Abstract
Salmonellae are facultative intracellular bacteria capable of surviving within macrophages. Salmonella pathogenicity island 2 (SPI-2) is required for growth within macrophages and for virulence in mice. In this study, we show the involvement of SPI-2 in a signal transduction pathway that induces cytokine expression in Salmonella-infected macrophages. High levels of interleukin-10 (IL-10) mRNA were induced in macrophages by infection with wild-type salmonellae compared to a strain carrying a mutation in the spiC gene, which is encoded within SPI-2. The two strains had the same effect on the expression of proinflammatory cytokines such as IL-1α, IL-6, and tumor necrosis factor alpha. IL-10 expression was dose dependently blocked by treatment of infected macrophages with the protein kinase A (PKA) inhibitor H-89, while IL-10 expression was increased by the PKA activator dibutyryl cyclic AMP. Cyclic AMP-dependent PKA activity was higher in macrophages infected with wild-type salmonellae compared to the spiC mutant, and Ser132 phosphorylation of cyclic AMP response element-binding protein (CREB), which is an important mediator of PKA activation, correlated with the levels of PKA activity. Taken together, these results indicate that salmonellae cause an SPI-2-dependent increase in PKA activity that leads to CREB phosphorylation, resulting in up-regulation of IL-10 expression in Salmonella-infected macrophages. Suppression of IL-10 expression by an antisense oligonucleotide did not affect the growth of wild-type salmonellae within macrophages, whereas growth was dose dependently inhibited by H-89, suggesting that the PKA signaling pathway plays a significant role in intramacrophage Salmonella survival.
Macrophages are essential to defense mechanisms against many infections. A number of cytokines are known to affect the function of macrophages. Gamma interferon (IFN-γ), produced by antigen-specific helper T cells, activates macrophages (33, 34), and this activation is critical for the host defense against intracellular pathogens that can survive within macrophages (5, 22, 46). Macrophage functions may, however, be blocked by interleukin-4 (IL-4), IL-10, and transforming growth factor beta (7, 8, 36, 38, 50). In particular, IL-10, which is a well-characterized anti-inflammatory cytokine (30, 32, 43), can inhibit the production of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) in activated macrophages (7, 21), and macrophages are a major source of IL-10 production. Some intracellular pathogens, including several Salmonella spp., Mycobacterium spp., Legionella pneumophila, and Listeria monocytogenes, target macrophages as a site of infection. Some of these intracellular pathogens have been reported to use IL-10 to survive within macrophages or to inhibit the host defense by deactivating macrophages in murine models of infection (1, 4, 12, 14, 16, 21, 39, 47). Thus, IL-10 appears to play a role in creating a favorable environment for intracellular pathogens within macrophages.
Salmonellae invade and destroy specialized epithelial cells (M cells) in the host's intestine; migrate to the mesenteric lymph nodes, where they encounter macrophages; and subsequently survive and replicate within infected macrophages. Specific virulence factors encoded within Salmonella pathogenicity islands (SPI) are required at various stages of Salmonella infection (23). SPI-1, located at centisome 63 on the Salmonella enterica serovar Typhimurium chromosome, is required for salmonellae to enter epithelial cells (18, 19) and to promote apoptosis in macrophages (26). In addition, Hobbie et al. (27) reported that proteins secreted by the type III secretion system encoded within SPI-1 can trigger host cell signal transduction pathways that activate transcription factors NF-κB and AP1. This activation induces production of proinflammatory cytokines such as IL-8, which are presumably important in the inflammatory diarrhea that follows Salmonella infection.
SPI-2, which is a 40-kb pathogenicity island located at centisome 30.7 on the S. enterica serovar Typhimurium chromosome, is required for the ability to grow within mammalian cells, such as macrophages or epithelial cells, and is required for virulence in mice (11, 23, 24, 25, 35, 45). Previous work showed that a mutant defective in the spiC gene, which lies adjacent to the spiR (ssrA)/ssrB two-component regulatory genes within SPI-2, was unable to survive within macrophages and unable to inhibit fusion between Salmonella-containing vacuoles (SCV) and lysosomal and endosomal compartments (48). SpiC protein is translocated into the cytosol of infected macrophages by the type III secretion system in SPI-2. Lee et al. (29) further reported that the SpiC protein interacts with a host protein homologous to the family of NIPSNAP proteins, which may be involved in intracellular vesicular trafficking. However, other researchers showed that SpiC is required for the translocation of SPI-2 effector proteins into target cells (15, 51). Thus, more research is needed to clarify the molecular function of SpiC. Recent studies (10, 20, 49) have shown that SPI-2 is required to inhibit fusion of SCV with vesicles of the endocytic pathway, such as inducible nitric oxide synthase-containing vesicles. SPI-2 is also involved in inhibiting recruitment of NADPH oxidase components to the phagosome. These observations indicate that a role of SPI-2 is to interfere with intracellular vesicular trafficking to avoid exposure to toxic agents such as ROI and RNI in infected macrophages.
In this study, we analyzed cytokine expression in Salmonella-infected macrophages to examine the mechanisms by which SPI-2 promotes intramacrophage survival and show the involvement of SPI-2 in Salmonella-induced expression of IL-10 in macrophages. Salmonellae cause an SPI-2-dependent increase in PKA activity that leads to cyclic AMP (cAMP) response element-binding protein (CREB) phosphorylation, resulting in up-regulation of IL-10 expression in Salmonella-infected macrophages. In addition, we discuss the roles of endogenous IL-10 and the PKA signaling pathway in intramacrophage survival of salmonellae. This is the first report that SPI-2 affects a signal transduction pathway that induces cytokine expression.
MATERIALS AND METHODS
Reagents.
Reagents for cell culture were purchased from Sigma (St. Louis, Mo.), and other reagents were purchased from the following sources: H-89 was from Seikagaku (Tokyo, Japan); PD98052, SB203580, and GF109203X were from Calbiochem (La Jolla, Calif.); SP600125 and dibutyryl cAMP (dbcAMP) were from Biomol (Plymouth Meeting, Pa.); and recombinant murine IFN-γ was from PharMingen (San Diego, Calif.). H-89, PD98052, SB203580, GF109203X, and dbcAMP were dissolved in dimethyl sulfoxide (DMSO). When these drugs were used, the final concentration of DMSO in the culture medium was 0.1%; this concentration of solvent did not affect the cell responses.
Bacterial strains and growth conditions.
The strains used in this study were derived from wild-type S. enterica serovar Typhimurium strain 14028s. The spiC::kan derivative EG10128 and purB::Tn10 strain EG9652 were described by Uchiya et al. (48). Bacteria were grown at 37°C in Luria broth (LB). Kanamycin and tetracycline were used at 50 and 15 μg/ml, respectively.
Macrophage survival assay.
The murine macrophage cell line Raw264.7 was maintained in a 37°C incubator with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum (HyClone, Logan, Utah), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. On the day before infection, the macrophages were plated at a density of 1.0 × 106/well in 6-well tissue culture plates (Falcon) or 0.4 × 106/well in 24-well plates in medium without antibiotics. The macrophage survival assay was conducted as described previously (48). A multiplicity of infection of 25 bacteria per macrophage was used.
Semiquantitative reverse transcription (RT)-PCR and Northern blot analysis.
Total RNA was extracted from macrophages in six-well plates infected with bacteria (1:25) by using RNAzol B (TEL-TEST, Friendswood, Tex.) in accordance with the protocol recommended by the manufacturer and then digested with DNase I (Stratagene). RNA (2 μg) was reverse transcribed with SuperScript II reverse transcriptase (Life Technologies, Rockville, Md.) by using an oligo(dT) primer. PCR was conducted in 20-μl reaction mixtures consisting of reaction buffer (Perkin-Elmer, Forester City, Calif.), 0.5 μM deoxynucleoside triphosphate, each primer at 1 μM, 1 μl of cDNA, and 1 U of Taq DNA polymerase (Perkin-Elmer) in cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplification was carried out for 30 cycles for IL-1α, 35 cycles for IL-6, 25 cycles for IL-10, 30 cycles for IL-12, 20 cycles for tumor necrosis factor alpha (TNF-α), and 17 cycles for β-actin, followed by a 7-min final extension at 72°C. In each case, the number of amplification cycles achieved exponential amplification, in which product formation was proportional to the starting cDNA (data not shown). The PCR products were subjected to electrophoresis in a 1.5% agarose gel and visualized by ethidium bromide staining. Visual analysis and image-analyzing software (Gel-Doc 2000 System; Bio-Rad, Hercules, Calif.) were used for comparison of band intensities. The primer pairs were as follows: IL-1α, 5′-CTC TAG AGC ACC ATG CTA CAG-3′ and 5′-TGG AAT CCA GGG GAA ACA CTG-3′ (309-bp fragment); IL-6, 5′-TGG AGT CAC AGA AGG AGT GGC TAA-3′ and 5′-TCT GAC CAC AGT GAG GAA TGT CCA-3′ (155-bp fragment); IL-10, 5′-TAC CTG GTA GAA GTG ATG CCC-3′ and 5′-CAT CAT GTA TGC TTC TAT GCA-3′ (252-bp fragment); IL-12(p40), 5′-CGT GCT CAT GGC TGG TGC AAA-3′ and 5′-CTT CAT CTG CAA GTT CTT GGG-3′ (314-bp fragment); TNF-α, 5′-GGC AGG TCT ACT TTG GAG TCA-3′ and 5′-ACA TTC GAG GCT CCA GTG AAT-3′ (300-bp fragment); β-actin, 5′-TGG AAT CCT GTG GCA TCC ATG AAA-3′ and 5′-TAA AAC GCA GCT CAG TAA CAG TCC-3′ (350-bp fragment).
For Northern blot analysis of IL-10 mRNA, 20 μg of total RNA was electrophoresed in a 1% formaldehyde-agarose gel and transferred to nylon membranes (Duralon-UV; Stratagene) in 10× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The 32P-labeled probes for IL-10 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were incubated with the filters for 18 h at 42°C, followed by two washes with 2× SSC containing 0.1% sodium dodecyl sulfate (SDS) at 37°C for 15 min. Final washes were carried out at 55°C for 15 min with 0.2× SSC containing 0.1% SDS. Bands were visualized by autoradiography and analyzed with a GS-800 calibrated densitometer (Bio-Rad). The probe for IL-10 corresponds to bases 1 to 636 of the murine IL-10-coding region. GAPDH was used as an internal standard for quantification of total RNA in each lane of the gel.
Quantification of IL-10.
The amount of IL-10 present in culture supernatants was determined by a murine IL-10 capture enzyme-linked immunosorbent assay (ELISA) with IL-10-specific MAb (monoclonal antibody [Ab]) JES3-9D7 as the capture Ab and biotinylated anti-IL-10 MAb JES-12G8 as the detection Ab (PharMingen). The assay was performed in accordance with the manufacturer's recommendations and standardized with murine recombinant IL-10 (PharMingen). The sensitivity of the IL-10 ELISA was 10 pg/ml.
Western blot analysis.
Macrophages in six-well plates were changed to serum-free medium and then 5 h later were infected with bacteria (1:25). At the indicated times postinfection, the cells were washed twice with phosphate-buffered saline and homogenized in lysis buffer (50 mM Tris-HCl [pH 7.5], 1% Triton X-100, 1 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 20 μM aprotinin), and protein concentrations were determined with a protein assay kit (Bio-Rad) with bovine serum albumin as the standard. Total proteins (50 μg) were fractionated in SDS-10% polyacrylamide gels and then electrotransferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). After blocking with Tris-buffered saline containing 3% bovine serum albumin and 0.05% Tween 20, the membranes were incubated sequentially with primary Ab and horseradish peroxidase-conjugated secondary Ab. Bands were detected by chemiluminescence (ECL; Amersham Pharmacia Biotech) and analyzed with a GS-800 calibrated densitometer. Phosphorylation of p38 mitogen-activated protein kinase (MAPK) and CREB was determined with the PhosphoPlus p38 MAPK (Tyr182) and PhosphoPlus CREB (Ser132) kits from New England Biolabs (Beverly, Mass.), respectively.
PKA assay.
Protein kinase A (PKA) activation was assayed as described by McKenzie and Pouyssegur (31), with minor modifications. After infection with bacteria, the cells were washed twice with cold phosphate-buffered saline and scraped in a buffer (50 mM Tris-HCl [pH 7.5], 0.25 M sucrose, 5 mM EDTA, 10 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 20 μM aprotinin, 50 μM β-glycerophosphate, 200 μM sodium orthovanadate, 50 mM NaF). The scraped cells were sonicated and centrifuged at 100,000 × g for 30 min at 4°C. After determination of protein concentrations, the supernatants were assayed for PKA activity with Kemptide (Sigma) as the substrate. Total PKA activity was measured in the presence of 10 μM cAMP. PKA activity was calculated from the difference in ATP incorporation in the absence or presence of 1 μM PKA inhibitor peptide (Calbiochem) and expressed as a percentage of the total PKA activity.
Transfection with oligonucleotides (ODNs).
An antisense ODN (AS-ODN; nucleotide positions 637 to 654) directed against the sequence of murine IL-10 mRNA and a corresponding sense ODN (S-ODN) were synthesized, and transfections of macrophages with ODNs were conducted as described previously (2).
Statistical analysis.
Each experiment was performed at least three times. The results are expressed as means ± standard deviations (SD). Data on inhibitor effects on macrophage survival were analyzed with the unpaired two-tailed Student t test, and other data were analyzed by analysis of variance with Dunnett's test. A P value of <0.05 was considered statistically significant.
RESULTS
The spiC and purB mutants are defective for intramacrophage survival.
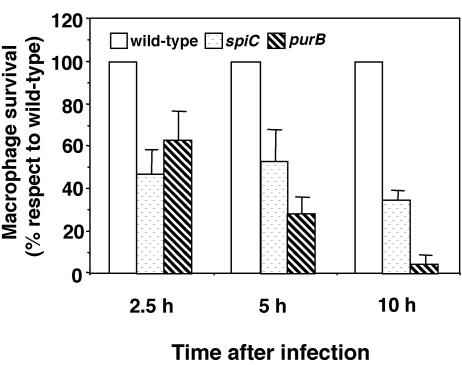
We previously reported that a strain carrying a nonpolar mutation in the spiC gene is defective for intramacrophage survival when assayed at 18 h postinfection (48). In this study, we examined in detail the levels of intramacrophage survival of wild-type, spiC mutant, and purB mutant salmonellae at 2.5, 5, and 10 h after infection with the macrophage-like cell line Raw264.7. The purB mutant, which is deficient in purine metabolism, is known to be defective for intramacrophage survival (6). As shown in Fig. 1, the level of intramacrophage survival of both spiC and purB mutants was about 50% of the wild-type level at 2.5 h postinfection. At 5 h postinfection, survival of the spiC mutant was unchanged, but that of the purB mutant was 30% of the wild-type level. At 10 h postinfection, the levels of the spiC and purB mutants were about 40 and 5% of the wild-type level, respectively. Because the spiC mutant is less defective in intramacrophage survival than is the purB mutant, the purB mutant can serve as a control to test whether spiC defects result simply from poor intramacrophage survival.
FIG. 1.
Salmonella growth levels within macrophages. Raw264.7 macrophages in 24-well tissue culture plates were infected at a ratio of 10 bacteria per cell, centrifuged, and incubated for 20 min to permit phagocytosis. Macrophages were lysed at 0, 2.5, 5, and 10 h and plated onto LB agar to determine the number of viable bacteria. For each mutant, the percent survival relative to that of the wild-type strain (100%) is presented. Data presented are the mean and SD of three independent experiments.
Expression of cytokine mRNAs in macrophages infected with salmonellae.
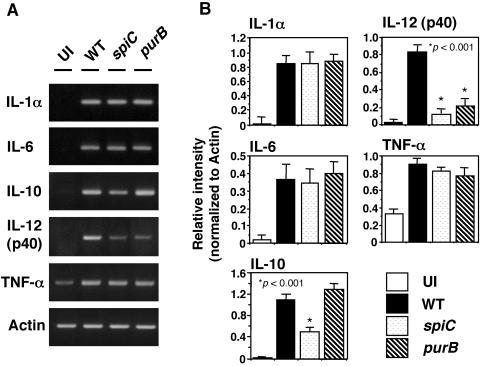
We next examined cytokine expression in macrophages infected with wild-type, spiC mutant, and purB mutant salmonellae. Expression was analyzed by RT-PCR of total RNA extracted from macrophages at 5 h postinfection. As shown in Fig. 2, the level of IL-10 mRNA was lower in spiC mutant-infected macrophages than in wild-type-infected macrophages. The purB mutation did not affect IL-10 levels, indicating that the lower level of IL-10 expression induced by spiC mutant salmonellae is due to defective SpiC function and is not a result of reduced replication within macrophages. On the other hand, the levels of IL-12(p40) expression induced by the spiC and purB mutants were lower than that induced by wild-type salmonellae, indicating that IL-12(p40) expression is dependent on the level of intracellular proliferation of salmonellae. The expression of proinflammatory cytokines IL-1α, IL-6, and TNF-α showed no difference following infection with wild-type salmonellae and the spiC mutant, suggesting that the spiC gene could be specifically influencing the signal transduction pathway involved in IL-10 expression.
FIG. 2.
Expression of cytokine mRNAs in macrophages infected with salmonellae. (A) At 5 h postinfection with wild-type (WT), spiC mutant, or purB mutant salmonellae, total RNA extracted from the pooled macrophages was reverse transcribed into cDNA and amplified by PCR with primer pairs specific to each cytokine. The PCR products were run on 1.5% agarose gels. (B) Expression of cytokines, normalized to actin expression. Data are the mean and SD of three independent experiments. The spiC mutant IL-10 level is significantly lower than that of wild-type salmonellae. *, P < 0.001 compared to macrophages infected with wild-type salmonellae. UI, uninfected.
Up-regulation of IL-10 expression is dependent on SPI-2.
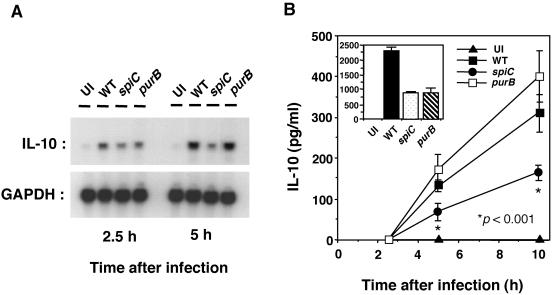
To confirm the RT-PCR analysis results, IL-10 mRNA in Salmonella-infected macrophages was quantified by Northern blot analysis. The expression of IL-10 mRNA in wild-type Salmonella-infected macrophages at 2.5 h postinfection was increased 1.4-fold compared with that in spiC mutant-infected macrophages (Fig. 3A). At 5 h postinfection, the difference in IL-10 mRNA expression was 2.5-fold. In accord with the RT-PCR analysis results, the purB mutant induced the same levels of IL-10 expression as did the wild type.
FIG. 3.
IL-10 expression in macrophages infected with salmonellae. (A) Northern blot analysis of IL-10 mRNAs in macrophages infected with wild-type (WT), spiC mutant, or purB mutant salmonellae. At 2.5 and 5 h postinfection, total RNA was extracted as described in Materials and Methods, run on 1.5% agarose gels, transferred to membranes, and hybridized with 32P-labeled DNA probes for IL-10 and GAPDH. The results of one of three experiments performed are presented. (B) Time course studies of Salmonella-induced IL-10 production by macrophages. Macrophages were infected with wild-type, spiC mutant, or purB mutant salmonellae. At the time points indicated, supernatants were harvested and tested for IL-10 content by ELISA. The inset shows the result at 20 h postinfection. Data are the mean and SD of three independent experiments. The spiC mutant IL-10 level is significantly lower than that of wild-type salmonellae. *, P < 0.001 compared to macrophages infected with wild-type salmonellae. UI, uninfected.
We next used an ELISA to examine the levels of IL-10 in culture supernatants of Salmonella-infected macrophages. As shown in Fig. 3B, IL-10 production increased during the course of infection. IL-10 levels were higher in macrophages infected with wild-type salmonellae or with the purB mutant than in those infected with the spiC mutant up to 10 h postinfection. At 20 h postinfection, however, IL-10 production in wild-type Salmonella-infected macrophages was threefold higher than that in spiC or purB mutant-infected macrophages, presumably because the purB mutant had little or no capacity to survive within macrophages at 20 h postinfection. These results show that the spiC gene is involved in the up-regulation of IL-10 production in Salmonella-infected macrophages.
Raw264.7 macrophages were used in this study because the levels of IL-10 expression in Raw264.7 macrophages infected with salmonellae were higher than those in J774 macrophages or in peritoneal macrophages obtained from female BALB/c mice. Nevertheless, the three types of macrophages had the same pattern of IL-10 expression after infection with wild-type, spiC mutant, or purB mutant salmonellae (data not shown).
Involvement of PKA and p38 MAPK in Salmonella-induced expression of IL-10.
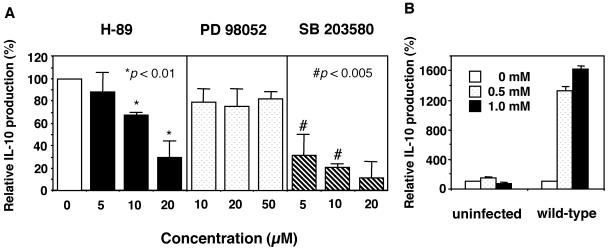
To understand the signal transduction pathways involved in Salmonella-induced expression of IL-10, we tested the effects of several protein kinase inhibitors on IL-10 induction. As shown in Fig. 4A, IL-10 production in wild-type Salmonella-infected macrophages was dose dependently blocked by the PKA inhibitor H-89 or the p38 MAPK inhibitor SB203580. In contrast, addition of the extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) inhibitor PD98052, the PKC inhibitor GF109203X, or the JNK inhibitor SP600125 did not inhibit IL-10 production (data not shown). These results indicate that both the PKA and p38 MAPK signaling pathways could participate in Salmonella-induced expression of IL-10. To examine further the involvement of PKA in IL-10 production, we used an activator of PKA, dbcAMP. The level of IL-10 production was increased 16-fold when Salmonella-infected macrophages were treated with 1.0 mM dbcAMP (Fig. 4B), whereas treatment of uninfected macrophages with dbcAMP had no effect on IL-10 production. These results show that up-regulation of IL-10 in Salmonella-infected macrophages may depend on the activation of PKA.
FIG. 4.
Involvement of PKA and p38 MAPK in Salmonella-induced IL-10 production by macrophages. (A) Effects of inhibitors of PKA (H-89), ERK1/2 (PD98052), and p38 MAPK (SB203580) on Salmonella-induced production of IL-10. Macrophages were infected with wild-type salmonellae, and an inhibitor at the indicated concentrations or a 0.1% DMSO solvent control was added simultaneously. At 18 h postinfection, supernatants were harvested and tested for IL-10 by ELISA. The graph shows percent IL-10 production by macrophages treated with inhibitors compared to that in untreated macrophages. Data are the mean and SD of three independent experiments. Both H-89 and SB203580 caused a significant reduction in the level of IL-10. *, P < 0.01; #, P < 0.005 (compared to macrophages treated with 0.1% DMSO solvent). (B) Effect of a PKA activator (dbcAMP) on Salmonella-induced production of IL-10. Macrophages were infected with wild-type or spiC salmonellae, and dbcAMP at the indicated concentrations or a 0.1% DMSO solvent control was added simultaneously. At 18 h postinfection, supernatants were harvested and tested for IL-10 content by ELISA. The graph shows percent IL-10 production by macrophages treated with dbcAMP compared to that by untreated macrophages. Data are the mean and SD of three independent experiments.
Salmonellae induce SPI-2-dependent PKA activation, resulting in up-regulation of IL-10 expression.
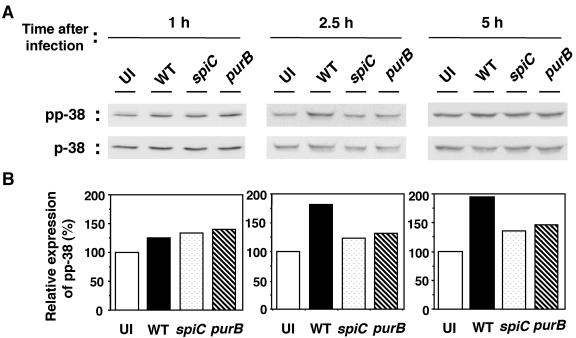
To examine the roles of p38 MAPK and PKA in Salmonella-mediated IL-10 induction, the levels of p38 MAPK phosphorylation and PKA activity were measured. p38 MAPK was assayed by Western blotting with phosphospecific antibodies. As shown in Fig. 5, infection with wild-type salmonellae increased p38 MAPK phosphorylation. The increase was detectable 1 h postinfection, and by 5 h postinfection, phosphorylation levels were 1.9-fold higher than in uninfected cells. At 10 h postinfection, the levels of p38 MAPK phosphorylation in Salmonella-infected macrophages returned to the basal level (data not shown). In contrast, the phosphorylation levels in spiC and purB mutant-infected macrophages were only slightly increased compared with those in uninfected macrophages, but there was no significant difference between the two mutant strains during the course of infection. Together with results obtained with the p38 MAPK inhibitor, these results indicate that, although p38 MAPK is involved in Salmonella-induced expression of IL-10, the increase in p38 MAPK phosphorylation is a result of the increased intracellular proliferation of wild-type salmonellae and may not depend on SpiC function.
FIG. 5.
Western blot analysis of phospho-p38 (pp-38) in macrophages infected with salmonellae. A Western blot analysis of cytosolic extracts from macrophages infected with wild-type (WT), spiC mutant, or purB mutant salmonellae is shown. At the indicated times postinfection, cytosolic extracts were prepared as described in Materials and Methods, run on an SDS-10% polyacrylamide gel, transferred to PVDF membranes, and developed with anti-phospho-p38 and anti-p38 Abs. Panel A shows the original blots. After analysis with an anti-phospho-p38 Ab (top), the membranes were stripped and reprobed with an Ab directed to p38 (bottom). (B) Densitometric analysis of the amount of phospho-p38 normalized to the amount of p38 in the same sample. The graphs show percentages of the value in uninfected macrophages. Similar results were obtained in three separate experiments. UI, uninfected.
PKA activity in wild-type Salmonella-infected macrophages was higher than that in uninfected macrophages (Table 1). In contrast, the levels of PKA activity in spiC mutant-infected macrophages were the same as those in uninfected macrophages. In addition, purB mutant-infected cells displayed wild-type levels of PKA activity up to 5 h postinfection, indicating that activation of PKA in wild-type Salmonella-infected macrophages depends on SpiC function.
TABLE 1.
PKA activity in macrophages infected with salmonellaea
| Strain used for infection | PKA activity (% of total)
|
||
|---|---|---|---|
| 2.5 h | 5 h | 10 h | |
| None (uninfected control) | 6.33 ± 0.70 | 7.63 ± 0.61 | 7.99 ± 0.66 |
| Wild type | 10.59 ± 0.26b | 12.58 ± 1.22b | 11.90 ± 0.38b |
| spiC mutant | 7.12 ± 0.57 | 6.42 ± 1.74 | 8.32 ± 0.74 |
| purB mutant | 9.42 ± 0.24b | 10.70 ± 0.59b | 9.46 ± 0.48 |
Macrophages were infected with wild-type, spiC mutant, or purB mutant salmonellae for 2.5, 5, and 10 h. Cell lysates were prepared and assayed for PKA activity as described in Materials and Methods. Results are expressed as the mean ± SD of three independent experiments.
P < 0.01 compared to macrophages infected with the spiC mutant.
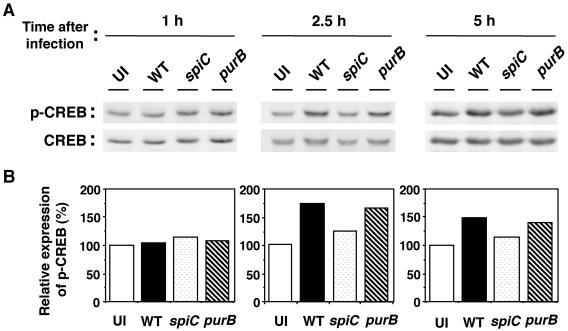
Since the spiC gene is involved in PKA activation, it was of interest to assay the phosphorylation of CREB. The catalytic subunit of activated PKA can phosphorylate CREB in the nucleus, which regulates the ability of CREB to activate transcription. Platzer et al. (40) reported that the IL-10 promoter contains a cAMP response element, where CREB binds, strongly suggesting that the phosphorylation of CREB is important for IL-10 expression. Thus, we measured the phosphorylation of CREB at Ser133 by Western blot analysis. As shown in Fig. 6, increased levels of CREB phosphorylation in Salmonella-infected macrophages were first observed at 2.5 h postinfection, and the elevation continued at 5 h. At 10 h postinfection, CREB phosphorylation returned to the basal level (data not shown). The purB mutant showed the same effect on CREB phosphorylation as the wild type. The spiC mutant, however, induced approximately 1.4-fold lower CREB phosphorylation at 2.5 and 5 h postinfection. Taken together, these results demonstrate a role for the spiC gene in the activation of PKA, but not p38 MAPK, that leads to the phosphorylation of CREB, resulting in increased IL-10 production in Salmonella-infected macrophages.
FIG. 6.
Western blot analysis of phospho-CREB (p-CREB) in macrophages infected with salmonellae. Macrophages were infected with wild-type (WT), spiC mutant, or purB mutant salmonellae. At the indicated times postinfection, cytosolic extracts were prepared as described in Materials and Methods, run on an SDS-10% polyacrylamide gel, transferred to PVDF membranes, and developed with anti-phospho-CREB and anti-CREB Abs. Panel A shows the original blots. Following analysis with an anti-phospho-CREB Ab (top), the membranes were stripped and reprobed with an Ab directed to CREB (bottom). (B) Densitometric analysis of amounts of phospho-CREB normalized to the amount of CREB in the same sample. The graphs show percentages of the value in uninfected macrophages. Similar results were obtained in three separate experiments. UI, uninfected.
The PKA signaling pathway plays a significant role in the intramacrophage survival of salmonellae.
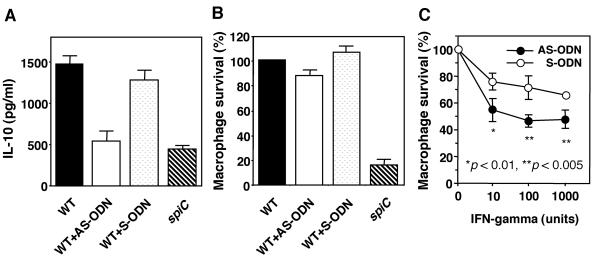
Having shown that salmonellae significantly induce production of IL-10 from macrophages, we tested whether increased IL-10 production is important for intramacrophage survival of salmonellae. For this purpose, an AS-ODN complementary to IL-10 mRNA was used to inhibit IL-10 production; a corresponding S-ODN served as a control. As shown in Fig. 7A, treatment with the AS-ODN (10 μM) decreased IL-10 production in wild-type Salmonella-infected macrophages to the level of IL-10 production in spiC mutant-infected macrophages. However, suppression of IL-10 production by the AS-ODN had little effect on the growth of wild-type salmonellae within macrophages (Fig. 7B). In addition, treatment of macrophages with recombinant IL-10 at 1 to 10 ng/ml had no significant effect on the intracellular growth of salmonellae (data not shown), indicating that IL-10 may not be required to mediate the role of SPI-2 in intramacrophage survival. To examine further the effect of IL-10 on the intramacrophage survival of salmonellae, macrophages activated by recombinant IFN-γ were used. Treatment with IFN-γ (10 to 1,000 U) significantly decreased the survival level of wild-type salmonellae in macrophages treated with the AS-ODN compared with that of macrophages treated with the S-ODN (P < 0.01 or P < 0.005, Fig. 7C). This result suggests that IL-10 could be inhibiting the cytotoxic mechanisms of macrophages activated by IFN-γ and shows the possibility that IL-10 plays an important role in the growth of salmonellae within activated macrophages in murine Salmonella infection.
FIG. 7.
Effects of AS-ODN directed against IL-10 mRNA on Salmonella survival within macrophages. (A) Effect of AS-ODN on IL-10 production. Macrophages were treated for 4 h with 10 μM AS-ODN or S-ODN and then infected with wild-type (WT) salmonellae. At 18 h postinfection, supernatants were harvested and tested for IL-10 by ELISA. Data are the mean and SD of three independent experiments. (B) Effect of AS-ODN on Salmonella survival within macrophages. Macrophages were treated for 4 h with AS-ODN or S-ODN (10 μM) and then infected with wild-type salmonellae. Samples were taken at 18 h and plated onto LB agar to determine the number of viable bacteria. The percent survival shown is relative to that of the wild-type strain within untreated macrophages. Data are the mean and SD of three independent experiments. (C) Effect of AS-ODN on Salmonella survival within IFN-γ-activated macrophages. Macrophages treated for 4 h with AS-ODN or S-ODN (10 μM) were infected with wild-type salmonellae, and IFN-γ at the indicated concentrations was added simultaneously. Samples were taken at 18 h and plated onto LB agar to determine the number of viable bacteria. The percent intramacrophage survival shown is relative to that of the wild-type strain within untreated macrophages. Data are the mean and SD of three independent experiments. AS-ODN caused a significant reduction on Salmonella survival within macrophages. *, P < 0.01; **, P < 0.005 (compared to macrophages treated with S-ODN).
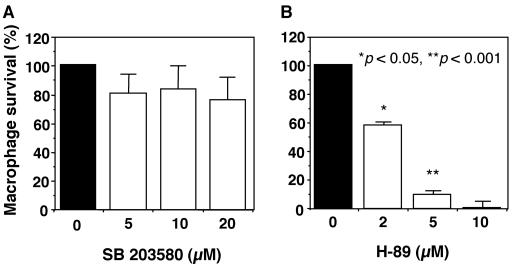
We next examined the influence of the PKA and p38 MAPK signaling pathways on the intramacrophage survival of salmonellae. As shown in Fig. 8, treatment with the p38 MAPK inhibitor SB203580 had little inhibitory effect on the intracellular growth of salmonellae, whereas growth was strongly decreased by treatment with the PKA inhibitor H-89 in a dose-dependent manner. These results indicate that the PKA signaling pathway plays a significant role in the intramacrophage survival of salmonellae and suggest that the role of SPI-2 in the intramacrophage survival of salmonellae is at least partly mediated by the PKA signaling pathway.
FIG. 8.
Effects of SB203580 (a p38 MAPK inhibitor) and H-89 (a PKA inhibitor) on Salmonella survival within macrophages. Macrophages were infected with the wild-type strain, and SB203580 (A) or H-89 (B) at the indicated concentrations or a 0.1% DMSO solvent control was added simultaneously. Samples were taken at 0 and 18 h and plated onto LB agar to determine the number of viable bacteria. The percent survival shown is relative to that of the wild-type strain within untreated macrophages. Data are the mean and SD of three independent experiments. H-89 caused a significant reduction in Salmonella survival within macrophages. *, P < 0.05; **, P < 0.001 (compared to macrophages treated with 0.1% DMSO solvent).
DISCUSSION
Here we show the involvement of SPI-2 in the signal transduction pathway that induces IL-10 expression in Salmonella-infected macrophages. Salmonellae cause activation of the PKA signaling pathway in a manner dependent on SPI-2, resulting in up-regulation of IL-10 expression. In addition, we also found that the PKA signaling pathway plays a significant role in the intramacrophage survival of salmonellae.
Previous work has shown roles for SPI-2 in inhibiting fusion of SCV with vesicles of the endocytic pathway, such as inducible nitric oxide synthase-containing vesicles, and in inhibiting recruitment of NADPH oxidase components to the phagosome (10, 20, 48, 49). To examine the mechanisms by which SPI-2 promotes intramacrophage survival of salmonellae, we focused on the cytokines expressed in Salmonella-infected macrophages, since a number of cytokines have been reported to affect the function of macrophages. In particular, IL-10 is known as a macrophage-deactivating factor, as are transforming growth factor beta and IL-4. Park et al. (39) reported that Legionella pneumophila, a facultative intracellular pathogen, stimulates production of IL-10 in human alveolar macrophages and blood monocytes, and IL-10 treatment enhances the growth of L. pneumophila in monocytes. Moreover, IL-10 may down-regulate the production of chemical mediators involved in inflammation, such as nitric oxide and ROI (7, 21). Certain viruses also can induce cellular IL-10 in macrophages (42, 44), whereas other viruses encode their own IL-10 homologs (28). Thus, IL-10 appears to create a favorable environment for pathogen survival in macrophages. These observations prompted us to examine the role of IL-10 in the intramacrophage survival of salmonellae. Unexpectedly, growth of salmonellae within macrophages was not affected either by antisense suppression of IL-10 production or by exogenously added IL-10. However, in macrophages activated by recombinant IFN-γ, the AS-ODN did inhibit the intracellular growth of salmonellae. Taken together, these results indicate that IL-10 does not directly mediate the role of SPI-2 in promoting intramacrophage survival of salmonellae. The results nevertheless raise the possibility that IL-10 plays an important role in the establishment of systemic infection by blocking the cytotoxic mechanisms of activated macrophages. Indeed, Arai et al. (1) showed that neutralization of endogenous IL-10 by anti-IL-10 MAb accelerates macrophage functions, enhancing the host defense against murine Salmonella infection.
Our results showed that SPI-2 is involved in the up-regulation of IL-10 expression in Salmonella-infected macrophages, but it does not affect the expression of proinflammatory cytokine IL-1α, IL-6, or TNF-α, suggesting that SPI-2 could influence the signal transduction pathway involved in IL-10 expression in macrophages. Therefore, we examined which signal transduction pathways are involved in Salmonella-mediated IL-10 induction. We found that inhibition of p38 MAPK and PKA blocked this induction, supporting the involvement of both the p38 MAPK and PKA signaling pathways in this process. p38 MAPK is commonly activated by inflammatory cytokines and stress stimuli (37). To examine further the role of p38 MAPK in Salmonella-induced expression of IL-10, we measured the phosphorylation of p38 MAPK. Wild-type salmonellae induced higher phosphorylation of p38 MAPK in macrophages than did spiC mutant salmonellae, but there was no significant difference between spiC mutant and purB mutant salmonellae, which, like spiC salmonellae, are defective for intramacrophage survival. The purB mutant thus tests whether the results obtained are due to the levels of intracellular growth of salmonellae or directly related to SpiC function. Our results imply that although p38 MAPK is involved in Salmonella-induced expression of IL-10, the increase in p38 MAPK phosphorylation is a result of the increased intracellular proliferation of wild-type salmonellae and is not dependent on SpiC function.
To confirm the involvement of the PKA signaling pathway in Salmonella-induced expression of IL-10, we measured PKA activity in infected macrophages. While wild-type salmonellae increased PKA activity in infected macrophages, the spiC mutant failed to induce any increase over the level in uninfected cells. Results obtained with the purB mutant control indicated that the increase in PKA activity depends on SpiC function and that the up-regulation of IL-10 production induced by wild-type salmonellae could occur through the activation of PKA. This conclusion is also supported by the fact that dbcAMP, which stimulates the PKA enzyme directly, enhanced IL-10 production in Salmonella-infected macrophages. Indeed, PKA activation leads to translocation of the catalytic subunit of PKA enzyme to the nucleus, where the phosphorylation of CREB takes place. A cAMP response element, where CREB binds, is present in the promoter region of the gene for IL-10 (40). Eigler et al. (13) reported that the activation of PKA by cAMP enhances IL-10 production in peripheral blood monocytes. Thus, since phosphorylation of CREB via PKA activation is important for the up-regulation of IL-10 production, we measured the phosphorylation levels of CREB in Salmonella-infected macrophages and found that they correlated with the results of PKA activity.
Given our finding that activation of PKA by salmonellae is dependent on SPI-2, we examined the role of the PKA signaling pathway in the intramacrophage survival of salmonellae. The intracellular growth of wild-type salmonellae was dose dependently decreased by treatment with a PKA inhibitor, suggesting that PKA activation could be a critical mechanism that salmonellae exploit to survive within macrophages. This conclusion is also supported by the following observations. PKA down-regulates the activation of the NADPH oxidase component p47phox in stimulated neutrophils, and it directly phosphorylates Rap1A, a low-molecular-weight GTP-binding protein (3, 17, 41). As phosphorylated Rap1A cannot bind to the flavocytochrome b558 component of the NADPH oxidase complex, no respiratory burst is elicited (9). Thus, PKA affects components of the NADPH oxidase complex and reduces its ability to produce superoxide radicals, indicating that PKA plays a significant role in superoxide-dependent mechanisms that kill ingested bacteria in phagocytes. Recent studies have shown that one of the mechanisms by which SPI-2 promotes the intramacrophage survival of salmonellae is interference with intracellular vesicular trafficking so as to avoid exposure to toxic agents such as ROI or RNI. To our knowledge, there are no reports that describe the relationship between the PKA signaling pathway and intracellular trafficking of vesicles. Taken together, these facts suggest that, in addition to inhibiting intracellular vesicular trafficking, SPI-2 also inhibits superoxide production by affecting components of the NADPH oxidase complex on the membrane of SCV through the activation of PKA. However, more research is needed to clarify the involvement of the PKA signaling pathway in the intramacrophage survival of salmonellae.
In conclusion, our study shows that Salmonella infection of macrophages causes activation of the PKA signaling pathway in a manner dependent on SPI-2, which leads to CREB phosphorylation and results in up-regulation of IL-10 expression. Although the mechanism by which SPI-2 affects the PKA signaling pathway remains unknown, our results show that this pathway plays a significant role in the intramacrophage survival of salmonellae.
Acknowledgments
We thank Ayako Terakado for technical assistance.
This work was supported by Grants-in-Aid for Specially Promoted Research of the Meijo University Research Institute, for the Scientific Frontier Research Project of Meijo University from the Ministry of Education, Culture, Sports, Science and Technology, and for Scientific Research (C) 11670277 from the Japan Society for the Promotion of Science to K.U.
Editor: A. D. O'Brien
REFERENCES
- 1.Arai, T., K. Hiromatsu, H. Nishimura, Y. Kimura, N. Kobayashi, H. Ishida, Y. Nimura, and Y. Yoshikai. 1995. Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology 85:381-388. [PMC free article] [PubMed] [Google Scholar]
- 2.Arima, H., M. Takahashi, Y. Aramaki, T. Sakamoto, and S. Tsuchiya. 1998. Specific inhibition of interleukin-10 production in murine macrophage-like cells by phosphorothioate antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 8:318-327. [DOI] [PubMed] [Google Scholar]
- 3.Bengis-Garber, C., and N. Gruener. 1996. Protein kinase A downregulates the phosphorylation of p47phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell. Signal. 8:291-296. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., and J. Champsi. 1993. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect. Immun. 61:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj, N., T. W. Nash, and M. A. Horwitz. 1986. Interferon-γ-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 137:2662-2669. [PubMed] [Google Scholar]
- 6.Blanc-Portard, A.-B., and E. A. Groisman. 1997. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16:5376-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin-10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdan, C., Y., and C. Nathan. 1993. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann. N. Y. Acad. Sci. 685:713-739. [DOI] [PubMed] [Google Scholar]
- 9.Bokoch, G. M., L. A. Quilliam, B. P. Bohl, A. J. Jesaitis, and M. T. Quinn. 1991. Inhibition of rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of rap1A. Science 254:1794-1796. [DOI] [PubMed] [Google Scholar]
- 10.Chakravortty, D., I. H. Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 12.Denis, M., and E. Ghadirian. 1993. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151:5425-5430. [PubMed] [Google Scholar]
- 13.Eigler, A., B. Siegmund, U. Emmerich, H. K. Baumann, G. Hartmann, and S. Endres. 1998. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 63:101-107. [DOI] [PubMed] [Google Scholar]
- 14.Flesch, I. E. A., and S. H. E. Kaufmann. 1994. Role of macrophages and αβ T lymphocytes in early interleukin 10 production during Listeria monocytogenes infection. Int. Immunol. 6:463-468. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, J. A., C. Rapple, V. Kuhle, M. Hensel, and S. I. Miller. 2002. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon proteins SseB and SseC. J. Bacteriol. 184:4971-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei, K., D. Nadal, H. W. Pfister, and A. Fontana. 1993. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J. Exp. Med. 178:1255-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabig, T. G., C. D. Cren, P. L. Mantel, and R. Rosli. 1995. Function of wild-type or mutant Rac2 and Rap1a GTPases in differentiated HL60 cell NADPH oxidase activation. Blood 85:804-811. [PubMed] [Google Scholar]
- 18.Galán, J. E., and R. Curtiss III. 1991. Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect. Immun. 59:2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galán, J. E., C. Ginocchio, and P. Costeas. 1992. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J. Bacteriol. 174:4338-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 21.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 22.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 23.Groisman, E. A., A.-B. Blanc-Portard, and K. Uchiya. 1999. Pathogenicity islands and the evolution of Salmonella virulence, p. 127-150. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 24.Hensel, M., J. E. Shea, B. Raupach, D. Monack, S. Falkow, C. Gleeson, T. Kubo, and D. F. Holden. 1997. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella pathogenicity island 2. Mol. Microbiol. 24:155-167. [DOI] [PubMed] [Google Scholar]
- 25.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 26.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychkinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbie, S., L. M. Chen, R. Davis, and J. E. Galán. 1997. Involvement of the mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 28.Kotenko, S., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, A. H., M. P. Zareei, and S. Daefler. 2002. Identification of a NIPSNAP homologue as host cell target for Salmonella virulence protein SpiC. Cell. Microbiol. 4:739-750. [DOI] [PubMed] [Google Scholar]
- 30.Lee, T.-S. and L.-Y. Chau. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 8:240-246. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie, F. R., and J. Pouyssegur. 1996. cAMP-mediated growth inhibition in fibroblasts is not mediated via mitogen-activated protein (MAP) kinase (ERK) inhibition. J. Biol. Chem. 271:13476-13483. [DOI] [PubMed] [Google Scholar]
- 32.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 33.Murray, H. W. 1988. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann. Intern. Med. 108:595-608. [DOI] [PubMed] [Google Scholar]
- 34.Nathan, C. R., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Farrell, A. M., Y. Liu, K. W. Moore, and A. L.-F. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for stat 3-dependent and -independent pathways. EMBO J. 17:1006-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono, K., and J. Han. 2000. The p38 signal transduction pathway activation and function. Cell. Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 38.Oswald, I. P., R. T. Gazzinelli, A. Sher, and S. L. James. 1992. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J. Immunol. 148:3578-3582. [PubMed] [Google Scholar]
- 39.Park, D. R., and S. J. Skerrett. 1996. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ. J. Immunol. 157:2528-2538. [PubMed] [Google Scholar]
- 40.Platzer, C., C. Meisel, K. Vogt, M. Platzer and H.-D. Volk. 1995. Up-regulation of monocytic IL-10 by tumor necrosis factor-α and cAMP elevating drugs. Int. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 41.Qilliam, L., H. Mueller, B. Bohl, V. Prossnitz, L. Sklar, C. Der, and G. Bokoch. 1991. Rap1A is a substrate for cyclic AMP-dependent protein kinase in human neutrophils. J. Immunol. 147:1628-1635. [PubMed] [Google Scholar]
- 42.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 43.Riley, J. K., K. Takeda, S. Akira, and R. D. Schreiber. 1999. Interleukin-10 receptor signaling through the Jak-STAT pathway: requirement for two distinct receptor-derived signals for anti-inflammatory action. J. Biol. Chem. 274:16513-16521. [DOI] [PubMed] [Google Scholar]
- 44.Schols, D., and E. De Clercq. 1996. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J. Virol. 70:4953-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 47.Tripp, C. S., S. F. Wolf, and R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 50.Wahl, S. M. 1992. Transforming growth factor beta: a cause and a cure. J. Clin. Immunol. 2:61-71. [DOI] [PubMed] [Google Scholar]
- 51.Yu, X.-J., J. Ruiz-Albert, K. E. Unsworth, S. Garvis, M. Liu, and D. W. Holden. 2002. SpiC is required for secretion of Salmonella pathogenicity island 2 type III secretion system proteins. Cell. Microbiol. 4:531-540. [DOI] [PubMed] [Google Scholar]