Abstract
Clostridium perfringens iota-toxin consists of two separate proteins identified as a cell binding protein, iota b (Ib), which forms high-molecular-weight complexes on cells generating Na+/K+-permeable pores through which iota a (Ia), an ADP-ribosyltransferase, presumably enters the cytosol. Identity of the cell receptor and membrane domains involved in Ib binding, oligomer formation, and internalization is currently unknown. In this study, Vero (toxin-sensitive) and MRC-5 (toxin-resistant) cells were incubated with Ib, after which detergent-resistant membrane microdomains (DRMs) were extracted with cold Triton X-100. Western blotting revealed that Ib oligomers localized in DRMs extracted from Vero, but not MRC-5, cells while monomeric Ib was detected in the detergent-soluble fractions of both cell types. The Ib protoxin, previously shown to bind Vero cells but not form oligomers or induce cytotoxicity, was detected only in the soluble fractions. Vero cells pretreated with phosphatidylinositol-specific phospholipase C before addition of Ib indicated that glycosylphosphatidyl inositol-anchored proteins were minimally involved in Ib binding or oligomer formation. While pretreatment of Vero cells with filipin (which sequesters cholesterol) had no effect, methyl-β-cyclodextrin (which extracts cholesterol) reduced Ib binding and oligomer formation and delayed iota-toxin cytotoxicity. These studies showed that iota-toxin exploits DRMs for oligomer formation to intoxicate cells.
Clostridium perfringens is a ubiquitous, gram-positive bacillus that produces numerous toxins involved in various human and animal diseases (21, 33). Iota-toxin, one of the four major lethal and dermonecrotic proteins produced by C. perfringens, shares many interesting attributes with other bacterial binary toxins synthesized by spore-forming bacilli such as Clostridium spiroforme (iota-like toxin), Clostridium botulinum (C2 toxin), Bacillus anthracis (lethal and edema toxins), and Bacillus cereus (vegetative insecticidal proteins) (13, 16, 18, 28). Iota-toxin consists of a 45-kDa ADP-ribosyltransferase, iota a (Ia), and an 81-kDa cell-binding protein, iota b (Ib), that are transcribed as separate proteins (27, 37). Ib is produced by C. perfringens as a biologically inactive 100-kDa protoxin (Ibp) capable of binding to cells but unable to form oligomers or interact with Ia (11, 36). In solution, Ibp is cleaved by serine-type proteases into a 20-kDa N-terminal peptide and the 81-kDa biologically active Ib. Ib binds to the cell, forming heptamers and Na+/K+-permeable pores that promote Ia internalization into the cytosol (7, 20, 25, 36). Once inside, Ia ADP-ribosylates G-actin at arginine-177 and disrupts the cytoskeleton (40).
In addition to iota-toxin, C. perfringens produces other toxins that also form large complexes and pores in cell membranes (23, 29, 31, 34). The enterically-acting protein, epsilon-toxin, forms surface-associated heptamers that have recently been shown to localize in cholesterol-rich, detergent-resistant membrane microdomains (DRMs) (24). DRMs represent specialized membrane entities on the cell that concentrate lipids and proteins into unique domains, thus facilitating internalization of receptors and other molecules (8, 30).
Other bacterial toxins, such as the C. perfringens perfringolysin O, Aeromonas hydrophila aerolysin, Clostridium septicum alpha-toxin, and B. anthracis lethal and edema toxins are found in DRMs, forming large membrane-associated complexes that generate ion-permeable pores through the cell membrane (2, 3, 12, 29). Since DRMs are involved in the binding and internalization of many different bacterial toxins, the following study investigated the potential role DRMs play in C. perfringens iota-toxin cytotoxicity, in particular focusing upon Ib binding and subsequent oligomer formation on cell membranes.
MATERIALS AND METHODS
Iota-toxin and antisera.
Purified components of C. perfringens iota-toxin (Ia, Ib, and Ibp), as well as rabbit anti-Ib serum, were produced as described previously (28). Goat C. spiroforme and C. perfringens type C antisera were purchased from TechLab, Inc. (Blacksburg, Va.). Mouse monoclonal antibodies against clathrin heavy chain and α-1 Na+,K+-ATPase as well as affinity-purified rabbit antibody against dynamin II were purchased from Affinity BioReagents (Golden, Colo.). Affinity-purified antibodies against flotillin-1 and caveolin-1 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). B. anthracis PA components (the protoxin PA83 and the enzymatically cleaved PA63), as well as rabbit anti-PA serum, were kind gifts from Stephen Little (U.S. Army Medical Research Institute of Infectious Diseases).
Cells and DRM isolation.
MRC-5 (human lung), Vero (African green monkey kidney), SW-13 (human adenocarcinoma), and PC12 (rat pheochromocytoma) cells were obtained from the American Type Culture Collection (Manassas, Va.). Cells were detached from culture flasks with 50 mM EDTA (Sigma, St. Louis, Mo.) in Hanks balanced salt solution (HBSS) lacking Ca2+ and Mg2+. As described previously (35), single-cell suspensions (5 × 107/ml) were prepared and incubated with 125 ng of Ib or Ibp in 50 μl of HBSS containing 0.2% bovine serum albumin (HBSS-BSA) for 10 min at 37°C, and then unbound Ib or Ibp was removed by three washes with HBSS-BSA.
To isolate DRMs, we used a procedure developed by Aman and Ravichandran (5) in which cells were solubilized in 750 μl of cold (4°C) 25 mM Tris-hydroxymethylaminomethane hydrochloride (Tris-HCl) buffer, pH 7.6, containing 0.5% Triton X-100, 10 mM sodium fluoride, 30 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 150 mM sodium chloride, and 0.1% (vol/vol) phosphatase inhibitor cocktail (Sigma). Aprotinin, leupeptin, pepstatin, and Prefabloc SC (Roche, Indianapolis, Ind.) were also added (60 ng each). Cell suspensions were then incubated at 4°C for 60 min. The lysate was mixed with an equal volume of 80% sucrose, placed in a polyallomer centrifuge tube (Beckman-Coulter, Fullerton, Calif.), and overlaid with 1.5 ml each of 30% and 5% sucrose solutions. After a 17-h centrifugation (215,000 × g), eight 500-μl fractions were collected, starting from the top of the tube. The DRMs, visible as a white flocculent material near the 5-to-30% interface, were collected in fractions 2 to 4, while the detergent-soluble proteins were collected in fractions 6 to 8. All procedures were performed at 4°C, and fractions were subsequently stored at −70°C until analyzed.
SDS-polyacrylamide gel electrophoresis and Western blot analysis.
Protein concentrations of fractions were determined by a micro-BCA assay (Pierce, Rockford, Ill.). All gel electrophoresis was performed using NuPAGE 4-to-12% bis-Tris polyacrylamide gels in morpholinepropanesulfonic acid-sodium dodecyl sulfate (SDS) running buffer (Invitrogen, Carlsbad, Calif.). For detection of Ib binding and oligomers, samples (20 μg each) were mixed in SDS-polyacrylamide gel electrophoresis sample buffer (Invitrogen) and electrophoresed at a 30-mA constant current. For detection of specific cellular proteins, samples (20 μg each) were mixed with sample buffer containing NuPAGE reducing agent (Invitrogen) and heated for 10 min at 70°C before electrophoresis at a constant 200 V. Rainbow protein molecular weight markers were purchased from Amersham Pharmacia Biotech (Piscataway, N.J.). Separated proteins were electrophoretically transferred to nitrocellulose and then placed in phosphate-buffered saline (PBS) containing 5% skim milk for 18 h at 4°C. Blots were then probed for 1 h (25°C) in PBS containing 0.1% Tween 20 (PBST), 3% skim milk, and the primary antibody (rabbit anti-Ib or rabbit anti-PA serum or antibodies against specific proteins). Blots were rinsed in PBST and incubated for 1 h (25°C) in PBST containing 3% skim milk and anti-immunoglobulin G (IgG; mouse or rabbit, as appropriate) conjugated to horseradish peroxidase (Amersham Pharmacia Biotech). The blots were rinsed in PBST, and immunoreactive bands were detected using ECL chemiluminescent Western blotting detection reagents (Amersham Pharmacia Biotech).
PI-PLC treatment.
In order to determine whether glycosylphosphatidyl inositol (GPI)-anchored proteins are involved in Ib binding and oligomer formation, Vero cells were resuspended for 30 min at 37°C in HBSS containing phosphatidyl inositol-specific phospholipase C (PI-PLC; 10 U) and cycloheximide (10 μg/ml) (1). Cells were washed in HBSS-BSA, incubated with Ib, and solubilized in Triton X-100 as described in the preceding sections.
To detect Ib binding on cells, PI-PLC-treated and untreated cells were incubated with Ib for 10 min at 37°C, and unbound Ib was removed by three washes with HBSS-BSA (35). Rabbit anti-Ib serum (1:400 dilution) was mixed with the cells and placed at 4°C for 30 min. Cells were washed three times in HBSS-BSA and then incubated for 30 min at 4°C in HBSS-BSA with goat anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC). After being washed, cells (4 × 105/ml) were analyzed by fluorescence-activated cell sorting (FACS) cytometry using a FACSort flow cytometer (Becton Dickinson, Mountain View, Calif.).
CD and filipin treatment.
To remove or sequester cholesterol from the Vero cell membranes before incubation with Ib, cells were pretreated for 1 h (37°C) with Eagle's minimal essential medium supplemented with nonessential amino acids and 0.2% BSA (EMEM-BSA) containing either 5 μg of filipin complex/ml or 5 mM methyl-β-cyclodextrin (CD; Sigma), according to the procedure of Smart and Anderson (32). After being washed in EMEM-BSA, cells were incubated with Ib (37°C for 10 min), washed in HBSS-BSA to remove unbound Ib, and then solubilized in Triton X-100 as described in the preceding sections.
Total cholesterol in untreated (control) cells and those pretreated with filipin complex or CD was measured colorimetrically using the Infinity cholesterol reagent (ThermoDMA, Louisville, Colo.). Briefly, 105 cells were resuspended in liquid reagent and incubated for 30 min at 37°C, after which absorbance was measured at 600 nm. Data were expressed as percentages of control values [(treated)/(untreated) × 100]. Results represent the average ± standard deviation from three separate experiments.
Biological activity of iota-toxin.
In order to determine whether cholesterol or GPI-anchored proteins were required for iota-toxin cytotoxicity, Vero cells grown in a 96-well plate were treated with filipin, CD, or PI-PLC as described previously. After treatment, the media containing compounds were removed, cells were washed twice, and 50 μl of medium containing 125 ng each of Ia and Ib was added per well. After 1 h of incubation (37°C), an additional 150 μl of medium was added to the wells and incubated at 37°C for 24 h, during which cells were examined microscopically for iota-toxin cytotoxicity (35). Cells affected by iota-toxin were rounded and detached from the plate while untreated cells, or those incubated with either Ia or Ib, retained a normal fibroblastic shape.
Cell viability.
Cell viability was monitored microscopically throughout all procedures using trypan blue dye exclusion (Life Sciences, Gaithersburg, Md.) to ensure that the percentage of live cells was ≥95%.
RESULTS
Ib oligomers are found in DRMs of iota-toxin-susceptible cells.
Iota-toxin-susceptible Vero cells were incubated with Ib and solubilized in cold (4°C) Triton X-100, and membrane components were separated by sucrose gradient centrifugation into detergent-resistant and -soluble fractions. Analysis by Western blotting showed that oligomeric Ib preferentially concentrated within the less-dense DRM fractions (Fig. 1A, lanes 2 to 4), while monomeric Ib appeared in the more-dense, soluble fractions (Fig. 1A, lanes 6 to 8). Additionally, previous investigations showed that Ibp binds to cells but does not form oligomers or facilitate iota-toxin cytotoxicity (35). Therefore, when Vero cells were incubated with Ibp and then subjected to Triton X-100 solubilization and gradient fractionation, oligomers were not detected but monomeric Ibp was found in the soluble fractions (Fig. 1B).
FIG. 1.
Western blotting detection of monomeric and oligomeric Ib in Triton X-100-solubilized Vero and MRC-5 cells. Lanes 1 to 8 represent fractions from a 5, 30, and 40% discontinuous sucrose density gradient. (A) Vero cells incubated with Ib; (B) Vero cells incubated with Ibp; (C) MRC-5 cells incubated with Ib; (D) Vero cells incubated without Ib. The last lane contains 5 ng of Ib only (A and C), 8 ng of Ibp only (B), and 8 ng of Ib only (D).
Previous investigations noted that, unlike Vero cells, in which Ib binding is detected when analyzed by FACS, Ib was not detected on MRC-5 cells which are resistant to iota-toxin cytotoxicity (35). However, further studies by Western blotting have shown that monomeric, not oligomeric, Ib can be detected on these cells (36). When MRC-5 cells were analyzed in the present investigation, Ib oligomers were not found in DRMs (Fig. 1C, lanes 2 to 4), although monomeric Ib was detected in the soluble fractions (Fig. 1C, lanes 6 to 8).
Since MRC-5 cells are not susceptible to iota-toxin and Ib binding is not observed by FACS analysis, Western blotting experiments were performed in order to determine whether the Ib binding detected in MRC-5 membrane preparations was specific. Previous studies showed that the binary C. spiroforme toxin shares common epitopes with iota-toxin, and antisera prepared against C. spiroforme toxin will neutralize the activity of iota-toxin (28). Conversely, since only the type E strains of C. perfringens produce iota-toxin, antiserum prepared against one of the other four major toxin types, like type C, will not neutralize iota-toxin cytotoxicity (21). In the present study, preincubation of Ib with C. spiroforme antiserum prevented Ib binding, unlike incubation with C. perfringens type C antiserum, thus indicating that Ib interactions with MRC-5 cells were specific (data not shown).
In addition to what occurred in experiments with Vero and MRC-5 cells, iota-toxin-susceptible PC12 and SW13 cells generated Ib oligomers that localized in DRMs, while monomeric Ib was detected in the soluble fraction (data not shown). Western blot analysis revealed a protein (220 kDa) consistently present in the soluble fractions of cells incubated with Ib (Fig. 1, 3, and 5), as well as those not incubated with Ib (Fig. 1D), thus indicating that this protein was not Ib specific. To a lesser degree than with anti-Ib serum, the band was detected by rabbit anti-PA serum, although not with normal rabbit serum (data not shown).
FIG. 3.
Effects of PI-PLC on Vero cells before incubation with Ib. (A) Ib oligomers from Triton X-100-solubilized cells were detected by Western blotting. Lanes 1 to 8 represent fractions from a 5, 30, and 40% discontinuous sucrose density gradient. The last lane contains 5 ng of Ib only. (B) Cell-bound Ib was detected using rabbit anti-Ib serum and FITC-conjugated goat anti-rabbit IgG. FACS histogram panels are as follows: 1, cells only; 2, cells plus Ib; 3, PI-PLC-treated cells only; 4, PI-PLC-treated cells plus Ib.
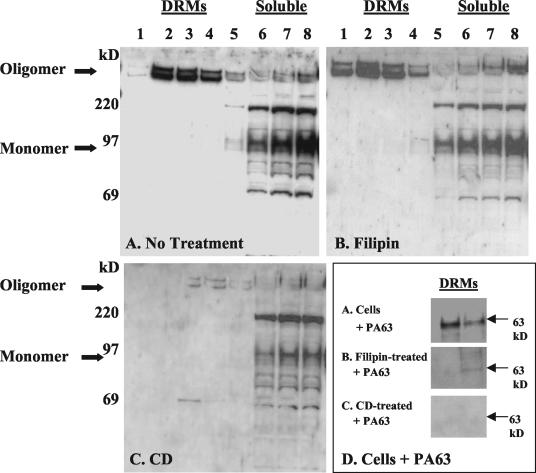
FIG. 5.
Western blotting detection of monomeric and oligomeric Ib on Vero cells pretreated with compounds that affect membrane cholesterol. After treatment, cells were solubilized in Triton X-100 and fractions were separated by sucrose discontinuous density gradient centrifugation. (A) No treatment; (B) filipin; (C) CD. For panels A to C, lanes 1 to 8 represent fractions from a 5, 30, and 40% discontinuous sucrose density gradient. (D) Western blotting detection in DRMs (fractions 2 and 3) of PA63 on Vero cells pretreated as for panels A to C.
Cellular proteins detected in DRMs from Ib-treated Vero cells.
Upon detergent solubilization and subsequent isolation, specific marker proteins localize in DRMs (caveolin-1 and flotillin-1) while others remain in the soluble fraction (α-1 Na+,K+-ATPase) (2, 24). When DRMs and detergent-soluble fractions prepared from Vero cells incubated with Ib were analyzed by Western blotting for distinct marker proteins, caveolin-1 and flotillin were found in DRMs while α-1 Na+,K+-ATPase was detected in the soluble fraction (Fig. 2). Two other proteins, clathrin and dynamin, often involved in endocytosis, were found in the soluble fraction (2, 39). DRMs prepared from Vero cells not incubated with Ib also contained caveolin-1 and flotillin-1 in DRMs and α-1 Na+,K+-ATPase, clathrin, and dynamin in the soluble fraction (data not shown).
FIG. 2.
Western blotting detection of specific cellular proteins in Vero cells incubated with Ib, solubilized in Triton X-100, and separated on a 5, 30, and 40% discontinuous sucrose density gradient. DRMs (pooled fractions 2 and 3) and soluble fractions (pooled fractions 6 and 7) were used in this study.
GPI-anchored proteins do not represent a receptor for Ib.
Previous investigations have shown that the Ib cell receptor consists of a protein (35). Often associated with DRMs and used by many bacterial toxins as cell receptors, GPI-anchored proteins are easily discerned by treating cells with PI-PLC, an enzyme that specifically cleaves the GPI anchor, thus releasing proteins from membranes (1, 9, 12, 41). In order to determine whether the receptor for Ib was a GPI-anchored protein, the effects of PI-PLC treatment on Ib binding and oligomer formation were investigated. Although Ib binding and oligomer formation were not abolished by pretreatment of cells with the enzyme, there appeared to be a slight shift of oligomeric Ib from the DRMs to monomeric Ib detected in the soluble fraction (Fig. 3A). In both control and enzyme-treated cells, however, Ib oligomers were detected in DRMs while monomeric Ib was located in the soluble fractions. In addition, when Ia and Ib were added to the cells, with or without PI-PLC pretreatment, there was similar sensitivity to iota-toxin, thus demonstrating that PI-PLC treatment did not alter iota-toxin cytotoxicity (data not shown).
When Vero cells were analyzed for Ib binding by FACS, pretreatment of cells with PI-PLC did not alter the number of Ib-positive cells (Fig. 3B). Histograms (Fig. 3B) showed the relative number of cells binding to Ib, as reflected by an increase in fluorescence from negative to positive when the cells were incubated without (panels 1 and 3) or with (panels 2 and 4) Ib. The amount of Ib binding to PI-PLC-treated cells (panels 3 and 4) did not differ from that in the untreated controls (panels 1 and 2).
Effects of compounds that remove or sequester cholesterol on Ib binding and iota-toxin cytotoxicity.
In order to determine whether cholesterol was required for iota-toxin cytotoxicity, Ia and Ib were added to Vero cells pretreated with filipin (which sequesters cholesterol) or CD (which removes cholesterol). Then, at various times after intoxication, cells were examined microscopically. When Ia plus Ib were added to cells either untreated (control) or pretreated with filipin, they uniformly rounded and detached within 2 h after addition of toxin (data not shown). If cells were pretreated with CD prior to iota-toxin, there was a noticeable delay in cytotoxicity. Examination by phase-contrast microscopy (Fig. 4) showed that 2 h after the addition of Ia and Ib, control cells were rounded and detached, while CD-treated cells did not show rounding until 6 h postintoxication. By 24 h, there was no difference between control and CD-treated cells incubated with Ia plus Ib, as all the cells were rounded and detached (data not shown).
FIG. 4.
Phase-contrast microscopy showing delay of iota-toxin cytotoxicity in Vero cells pretreated with or without CD. Ia plus Ib (125 ng of each) were incubated with Vero cells (left side) and CD-treated Vero cells (right side). Photographs were taken immediately (0 h), 2 h, and 6 h after addition of toxin.
As might be expected from the cytotoxicity results, when DRM fractions were compared with control cells incubated with Ib (Fig. 5A), pretreatment of Vero cells with filipin had little effect on either Ib binding or oligomer formation (Fig. 5B). Pretreatment of Vero cells with CD, however, decreased Ib binding as well as oligomer formation (Fig. 5C). Since pretreatment of CHO cells with filipin or CD affects PA63 binding in DRMs (2), PA63 was incubated with a portion of Vero cells as a control for these experiments. Similar to what occured with CHO cells, PA63 binding was abolished in filipin-treated Vero cells and greatly reduced in those preincubated with CD (Fig. 5D). Compared to the total cholesterol in untreated (control) cells, those levels in cells treated with CD were reduced to 47% ± 3%, while cholesterol levels in cells treated with filipin were 90% ± 5%.
DISCUSSION
Specific plasma membrane lipids and proteins partition into distinct microdomains that function as platforms for cell signaling and receptor internalization (4, 8). Bacterial toxins have also adopted these DRMs for use as portals of entry into the cell (10). Recent investigations have shown that DRMs are involved in the binding and oligomer formation of many bacterial pore-forming toxins, including A. hydrophila aerolysin and C. perfringens epsilon-toxin, as well as the PA component of B. anthracis lethal and edema toxins (2, 3, 24). In the investigations presented here, the cell-binding protein of C. perfringens iota-toxin, Ib, was also shown to associate with DRMs, exhibiting similarities but also distinct differences in cell binding and oligomer formation from these other toxins.
With toxins such as aerolysin, epsilon-toxin, or a toxin component like PA, monomers are enriched in DRMs that facilitate oligomer formation and subsequent anchoring of the pore-forming oligomers into the cell membrane (2, 3, 24). However, monomeric Ib detected in the soluble fraction, not in DRMs, indicated that Ib initially bound to a receptor located outside DRMs and subsequently coalesced into these microdomains.
The initial and critical step in binding and subsequent oligomer formation for these pore-forming toxins is the generation of a biologically active molecule from the inactive protoxin. Ibp shares similarities with the epsilon equivalent in that both are proteolytically cleaved by serine-type proteases before cell binding and subsequent oligomer formation (24, 36). Ibp and epsilon protoxins bind to cells but do not form cell-associated oligomers and do not facilitate cytotoxicity (23, 36). Unlike epsilon-toxin, where both the protoxin and proteolytically activated forms associate with DRMs, Ibp was detected in only the soluble fractions.
Although Ibp resembles the epsilon protoxin, in that both are cleaved prior to cell binding, the cellular association of Ibp more closely resembles that of the PA protoxin (PA83), which binds to a non-DRM protein, the anthrax toxin receptor (2). Cell surface activation of PA83 by furin then leads to monomeric PA63, and the cell receptor is seemingly concentrated into DRMs that facilitate rapid formation of oligomers (3, 17, 19, 22). When Vero cells were incubated with PA83 or PA63 and subjected to Triton X-100 solubilization and sucrose gradient fractionation, analysis by Western blotting detected PA83 in the soluble fractions and PA63 primarily in DRMs.
Previous investigations have indicated that the cellular receptor for Ib is a protein because pronase pretreatment of Vero cells prevents Ib binding (35). GPI-anchored proteins, normally involved in cell signal transduction, associate with DRMs and are often used by bacterial toxins as receptors for binding to cells (9, 12, 41). The toxins may bind to specific GPI-anchored proteins or, as with aerolysin, bind to glycan regions conserved in several different GPI-anchored proteins. Involvement of these proteins as toxin receptors is easily determined, since most GPI-anchored proteins are released from DRMs when cells are treated with PI-PLC (1, 9). While PI-PLC treatment of Vero cells did not inhibit Ib binding and oligomer formation, the treatment resulted in a slight reduction of oligomeric Ib detected in DRMs and an increase of monomeric Ib in the soluble fractions. FACS analysis would not detect this shift, because the rabbit Ib antiserum does not differentiate between Ib oligomers or monomers bound to the cell surface (35).
Since PI-PLC treatment of Vero cells did not alter iota-toxin cytotoxicity and did not inhibit oligomer formation in DRMs, the Ib receptor was apparently not a GPI-anchored protein. The slight shift from oligomeric to monomeric Ib, however, indicated that GPI-anchored proteins could be involved, either directly or indirectly, with Ib forming oligomers in DRMs. While specific for cleavage of a GPI anchor, PI-PLC is not specific in terms of the protein moiety, and the enzyme will release these anchors from various proteins that are normally contained within DRMs, the removal of which could influence DRM formation and stability (9). Further investigation is needed to understand the role these proteins may play in Ib binding and oligomer formation.
Removal or sequestration of cholesterol results in dispersal of DRM-associated components so that certain protein toxins can no longer bind to normally targeted cells (24, 26). Although filipin and CD reduce the availability of membrane-associated cholesterol, their mode of action differs and often results in differential effects on receptor binding and signaling processes within the membrane (6, 15, 38). Filipin, a polyene macrolide antibiotic, inserts into the membrane and sequesters cholesterol, thus inducing structural disorder that distorts caveolar domains and inhibits caveolar function. The drug preferentially binds to cholesterol within caveolae and has been used to identify caveola-associated processes (32). Pretreatment of Vero cells with filipin did not inhibit iota-toxin cytotoxicity or alter either Ib binding or oligomer formation, thus indicating no involvement by caveolae (26). These results are supported by the findings of M. Gibert et al. (unpublished data), showing that cells pretreated with filipin or a related compound, nystatin, did not prevent iota-toxin cytotoxicity as measured by actin filament depolymerization. Further analysis also suggested that caveolae are not involved in Ib oligomer formation because caveolin-1, a major component of caveolae, was detected in DRMs isolated from MRC-5 cells that are toxin resistant and do not form oligomers (36). Conversely, Ib oligomers were found in DRMs from toxin-sensitive SW-13 cells after incubation with Ib, but caveolin-1, as initially described by Holwell and coworkers (14), was not detected (data not shown).
While filipin selectively complexes with membrane cholesterol, CD is a small cyclic oligosaccharide that concentrates at the cell surface and extracts cholesterol from the membrane (6, 42). Recent investigations note that the membrane contains both CD-sensitive and -resistant cholesterol pools, primarily located outside DRMs (15, 42). Since monomeric Ib was not detected in DRMs and CD treatment diminished Ib binding, the cell receptor for Ib more than likely associated with CD-sensitive cholesterol pools, and cholesterol removal resulted in membrane release or conformational distortion of the receptor (15). Additionally, cholesterol extraction by CD could also affect accessibility of the Ib cell receptor, leading to decreased binding of Ib, fewer Ib-Ib interactions, and ultimately decreased, slower oligomer formation (3). As suggested by the delay in time to cell death, however, sufficient oligomers were ultimately formed to facilitate the entry of Ia. Very few molecules of Ia in the cytosol are likely needed to cause cell death, but this aspect of iota-toxin cytotoxicity remains largely unexplored. The CD effect was specific, because both the delay in cytotoxicity and the decrease in oligomer formation did not occur if cholesterol was added to the medium after CD pretreatment (data not shown). The results from cholesterol-reducing agents indicated that while the cell receptor was not found in DRMs, the receptor probably resided in an area associated with cholesterol that, upon Ib binding, helped to facilitate oligomer formation within DRMs.
Oligomer formation dependence upon an acidic microenvironment and a reduction of PA binding in cells that lack functional clathrin-dependent endocytosis led Abrami and colleagues (2) to conclude that the initial entry of PA into acidic endosomes is by a clathrin-dependent process. Cells lacking functional clathrin-dependent endocytosis or pretreatment of Vero cells with chlorpromazin, a drug that blocks endocytosis through clathrin-coated pits, does not alter Ib binding or iota-toxin cytotoxicity (Gibert et al., unpublished). While caveolin-1 and flotillin-1, proteins commonly found in DRMs, were detected in DRMs extracted from Vero cells incubated with or without Ib, clathrin and dynamin were found in the soluble fractions, thus indicating again that Ib did not use a clathrin-dependent route for endocytosis (2). Additional experiments showed that pretreatment of cells with antibodies prepared against these proteins did not prevent or delay iota-toxin cytotoxicity (data not shown), thus providing further evidence that Ib does not use clathrin-coated pits or caveolae as an entry portal.
Finally, evidence that the cell likely plays an active role in oligomerization is also suggested by experiments with paraformaldehyde-fixed Vero cells that bind Ib but do not form Ib oligomers (B. G. Stiles et al., unpublished data). Ib oligomers rapidly form (in less than 1 min) on Vero cells at 37°C, but oligomers and increased ion permeability are not evident at 4°C (36). Likewise, oligomers were observed in DRMs isolated from cells incubated with Ib at 37°C but not 4°C (data not shown). These studies indicate that Ib oligomer formation is not a random event and requires a metabolically active cell.
In summary, the investigations presented here suggest that Ib binds to a specific protein located outside DRMs. Although the Ib receptor does not reside in DRMs, association with DRMs likely facilitates Ib-Ib interactions and stabilizes nascent oligomer formation. These investigations showed that while the binary bacterial toxins share many similarities, there are also marked differences in binding and subsequent oligomerization. Further investigations will provide a greater understanding of how iota-toxin and other binary toxins bind to cells, form oligomeric complexes, and facilitate protein transport across cell membranes.
Acknowledgments
We thank S. Little for his generous gifts of recombinant B. anthracis PA proteins (PA63 and PA83) and rabbit anti-PA serum. We thank Y. Campbell, M. Gibert, and M. Guetthoff for their kind and competent technical assistance.
The opinions, interpretations, and conclusions are those of the authors and are not necessarily endorsed by the U.S. Army.
Editor: J. T. Barbieri
REFERENCES
- 1.Abrami, L., M. Fivaz, P. Glauser, N. Sugimoto, C. Zurzolo, and F. G. van der Goot. 2003. Sensitivity of polarized epithelial cells to the pore-forming toxin aerolysin. Infect. Immun. 71:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrami, L., and F. G. van der Goot. 1999. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J. Cell Biol. 147:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 36:10944-10953. [DOI] [PubMed] [Google Scholar]
- 5.Aman, M. J., and K. S. Ravichandran. 2000. A requirement for lipid rafts in B cell receptor induced Ca++ flux. Curr. Biol. 10:393-396. [DOI] [PubMed] [Google Scholar]
- 6.Awasthi-Kalia, M., P. P. M. Schnetkamp, and J. P. Deans. 2001. Differential effects of filipin and methyl-β-cyclodextrin on B cell receptor signaling. Biochem. Biophys. Res. Commun. 287:77-82. [DOI] [PubMed] [Google Scholar]
- 7.Blöcker, D., J. Behlke, K. Aktories, and H. Barth. 2001. Cellular uptake of the Clostridium perfringens binary iota-toxin. Infect. Immun. 69:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 9.Diep, D. B., K. L. Nelson, S. M. Raja, R. W. McMaster, and J. T. Buckley. 1998. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J. Biol. Chem. 273:2355-2360. [DOI] [PubMed] [Google Scholar]
- 10.Fivaz, M., L. Abrami, and F. G. van der Goot. 1999. Landing on lipid rafts. Trends Cell Biol. 9:212-213. [DOI] [PubMed] [Google Scholar]
- 11.Gibert, M., L. Petit, S. Raffestin, A. Okabe, and M. R. Popoff. 2000. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity. Infect. Immun. 68:3848-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon, V. M., K. L. Nelson, J. T. Buckley, V. L. Stevens, R. K. Tweten, P. C. Elwood, and S. H. Leppla. 1999. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J. Biol. Chem. 38:27274-27280. [DOI] [PubMed] [Google Scholar]
- 13.Han, S., J. A. Craig, C. D. Putnam, N. B. Carozzi, and J. A. Tainer. 1999. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat. Struct. Biol. 6:932-936. [DOI] [PubMed] [Google Scholar]
- 14.Holwell, T. A., S. C. Schweitzer, M. E. Reyland, and R. M. Evans. 1999. Vimentin-dependent utilization of LDL-cholesterol in human adrenal tumor cells is not associated with the level of expression of apoE, sterol carrier protein-2, or caveolin. J. Lipid Res. 40:1440-1452. [PubMed] [Google Scholar]
- 15.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki, M., I. Ohishi, and G. Sakaguchi. 1980. Evidence that botulinum C2 toxin has two dissimilar components. Infect. Immun. 29:390-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimpel, K. R., S. S. Molloy, G. Thomas, and S. H. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell-surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 89:10277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppla, S. H. 1999. The bifactorial Bacillus anthracis lethal and oedema toxins, p. 243-263. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, London, United Kingdom.
- 19.Liu, S., and S. H. Leppla. 2003. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J. Biol. Chem. 278:5227-5234. [DOI] [PubMed] [Google Scholar]
- 20.Marvaud, J. C., T. Smith, M. L. Hale, M. R. Popoff, L. A. Smith, and B. G. Stiles. 2001. Clostridium perfringens iota-toxin: mapping of receptor binding and Ia docking domains on Ib. Infect. Immun. 69:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonel, J. L. 1986. Toxins of Clostridium perfringens A, B, C, D, E, p. 477-517. In F. Dorner and J. Drews (ed.), Pharmacology of bacterial toxins. Pergamon Press, Oxford, United Kingdom.
- 22.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 269:20607-20612. [PubMed] [Google Scholar]
- 23.Miyata, S., O. Matsushita, J. Minami, S. Katayama, S. Shimamoto, and A. Okabe. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon toxin in the synaptosomal membrane. J. Biol. Chem. 276:13778-13783. [DOI] [PubMed] [Google Scholar]
- 24.Miyata, S., J. Minami, E. Tamai, O. Matsushita, S. Shimamoto, and A. Okabe. 2002. Clostridium perfringens ɛ-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463-39468. [DOI] [PubMed] [Google Scholar]
- 25.Nagahama, M., K. Nagayasu, K. Kobayashi, and J. Sakurai. 2002. Binding component of Clostridium perfringens iota-toxin induces endocytosis in Vero cells. Infect. Immun. 70:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orlandi, P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perelle, S., M. Gibert, P. Boquet, and M. R. Popoff. 1993. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect. Immun. 61:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perelle, S., S. Scalzo, S. Kochi, M. Mock, and M. R. Popoff. 1997. Immunological and functional comparison between Clostridium perfringens iota toxin, C. spiroforme toxin, and anthrax toxins. FEMS Microbiol. Lett. 146:117-121. [DOI] [PubMed] [Google Scholar]
- 29.Rossjohn, J., S. C. Feil, W. J. McKinstry, R. K. Tweten, and M. W. Parker. 1997. Structure of a cholesterol-binding thiol-activated cytolysin and a model of its membrane form. Cell 89:685-692. [DOI] [PubMed] [Google Scholar]
- 30.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 31.Singh, U., C. M. Van Itallie, L. L. Mitic, J. M. Anderson, and B. A. McClane. 2000. CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J. Biol. Chem. 275:18407-18417. [DOI] [PubMed] [Google Scholar]
- 32.Smart, E. J., and R. G. W. Anderson. 2002. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 353:131-139. [DOI] [PubMed] [Google Scholar]
- 33.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinthorsdottir, V., H. Halldorsson, and O. S. Andresson. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 28:45-50. [DOI] [PubMed] [Google Scholar]
- 35.Stiles, B. G., M. L. Hale, J. C. Marvaud, and M. R. Popoff. 2000. Clostridium perfringens iota toxin: binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect. Immun. 68:3475-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiles, B. G., M. L. Hale, J. C. Marvaud, and M. R. Popoff. 2002. Clostridium perfringens iota toxin: characterization of the cell-associated complex. Biochem. J. 367:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiles, B. G., and T. D. Wilkins. 1986. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect. Immun. 54:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, C. M., T. Coetzee, and S. E. Pfeiffer. 2002. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J. Neurochem. 81:993-1004. [DOI] [PubMed] [Google Scholar]
- 39.van Dam, E. M., and W. Stoorvogel. 2002. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. 13:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandekerckhove, J., B. Schering, M. Barmann, and K. Aktories. 1987. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 225:48-52. [DOI] [PubMed] [Google Scholar]
- 41.Wang, A., A. Wada, K. Yahiro, T. Nomure, Y. Fujii, K. Okamoto, Y. Mizuta, S. Kohno, J. Moss, and T. Hirayama. 1999. Identification and characterization of the Aeromonas sobria hemolysin glycoprotein receptor on intestine 407 cells. Microb. Pathog. 27:215-221. [DOI] [PubMed] [Google Scholar]
- 42.Yancey, P. G., W. V. Rodrigueza, E. P. C. Kilsdonk, G. W. Stoudt, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1996. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271:16026-16034. [DOI] [PubMed] [Google Scholar]