Abstract
The mycobacterial cell wall component lipoarabinomannan (LAM) has been described as a virulence factor of Mycobacterium tuberculosis, and modification of the terminal arabinan residues of this compound with mannose caps (producing mannosyl-capped LAM [ManLAM]) in M. tuberculosis or with phosphoinositol caps (producing phosphoinositol-capped LAM [PILAM]) in Mycobacterium smegmatis has been implicated in various functions associated with these lipoglycans. A structure-function analysis was performed by using LAMs and their biosynthetic precursor lipomannans (LMs) isolated from different mycobacterial species on the basis of their capacity to induce the production of interleukin-12 (IL-12) and/or apoptosis of macrophage cell lines. Independent of the mycobacterial species, ManLAMs did not induce IL-12 gene expression or apoptosis of macrophages, whereas PILAMs induced IL-12 secretion and apoptosis. Interestingly, uncapped LAM purified from Mycobacterium chelonae did not induce IL-12 secretion or apoptosis. Furthermore, LMs, independent of their mycobacterial origins, were potent inducers of IL-12 and apoptosis. The precursor of LM, phosphatidyl-myo-inositol dimannoside, had no activity, suggesting that the mannan core of LM was required for the activity of LM. The specific interaction of LM with Toll-like receptor 2 (TLR-2) but not with TLR-4 suggested that these responses were mediated via the TLR-2 signaling pathway. Our experiments revealed an important immunostimulatory activity of the biosynthetic LAM precursor LM. The ratio of LAM to LM in the cell wall of mycobacteria may be an important determinant of virulence, and enzymes that modify LM could provide targets for development of antituberculosis drugs and for derivation of attenuated strains of M. tuberculosis.
Mycobacterium tuberculosis is the causative agent of pulmonary tuberculosis in humans and leads to an estimated 2 million to 3 million deaths worldwide each year. This organism is one of the most successful bacterial pathogens, having infected and persisted in an estimated 2 billion humans (8). In order to evade the host's immune response, M. tuberculosis has evolved multiple strategies to manipulate infected host cells (9). Two important mechanisms are the suppression of interleukin-12 (IL-12) production and the inhibition of infection-induced apoptosis in macrophages. IL-12 is a key cytokine in the host defense against mycobacterial infections, and humans and mice deficient in IL-12 responses are highly susceptible to mycobacterial infections (2, 17). The importance of apoptosis in the host's innate immune response is suggested by the correlation of the virulence of mycobacterial strains with their capacity to inhibit apoptosis (18, 29). In addition, macrophages from mouse strains susceptible to mycobacterial infections undergo less apoptosis after infection than macrophages from a mouse strain that is resistant to mycobacterial infections undergo (29). However, the mechanisms by which M. tuberculosis manipulates the host cell responses to infection are only poorly understood.
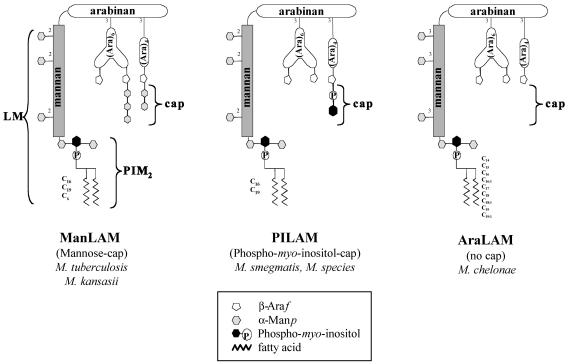
Lipoarabinomannan (LAM) is a cell wall lipoglycan, and to date three families of LAMs isolated from mycobacteria have been described (24). These lipoglycans are composed of three structural domains, a glycosylphosphatidyl anchor, a d-mannan core, and a large terminal d-arabinan. d-Mannan and d-arabinan represent the carbohydrate skeleton; the mannan core is terminated by the glycosylphosphatidyl anchor at its reducing end, whereas the arabinan domain is capped by either mannosyl (7, 19, 28, 33) or phosphoinositol residues (12, 19) (Fig. 1). The presence and the structure of capping allow classification of LAM molecules into three major classes (24). First, mannosylated LAMs (ManLAMs), which are characterized by the presence of mannosyl caps, are found in pathogenic species, including M. tuberculosis and Mycobacterium kansasii (14). Second, phosphoinositol-capped LAMs (PILAMs), which are characterized by the presence of phosphoinositol caps, have been isolated from nonpathogenic species, such as Mycobacterium smegmatis and another unidentified fast-growing mycobacterial species (Mycobacterium sp.) (12, 19). Finally, uncapped LAM (AraLAM) isolated from Mycobacterium chelonae, which lacks both mannose and phosphoinositol caps, is representative of the newly discovered family of LAM molecules (15, 24) (Fig. 1).
FIG. 1.
Structures of the three representative families of LAM molecules isolated from different mycobacterial species and used in this study.
Mycobacterial LAMs are major virulence factors and can be considered modulins because of their capacity to manipulate the host immune system (3, 6, 24). LAMs have been implicated in triggering multiple signaling pathways that modulate the apoptosis and IL-12 production of macrophages and dendritic cells. ManLAM isolated from M. tuberculosis does not induce IL-12 and apoptosis and may actually inhibit the induction of these two pathways via agonists such as lipopolysaccharide (LPS) or bacterial infection (25, 26, 30). In contrast, the PILAMs from the nonpathogenic organisms M. smegmatis and Mycobacterium sp. are potent inducers of IL-12 and apoptosis (11, 24, 25, 35). These observations suggested that the mannose caps of M. tuberculosis ManLAM are important for modulating the induction of proinflammatory signaling on macrophages and dendritic cells. Nevertheless, these structure-function studies were limited because of technical difficulties that prevented specific removal of the mannose caps from the isolated M. tuberculosis ManLAM and by the limited number of structural LAM variants available for study.
The recent isolation of and structural data for LAMs from M. kansasii and M. chelonae (14, 15) allowed us to directly compare the effects of the three families of LAM molecules (ManLAM, PILAM, and AraLAM), as well as their corresponding biosynthetic precursors (lipomannan [LM] and phosphatidyl-myo-inositol dimannoside [PIM2]), on IL-12 and apoptosis induction in macrophages (Fig. 1). In this study we demonstrated that the mannose caps of ManLAMs are most likely not necessary to modulate the induction of IL-12 and apoptosis. Furthermore, we present evidence here that LM, but not PIM2, strongly induces IL-12 production and apoptosis. Since LM and LAM coexist in the mycobacterial cell wall, the ratio of LAM to LM in M. tuberculosis may be important for the capacity of this organism to circumvent IL-12 production and inhibit apoptosis, thereby directly influencing the outcome of the infection.
MATERIALS AND METHODS
Lipoglycans.
Purified LAM, LM, and PIM2 from M. chelonae ATTC 19538 or M. kansasii PHRI 901 (clinical isolate) were obtained as described by Guerardel et al. (14, 15). PILAM from an unidentified, fast-growing mycobacterium (Mycobacterium sp.) and lipoglycans from M. tuberculosis strain H37Rv were obtained from J. T. Beslile (Colorado State University). The amount of LPS contamination in each preparation was determined by the Limulus lysate assay (BioWittaker) and was found to be below the threshold of detection in all of the assays performed.
Apoptosis assay.
The apoptosis assay was performed as described by Guerardel et al. (14). The human monocytic cell line THP-1 (ATCC TIB202) was differentiated by using phorbol 12-myristate 13-acetate (Sigma) at a final concentration of 20 ng/ml for 20 h. Next, cells were incubated in medium with 5% human serum and the different lipoglycans (LAMs at a concentration of 20 μg/ml and LMs and PIM2 at a concentration of 10 μg/ml) for 24 h. The percentage of apoptotic cells was determined by using AnnexinV-Alexa488 conjugates (Molecular Probes) and propidium iodide staining of dead cells. For flow cytometry analysis, 10,000 events were collected for each condition, and the percentages of AnnexinV-positive and propidium iodide-negative cells were determined. Experiments were performed three to five times, and average values and standard deviations are shown below. The significance of differences in comparisons with untreated cells was calculated by using the unpaired t test, and two-tailed P values are given below.
RAW/pIL-12-GFP cell line-based IL-12 gene expression assay.
The minimal mouse IL-12p40 promoter (23, 27) (positions −348 to 8 relative to the transcription start) was amplified from C57B6 mouse genomic DNA by PCR by using upstream primer 5′GCAAGTCCTTCCTTTTTCTGCAGTCT 3′ and downstream primer 5′ CACCCACTGTTCCTTCTGCTGC 3′ and was inserted into the TOPO cloning vector (Invitrogen). A HindIII/PstI IL-12p40 promoter-containing fragment isolated from the TOPO cloning vector was cloned into the HindIII/PstI site of a green fluorescent protein (GFP) reporter vector, pEGFP-1 (BD Bioscience). This vector, p348+8mIL12p40.EGFP-1, was used to transfect the murine RAW 264.7 macrophage cell line to detect IL-12p40 promoter activity.
RAW 264.7 cells (ATCC TIB-71) were transfected with p348+8mIL12p40.EGFP-1 by using the Superfect reagent according to the manufacturer's protocol (Invitrogen). Briefly, 5 × 106 cells were inoculated into a 100-mm tissue culture plate. Ten micrograms of DNA was mixed with the reagent and incubated for 5 to 10 min to allow SuperFect-DNA complexes to form and then was added directly to the cells. After 2 to 3 h of incubation the medium was replaced with Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum, and 5% NCTC-109 medium, 10 mM HEPES, and 1 mg of neomycin per ml were added the next day. After this all manipulations were done in this medium. Clones of cells resistant to neomycin (designated RAW/pIL-12-GFP) were isolate after 2 weeks and were then tested for the ability to respond to known inducers of IL-12p40, including 0.1 μg of LPS from Salmonella typhosa (L7895; Sigma) per ml and 1 μM mouse CpG DNA (HC4033; Cell Sciences).
To analyze the capacity of the lipoglycans to activate the IL-12 promoter, 2 × 105 RAW/pIL-12-GFP cells were seeded into each well of 96-well plates overnight. The next day 200-μl portions of medium containing the different lipoglycans or LPS were added, and the cells were incubated for 16 h. The expression of GFP was detected with a Becton Dickinson FACScan, and data for 5,000 collected events were analyzed by using CellQuest software (BD Bioscience). Untreated and treated cells were gated by forward and side scatter. GFP-positive cells were considered to be cells that had greater fluorescence than untreated cells. Experiments were performed three to five times, and average values and standard deviations are shown below. The significance of differences in comparisons with untreated cells was calculated by using the unpaired t test, and two-tailed P values are indicated below.
Analysis of IL-12 secretion from bone marrow-derived macrophages.
Bone marrow-derived macrophages were prepared as described previously (5). Briefly, bone marrow cells from 6- to 8-week-old BALB/c mice were flushed from the tibia and femur with DMEM (Invitrogen) containing 100 U of penicillin per ml and 100 μg of streptomycin (Invitrogen) per ml. Red blood cells were eliminated by using lysis buffer (00-4333-57; eBioscience) as recommended by the manufacturer. Then 1 × 10 6 to 2 × 106 cells were inoculated into a 100-mm petri dish at a density of 4 × 105 cells/ml. Cells were differentiated into macrophages by supplementing DMEM with 10% fetal calf serum and 20% day 7 L-929 cell (ATCC CCL-1) supernatant as a source of macrophage differentiation factor. On day 6, adherent cells were harvested with cold phosphate-buffered saline containing 0.5 mM EDTA for analysis of the secretion of IL-12 induced by lipoglycans. Therefore, triplicate preparations containing 2 × 105 bone marrow-derived macrophages were cultured in 96-well plates either with lipoglycans or with medium alone for 24 h in DMEM. The supernatants from 16- to 24-h cultures were analyzed for mouse IL-12p40 by an enzyme-linked immunosorbent assay (ELISA) by using an mIL12p40 ELISA kit (KMCO122) from Biosource International. The assay was performed as suggested by the manufacturer.
TLR interaction assay.
Chinese hamster ovary (CHO) cells transfected with the inducible membrane protein CD25 under control of a region from the human E-selectin promoter containing nuclear factor-κB binding sites and expressing CD14 and either human Toll-like receptor 2 (TLR-2) or human TLR-4 (21) were kindly provided by D. T. Golenbock (University of Massachusetts). The cells were maintained as described previously (21). A total of 5 × 105 cells were treated with lipoglycans for 24 h in 250 μl (final volume) of medium per well. The cells were harvested and stained with anti-human CD25 antibody (5 μg/ml; BD Bioscience) for 30 min, washed, and stained with Alexa633-conjugated goat anti-mouse antibody (5 μg/ml; Molecular Probes). The induction of CD25 cell surface expression was analyzed by examining 10,000 cells per condition by using a FACS-Calibur (BD Bioscience) in the FL-4 channel. To quantify the percentage of CD25-positive cells induced by the lipoglycan treatment, a gate was defined on the medium-treated CHO cells to include only the highest 5% expressing cells. The same gate was applied to all other conditions, and the percentage of positive cells was determined. The average for three independent experiments was calculated. The significance of differences in comparisons with untreated cells was calculated by using the unpaired t test, and two-tailed P values are indicated below.
RESULTS AND DISCUSSION
Lack of requirement for mannose caps for modulation of the inflammatory response of macrophages to LAM.
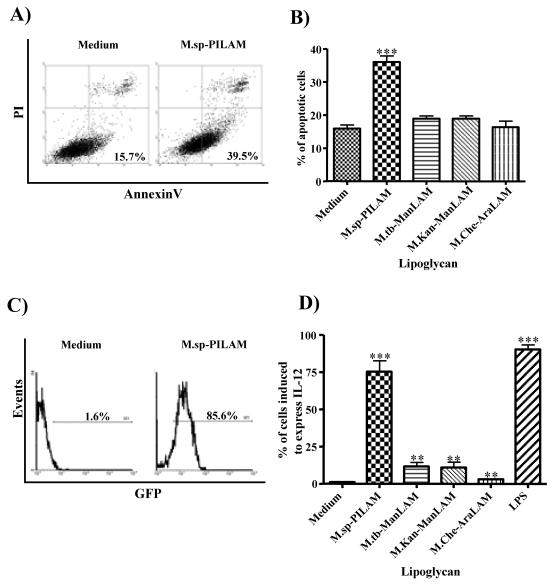
The effect of PILAM isolated from a fast-growing, unidentified Mycobacterium species on the induction of an inflammatory response has been described previously (24). Here, we reexamined the capacity of PILAM to modulate apoptosis and IL-12 gene expression using a variety of specific assays. As expected, the PILAM induced IL-12p40 gene expression and apoptosis of macrophages in a dose-dependent manner, whereas ManLAM from M. tuberculosis had no activity (data not shown). In order to address the hypothesis that the mannose caps of ManLAM are important for the modulation of apoptosis and/or IL-12 gene expression, macrophages were stimulated with ManLAMs isolated from M. kansasii and M. tuberculosis, PILAM isolated from Mycobacterium sp., and AraLAM isolated from M. chelonae at a concentration of 20 μg/ml for 24 h. Interestingly, neither the ManLAMs nor the AraLAM induced apoptosis at a level above the background level in untreated cells, as measured by AnnexinV staining and flow cytometry analysis (Fig. 2). In contrast, addition of PILAM to the macrophages increased the percentage of apoptotic cells from 15% in untreated controls to about 35% (Fig. 2A and B), suggesting that the phosphoinositol residues may participate in induction of apoptosis.
FIG. 2.
PILAM induces apoptosis and IL-12 gene expression in macrophages. (A) Differentiated human THP-1 cells either were not treated (left panel) or were incubated with PILAM (20 μg/ml) for 24 h and then stained with AnnexinV-Alexa488 and propidium iodide and analyzed by flow cytometry to determine the induction of apoptosis. (B) Percentage of apoptotic THP-1 cells treated with different LAM molecules which are representative of the three LAM families (ManLAM, PILAM, and AraLAM) and were isolated from various mycobacterial species. The percentage of apoptotic cells was determined as the number of AnnexinV-Alexa488-positive and propidium iodide-negative cells in a population of 10,000 cells analyzed by flow cytometry. M.sp.-PILAM, Mycobacterium sp. PILAM; M.tb-ManLAM, M. tuberculosis ManLAM; M.Kan-ManLAM, M. kansasii ManLAM; M.Che-AraLAM, M. chelonae AraLAM. (C) RAW cells transfected with an IL-12p40 gene promoter-GFP gene fusion plasmid (RAW/pIL-12-GFP) either were not treated (left panel) or were treated with 20 μg of PILAM per ml (right panel) for 16 h. Activation of the IL-12p40 promoter was determined by flow cytometry analysis of 5,000 events per condition, and the percentage of GFP-positive cells is indicated. (D) Induction of IL-12 expression by different lipoglycans analyzed as described above. The percentages of cells induced to express IL-12 are indicated. For panels B and D the means and standard deviations for three to five experiments are shown, and statistical analyses were performed by using the unpaired t test to compare treated cells to untreated cells; three asterisks indicate that the P value is <0.001, and two asterisks indicate that the P value is <0.01.
Interestingly, the ManLAMs and AraLAM induced a slight increase in IL-12p40 gene expression, as determined by the increased fraction of GFP-positive RAW cells transfected with an IL-12p40 promoter-GFP fusion construct (RAW/pIL-12-GFP). The AraLAM induced IL-12 expression in about 3% of the cells, whereas approximately 10% of the cells were positive when they were treated with the ManLAMs. Statistical analysis revealed that these levels of induction were significantly different (P < 0.05) than the levels for untreated cells (∼1.5% GFP-positive cells) (Fig. 2D). Nevertheless, these levels are most likely not biologically relevant compared to the strong activity of PILAM, which was found to induce IL-12 expression in more than 70% of the cells, which approached the level of induction observed after LPS stimulation (∼85%) (Fig. 2C and D). Altogether, these results suggest that the mannose caps of ManLAM from M. tuberculosis or M. kansasii are not required for modulation of the proinflammatory activities. Instead, since AraLAM also showed no activity, it appears more likely that the phosphoinositol capping was responsible, either directly or indirectly, for inducing an inflammatory response of stimulated macrophages and that AraLAM and ManLAM simply do not induce proinflammatory responses.
It should be pointed out that our results were obtained by using purified LAM preparations, and it is likely that the mannose caps of ManLAMs have significant effects on modulation of the responses of macrophages and dendritic cells in the context of concurrent stimuli as they occur during infection with whole bacteria. For instance, it has been demonstrated that ManLAM mediates the inhibition of the LPS-induced IL-12 response of human dendritic cells (10, 25, 26, 31) and the induction of apoptosis mediated by M. tuberculosis infection (26, 30). This activity was proposed to require the interaction of ManLAM with the mannose receptor and/or DC-SIGN (26, 31). Therefore, the mannose caps may prove to be important for the capacity to inhibit a receptor-mediated signaling event.
Potent inflammatory responses to the LAM precursor LM.
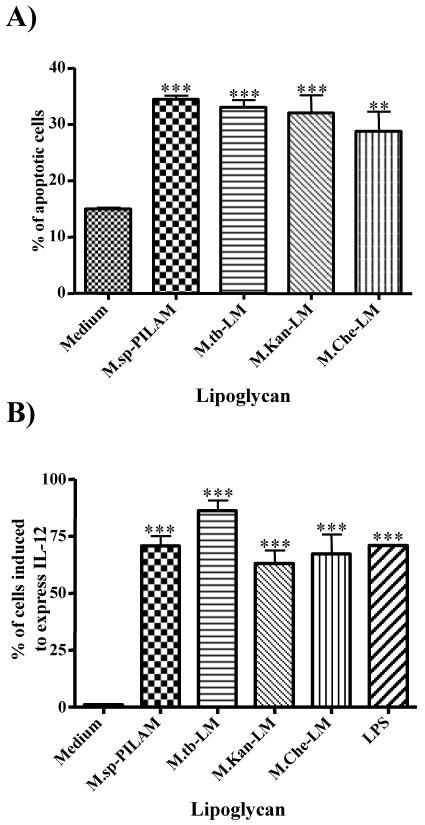
It was demonstrated previously that M. kansasii LM is a potent inducer of apoptosis of macrophages (14), and since the molecules are structurally similar, we hypothesized that this activity may be a common feature of all LMs, regardless of mycobacterial origin. To address this hypothesis, we compared the abilities of LMs isolated from M. chelonae, M. kansasii, and M. tuberculosis to induce IL-12p40 gene expression and apoptosis of macrophages. As shown in Fig. 3A, all of the LMs induced apoptosis at similar levels compared to the levels induced by PILAM, and treatment resulted in about 30 to 35% apoptotic cells, compared to the 15% apoptotic cells in untreated cultures. In addition, all the LMs induced significant IL-12p40 transcription, with M. chelonae LM and M. kansasii LM inducing gene expression in about 60% of the cells and M. tuberculosis LM inducing gene expression in about 80% of the cells (Fig. 3B). Only 1% of the cells were positive in untreated cultures. As a positive control we used LPS and PILAM, which induced IL-12 expression in about 70% of the cells (Fig. 3B). Our findings are consistent with the recent study of Vignal et al. (34), which demonstrated that LMs, but not LAMs isolated from M. kansasii and M. chelonae, induce tumor necrosis factor alpha (TNF-α) and IL-8 secretion. In the present study we extended this analysis and found that LMs from all species tested, including virulent M. tuberculosis, were potent inducers of apoptosis and IL-12 expression.
FIG. 3.
LM induces apoptosis and IL-12 gene expression in macrophages. (A) Differentiated THP-1 cells were incubated with either PILAM (20 μg/ml) or with LMs isolated from M. tuberculosis, M. kansasii, and M. chelonae (10 μg/ml) for 24 h. The percentage of apoptosis was determined by flow cytometry analysis of 10,000 events after cells were stained with AnnexinV and propidium iodide. (B) RAW/pIL-12-GFP cells were incubated with wither PILAM (20 μg/ml), LMs (10 μg/ml), or LPS (100 ng/ml) for 16 h, and flow cytometry was used to determine the induction of GFP-positive cells in 5,000 events. The results are the means of three independent experiments. A statistical analysis was performed as described in the legend to Fig. 2; three asterisks indicate that the P value is <0.001, and two asterisks indicate that the P value is <0.01. M.sp.-PILAM, Mycobacterium sp. PILAM; M.tb-LM, M. tuberculosis LM; M.Kan-LM, M. kansasii LM; M.Che-LM, M. chelonae LM.
Together, the structure-function studies suggested that addition of the arabinan in LAM masks the activity of the mannan core in LM, and therefore, enzymes that mediate this event may be important in determining the virulence of a mycobacterium. At this time, very little is known regarding arabinan synthesis. However, recent work has suggested that an embC-deficient M. smegmatis mutant strain is affected in arabinosylation of LAM (36). Thus, EmbC, a protein that is highly homologous to EmbA and EmbB, appears to catalyze arabinosyltransferase activity in LAM arabinan biosynthesis. The availability of embC mutants may provide valuable tools for analyzing the in vivo effect of an altered LM-LAM balance on both IL-12 expression and apoptosis. In addition, a comparison of the LAM/LM ratio in the cell walls of different mycobacterial strains should allow assessment of the hypothesis that in virulent strains this balance is tipped towards LAM and in less virulent strains it is tipped towards LM.
LM precursor PIM2 does not induce IL-12 secretion.
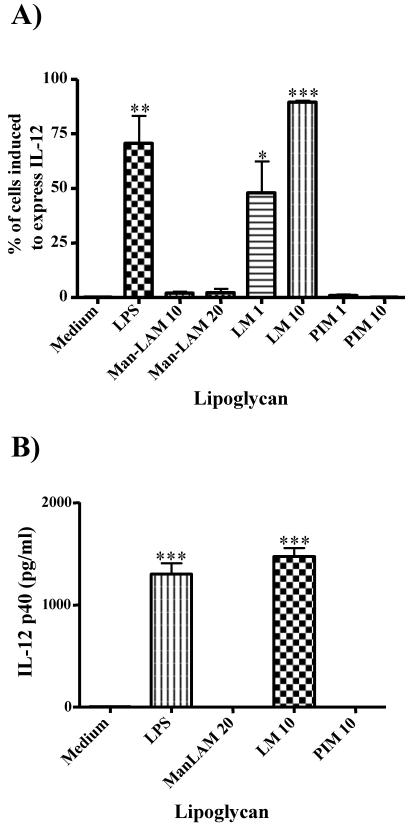
To address whether the mannan core of LM is essential for its biological activity, the biosynthetic precursor PIM2, containing only two mannose residues (Fig. 1), was used to stimulate macrophages. The percentage of cells expressing the IL-12p40 gene in untreated samples was less than 1% and was significantly increased by M. tuberculosis LM (90%) but neither by M. tuberculosis ManLAM (∼4%) nor by M. tuberculosis PIM2 (<1%) (Fig. 4A). The PIM2 of M. kansasii also did not induce IL-12 gene expression (<2%) when it was compared to M. kansasii LM (∼50%) (data not shown), which is consistent with the reported lack of an effect of M. kansasii PIM2 on induction of apoptosis (14) and IL-8 and TNF-α secretion (34). In addition, the ability of ManLAM, LM, and PIM2 from M. tuberculosis to induce secretion of IL-12 by bone marrow-derived macrophages from BALB/c mice was analyzed by an ELISA. In agreement with the results of the IL-12 reporter assay, only LM induced secretion of IL-12 (∼1.5 ng/ml); ManLAM and PIM2 did not induce secretion of this cytokine (Fig. 4B). These results confirmed the validity of the IL-12 reporter assay performed with a macrophage cell line for interpretation of the effect of LM isolated from M. tuberculosis on primary macrophages. Together, the experiments whose results are shown in Fig. 4 demonstrate that the mannan core is essential for the biological activity of LM and that the lipid anchor PIM2 may be necessary in the context of LM but has no activity on its own. Since PIM2 contains only two mannose residues, these results suggest that a higher mannose content is required to stimulate IL-12 expression. This is in agreement with previous results which showed that, in contrast to LM, PIM2 was unable to induce TNF-α and IL-8 production in THP-1 cells (34). These findings somewhat contradict a report on induction of TNF-α secretion by macrophages stimulated with PIM2 and PIM6 of Mycobacterium bovis BCG (13). The discrepancy may be explained by the use of very high concentrations of PIM2 (30 to 50 μg/ml) in the previous study (13). In addition, in the previous study the researchers did not compare the activity of PIM2 to the activity of LM, and, although they showed induction of TNF-α, the amount of TNF-α secreted was very low (0.2 to 0.4 ng/ml) compared to the amount of LPS-induced TNF-α secreted (∼10 ng/ml). Therefore, we propose that, although PIM2 may have residual activity when it is given in high doses, PIM2 alone is not sufficient to induce strong proinflammatory responses. The main activity for induction of cytokines and apoptosis is mediated by LM, presumably through an effect dependent on its mannan domain.
FIG. 4.
PIM2 does not induce IL-12 gene expression in macrophages. (A) RAW/p-IL-12-GFP cells were incubated with either LPS (100 ng/ml), ManLAM (10 or 20 μg/ml), LM (1 or 10 μg/ml), or PIM2 (PIM) (1 or 10 μg/ml) (all isolated from M. tuberculosis) for 16 h, and IL-12 gene induction was measured by flow cytometry analysis as described in the legend to Fig. 2. (B) Bone marrow-derived murine macrophages were incubated with lipoglycans as described above for 24 h, and the secretion of IL-12 into the supernatant was analyzed by an ELISA. The results are the means of three independent experiments, and a statistical analysis was performed as described in the legend to Fig. 2; three asterisks indicate that the P value is <0.001, two asterisks indicate that the P value is <0.01, and one asterisk indicates that the P value is <0.05.
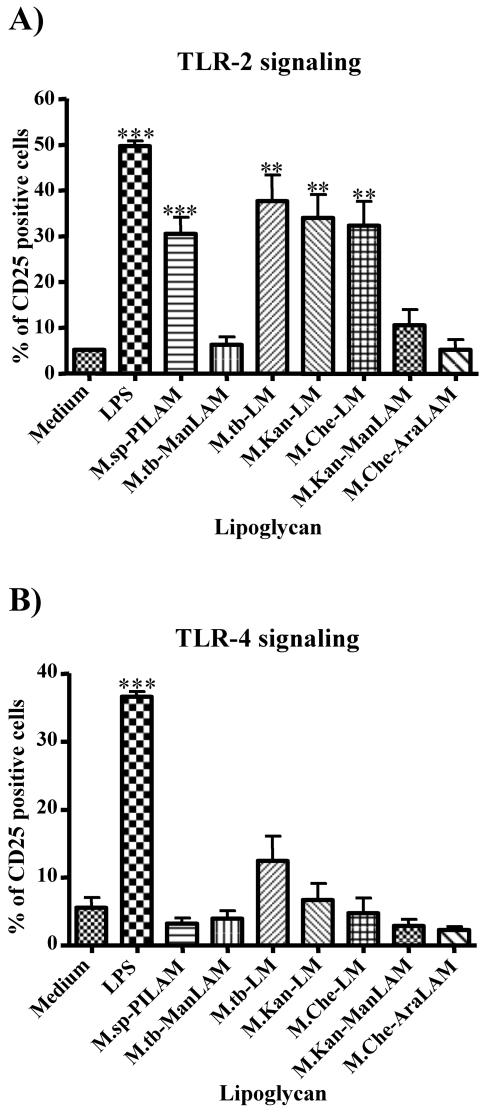
LM induces cell signaling through TLR-2 ligation but not through TLR-4 ligation.
TLRs are important pattern recognition receptors that have been implicated in the innate immune response (16, 20). Since the interaction of PILAM with TLR-2 has been documented (22) and the interaction of other bacterial ligands with TLR-2 has been shown to induce IL-12 secretion and apoptosis (1, 4), we hypothesized that the proinflammatory activity of LM may be TLR-2 dependent. Therefore, the interactions of the different LMs with TLR-2 and/or TLR-4 were analyzed by using transfected CHO cells as described previously (21, 22). Interestingly, all the lipoglycans that had IL-12- and apoptosis-inducing activity strongly interacted with TLR-2 but not with TLR-4. Thus, PILAM from Mycobacterium sp. and LMs from M. tuberculosis, M. kansasii, and M. chelonae all induced about six- to sevenfold increases in expression of the reporter gene (CD25), as demonstrated by flow cytometry analysis measuring the percentages of CD25-positive cells of lipoglycan-treated and untreated cells (Fig. 5A). M. tuberculosis ManLAM and M. chelonae AraLAM did not stimulate cells via TLR-2, and M. kansasii ManLAM showed a reproducible but weak interaction (<twofold increase) which was not statistically significantly different from CD25 expression in untreated cells (Fig. 5A). Interestingly, none of the lipoglycans showed strong interactions with TLR-4, although M. tuberculosis LM had a consistent but very weak effect on CD25 expression mediated by TLR-4 that proved to be not statistically significant (P > 0.05) (Fig. 5B). Therefore, it appears that like PILAM, LM mediates its proinflammatory activity via interaction with TLR-2 on the surface of macrophages. However, it is noteworthy that when this assay is used, it is possible that other TLRs may be implicated in association with TLR-2, as has recently been shown for TLR-1 in the binding of PILAM to TLR-2 (32). The intriguing question remains why LMs but not ManLAMs and AraLAMs bind to TLR-2, although they all contain a precursor LM that by itself may interact with TLR-2. One compelling hypothesis is that the arabinan core of ManLAM and AraLAM masks the mannan core from recognition by TLR-2 and in the case of PILAM the phosphoinositol residues that are attached to the arabinan core may induce conformational changes, which would expose the presumably hidden mannan core for binding to TLR-2. Indeed, it was recently shown that chemical degradation of the arabinan domain of ManLAM from M. kansasii, which failed to induce TNF-α secretion, restored the cytokine-inducing activity to a level similar to that of LM isolated from M. kansasii (34). These results support the notion that the presence of the arabinan in LAM prevents an interaction with TLR-2.
FIG. 5.
LM activates cells in a TLR-2-dependent manner. CHO/CD14/TLR-2 (A) and CHO/CD14/TLR-4 (B) reporter cell lines were incubated either with LAM (20 μg/ml), with LMs (10 μg/ml) isolated from various mycobacterial species, or with LPS (1 μg/ml) for 16 h. Cellular activation was measured by determining the expression of CD25 at the cell surface by using anti-CD25 monoclonal antibody and flow cytometry analysis. The percentage of CD25-positive cells was determined, and the averages of three independent experiments are shown. A statistical analysis was performed as described in the legend to Fig. 2; three asterisks indicate that the P value is <0.001, and two asterisks indicate that the P value is <0.01. M.sp.-PILAM, Mycobacterium sp. PILAM; M.tb-ManLAM, M. tuberculosis ManLAM; M.tb-LM, M. tuberculosis LM; M.Kan-LM, M. kansasii LM; M.Che-LM, M. chelonae LM; M.Kan-ManLAM, M. kansasii ManLAM; M.Che-AraLAM, M. chelonae AraLAM.
In conclusion, we demonstrated that LM, the biosynthetic precursor of LAM, has strong inflammatory activity regardless of the species of Mycobacterium from which it is isolated. These results point to an important function of the arabinan core that may mask the proinflammatory capacity of the mannan core of LM. Therefore, the expression and activity of enzymes such as arabinosyltransferases that modify the mannan core of the LM may be important in determining the relative virulence of the bacteria by modifying the LAM/LM ratio within the cell wall. Thus, it should be interesting to determine how closely the ratio of LAM to LM in the cell wall of mycobacteria correlates with virulence. Our results suggest that enzymes involved in adding arabinan to LM are interesting potential targets for antituberculosis drugs and for generation of attenuated M. tuberculosis strains by targeted gene disruption.
Acknowledgments
This study was supported by grants from the IDSA and by NIH grant AI51696-01 to V.B., by INSERM and CNRS grants to L.K. and Y.G., respectively, by NIH grant AI26170 to W.R.J., by NIH grant T32CA9173-28 to D.N.D., by NIH grants AI48933 and AI45889 to S.A.P., and by a Heiser Foundation Fellowship Award to A.M.
Editor: D. L. Burns
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 3.Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 83:91-97. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 160:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee, D., and K. H. Khoo. 1998. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8:113-120. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, D., K. Lowell, B. Rivoire, M. R. McNeil, and P. J. Brennan. 1992. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 267:6234-6239. [PubMed] [Google Scholar]
- 8.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, J. L., and J. Chan. 2003. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 15:450-455. [DOI] [PubMed] [Google Scholar]
- 10.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh, S., S. Pal, S. Das, S. K. Dasgupta, and S. Majumdar. 1998. Lipoarabinomannan induced cytotoxic effects in human mononuclear cells. FEMS Immunol. Med. Microbiol. 21:181-188. [DOI] [PubMed] [Google Scholar]
- 12.Gilleron, M., N. Himoudi, O. Adam, P. Constant, A. Venisse, M. Riviere, and G. Puzo. 1997. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localization of alkali-labile and alkali-stable phosphoinositides. J. Biol. Chem. 272:117-124. [DOI] [PubMed] [Google Scholar]
- 13.Gilleron, M., V. F. Quesniaux, and G. Puzo. 2003. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis Bacillus Calmette Guerin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 278:29880-29889. [DOI] [PubMed] [Google Scholar]
- 14.Guerardel, Y., E. Maes, V. Briken, F. Chirat, Y. Leroy, C. Locht, G. Strecker, and L. Kremer. 2003. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: novel structural features and apoptosis-inducing properties. J. Biol. Chem. 278:36637-36651. [DOI] [PubMed] [Google Scholar]
- 15.Guerardel, Y., E. Maes, E. Elass, Y. Leroy, P. Timmerman, G. S. Besra, C. Locht, G. Strecker, and L. Kremer. 2002. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J. Biol. Chem. 277:30635-30648. [DOI] [PubMed] [Google Scholar]
- 16.Heldwein, K. A., and M. J. Fenton. 2002. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 4:937-944. [DOI] [PubMed] [Google Scholar]
- 17.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 18.Keane, J., H. G. Remold, and H. Kornfeld. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164:2016-2020. [DOI] [PubMed] [Google Scholar]
- 19.Khoo, K. H., A. Dell, H. R. Morris, P. J. Brennan, and D. Chatterjee. 1995. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J. Biol. Chem. 270:12380-12389. [DOI] [PubMed] [Google Scholar]
- 20.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 21.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 22.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 23.Murphy, K. M., T. L. Murphy, S. J. Szabo, N. G. Jacobson, M. L. Guler, J. D. Gorham, and U. Gubler. 1997. Regulation of IL-12 receptor expression in early T-helper responses implies two phases of Th1 differentiation: capacitance and development. Chem. Immunol. 68:54-69. [DOI] [PubMed] [Google Scholar]
- 24.Nigou, J., M. Gilleron, and G. Puzo. 2003. Lipoarabinomannans: from structure to biosynthesis. Biochimie 85:153-166. [DOI] [PubMed] [Google Scholar]
- 25.Nigou, J., M. Gilleron, M. Rojas, L. F. Garcia, M. Thurnher, and G. Puzo. 2002. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 4:945-953. [DOI] [PubMed] [Google Scholar]
- 26.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 27.Plevy, S. E., J. H. Gemberling, S. Hsu, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 17:4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prinzis S., D. Chatterjee, and P. J. Brennan. 1993. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J. Gen. Microbiol. 139:2649-2658. [DOI] [PubMed] [Google Scholar]
- 29.Rojas, M., L. F. Barrera, G. Puzo, and L. F. Garcia. 1997. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J. Immunol. 159:1352-1361. [PubMed] [Google Scholar]
- 30.Rojas, M., L. F. Garcia, J. Nigou, G. Puzo, and M. Olivier. 2000. Mannosylated lipoarabinomannan antagonizes Mycobacterium tuberculosis-induced macrophage apoptosis by altering Ca+2-dependent cell signaling. J. Infect. Dis. 182:240-251. [DOI] [PubMed] [Google Scholar]
- 31.Tailleux, L., O. Schwartz, J. L. Herrmann, E. Pivert, M. Jackson, A. Amara, L. Legres, D. Dreher, L. P. Nicod, J. C. Gluckman, P. H. Lagrange, B. Gicquel, and O. Neyrolles. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapping, R. I., and P. S. Tobias. 2003. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J. Endotoxin Res. 9:264-268. [DOI] [PubMed] [Google Scholar]
- 33.Venisse, A., J. M. Berjeaud, P. Chaurand, M. Gilleron, and G. Puzo. 1993. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 268:12401-12411. [PubMed] [Google Scholar]
- 34.Vignal, C., Y. Guerardel, L. Kremer, M. Masson, D. Legrand, J. Mazurier, and E. Elass. 2003. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-alpha and IL-8 secretion by a CD14-Toll-like receptor 2-dependent mechanism. J. Immunol. 171:2014-2023. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, A., and Y. Koide. 1997. Arabinofuranosyl-terminated and mannosylated lipoarabinomannans from Mycobacterium tuberculosis induce different levels of interleukin-12 expression in murine macrophages. Infect. Immun. 65:1953-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, N., J. B. Torrelles, M. R. McNeil, V. E. Escuyer, K. H. Khoo, P. J. Brennan, and D. Chatterjee. 2003. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol. Microbiol. 50:69-76. [DOI] [PubMed] [Google Scholar]