Abstract
Purpose of review
The pleotropic effects of vitamin D on chronic diseases have received significant attention; however, its role in acute illness is less understood. The purpose of this review is to summarize the current evidence regarding the role of vitamin D in acute stress and critical illness.
Recent findings
25-Hydroxyvitamin D levels may affect risk of developing acute illnesses (e.g. respiratory infections), and low concentrations are associated with unfavorable outcomes during critical care. Inflammatory changes alone do not explain the observed deterioration in vitamin D status following acute stress. Hemodilution, interstitial extravasation, decreased synthesis of binding proteins, and renal wasting of 25-hydroxyvitamin D, all appear to play a more significant role in the regulation of vitamin D status during critical illness.
Summary
Single-point assessments of 25-hydroxyvitamin D following acute stress may provide an inaccurate assessment of vitamin D status. In such cases, measurement of binding proteins and free vitamin D metabolites may be essential to create a more realistic approximation of vitamin D status. Variations in patient responses to acute stress and critical illness may depend not only on the degree of systemic vitamin D insufficiency, but also on the individual tissue requirements.
Keywords: 25(OH)D, acute illness, critical care, vitamin D
INTRODUCTION
The prevalenceof low vitamin D status has increased in the general population [1]. This is concerning given the growing recognition of the association between vitamin D insufficiency and increased risk of cardiovascular disease, cancer, and pulmonary ailments [2–7]. Indeed, preliminary trials – although not uniform in their results – suggest that vitamin D supplementation may mitigate the incidence and adverse outcomes of these diseases and may reduce all-cause mortality [8–11, 12■]. Although its pleotropic effects on chronic diseases have received significant attention, the role of vitamin D in acute illness is less understood. In this review, we summarize current evidence and conclude that vitamin D status may play an important role in acute stress and critical illness.
MEASURING VITAMIN D STATUS
Vitamin D, a 27-carbon secosteroid, is unlike most other hormones. Differences in source (endogenous vs. exogenous prehormone), extensive need for tissue modification (skin vs. liver vs. kidney), an active intermediary prohormone {25-hydroxy-vitamin D [25(OH)D]}, and a critical set of modulators [parathyroid hormone (PTH), calcium, phosphorus, and fibroblast growth factor-23 (FGF-23)] add to the complexity of vitamin D regulation [13]. Taken together, these factors complicate the direct measurement of vitamin D, as well as which parameter would best reflect overall vitamin D status [14].
Upon exposure to ultraviolet B irradiation, endogenous synthesis of vitamin D3 starts with a photochemical reaction in the epidermis (Fig. 1). Alternatively, vitamin D3 can be supplied exogenously as a nutrient or obtained through a dietary intake of fatty fish and vitamin D-enriched foods. Specially treated plant materials can also contribute to vitamin D intake in the form of vitamin D2 (ergocalciferol). Although their metabolic pathways are identical and both may contribute to vitamin D adequacy, vitamin D3 appears to be more efficiently metabolized [12■, 15, 16].
FIGURE 1.
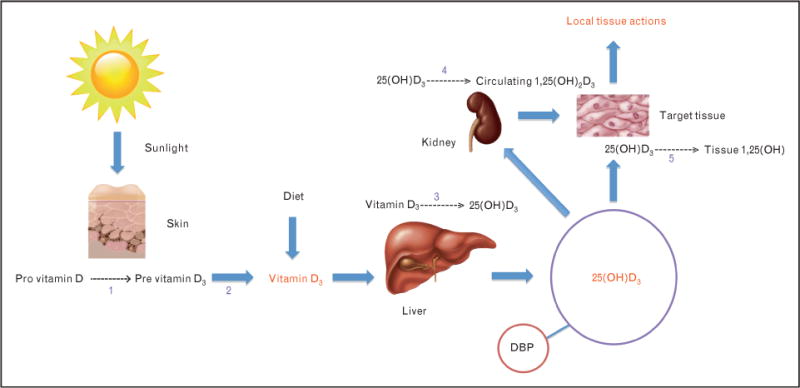
Synthetic pathway of the major vitamin D metabolites. (1) Reaction catalyzed by UVB (290–310 nm). (2) Isomerization reaction catalyzed by heat. (3) Reaction catalyzed by 25-hydroxylase. (4) Reaction catalyzed by renal 1-a-hydroxylase. (5). Reaction catalyzed by tissue 1-a-hydroxylase. 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; DBP, vitamin D-binding protein.
Vitamin D traverses the systemic circulation after binding to vitamin D-binding protein (DBP) and is hydroxylated in the liver to 25(OH)D. 25(OH)D is rapidly released by the liver into the circulation, where under normal circumstances, it exhibits a biological half-life of approximately 2–3 weeks [17]. In the kidney, 25(OH)D is enzymatically converted to the vitamin D hormone calcitriol {1,25-dihydroxyvitamin D [1,25(OH)2D]}, which is the most biologically active metabolite of the vitamin D synthesis pathway. This final step is rigidly controlled by the stimulus of PTH, inhibition by FGF-23, and circulating levels of calcium, phosphorus, and 1,25(OH)2D itself [13]. Circulating 25(OH)D levels are, however, approximately 500–1000 times higher than 1,25(OH)2D levels, and both 25(OH)D and 1,25(OH)2D are predominantly protein-bound in circulation. Indeed, only 0.03% of 25(OH)D is free, with close to 88% bound to DBP and the remainder to albumin [18■].
Serum 25(OH)D levels reflect overall vitamin D body stores from sunlight exposure and dietary intake [14]. Under normal circumstances, it is the best indicator of vitamin D status [19■, 20]. This is unusual in the sense that a metabolite one step removed from the most biologically active form is used to assess adequacy. Nonetheless, 25(OH)D is the most abundant vitamin D metabolite and its relative stability in the systemic circulation makes it a good indicator of vitamin D stores in the general population. However, recent evidence suggests that significant variation in 25(OH)D levels may occur from hour to hour in acutely ill patients and that single-point assessments may be inaccurate estimates of vitamin D status in such patients [21■■]. On the contrary, 1,25(OH)2D is present in picomolar concentrations and, because it is tightly regulated, the concentration can remain normal or even be elevated, despite evidence of deficiency [17]. Moreover, in stark contrast to 25(OH)D, the half-life of 1,25(OH)2D is only a few hours [18■]. Furthermore, measurement of 1,25(OH)2D maybe confounded by the greater technical difficulty in performing the assay and by conditions such as renal insufficiency or advanced aging that reduce 1-a-hydroxylase activity, which is a major cause of low levels of 1,25(OH)2D independent of vitamin D stores [14, 22].
25-HYDROXYVITAMIN D AS A BIOMARKER
The role of 25(OH)D levels as a biomarker for disease is controversial. Widespread utilization of thresholds to define disease risk has led to multiple issues from both a clinical and research perspective [23]. To date, there is no consensus about the optimal definitions of either vitamin D deficiency or insufficiency, and diverse cut points for 25(OH)D levels have been suggested, ranging from 16 to 48 ng/ml [19■, 24–26]. This uncertainty is likely to be multifactorial, including inadequate standardization of vitamin D assay methodologies [14] and differences in the measured functional endpoints used by various investigators, which arise from the classic and nonclassic effects of vitamin D.
The classic function of vitamin D is the control of extracellular calcium metabolism by regulating absorption in epithelia involved in calcium transport. Consequently, the traditional ‘low normal’ level for 25(OH)D was 10ng/ml (to convert to nmol/l, multiply by 2.496), as this threshold had the advantage of high specificity for rickets and osteomalacia [13, 17]. Since low vitamin D status stimulates PTH secretion to increase intestinal calcium absorption and bone resorption to maintain calcium balance, it has been proposed that vitamin D sufficiency be described as the concentration of 25(OH)D which achieves maximal PTH suppression [27]. In this regard, vitamin D sufficiency is defined by a 25(OH)D level of 28ng/ml [28]. On the basis of studies on fracture prevention, most investigators have adopted the definition of vitamin D insufficiency as 25(OH)D concentrations less than 30ng/ml and deficiency below 20ng/ml [17, 27, 29]. However, the 2011 report from the Institute of Medicine defined vitamin D adequacy as 25(OH)D levels between 20 and 50ng/ml, on the basis of an estimate that 25(OH)D levels of 20ng/ml would protect 97.5% of the healthy population from skeletal disorders and the higher risk of vitamin D toxicity at 25(OH)D levels above 50ng/ml [19■, 30■].
The nonclassic function of vitamin D includes regulation of cell proliferation and differentiation, regulation of hormone secretion, and the regulation of immune function [20, 31]. These effects take place on a cellular level and are directly dependent on 25(OH)D levels [32]. Indeed, cells of the neuromuscular, cardiovascular, endocrine, and immune system express the vitamin D receptor (VDR) [33]. Furthermore, most of these cells express the 25(OH)D-1-a-hydroxylase, to produce 1,25(OH)2D for autocrine and paracrine use within the target cell itself [13, 34]. The discovery of VDRs in activated immune cells has particularly stimulated research into the role of vitamin D in immune function [35■]. It is now recognized that vitamin D plays a critical role in the regulation of the innate and the adaptive immune systems [36, 37]. 1,25(OH)2D inhibits adaptive immunity by attenuating the proliferation and differentiation of both T and B lymphocytes, which is thought to ameliorate the severity of autoimmune diseases [38]. In contrast to its inhibitory role in adaptive immunity, 1,25(OH)2D is a potent activator of the innate immune system [39]. Innate immunity represents the first line of defense against microbial invasion and constitutes both epithelial and mucosal cells, as well as polymorphonuclear leukocytes, monocytes, and macrophages [38]. The central mechanism underlying microbial eradication is the activation of toll-like receptors in the host cell, which induces formation of potent antimicrobial peptides, such as cathelicidin [40]. Macrophages and epithelial cells respond to both circulating and local 1,25(OH)2D synthesized by 1-a-hydroxylase activity on 25(OH)D. Recent evidence suggests that 25(OH)D levels of approximately 30–35ng/ml might be sufficient for vitamin D to optimize its cathelicidin effects [41■].
25-HYDROXYVITAMIN D LEVELS AND ACUTE ILLNESS
Seasonal variations in influenza and pneumococcal community-acquired pneumonia suggest that vitaminD, through its antimicrobial, anti-inflammatory, and/or immunomodulatory actions, may play an important role in disease risk [42, 43]. Although a number of early clinical studies suggested anassociation between 25(OH)D levels below 10ng/ml and acute respiratory tract infections (ARIs) [44, 45], recent studies have furnished more compelling evidence (Table 1). Two large retrospective studies demonstrated that 25(OH)D levels below 30ng/ml are associated with increased risk for upper respiratory tract infections (URIs) [46, 47■■]. This evidence is further strengthened by a prospective, observational cohort study, which demonstrated an almost twofold reduction inviral URI risk when serum 25(OH)D levels were at least 38ng/ml [48]. Low 25(OH)D levels have also been shown to significantly increase the risk of work absenteeism owing to URIs [49]. Evidence from randomized clinical trials (RCTs) is limited, but in general, suggests that improved vitamin D status through supplementation is associated with a lower incidence of ARIs [50].
Table 1.
Evidence to support the association between 25(OH)D levels and acute respiratory illness
| Source | Study details | Main outcome(s) |
|---|---|---|
| Ginde et al. [46] | Design: Secondary analysis of the US NHANES III data (n = 18 883 adults). Objective: Investigated association between 25(OH)D levels and risk of URI. Reference group: 25(OH)D levels ≥30 ng/ml. | 25(OH)D levels <10ng/ml were associated with increased risk of URI (OR 1.36; 95% CI 1.01 – 1.84). 25(OH)D levels ≥10 to <30 ng/ml were associated with increased risk of URI (OR 1.24; 95% CI 1.07–1.43). |
| Berry et al. [47■■] | Design: Secondary analysis of the Nationwide 1958 British Birth Cohort data (n =6789 adults). Objective: Investigated association between 25(OH)D levels and risk of URI. Reference group: 25(OH)D levels <10ng/ml | Each 4ng/ml increase in 25(OH)D level was associated with reduction in risk of URIs (OR 0.93; 95% CI 0.89–0.97). |
| Sabetta et al. [48] | Design: Prospective cohort study (n =198 healthy adults). Objective: Investigated association between 25(OH)D levels and risk of URI during a single fall and winter season in New England. Reference group: 25(OH)D levels <38 ng/ml. | 25(OH)D levels >38 ng/ml were associated with reduction in risk of viral URI (OR 1.49; 95% CI 1.25–1.84). |
| Laaksi et al. [49] | Design: Prospective cohort study (n = 756 young males). Objective: Investigated association between 25(OH)D levels and risk of missed work days from URIs over a 6-month period in Finland. Reference group: 25(OH)D levels ≥16 ng/ml. | 25(OH)D levels <16ng/ml were associated with increased risk of missed work days from URIs (OR 1.63; 95% CI 1.15–2.24). |
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; OR, odds ratio; URI, upper respiratory infection; US NHANES, United States National Health and Nutrition Examination Survey.
Whereas the association between suboptimal vitamin D status and overall risk of cardiovascular disease has been well established [51■], hypovitaminosis D in the setting of acute myocardial infarction (AMI) is just starting to receive attention. Indeed, 25(OH)D levels below 30ng/ml have been observed in up to 96% of patients upon presentation for care of a confirmed AMI [51■, 52■]. Moreover, 25(OH)D levels at the time of index admission for AMI have been shown to be positively associated with in-hospital survival rate and inversely related to desired post-AMI biomarker profiles [53].
A growing body of evidence suggests that 25(OH)D may also influence the risk of mortality from critical illness. Though hypovitaminosis D in the ICU was reported sporadically between 1987 and 2003, Lee et al. recently described a disproportionately high incidence of vitamin D insufficiency in this population [54–58]. Although this led to new studies to detect associations between vitamin D status and survival from critical illness [46, 59], a single definitive threshold level for 25(OH)D and mortality benefit during critical illness remains elusive. The evidence does suggest, however, that the beneficial association of higher 25(OH)D levels with survival from critical illness is weaker in the setting of acute on chronic disease states (Table 2). Lower mortality risk in patients with multiple significant underlying comorbidities admitted for acute illness to the medical ICUs was observed at 25(OH)D levels between 12 and 20ng/ml [60■■, 61, 62■■] vs. observed benefit with levels around 26ng/ml in patients with a focused disease process admitted to the surgical ICU [63■■]. This may explain why increased mortality risk in a mixed ICU population (medical and surgical patients) is only observed at 25(OH)D levels 15ng/ml or less but not above 15 to below 30 ng/ml [64■■]. Although a limited number of RCTs have demonstrated an improvement in vitamin D status during critical illness through supplementation, the studies were underpowered to determine an effect on mortality [18■].
Table 2.
Evidence to support the association between 25(OH)D levels and critical illness
| Source | Study details | Main outcome(s) |
|---|---|---|
| Leow et al. [60■■] | Design: Prospective cohort study (n = 112 elderly patients admitted for community acquired pneumonia). Objective: Investigated association between admission 25(OH)D levels and 30-day mortality. Reference group: 25(OH)D levels >12 ng/mL. | 25(OH)D levels <12 ng/ml were associated with increased risk of 30-day mortality (OR 13.5; 95% CI 2.6–69.1). |
| Annweiler et al. [61] | Design: Prospective cohort study (n = 399 elderly patients admitted to an acute care geriatric unit). Objective: Investigated association between admission 25(OH)D levels and short-term (<14 days) mortality. Reference group: 25(OH)D levels ≤20 ng/mL. | 25(OH)D levels >20 ng/ml were associated with a reduction in risk of short-term mortality (OR 0.65; 95% CI 0.44–0.96). |
| Venkatram et al. [62■■] | Design: Retrospective cohort study (n = 437 patients admitted to a medical ICU). Objective: Investigated association between admission 25(OH)D levels and index in-hospital mortality. Reference group: 25(OH)D levels ≥20 ng/ml. | 25(OH)D levels <20 ng/ml were associated with increased risk of in-hospital mortality (OR 8.7; 95% CI 1.03–72.8). |
| Arnson et al. [65] | Design: Prospective cohort study (n = 130 patients admitted to a medical ICU). Objective: Investigated association between admission 25(OH)D levels and 60-day mortality. Reference group: 25(OH)D levels ≤20 ng/ml. | 25(OH)D levels >20 ng/ml were not associated with a reduction in risk of 60-day mortality. |
| Matthews et al. [63■■] | Design: Prospective cohort study (n = 258 patients admitted to a surgical ICU). Objective: Investigated association between admission 25(OH)D levels and ICU LOS, ICU-related costs, and ICU-related mortality. Reference group: 25(OH)D levels >26 ng/ml. | 25(OH)D levels <26 ng/ml were associated with longer ICU LOS (r = 0.194, P = 0.001), increased ICU-related costs (r = 0.194, P = 0.001), and a higher ICU-related mortality rate (r = 0.125, P = 0.023). |
| Braun et al. [64■■] | Design: Retrospective cohort study (n = 1325 patients admitted to a medical or surgical ICU). Objective: Investigated association between 25(OH)D levels ±7 days of ICU admission and in-hospital, 30-day, 90-day, and 1-year mortality. Reference group: 25(OH)D levels >15ng/ml. | 25(OH)D levels <15 ng/ml were associated with increased risk of in-hospital (OR 1.77; 95% CI 1.04–3.01), 30-day (OR 1.94; 95% CI 1.17–3.21), 90-day (OR 1.78; 95% CI 1.14–2.76), and 1-year mortality (OR 1.65; 95% CI 1.12–2.43). |
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; LOS, length of stay; OR, odds ratio.
25-HYDROXYVITAMIN D LEVELS AND INFLAMMATION
The ability to modulate inflammatory cytokines may explain many of the health benefits that some attribute to vitamin D [33, 36]. However, observational studies reveal variable results for the association between vitamin D status and inflammation [65–67]. Moreover, randomized controlled trials of vitamin D supplementation show inconsistent results, with some trials suggesting a decrease and others finding no effect on inflammatory biomarkers [68–71]. A recent secondary analysis on the US National Health and Nutrition Examination Survey (NHANES) data (2001–2006) investigated the relationship between 25(OH)D levels and C-reactive protein (CRP) in asymptomatic ambulatory patients [72]. And although an inverse relationship between 25(OH)D levels below 21ng/ml and CRP concentration was observed, 25(OH)D levels of at least 21ng/ml demonstrated a positive association with CRP concentrations.
In contrast, threshold-related associations between 25(OH)D levels and inflammatory markers are not as evident in symptomatic patients. Lucidarme et al. [59] were actually unable to demonstrate any association between 25(OH)D levels and CRP concentrations at the time of admission in a small ICU cohort. Similarly, Barth et al. [73] demonstrated that 25(OH)D levels did not change significantly in a small cohort of AMI patients despite a modest acute elevation in CRP concentrations. However, in another small cohort, Bang et al. [74■] demonstrated that the range of 25(OH)D levels maintained an inverse relationship with CRP concentrations between admission and day 2 in patients with acute pancreatitis. This inverse relationship between 25(OH)D and CRP in acute illness was further elucidated in a large, retrospective analysis of patients hospitalized throughout Scotland [75■].
Healthcare-related interventions are also often associated with profound inflammatory responses (Table 3). For example, the administration of zoledronic acid (nitrogen-containing bisphosphonate) for the treatment of osteoporosis typically induces a strong acute-phase response [79]. As such, declining 25(OH)D levels over the first three post-infusion days were shown to be inversely correlated with increasing CRP concentrations [76]. It is, however, noteworthy that when baseline 25(OH)D levels were above 20ng/ml, clinically significant elevations in CRP concentrations (i.e. ≥10mg/l) following administration of zoledronic acid did not occur. Vitamin D status is also deranged following surgical manipulation, but does not appear to be correlated with postoperative inflammatory responses [77■■, 78]. Although Reid et al. [77■■] demonstrated a generally inverse data trend between 25(OH)D levels and CRP concentrations up to the 5th postoperative day, a statistically significant correlation was not observed. More importantly, the fact that 25(OH)D and calculated free 25(OH)D levels remained low (relative to baseline) as far out as 3 months after surgery, despite normalized CRP concentrations, strengthens the argument that derangements in vitamin D status following acute stress are not completely explained by inflammatory changes. Indeed, Krishnan et al. [78] suggest that acute fluid loading, rather than inflammatory changes, may have a more significant effect on vitamin D status during the early resuscitative phases of critical illness.
Table 3.
The association between vitamin D status and inflammatory responses to healthcare-associated interventions
| Source | Study details | Main outcome(s) |
|---|---|---|
| Bertoldo et al. [76] | Design: Prospective cohort study (n = 90 elderly females with known diagnosis of osteoporosis). Intervention: Administration of 5 mg i.v. zoledronic acid. Objective: Investigated the association between 25(OH)D and APR following administration of zoledronic acid. Reference group: 25(OH)D levels > 12 ng/ml. | 25(OH)D levels <12 ng/ml were associated with increased risk of APR (OR 2.38; 95% CI 1.85–2.81). Mean 25(OH)D levels dropped 17.3% (±9.6%, P <0.02) by postinfusion day 3 (compared to baseline) and was inversely correlated with CRP levels (r = − 0.79, P < 0.001) |
| Reid et al. [77■■] | Design: Prospective cohort study (n = 33 adult surgical patients). Intervention: Knee arthroscopy. Objective: Investigated the association between vitamin D status and postoperative inflammatory response. Reference group: Preoperative laboratory assessments. | 25(OH)D levels dropped ~40% (P < 0.001) by POD2 and remained below baseline at POD5 (P < 0.001) and at 3-month follow-up (P = 0.003). DBP levels remained unchanged at POD2, POD5, and at 3-month follow-up. Albumin levels dropped ~20% (P < 0.001) by POD2, remained below baseline at POD5 (P < 0.001), but was back to baseline at 3-month follow-up. Free 25(OH)D dropped ~30% (P < 0.001) by POD2 and remained below baseline at POD5 (P < 0.001) and at 3-month follow-up (P = 0.006). CRP levels increased ~60-fold at POD2 (P < 0.001), remained elevated at POD5, but was back to baseline at 3-month follow-up. A correlation between perioperative 25(OH)D and CRP levels was not observed. |
| Krishnan et al. [78] | Design: Prospective cohort study (n = 19 adult surgical patients). Intervention: Cardiac surgery with intraoperative CPB. Objective: Investigated the association between vitamin D status and acute fluid loading. Reference group: Pre-CPB laboratory assessments. | 25(OH)D levels dropped ~35% (P < 0.0001) following CPB and returned to baseline at POD5. 1,25(OH)2 levels dropped ~45% (P < 0.0001) following CPB and overshot by ~115% (P < 0.0001) at POD5. Albumin levels dropped ~30% following CPB (P < 0.0001) and returned to baseline by POD5. CRP levels dropped ~30% (P = 0.04) following bypass and had increased ~20-fold by POD5. Fluid balance was inversely associated with both 25(OH)D (effect size −4.9: 95% CI −6.4 to −3.4, P < 0.0001) and 1,25(OH)2D levels (effect size −14.0: 95% CI −22 to −6, P < 0.001). CRP levels were association with both 25(OH)D (effect size 0.08: 95% CI, 0.02–0.14, P < 0.01) and 1,25(OH)2D (effect size 0.62: 95% CI 0.39–0.84, P < 0.0001) levels. |
25(OH)D, 25-hydroxyvitamin D; APR, acute-phase response; CI, confidence interval; CPB, cardiopulmonary bypass; CRP, C-reactive protein; DBP, vitamin D-binding protein; i.v., intravenous; POD, postoperative day.
INTERPRETTING 25-HYDROXYVITAMIN D LEVELS IN ACUTE STRESS
Interpretations of vitamin D status based on 25(OH)D measurement in acute illness should be performed with caution. It is essential to keep in mind that 25(OH)D levels vary with the assay method [14]. Reproducibility is poor and may vary from 10 to 100% when liquid chromatographytandem mass spectrometry is used as the reference. Furthermore, extraction from binding proteins and procedural losses may also contribute to measurement variations. But, it is unlikely that differences in assay methodologies and technical challenges can fully explain the changes in 25(OH)D levels in acute stress and critical illness. It does, however, underscore the need for a standardized approach to measuring vitamin D status in future studies.
Rapid fluid shifts may influence measured vitamin D status, since more than 99% of 25(OH)D is bound to DBP and albumin. Krishnan et al. [78] demonstrated that a 3 liter fluid load during cardiopulmonary bypass was not only associated with an approximate 35% reduction in circulating 25(OH)D levels, but also a concomitant 30% reduction in serum albumin concentration. On the contrary, Reid et al. [77■■] demonstrated that whereas patients received 3 liters of fluid over 24h after knee arthroscopy, a 40% reduction in 25(OH)D levels was accompanied by only a 15% reduction in albumin concentration and 10% reduction in DBP levels. As such, volume dilution does not completely explain sustained low levels of 25(OH)D following acute stress. Moreover, critically ill patients typically exhibit significantly lower DBP and albumin levels compared to the general population [56, 80]. The mechanism underlying such dramatic loss of binding proteins is still unknown, but it is thought to be related to interstitial extravasation from increased vascular permeability following inflammatory responses, decreased synthesis, and hemodilution during active resuscitation. Regardless, binding proteins are critical for efficient 25(OH)D reabsorption at the renal tubule [81]. Therefore, acute hemodilution, interstitial extravasation, or decreased synthesis of binding proteins augment renal wasting of 25(OH)D, which may explain the disproportionately low levels. Therefore, not only is it important to carefully time the measurement of vitamin D metabolites or to perform multiple assessments, but also the assessment of binding proteins and free vitamin D metabolites may be essential to create a more realistic approximation of vitamin D status in individual patients.
It has been proposed that a functional vitamin D deficiency may exist in acute stress and critical illness [82]. As such, the clinical consequences of vitamin D deficiency would not only be dependent on the severity of vitamin D depletion, but more importantly, would be related to tissue requirement (Fig. 2). In such a model, the circulating 25(OH)D pool represents a substrate reservoir for conversion to active metabolites at the tissue level during times of acute stress. It is conceivable that a regulatory system responds to local tissue signals,which in turn matches physiological needs with appropriate and adequate substrate [25(OH)D] for conversion to the active hormone [1,25(OH)2D]. In acute stress and critical illness, hypocalcemia is common and may lead to compensatory rise in PTH, which would heighten conversion of 25(OH)D to 1,25(OH)2D to maintain calcium homeostasis. The consumption of 25(OH)D in the setting of secondary hyper-parathyroidism would further exacerbate vitamin D insufficiency. Evidence in support of this hypothesis comes from studies that demonstrate secondary hyperparathyroidism with hypocalcaemia and low 25(OH)D levels in acutely ill patients [56, 59, 83, 84■■]. Furthermore, in contrast to the general population, in which 1,25(OH)2Dconcentrations are maintained within a normal range, calcitriol levels correlate positively with 25(OH)D levels and are up to 50% lower in critically ill patients [56, 85]. Vitamin D deficiency in acute stress and critical illness may therefore represent a state of mismatch between substrate supply and tissue requirement, in that despite maximal stimulation of 1-a-hydroxylase by PTH, local tissue is unable to generate adequate 1,25(OH)2D. PTH resistance, which occurs in hypo-magnesaemia, renal failure, and hypoparathyroidism, can further compromise 1,25(OH)2D formation [82]. Variations in individual patient responses to acute stress and critical illness may therefore depend on the degree of vitamin D insufficiency and extent of tissue requirement.
FIGURE 2.
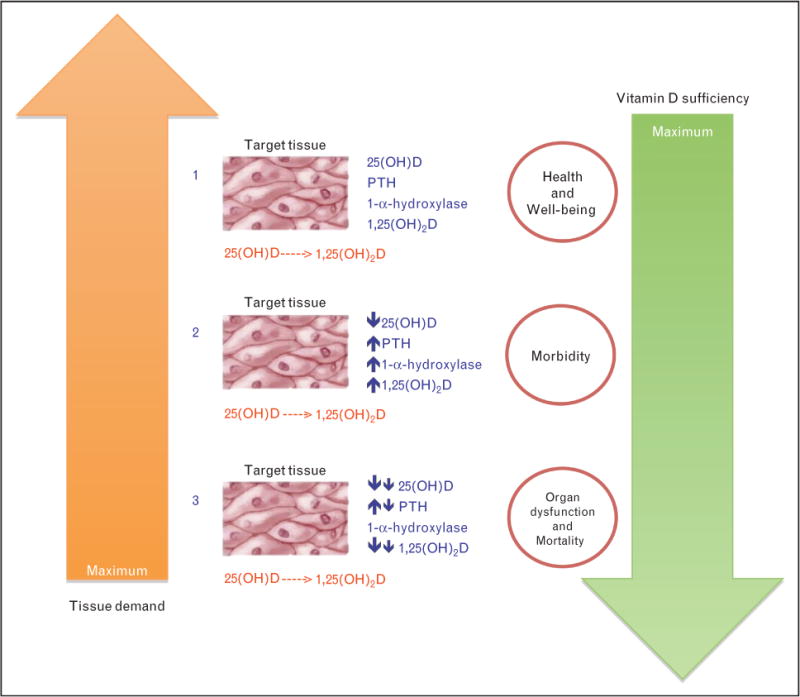
Functional model of vitamin D deficiency. (1) Sufficient vitamin D levels and relatively low tissue requirements facilitate normal organ function and maintain general health. (2) PTH stimulation ensures adequate generation of 1,25(OH)2D during times of moderate circulating vitamin D deficiency and moderate tissue requirements. Organ functions and general health are initially preserved as tissue requirements are met by an activated PTH axis. (3) Severe vitamin D deficiency and maximal tissue requirement in the setting of an insufficient PTH axis results in loss of homeostasis, which results in morbidity and eventually, mortality. Adapted with permission from [82].
CONCLUSION
We present preliminary evidence to suggest that vitamin D status plays an important role in acute stress and critical illness. Future studies should aim to characterize optimal markers of vitamin D status following acute stress and should focus on determining how these markers change over time to affect outcomes during critical illness. The development of novel approaches to determine tissue-specific vitamin D status would not only confirm a theoretically plausible hypothesis, but may also offer key insights on developing targeted therapies for individuals with functional vitamin D insufficiency.
KEY POINTS.
25(OH)D is generally the best indicator of vitamin D status, but single-point assessments in acutely ill patients may lead to inaccurate assessments of vitamin D status.
To date, there is no consensus on the optimal definitions of vitamin D deficiency, vitamin D insufficiency, or threshold levels to define optimal health benefits.
25(OH)D levels of 30–35 ng/ml appear to be necessary for vitamin D to optimize its cathelicidin effects and therefore maximize its potential anti-infective benefits during acute illness.
25(OH)D levels are associated with disease risk in the acute care setting (e.g. acute respiratory infections) and higher concentrations are associated with a reduction in mortality risk during critical illness.
Acute hemodilution, interstitial extravasation, decreased synthesis of binding proteins, and renal wasting of 25(OH)D, all appear to play an important role in the regulation of vitamin D status following acute stress.
Variations in patient responses to acute stress and critical illness may depend on the degree of vitamin D insufficiency and the extent of individual tissue requirements.
Acknowledgments
None.
Footnotes
Conflicts of interest
Dr Quraishi received support from the National Institutes of Health (NIH) grants 5T32GM007592-33 (Harvard Anaesthesia Center) and UL1 RR025758 (Harvard Scholars in Clinical Science Program). He also serves on the Board of Directors of the Vitamin D Council; whereas Dr Camargo received support from NIH grants R01 AI093723 and U01 AI087881.
Papers of particular interest, published within the annual period of review, have been highlighted as:
of special interest
of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 664–665).
REFERENCES AND RECOMMENDED READING
- 1.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, O’Keefe JH, Bell D, et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Lee JE. Circulating levels of vitamin D, vitamin D receptor polymorphisms, and colorectal adenoma: a meta-analysis. Nutr Res Pract. 2011;5:464–470. doi: 10.4162/nrp.2011.5.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J, Manson JE, Lee I-M, et al. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 6.Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semba RD, Chang SS, Sun K, et al. Serum 25-hydroxyvitamin D and pulmonary function in older disabled community-dwelling women. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vacek JL, Vanga SR, Good M, et al. Vitamin d deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109:359–363. doi: 10.1016/j.amjcard.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 10.Laaksi I, Ruohola JP, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;202:809–814. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 11.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 12■.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation forprevention of mortality in adults. Cochrane Database Syst Rev. 2011;7:CD007470. doi: 10.1002/14651858.CD007470.pub2. Suggests higher clinical efficacy when supplementation is with vitamin D3 vs. D2. [DOI] [PubMed] [Google Scholar]
- 13.de Paula FJA, Rosen CJ. Vitamin D safety and requirements. Arch Biochem Biophys. 2011 doi: 10.1016/j.abb.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai JKC, Lucas RM, Clements MS, et al. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54:1062–1071. doi: 10.1002/mnfr.200900468. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP, Recker RR, Grote J, et al. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 16.Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 18■.Amrein K, Venkatesh B. Vitamin D and the critically ill patient. Curr Opin Clin Nutr Metab Care. 2012;15:188–193. doi: 10.1097/MCO.0b013e32834f0027. Comprehensive review of vitamin D-related issues in critically ill patients. [DOI] [PubMed] [Google Scholar]
- 19■.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. Discusses the 2011 IOM vitamin D dietary reference guidelines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 21■■.Venkatesh B, Davidson B, Robinson K, et al. Do random estimations of vitamin D3 and parathyroid hormone reflect the 24-h profile in the critically ill? Intensive Care Med. 2012;38:177–179. doi: 10.1007/s00134-011-2415-x. Suggests that 25(OH)D levels fluctuate widely during critical illness. [DOI] [PubMed] [Google Scholar]
- 22.Passeri G, Pini G, Troiano L, et al. Low vitamin D status, high bone turnover, and bone fractures incentenarians. J Clin Endocrinol Metab. 2003;88:5109–5115. doi: 10.1210/jc.2003-030515. [DOI] [PubMed] [Google Scholar]
- 23.Cashman KD, Kiely M. Towards prevention of vitamin D deficiency and beyond: knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr. 2011;106:1617–1627. doi: 10.1017/S0007114511004995. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 25.Glendenning P, Inderjeeth CA. Screening for vitamin D deficiency: defining vitamin D deficiency, target thresholds of treatment and estimating the benefits of treatment. Pathology. 2012;44:160–165. doi: 10.1097/PAT.0b013e32834e8df6. [DOI] [PubMed] [Google Scholar]
- 26.Biesalski HK. Vitamin D recommendations: beyond deficiency. Ann Nutr Metab. 2011;59:10–16. doi: 10.1159/000332066. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 28.Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, et al. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr. 2010;140:595–599. doi: 10.3945/jn.109.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30■.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:455–457. doi: 10.1002/jbmr.328. Discusses the potential shortcomings of the 2011 IOM recommendations. [DOI] [PubMed] [Google Scholar]
- 31.Kulie T, Groff A, Redmer J, et al. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 32.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11:584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 33.White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord. 2011 doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 34.Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25:531–541. doi: 10.1016/j.beem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 35■.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. Comprehensive review of the immunomodulatory effects of vitamin D. [DOI] [PubMed] [Google Scholar]
- 36.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigmundsdottir H. From the bench to the clinic: new aspects on immunoregulation by vitamin D analogs. Dermatoendocrinol. 2011;3:187–192. doi: 10.4161/derm.3.3.15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White JH. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch Biochem Biophys. 2011 doi: 10.1016/j.abb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–335. [PMC free article] [PubMed] [Google Scholar]
- 40.Méndez-Samperio P. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–1798. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 41■.Bhan I, Camargo CA, Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127:1302–1304. e1. doi: 10.1016/j.jaci.2010.12.1097. Suggests cathelicidin effects are optimized at 25(OH)D levels of 30–35 ng/ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White ANJ, Ng V, Spain CV, et al. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 45.El-Radhi AS, Majeed M, Mansor N, Ibrahim M. High incidence of rickets in children with wheezy bronchitis in a developing country. J R Soc Med. 1982;75:884–887. doi: 10.1177/014107688207501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47■■.Berry DJ, Hesketh K, Power C, Hyppo¨nen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Brit J Nutr. 2011;106:1433–1440. doi: 10.1017/S0007114511001991. Suggests an association between 25(OH)D levels and risk of URI. [DOI] [PubMed] [Google Scholar]
- 48.Sabetta JR, DePetrillo P, Cipriani RJ, et al. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations 40nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 50.Camargo CA, Ginde AA, Masbach JM. Vitamin D, childhood wheezing, asthma, and chronic obstructive pulmonary disease. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3. London, UK: Elsevier; 2011. pp. 2005–2006. [Google Scholar]
- 51■.Lee JH, Gadi R, Spertus JA, et al. Prevalence of vitamin D deficiency in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1636–1638. doi: 10.1016/j.amjcard.2011.01.048. Suggests an association between 25(OH)D levels and AMI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52■.Wetmore JB, Gadi R, Lee JH, et al. Association of 25-hydroxyvitamin D deficiency with NT-pro BNP levels in patients with acute myocardial infarction: a cross-sectional analysis. BMC Res Notes. 2011;4:542. doi: 10.1186/1756-0500-4-542. Suggests an association between 25(OH)D levels and AMI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly D, Khan SQ, Thompson M, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–2124. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaloga GP, Chernow B. The multifactorial basis for hypocalcemia during sepsis. Ann Intern Med. 1987;107:36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]
- 55.Nierman DM, Mechanick JI. Bone hyperresorption is prevalent in chronically critically ill patients. Chest. 1998;114:1122–1128. doi: 10.1378/chest.114.4.1122. [DOI] [PubMed] [Google Scholar]
- 56.Van den Berghe G, Van Roosbroeck D, Vanhove P, et al. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- 57.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:191–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 58.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 2009;35:2028–2032. doi: 10.1007/s00134-009-1642-x. [DOI] [PubMed] [Google Scholar]
- 59.Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36:1609–1611. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 60■■.Leow L, Simpson T, Cursons R, et al. Vitamin D, innate immunity and out-comes in community acquired pneumonia. Respirology. 2011;16:611–616. doi: 10.1111/j.1440-1843.2011.01924.x. Suggests association between 25(OH)D levels and mortality in critical illness. [DOI] [PubMed] [Google Scholar]
- 61.Annweiler C, Pochic S, Fantino B, et al. Serum vitamin D concentration and short-term mortality among geriatric inpatients in acute care settings. Adv Ther. 2010;27:245–249. doi: 10.1007/s12325-010-0025-6. [DOI] [PubMed] [Google Scholar]
- 62■■.Venkatram S, Chilimuri S, Adrish M, et al. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. Suggests association between 25(OH)D levels and mortality in critical illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63■■.Matthews LR, Ahmed Y, Wilson KL, et al. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012 doi: 10.1016/j.amjsurg.2011.07.021. Suggests association between 25(OH)D levels and mortality in critical illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64■■.Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. Suggests association between 25(OH)D levels and mortality in critical illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnson Y, Gringauz I, Itzhaky D, Amital H. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. Q J Med. 2012 doi: 10.1093/qjmed/hcs014. [DOI] [PubMed] [Google Scholar]
- 66.Michos ED, Streeten EA, Ryan KA, et al. Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or C-reactive protein in the old order Amish. Calcif Tissue Int. 2009;84:195–202. doi: 10.1007/s00223-008-9209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jorde R, Sneve M, Torjesen PA, et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50:175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 69.Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a doubleblind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 70.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 71.Bjorkman M, Sorva A, Tilvis R. C-reactive protein and fibrinogen of bedridden older patients in a six-month vitamin D supplementation trial. J Nutr Health Aging. 2009;13:435–439. doi: 10.1007/s12603-009-0080-3. [DOI] [PubMed] [Google Scholar]
- 72.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin d and C-reactive protein in asymptomatic adults (from the continuous national health and nutrition examination survey 2001 to 2006) Am J Cardiol. 2012;109:226–230. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 73.Barth JH, Field HP, Mather AN, et al. Serum 25 hydroxy-vitamin D does not exhibit an acute phase reaction after acute myocardial infarction. Ann Clin Biochem. 2012 doi: 10.1258/acb.2011.011195. [DOI] [PubMed] [Google Scholar]
- 74■.Bang UC, Novovic S, Andersen AM, et al. Variations in serum 25-hydroxy-vitamin D during acute pancreatitis: an exploratory longitudinal study. Endocr Res. 2011;36:135–141. doi: 10.3109/07435800.2011.554937. Suggests association between 25(OH)D and CRP levels in acute pancreatitis. [DOI] [PubMed] [Google Scholar]
- 75■.Duncan A, Talwar D, McMillan DC, et al. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95:64–71. doi: 10.3945/ajcn.111.023812. Suggests that inflammatory responses influence the accuracy of vitamin D status assessments. [DOI] [PubMed] [Google Scholar]
- 76.Bertoldo F, Pancheri S, Zenari S, et al. Serum 25-hydroxyvitamin D levels modulate the acute-phase response associated with the first nitrogen-containing bisphosphonate infusion. J Bone Miner Res. 2010;25:447–454. doi: 10.1359/jbmr.090819. [DOI] [PubMed] [Google Scholar]
- 77■■.Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–1011. doi: 10.3945/ajcn.110.008490. Suggests that inflammatory responses alone do not explain postoperative derangements in vitamin D status. [DOI] [PubMed] [Google Scholar]
- 78.Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. 2010;14:R216. doi: 10.1186/cc9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strampel W, Emkey R, Civitelli R. Safety considerations with bisphosphonates for the treatment of osteoporosis. Drug Saf. 2007;30:755–763. doi: 10.2165/00002018-200730090-00003. [DOI] [PubMed] [Google Scholar]
- 80.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and antimicrobial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 82.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:769–781. doi: 10.1016/j.beem.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Kestenbaum B, Katz R, de Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58:1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84■■.Flynn L, Zimmerman LH, McNorton K, et al. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg. 2011;203:379–382. doi: 10.1016/j.amjsurg.2011.09.012. Suggests association between 25(OH)D levels and morbidity in critical illness. [DOI] [PubMed] [Google Scholar]
- 85.Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, et al. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121:452–455. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]


