PBS. Lane 2, OG1/pCF500 in THB plus cCF10. Lane 3, OG1/pCF500 in human urine. Lane 4, OG1/pCF500 in mouse urine. Lane 5, INY3000 in PBS. Lane 6, INY3000 in THB plus cCF10. Lane 7, INY3000 in human urine. Lane 8, INY3000 in mouse urine. Samples were assayed for expression of AS by Western blotting after incubation for 0, 1, 3, or 5 h. Positive control spots (pos. cntl.) (for orientation) are from purified Asc10 protein. Purified AS protein (Asc10) was spotted as a positive control and to mark the various lanes. The only expression of AS detected was with OG1/pCF500 in THB plus cCF10 (lane 2). Similar bacterial concentrations were present in all samples (data not shown).
Comparative urovirulence: recombinant strains.
To ensure AS expression in vivo, we used OG1SSp/pINY1801, which expresses AS constitutively (25, 29). OG1SSp/pINY1801 and isogenic (AS− EBS−) variant INY3000/pWM401 were compared for urovirulence by using a single-strain challenge model. No difference was detected between the two test strains in cultures of urine, bladders, and kidneys harvested (from 21 Swiss Webster mice per strain) at 24 h, 48 h, or 5 days after inoculation with approximately 108 CFU of either test strain (data not shown). However, extensive in vivo plasmid loss was evident, with approximately 50% of colonies recovered from mice having regained chloramphenicol susceptibility, which complicated the interpretation of results. Plasmid loss was also documented in vitro for both strains, with approximately 50% of the resistant population losing chloramphenicol resistance during each nonselective passage (data not shown). The fact that half of the organisms recovered from infected animals had lost the plasmid suggests that there was not strong selection for AS-expressing strains in vivo in this model, in marked contrast to the results obtained in experimental endocarditis, where AS-encoding plasmids impart a significant selective advantage to the infecting bacterial strain (11, 23).
Significance of findings.
We found no evidence that AS, in combination with constitutively expressed EBS, enhanced the urinary tract colonization ability of E. faecalis strain OG1SSp compared with that of its plasmidless, EBS-negative derivative INY3000. This fails to support the hypothesis that AS or EBS are urovirulence factors for E. faecalis. It is particularly noteworthy that INY3000 was equally virulent in this infection model, since the cell wall alterations in this strain have been shown to have major effects on the bacterium-host interactions in endocarditis (23).
The apparent lack of a role for AS in this UTI model was unexpected, since AS is epidemiologically associated with UTI (6), contributes to enterococcal adherence to cultured renal epithelial cells (14), and protects enterococci from killing by polymorphonuclear leukocytes (19). Conceivably, the expression of AS by pCF500 is not induced sufficiently within the urinary tract for AS to influence urovirulence, a hypothesis supported by our inability to demonstrate ex vivo expression of AS by strain OG1SSp/pCF500 after incubation in human or mouse urine (although this seemingly conflicts with a report of the induction of asa expression during ex vivo growth of E. faecalis in human urine, both in log phase and stationary phase [26]; different strains, culture methods, and detection systems were used in the two studies). Our attempt to more directly study the impact of AS on urovirulence by using a constitutively AS-expressing strain was confounded by extensive plasmid segregation. The fact that the frequency of plasmid loss in vivo in these experiments was similar to that observed during nonselective in vitro growth suggests that the expression of AS in vivo did not provide a selective pressure for the maintenance of the plasmid. This is further, albeit indirect, evidence against a major role for AS in this infection model.
It is conceivable that we might have detected an effect of AS and EBS on urovirulence had we used a different challenge dose for the infection experiments. However, this seems unlikely. The bacterial dose we administered yielded an event rate that was sufficiently high to provide adequate power for detecting a difference if one were present, yet it clearly had not saturated the system, since approximately one-third of postharvest cultures were negative. In addition, the competition model we used is highly sensitive to small differences in virulence, since each mouse (actually, each site cultured) serves as its own control.
To date, Esp is the only confirmed urovirulence factor of Enterococcus, and its effect was evident only for bladder, not kidney, infection (24). Yet, as demonstrated here and by others (24) for mice and as observed clinically in humans (9, 10), E. faecalis clearly has the ability to cause ascending renal infection. In our competition experiments, 61% of kidneys were culture positive, despite the initial delivery of bacteria only to the lower urinary tract. Thus, additional urovirulence factors in E. faecalis surely exist but remain to be identified.
Acknowledgments
This material is based upon work supported by the Department of Defense and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.R.J.), and NIH grant HL51980 (G.D.).
Dave Prentiss (Minneapolis VA Medical Center) helped prepare Fig. 1.
Editor: B. B. Finlay
REFERENCES
- 1.Bensing, B. A., and G. M. Dunny. 1993. Cloning and molecular analysis of genes affecting expression of binding substance, the recipient-encoded receptor(s) mediating mating aggregate formation in Enterococcus faecalis. J. Bacteriol. 175:7421-7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth, M. C., K. L. Hatter, D. Miller, J. Davis, R. Kowalski, D. W. Parke, J. Chodosh, B. D. Jett, M. C. Callegan, R. Penland, and M. S. Gilmore. 1998. Molecular epidemiology of Staphylococcus aureus and Enterococcus faecalis in endophthalmitis. Infect. Immun. 66:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, D. B. Clewell, and M. J. Zervos. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37:2474-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie, P. J., S.-M. Kao, J. C. Adsit, and G. M. Dunny. 1988. Cloning and expression of genes encoding pheromone-inducible antigens of Enterococcus (Streptococcus) faecalis. J. Bacteriol. 170:5161-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie, P. J., R. Z. Korman, S. A. Zahler, J. C. Adsit, and G. M. Dunny. 1987. Two conjugation systems associated with Streptococcus faecalis plasmid pCF10: identification of a conjugative transposon that transfers between S. faecalis and Bacillus subtilis. J. Bacteriol. 169:2529-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coque, T. M., J. E. Patterson, J. M. Steckelberg, and B. E. Murray. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 171:1223-1229. [DOI] [PubMed] [Google Scholar]
- 7.Dunny, G. M., B. A. Leonard, and P. J. Hedberg. 1995. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J. Bacteriol. 177:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont, H., P. Montravers, J. Mohler, and C. Carbon. 1998. Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infect. Immun. 66:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fierer, J. 1987. Acute pyelonephritis. Urol. Clin. North Am. 14:251-256. [PubMed] [Google Scholar]
- 10.Grover, S. A., A. L. Komaroff, M. Weisberg, E. F. Cook, and L. Goldman. 1987. The characteristics and hospital course of patients admitted for presumed acute pyelonephritis. J. Gen. Intern. Med. 2:5-10. [DOI] [PubMed] [Google Scholar]
- 11.Hirt, H., P. M. Schlievert, and G. M. Dunny. 2002. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect. Immun. 70:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huycke, M. M., and M. S. Gilmore. 1995. Frequency of aggregation substance and cytolysin genes among enterococcal endocarditis isolates. Plasmid 34:152-156. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, J. R., and J. J. Brown. 1996. Defining inoculation conditions for the mouse model for ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J. Infect. Dis. 173:746-749. [DOI] [PubMed] [Google Scholar]
- 14.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard, B. A. B., A. Podbielski, P. J. Hedberg, and G. M. Dunny. 1996. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 93:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mylonakis, E., M. Engelbert, Z. Qin, C. D. Sifri, B. E. Murray, F. M. Ausubel, M. S. Bilmore, and S. B. Calderwood. 2002. The Enterococcus faecalis fsr gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect. Immun. 70:4678-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olmsted, S. B., G. M. Dunny, S. L. Erlandsen, and C. L. Wells. 1994. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J. Infect. Dis. 170:1549-1556. [DOI] [PubMed] [Google Scholar]
- 19.Rakita, R. M., N. N. Vanek, K. Jacques-Palaz, M. Mee, M. M. Mariscalco, G. M. Dunny, M. Snuggs, W. B. van Winkle, and S. I. Simon. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 67:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice, L. B., L. Carias, S. Rudin, C. Vael, H. Goossens, C. Konstabel, I. Klare, S. R. Nallapareddy, W. Huang, and B. E. Murray. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508-512. [DOI] [PubMed] [Google Scholar]
- 21.Russo, T. A., U. B. Carlino, and J. R. Johnson. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlievert, P. M., G. M. Dunny, J. A. Stoehr, and A. P. Assimacopoulos. 1998. Aggregation and binding substances enhance pathogenicity in a rabbit model of Enterococcus faecalis endocarditis. Infect. Immun. 66:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankar, N., C. V. Lockatell, A. S. Baghdayan, C. Drachenberg, M. S. Gilmore, and D. E. Johnson. 2001. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect. Immun. 69:4366-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 70:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Submuth, S. D., M. Muscholl-Silberhorn, R. With, M. Susa, R. Marre, and E. Rozdzinski. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng, F., K. D. Jacques-Palaz, G. M. Weinstock, and B. Murray. 2002. Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect. Immun. 70:2010-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotter, K. M., and G. M. Dunny. 1990. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid 24:57-67. [DOI] [PubMed] [Google Scholar]
- 30.Vergis, E. N., N. Shankar, J. W. Chow, M. K. Hayden, D. R. Snydman, M. J. Zervos, P. K. Linden, M. M. Wagener, and R. R. Muder. 2002. Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due to Enterococcus faecalis. Clin. Infect. Dis. 35:570-575. [DOI] [PubMed] [Google Scholar]
- 31.Waar, K., A. B. Muscholl-Silberhorn, R. J. L. Willems, M. J. H. Slooff, H. J. M. Harmsen, and J. E. Degener. 2002. Genogrouping and incidence of virulence factors of Enterococcus faecalis in liver transplant patients differ from blood culture and fecal isolates. J. Infect. Dis. 185:1121-1127. [DOI] [PubMed] [Google Scholar]
- 32.Xu, Y., K. V. Singh, X. Qin, B. E. Murray, and G. M. Weinstock. 2000. Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect. Immun. 68:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]