Abstract
Cross-sectional studies show that higher blood concentrations of inflammatory markers tend to be more common in frail older people, but longitudinal evidence that these inflammatory markers are risk factors for frailty is sparse and inconsistent. We investigated the prospective relation between baseline concentrations of the inflammatory markers C-reactive protein (CRP) and fibrinogen and risk of incident frailty in 2,146 men and women aged 60 to over 90 years from the English Longitudinal Study of Ageing. The relationship between CRP and fibrinogen and risk of incident frailty differed significantly by sex (p for interaction terms <0.05). In age-adjusted logistic regression analyses, for a standard deviation (SD) increase in CRP or fibrinogen, odds ratios (95 % confidence intervals) for incident frailty in women were 1.69 (1.32, 2.17) and 1.39 (1.12, 1.72), respectively. Further adjustment for other potential confounding factors attenuated both these estimates. For an SD increase in CRP and fibrinogen, the fully-adjusted odds ratio (95 % confidence interval) for incident frailty in women was 1.27 (0.96, 1.69) and 1.31 (1.04, 1.67), respectively. Having a high concentration of both inflammatory markers was more strongly predictive of incident frailty than having a high concentration of either marker alone. In men, there were no significant associations between any of the inflammatory markers and risk of incident frailty. High concentrations of the inflammatory markers CRP and fibrinogen are more strongly predictive of incident frailty in women than in men. Further research is needed to understand the mechanisms underlying this sex difference.
Keywords: Frailty, Inflammation, C-reactive protein, Fibrinogen, Longitudinal study
Introduction
Frailty is a clinically recognisable syndrome observed in older people whose core feature is an increased vulnerability to stressors due to impairments in multiple, inter-related systems, decreased physiological reserves and a decline in the ability to maintain homeostasis (Bergman et al. 2007;Fried et al. 2001). It is common (Syddall et al. 2010) and has numerous adverse consequences, including disability, falls, morbidity, hospitalisation, institutionalisation and death. At present, there is no universally accepted model or definition of frailty (Bergman et al. 2007), but it is generally agreed that its causes are complex and likely to involve both biological and psychosocial mechanisms (Walston et al. 2006;Rockwood et al. 1994).
Linda Fried and John Walston hypothesised several years ago that inflammation, coupled with neuroendocrine dysregulation and sarcopenia, might underlie the onset of frailty (Fried and Walston 1998) but as yet empirical evidence for this is limited. Although several cross-sectional studies—primarily in women—have reported higher blood concentrations of various inflammatory or coagulation markers such as C-reactive protein (CRP), interleukin-6 (IL-6), factor VIII and D-dimer in older people who meet the criteria for physical frailty that Fried et al. devised (Bandeen-Roche et al. 2006), the design of these studies makes it impossible to establish whether inflammation precedes or follows the development of frailty (Collerton et al. 2012; Walston et al. 2002; Leng et al. 2007; Hubbard et al. 2009; Schmaltz et al. 2005). Longitudinal evidence on whether such biomarkers are predictive of incident frailty in men and women is sparse and inconsistent. Four prospective studies have examined the link between various inflammatory or coagulation markers and subsequent frailty to date. Of these, two—based on men and women from the Cardiovascular Health Study and the Longitudinal Ageing Study Amsterdam, respectively—found significant associations between raised concentrations of CRP at baseline and increased risk of incident frailty at follow-up, but raised levels of IL-6 were not predictive of increased risk (Barzilay et al. 2007; Puts et al. 2005). In the Hertfordshire Ageing Study, higher levels of a range of immune-endocrine markers, including differential white cell counts, were associated with increased risk of later frailty in both sexes, although no association was found between CRP levels and frailty risk (Baylis et al. 2012). A fourth study, based on the Women’s Health Initiative, found that higher D-dimer and tissue plasminogen activator (t-PAS) were associated with higher risk of incident frailty, but neither CRP, IL-6, factor VIII or fibrinogen were linked with frailty risk (Reiner et al. 2009). There is a need for more longitudinal studies, particularly in men, to establish which inflammatory or coagulation markers are predictive of incident frailty and whether these associations are the same in both sexes.
The English Longitudinal Study of Ageing (ELSA) is a large population-based sample of older men and women. We used these data to investigate the prospective relation between the inflammatory markers, CRP, fibrinogen and ferritin and risk of incident physical frailty in men and women aged 60 to over 90 years.
Methods
Participants
The data for this study come from the ELSA. The sample for ELSA was based on people aged ≥50 years who had participated in the Health Survey for England in 1998, 1999 or 2001 (Marmot et al. 2011; Steptoe et al. 2012). It was drawn by postcode sector, stratified by health authority and proportion of households in non-manual socioeconomic groups. There were 11,392 people who participated in Wave 1 in 2002–2003. At Wave 2 in 2004–2005 and at Wave 4 in 2008–2009, participants who completed the main interview were invited to have a visit from a nurse that included measurements of physical function, anthropometry and blood sampling. Ethical approval was obtained from the Multicentre Research and Ethics Committee. Participants gave written informed consent.
Measures
Maximum handgrip strength was measured three times on each side using a dynamometer; the best of these measurements was used for analysis. Height and weight were measured with a portable stadiometer and electronic scales, respectively. Body mass index (BMI) was calculated as weight (in kilograms)/height (in meters)2. Gait speed was assessed in participants aged 60 and over by measuring the time taken to walk a distance of 8 ft at usual pace; the timed walk was repeated and the mean of the two measurements was calculated. Participants responded to three questions about the frequency with which they did vigorous, moderate or mild exercise. We ranked the combinations of responses to these questions according to the amount and intensity of exercise involved to provide an estimate of usual physical activity. Symptoms of depression were assessed using the eight-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (Steffick and The HRS Working Group 2000). We used these data, together with information on participants’ weight at the initial survey, to derive an indicator of physical frailty at Wave 2 and at Wave 4 in men and women aged ≥60 years using the Fried criteria (Fried et al. 2001). These criteria define physical frailty as the presence of three or more of the following: unintentional weight loss, weakness, self-reported exhaustion, slow walking speed and low physical activity. We operationalised these criteria using definitions very similar to those used in the original phenotype of frailty studies (Bandeen-Roche et al. 2006; Fried et al. 2001): weight loss was defined as either loss of ≥10 % of body weight since the initial survey (for frailty at Wave 2) or since Wave 2 (for frailty at Wave 4), or current BMI <18.5 kg/m2; weakness was defined as maximum grip strength in the lowest 20 % of the distribution, after taking sex and BMI into account; exhaustion was considered present if the participant gave a positive response to either of the CES-D questions ‘Felt that everything I did was an effort in the last week’ or ‘Could not get going in the last week’; slow walking speed was defined as a walking speed in the lowest 20 % of the distribution, after taking account of sex and height; and low physical activity was defined as physical activity in the lowest sex-specific 20 % of the distribution.
Participants were asked whether a doctor had ever told them that they had any of the following conditions: high blood pressure/hypertension, angina, heart attack, congestive heart failure, diabetes or high blood sugar, a stroke, chronic lung disease, arthritis or rheumatism, or cancer. For the purposes of these analyses, we created a single variable to indicate history of cardiovascular disease from the variables on angina, heart attack, congestive heart failure and stroke.
Cognitive function was assessed using tests of immediate and delayed verbal memory (recall of 10 aurally presented nouns), prospective memory (remembering to do a specific task), verbal (semantic) fluency (naming as many animals as possible in 60 s) and attention (letter cancellation task) (Marmot et al. 2011). A total cognitive function score was calculated by summing scores on these tests. Socioeconomic status was indexed by total household wealth, including savings and investments, value of any property or business assets, and net of debt, excluding pension assets. Household wealth has been identified as the most accurate indicator of long-term socioeconomic circumstances in ELSA (Banks et al. 2003).
Blood samples were taken from all participants except those who were not willing to give written consent, those with clotting or bleeding disorders and those taking anti-coagulant drugs. Fasting samples (defined as no food or drink except water for the past 5 h) were taken where possible (67 % of participants). Samples were assayed for C-reactive protein and fibrinogen at the Royal Victoria Infirmary, Newcastle-upon-Tyne, UK. Detailed information on the technicalities of the blood analysis, the internal quality control and the external quality assessment for the laboratory that carried it out are given in the 2004 Health Survey for England technical report as both the Health Survey for England and ELSA used the same laboratory, and the same guidelines and protocols for blood analysis (Graig et al. 2006).
Analytical sample
Of the 5,918 study members aged ≥60 years who were interviewed at Wave 2 in 2004–2005, 5,377 agreed to be visited by a nurse (91 %) and 4,571 (77 %) agreed to give a blood sample. Of these 4,571 people, 2,168 (47 %) were interviewed and visited by a nurse at Wave 4 in 2008–2009. The present analysis is based on 2,146 people who had complete data on baseline covariates at Wave 2 and frailty at Wave 4 and who were not frail at baseline.
Statistical analysis
The distribution of blood concentrations of CRP and fibrinogen was skewed and was transformed to normality using logarithms. We used the t test and chi-square test to examine differences in baseline characteristics between men and women according to the presence of incident frailty at follow-up. We used logistic regression to examine the relation between each inflammatory marker and risk of incident frailty in men and women separately. Initially, we examined the unadjusted risk of incident frailty according to thirds of the distribution of CRP and fibrinogen, using these variables in their original untransformed state. Having established that there was no indication that the associations were non-linear, we then examined risk of incident frailty according to a standard deviation (SD) increase in log CRP and log fibrinogen, controlling for potentially confounding factors (age, household wealth, smoking status, BMI, depressive symptom score, cognitive function, history of cardiovascular disease, diabetes, hypertension, chronic lung disease, arthritis, cancer and number of frailty criteria present at baseline). All analyses were weighted to account for non-response and the complex survey design. Finally, we examined whether the risk of incident frailty associated with the combination of CRP and fibrinogen was greater than the risk associated with each inflammatory marker individually. For this analysis—which was carried out in women only—we divided participants into four categories: those in the upper third of the distribution for both inflammatory markers, those in the upper third for CRP but not fibrinogen, those in the upper third for fibrinogen but not CRP and the reference category of those who were not in the upper third for either marker.
Results
Table 1 shows the baseline characteristics of the 2,146 men and women in the study according to whether they became frail during the follow-up period. The weighted percentage of men and women who became frail during follow-up was 11 % and 16 %, respectively. In both men and women, incident cases of frailty tended to be older, to have poorer cognitive function, to have a history of diabetes, arthritis and chronic lung disease, and to meet more of Fried’s criteria for frailty at baseline. In both sexes, incident frailty occurred more commonly in those who were in the lowest quintile for household wealth at baseline, but lack of wealth was much more strongly predictive of incident frailty in women than in men; in men, the relation was of borderline statistical significance only. In women only, incident cases of frailty were associated with being more depressed, having a higher BMI, having a history of cardiovascular disease and hypertension, and having higher blood concentrations of CRP and fibrinogen at baseline.
Table 1.
Baseline characteristics of the men (n = 988) and women (n = 1,158) in the study according to incident frailty at follow-up
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Frail (n = 80) | Not frail (n = 908) | p value | Frail (n = 150) | Not frail (n = 1,008) | p value | |
| Age (years), mean (SE) | 76.2 (1.17) | 69.2 (0.24) | <0.0001 | 76.7 (0.87) | 69.7 (0.28) | <0.0001 |
| Lowest quintile of wealth (%) | 21.1 | 13.1 | 0.09 | 34.3 | 17.0 | <0.0001 |
| Current smoker (%) | 17.4 | 11.2 | 0.16 | 12.0 | 8.8 | 0.25 |
| History of cardiovascular disease (%) | 21.5 | 18.5 | 0.52 | 19.4 | 10.4 | 0.004 |
| History of diabetes (%) | 21.1 | 6.49 | <0.0001 | 13.3 | 5.05 | 0.0003 |
| History of chronic lung disease (%) | 10.9 | 5.67 | 0.07 | 14.2 | 5.24 | 0.0002 |
| History of arthritis (%) | 39.1 | 27.1 | 0.03 | 69.9 | 42.0 | <0.0001 |
| History of cancer (%) | 9.86 | 7.48 | 0.528 | 10.6 | 8.51 | 0.44 |
| History of hypertension (%) | 48.7 | 40.5 | 0.17 | 63.8 | 43.0 | <0.0001 |
| No. of frailty criteria present, mean (SE) | 1.03 (0.09) | 0.50 (0.03) | <0.0001 | 1.13 (0.07) | 0.46 (0.02) | <0.0001 |
| Depressive symptom score ≥4 (%) | 4.47 | 4.13 | 0.89 | 20.0 | 9.90 | 0.001 |
| Cognitive function, mean (SE) | 24.8 (0.52) | 28.3 (0.19) | <0.0001 | 25.8 (0.53) | 29.3 (0.21) | <0.0001 |
| BMI, mean (SE) | 27.0 (0.54) | 27.1 (0.12) | 0.37 | 28.9 (0.46) | 27.4 (0.16) | 0.002 |
| C-reactive protein (mg/l), geometric mean (SE) | 2.14 (1.13) | 1.90 (1.03) | 0.36 | 2.84 (1.09) | 2.05 (1.03) | 0.002 |
| Fibrinogen (g/l), geometric mean (SE) | 3.12 (1.03) | 3.09 (1.01) | 0.85 | 3.36 (1.02) | 3.18 (1.01) | 0.01 |
Preliminary analyses showed that the relationships between baseline concentrations of CRP and fibrinogen and risk of incident frailty differed significantly between the sexes (p for interaction terms both <0.05). Logistic regression analyses of frailty risk were therefore carried out in men and women separately.
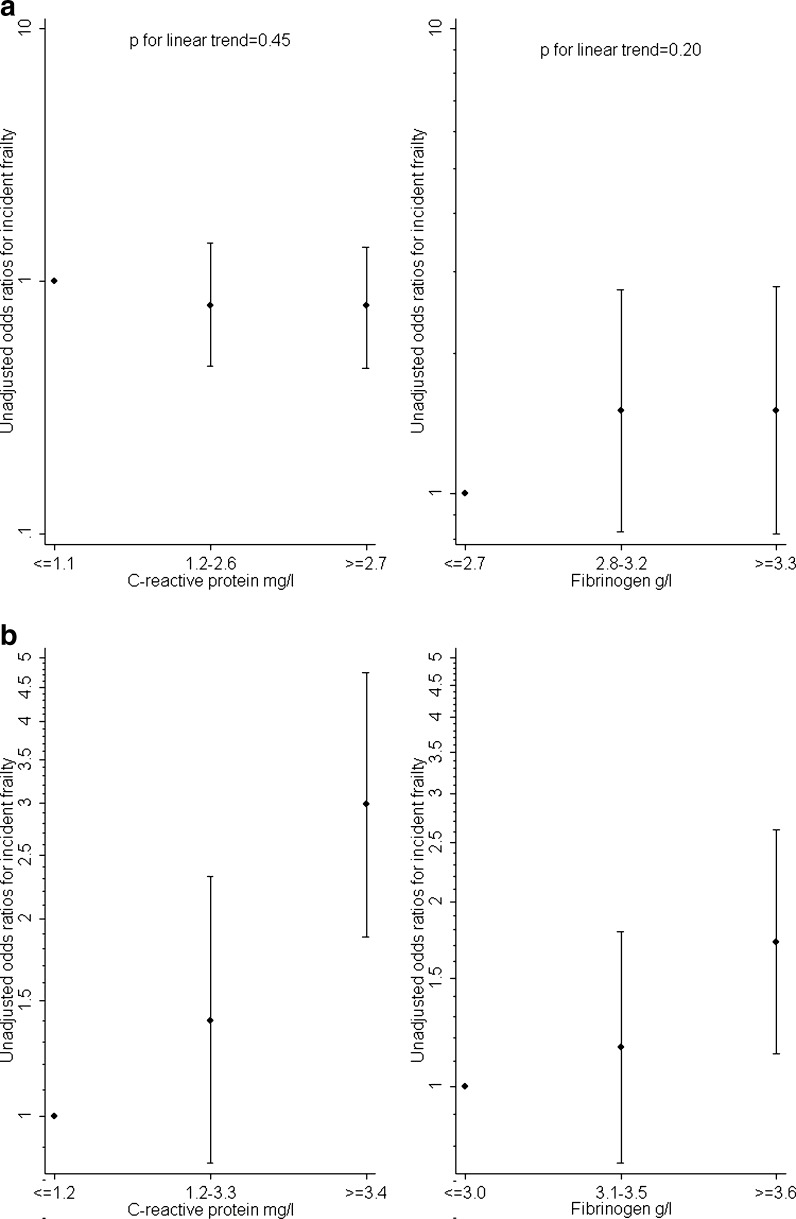
Figure 1a and b shows unadjusted odds ratios for incident frailty according to thirds of the distribution of concentrations of CRP and fibrinogen in men and women using the lowest third as the reference category. In men, there were no significant linear trends between concentrations of either inflammatory marker and risk of incident frailty. In women, risk of incident frailty rose with increasing concentrations of both CRP and fibrinogen (p for linear trend <0.0001).
Fig. 1.
a, b Unadjusted odds ratios for incident frailty in men (a) and women (b) according to thirds of the distribution of blood concentrations of CRP and fibrinogen at baseline
As there was no indication that the relationships between inflammatory markers and risk of incident frailty were non-linear, we examined risk of frailty for a standard deviation increase in CRP and fibrinogen to give us greater statistical power. Table 2 shows adjusted odds ratios from these analyses. Results are shown adjusted first for age and then with further adjustment for the other potential confounding variables measured at baseline, household wealth, depressive symptoms, cognitive function, BMI, smoking, number of frailty criteria present, history of cardiovascular disease, diabetes, hypertension, chronic lung disease, arthritis and cancer. In men, there were no significant associations between the inflammatory markers and risk of incident frailty in age-adjusted analyses. Further adjustment for the potential confounding variables had little effect on these estimates. In women, in models adjusting for age alone there were significant associations between raised concentrations of CRP and fibrinogen at baseline and risk of incident frailty. For an SD increase in CRP and fibrinogen, the age-adjusted odds ratio (95 % confidence interval) for incident frailty was 1.69 (1.32, 2.17) and 1.39 (1.12, 1.72), respectively. Further adjustment for other potential confounding factors attenuated both these estimates. For an SD increase in CRP and fibrinogen, the fully adjusted odds ratio (95 % confidence interval) for incident frailty was 1.27 (0.96, 1.69) (p = 0.09) and 1.31 (1.04, 1.67) (p = 0.03), respectively.
Table 2.
Adjusted odds ratios (95 % CI) for incident frailty in men and women according to concentrations of CRP and fibrinogen at baseline
| Inflammatory marker | Men | Women | ||
|---|---|---|---|---|
| OR (95 % CI), adjusted for age | OR (95 % CI), fully adjusteda | OR (95 % CI), adjusted for age | OR (95 % CI), fully adjustedb | |
| C-reactive protein, per SD | 0.98 (0.72, 1.32) | 0.93 (0.66, 1.30) | 1.69 (1.32, 2.17) | 1.27 (0.96, 1.69) |
| Fibrinogen, per SD | 0.92 (0.70, 1.21) | 0.89 (0.67, 1.18) | 1.39 (1.12, 1.72) | 1.31 (1.04, 1.67) |
aThe SD of natural log C-reactive protein is 1.5 in men and women. The SD of natural log fibrinogen is 0.21 in men 0.20 in women
bAdjusted for age, household wealth, depressive symptoms, cognitive function, body mass index, smoking, number of frailty criteria present, history of cardiovascular disease, diabetes, hypertension, chronic lung disease, arthritis and cancer
We repeated these multivariate analyses to examine risk of incident frailty according to thirds of the distribution of CRP and fibrinogen. In men, compared to those in the lowest third of the distribution of CRP, the fully adjusted odds ratios (95 % confidence interval) for incident frailty for those in the middle and top thirds of the distribution were 1.20 (0.63, 2.50) and 0.94 (0.49, 1.80), respectively; the equivalent figures for those in the middle and top thirds of the distribution of fibrinogen compared to those in the lowest third were 1.30 (0.66, 2.62) and 0.90 (0.44, 1.84), respectively. In women, those in the top third of the distribution of either CRP or fibrinogen had an increased risk of incident frailty compared to those in the bottom third, but these associations did not reach statistical significance: the fully adjusted odds ratios (95 % confidence interval) for incident frailty for those in the middle and top thirds of the CRP distribution were 0.81 (0.45, 1.44) and 1.41 (0.80, 2.49), respectively; the equivalent figures for those in the middle and top thirds of the distribution of fibrinogen compared to those in the lowest third were 0.99 (0.63, 1.62) and 1.32 (0.81, 2.17), respectively.
Finally, we examined whether women who had high blood concentrations of both fibrinogen and CRP (defined here as being in the top third of the distribution of both these inflammatory markers) had a greater risk of incident frailty than those who had high blood concentrations of either of these markers alone (defined as being in the top third of either fibrinogen or CRP only). Results of this analysis are shown in Table 3. Risk of incident frailty was greatest in women with high concentrations of both inflammatory markers. Having high concentrations of either marker alone was not associated with increased risk, although it is important to note that confidence intervals around these latter estimates were wide and numbers of cases of frailty in the group with high concentrations of fibrinogen alone were small.
Table 3.
Adjusted odds ratios (95 % CI) for incident frailty in women: comparing the effect of high fibrinogen alone, high CRP alone, or high fibrinogen and CRP combined
| Inflammatory marker | No. of cases/no at risk | OR (95 % CI), adjusted for age | OR (95 % CI), fully adjusteda |
|---|---|---|---|
| Neither fibrinogen nor CRP in upper third of distribution | 60/635 | 1.0 | 1.0 |
| Only fibrinogen in upper third of distribution | 10/122 | 0.67 (0.32, 1.40) | 0.63 (0.28, 1.39) |
| Only CRP in upper third of distribution | 31/174 | 1.93 (1.18, 3.17) | 1.10 (0.62, 1.95) |
| Both fibrinogen and CRP in upper third of distribution | 45/203 | 2.82 (1.80, 4.42) | 1.89 (1.12, 3.21) |
aAdjusted for age, household wealth, depressive symptoms, cognitive function, body mass index, smoking, number of frailty criteria present, history of cardiovascular disease, diabetes, hypertension, chronic lung disease, arthritis and cancer
Discussion
In this large longitudinal study of people aged 60 to over 90 years, higher baseline concentrations of the inflammatory markers CRP and fibrinogen were each separately associated with an increased risk of incident frailty in women. After adjustment for potential confounding factors, the association between CRP and risk of incident frailty was attenuated such that it became of borderline statistical significance, but that between fibrinogen concentration and risk of incident frailty remained significant. Having a high concentration of both inflammatory markers was more strongly predictive of incident frailty than having a high concentration of either marker alone. In men, by contrast, there was no evidence that higher concentrations of either of these inflammatory markers were associated with increased risk.
Existing evidence on whether the relationship between inflammatory markers and risk of frailty is the same in men and women is sparse. Of the three previous prospective studies that have examined the link between inflammation and risk of frailty in mixed sex populations, all have analysed men and women together (Puts et al. 2005; Baylis et al. 2012; Barzilay et al. 2007). One of these studies reported that associations between a range of immune-endocrine markers—among them differential white cell counts—and later risk of frailty were similar in men and women (Baylis et al. 2012). In contrast to the present study, no association was found between CRP concentration and subsequent frailty in either sex (Baylis et al. 2012). Another study reported that the association found between CRP levels, categorised into three groups, and subsequent incident frailty did not differ significantly between the sexes (Puts et al. 2005); whether treating CRP as a categorical rather than continuous variable in this analysis affected the authors’ ability to detect any interaction by sex is uncertain. Our finding that the relationship between the inflammatory factors CRP and fibrinogen and risk of incident frailty was markedly stronger in women is therefore novel. However, previous studies have found evidence of a sex difference in associations between inflammatory markers and outcomes other than frailty. For instance, concentrations of CRP or fibrinogen have been much more strongly associated with type 2 diabetes (Thorand et al. 2007; Lee et al. 2009), the metabolic syndrome (Rudnicka et al. 2011), blood pressure (Pruijm et al. 2013) and mortality (Jenny et al. 2007) in women than in men.
The reasons why inflammatory markers were more strongly associated with frailty in women than in men in our study are unclear. One potential explanation may lie in differences in distribution of body fat. Women’s tendency to have more adipose tissue than men results in greater hepatic production of CRP. As a result, associations between CRP and body size or fat mass tend to be stronger in women than in men (Khera et al. 2009; Bowles et al. 2003; Thorand et al. 2006). In the present study, correlations between CRP and BMI were markedly greater in women than in men (r = 0.42 vs. r = 0.22); a similar difference was observed in the case of correlations between fibrinogen and BMI (r = 0.19 vs. r = 0.06). Obesity-triggered inflammation could have a greater influence on the onset of frailty in women. In this study, high BMI was associated with an increased risk of incident frailty in women, but not in men. We examined whether BMI accounted for the interaction between sex and inflammatory factors (data not shown). Adjustment for BMI weakened the interaction term, but it remained statistically significant. We lacked specific data on fat mass so we were not able to investigate what role it might play in the interaction by sex. Another possibility may be that endogenous sex hormone levels or HPA axis hormones such as dehydroepiandosterone interact with inflammatory markers to influence risk of frailty (Voznesensky et al. 2009), but no data were available to explore this. There are indications that lower levels of physical activity may be more strongly linked to poorer physical function in women than in men (Martin et al. 2008), but, to our knowledge, there have been no reports of a sex difference in the inverse association between physical activity and levels of inflammatory markers (Klenk et al. 2013; Rothenbacher et al. 2003) so this is unlikely to explain our results. Our findings could perhaps have arisen if men with high levels of inflammatory markers were less likely to take part in the follow-up survey due to death or non-response than women with high levels of these markers, but we found no evidence of this. However, it is important to note that the confidence intervals around the multivariate-adjusted estimates in our sample of 988 men were very wide. For a SD increase in baseline CRP in men, for instance, our results were statistically compatible with a reduction of as much as 53 % in the risk of incident frailty or with an increase in risk of up to 29 %. Further large studies specifically in men are needed to investigate the nature of the relation between inflammatory markers and risk of incident frailty in this group.
The strengths of our study include the large sample size, the fact that it is representative of the community-dwelling English population aged 60 and over (Taylor et al. 2003), and our use of a prospective study design in which we assessed incident cases of frailty that occurred after the measurement of inflammatory markers. There are also some weaknesses. Firstly, not all the participants in the baseline survey gave a blood sample (77 % of those interviewed); non-responders to the blood sample tended to be older and in poorer health. However, all analyses have been weighted to reduce any potential bias arising from differential non-response. Secondly, there was limited characterisation of inflammatory markers or body composition at baseline and no information on the endocrine axis. Thirdly, it is possible that results of our multivariate analyses might be affected by residual confounding as we lacked detailed data on the medications that participants were taking at baseline and on the severity of the specific chronic diseases that they reported; furthermore, information on the prevalence of such diseases may have been incomplete.
In this prospective study of people aged 60 to over 90 years, higher concentrations of the inflammatory markers CRP and fibrinogen were predictive of incident frailty in women but not in men. Future research needs to investigate the mechanisms underlying this sex difference.
Acknowledgement
We are grateful to the UK Data Archive for supplying the ELSA data. The original data creators, depositors or copyright holders, the funders of the data collections and the UK Data Archive bear no responsibility for the analysis or interpretation presented here.
Footnotes
Cyrus Cooper and Avan Aihie Sayer are joint senior authors.
References
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Banks J, Karlsen S, Oldfield Z. Socio-economic position. In: Marmot M, Banks J, Blundell R, Lessof C, Nazroo J, editors. Health, wealth and lifestyles of the older population in England. London: Institute of Fiscal Studies; 2003. pp. 71–125. [Google Scholar]
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA (2012) Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr) [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles LK, Cooper JA, Howarth DJ, Miller GJ, MacCallum PK. Associations of haemostatic variables with body mass index: a community-based study. Blood Coagul Fibrinolysis. 2003;14:569–573. doi: 10.1097/00001721-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, Von ZT, Kirkwood TB. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard WR, Blass J, Ettinger WH, Halter J, Ouslander J, editors. Principles of geriatric medicine and gerontology. New York: McGraw-Hill; 1998. pp. 1387–1402. [Google Scholar]
- Graig R, Deverill C, Pickering K. Quality control of blood, saliva and urine analytes. In: Spronston K, Mindell J, editors. Health Survey for England 2004, methodology and documentation. London: The Information Centre; 2006. pp. 34–41. [Google Scholar]
- Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation biomarkers and near-term death in older men. Am J Epidemiol. 2007;165:684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, de Lemos JA. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk J, Denkinger M, Nikolaus T, Peter R, Rothenbacher D, Koenig W (2013) Association of objectively measured physical activity with established and novel cardiovascular biomarkers in elderly subjects: every step counts. J Epidemiol Community Health 67:194–197 [DOI] [PubMed]
- Lee CC, Adler AI, Sandhu MS, Sharp SJ, Forouhi NG, Erqou S, Luben R, Bingham S, Khaw KT, Wareham NJ. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52:1040–1047. doi: 10.1007/s00125-009-1338-3. [DOI] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Marmot M, Nazroo J, Banks J, Blundell R, Erens B, Lessof C, Huppert FA. English Longitudinal Study of Ageing: Wave 0 (1998, 1999 and 2001) and Waves 1–4 (2002–2009) [computer file] 15. Colchester, Essex: UK Data Archive [distributor]; 2011. [Google Scholar]
- Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA. Relationship between customary physical activity, muscle strength and physical performance in older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2008;37:589–593. doi: 10.1093/ageing/afn148. [DOI] [PubMed] [Google Scholar]
- Pruijm M, Vollenweider P, Mooser V, Paccaud F, Preisig M, Waeber G, Marques-Vidal P, Burnier M, Bochud M. (2013) Inflammatory markers and blood pressure: sex differences and the effect of fat mass in the CoLaus Study. J Hum Hypertens 27:169–175 [DOI] [PubMed]
- Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB, Kooperberg CL, Woods NF, LaCroix AZ. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med. 2009;122:947–954. doi: 10.1016/j.amjmed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150:489–495. [PMC free article] [PubMed] [Google Scholar]
- Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163:1200–1205. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Rumley A, Whincup PH, Lowe GD, Strachan DP. Sex differences in the relationship between inflammatory and hemostatic biomarkers and metabolic syndrome: British 1958 Birth Cohort. J Thromb Haemost. 2011;9:2337–2344. doi: 10.1111/j.1538-7836.2011.04517.x. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Steffick DE, The HRS working group (2000) Documentation of affective functioning measures in the Health and Retirement Study. HRS/AHEAD Documentation Report DR-005 [online report]
- Steptoe A, Breeze E, Banks J, Nazroo J. (2012) Cohort Profile: The English Longitudinal Study of Ageing. Int J Epidemiol [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie SA. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39:197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Conway L, Calderwood L, Lessof C. Methodology. In: Marmot M, Banks J, Blundell R, Lessof C, Nazroo J, editors. Health, wealth and lifestyles of the older population in England: the 2002 English Longitudinal Study of Ageing. London: Institute of Fiscal Studies; 2003. pp. 357–374. [Google Scholar]
- Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, Giani G, Koenig W. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184:216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Kolb H, Meisinger C, Chambless L, Koenig W, Herder C. Sex differences in the prediction of type 2 diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetes Care. 2007;30:854–860. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009;38:401–406. doi: 10.1093/ageing/afp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]