Abstract
The Norwegian Breast Cancer Screening Program was rolled out county by county over the course of a decade, from 1996 to 2005, and now encompasses all Norwegian women aged 50–69 years. We aim to compare DCIS and stage-specific invasive breast cancer incidence rates among participants, non-participants, and women not yet invited to the screening program over this entire implementation period. We estimate stage-specific breast tumor incidence rates for 640,347 women 50–69 years of age invited to the screening program between 1996 and 2007. We compare incidence rates and stage distribution among women diagnosed with breast cancer who were invited and participated, invited but not participated, and women not yet invited to the screening program using two-sided Chi-squared tests to determine statistical significance between groups. The incidence of ductal carcinoma in situ (DCIS) was 3.0 times higher and invasive breast cancer was 1.5 times higher for invited participants compared to invited non-participants (p < 0.001). While the incidence of Stage I cancer was two times higher among participants compared to non-participants (p < 0.001), the incidences of Stages III and IV cancer were two and three times lower, respectively, among participants compared to non-participants (p < 0.001 for both). No significant differences in stage-specific incidence or treatment utilization rates were observed between invited non-participants and not yet invited women, except for stage IV cancers, which were detected at a higher rate among women who were not yet invited (7.5 vs. 4.6 %, p = 0.001). Compared with women invited who did not participate, participants in the screening program are more likely to be diagnosed with DCIS and early stage invasive breast cancer and are less likely to be diagnosed with advanced stage breast cancer. More research is required to determine whether these differences in stage-specific incidences among invited participants and non-participants are associated with differences in mortality rates.
Keywords: Breast cancer, Population screening, Screening mammography, Cancer incidence, Stage-specific incidence, Mastectomy rate
Introduction
The main goal of screening mammography is to reduce breast cancer mortality and morbidity by detecting the disease at its earliest stage [1–3]. Despite several randomized trials and evaluations of large screening programs the trade-offs between the benefits and the harms of screening mammography are still heavily debated [4–9]. The 2009 revised breast cancer screening recommendations by the US Preventive Services Task Force have caused even more confusion among women in the target age population [2, 3].
The reported effectiveness of population-wide mammographic screening programs is highly dependent upon the methods and definitions used for their estimation [10,11]. It is not possible to interpret effectiveness without determining how the screening group is defined and without accounting for possible differences in health care system-specific screening techniques, available treatments, and systemic workflow [7, 11–19]. These complexities make general conclusions about the effectiveness of population-wide mammographic screening programs difficult.
The Norwegian Breast Cancer Screening Program, administered by the Cancer Registry of Norway, started as a pilot program in four counties in November 1995 and was gradually expanded and covered all 19 counties by 2005 [20]. While long-term mortality data are not yet available from the screening program, making an evaluation of its effectiveness difficult, data on breast cancer stage at diagnosis are now available for a growing number of women. Higher breast cancer stage at diagnosis is a risk factor for subsequent breast cancer mortality [21–23]. Therefore, an analysis of stage-specific incidence rates in the screening versus non-screening populations is an important step towards evaluating the population-wide screening program.
The national screening program tracks all breast cancers diagnosed in women who participate in the program, while the Cancer Registry records all cases of breast cancer regardless of program enrollment status. Merging the two databases allows for the categorization of all ductal carcinoma in situ (DCIS) and invasive breast cancer cases diagnosed in a national population into three subpopulations: (1) breast tumors (DCIS and invasive breast cancer) diagnosed in women who were invited and participated in the screening program, (2) breast tumors diagnosed in those who were invited to screen but did not participate, and (3) those women who had not yet been invited into the screening program at the time of breast cancer diagnosis. This latter group has been neglected in previous mammography-related, population-wide breast cancer incidence reports and is comprised women in the target group of the screening program, whose breast cancer was diagnosed in the period before they had received any invitation to participate in the screening program.
By merging two unique national data resources, we aim to provide a descriptive comparison of stage-specific breast cancer incidence between the three distinct subpopulations of Norwegian women aged 50–69 years: invited participants, invited non-participants, and women not yet invited to be screened. We hypothesize that the incidence of advanced stage breast cancer is lower among women who choose to participate in the national screening program compared to those who do not participate and those not yet invited.
Methods
Data source
We obtained anonymous merged data from the incidence database and the screening database, both at the Cancer Registry of Norway. The incidence database has been in effect since 1953 and is 99 % complete for the recording of all newly diagnosed solid tumors in Norway, including breast cancer [24]. The screening database includes information about the women’s screening invitation status and their history of screening activity and screening outcome. The collection of Norwegian cancer data is authorized by the Cancer Registry Regulations on the collection and processing of personal health data in the Cancer Registry [25]. As we obtained data in an anonymous form, no Institutional Review Board or Data Inspectorate approval was required.
Study population
In Norway, all inhabitants are given a unique 11-digit personal identification number (PIN) by birth or immigration, allowing for identification of women of age-appropriate cohorts. During the implementation phase, 1996–2005, women aged 50–69 years received a personal invitation letter with a specified time and place for screening examination during the 2-year start-up period for the actual county the women resided. After the implementation phase, the women receive a personal invitation biennially. The screening database keeps records of all women in the target population and the outcome of the screening examination including breast cancer detection. The incidence database keeps records of all breast cancer cases regardless of whether or not the women participated in the screening program. DCIS and invasive breast cancer registered in the screening and incidence databases during the study period, January 1, 1996 through December 31, 2007, were included in our analysis.
The screening data collection system tracks postal dates of invitation, actual date of screening, and the outcomes of the screening examinations, including the diagnosis dates for breast tumors. Based on this information, we stratified the population of women receiving screening invitations into two groups: those resulting in participation (e.g., attendance in the national screening program during the next 2 years) and those resulting in non-participation in the program. We followed all women for 2 years (730 days) after postal date of invitation, irrespective of whether or not they chose to participate. A woman could be part of the participant group for one 2-year period but a part of the non-participant group during another 2-year period.
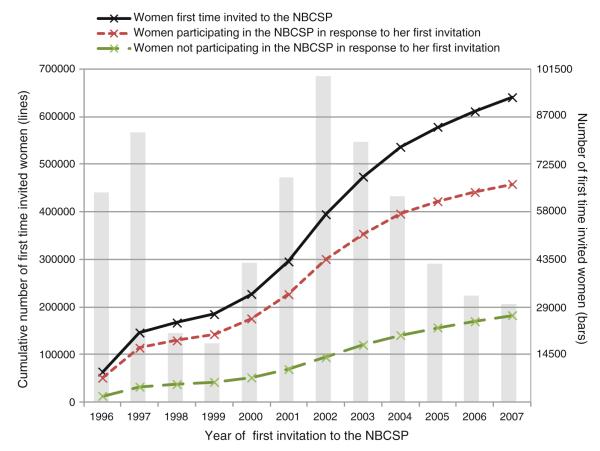
A total of 640,347 women received 1,925,725 invitations to screening during the study period. The invitations resulted in 1,475,978 screening examinations (participations) and 449,747 absences of participation (non-participation) during the study period (Fig. 1). The number of invitations women received ranged from one to seven with three being the average. The number of invitation varied depending on age and county of residence. The cumulative number of first-time invited women to the NBCSP increased from 64,000 in 1996 to 640,000 in 2007, while the cumulative number of women screened as a response to the first invitation received increased from 50,000 to 460,000 (Fig. 2). The overall screening participation rate was 76 % (1,475,978/1,925,725) during the study period. Full screening compliance was achieved by 60 % of the women, while 17 % chose never to participate. About 23 % of women participated irregularly.
Fig. 1.
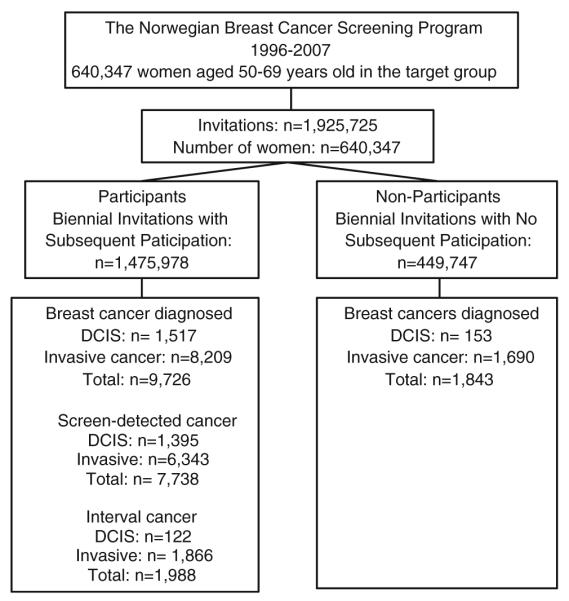
The study population
Fig. 2.
Cumulative and annual number of first time invited, participating, and non-participating women in the Norwegian Breast Cancer Screening Program, 1996–2007
Women are invited to participate in the screening program by a posted personal letter where time and place for the screening test is given. Due to the 2-year screening interval, some women had to wait almost 2 years after the screening program started in the actual county, to receive an invitation.
Statistical methods
All statistical analyses were conducted using SPSS (SPSS, version 17.0.1 for Windows, SPSS Inc, Chicago, Illinois). Two-sided Chi-squared tests were used to determine statistical significance between groups. A p value ≤0.05 was considered statistically significant.
In this non-experimental retrospective observational study, the outcome of participating in the screening program after the biennial invitations constitutes the basis for incidence rate estimates. Incidence rates are estimated for women aged 50–69 years at postal date of invitation. Postal date was, on average, 25 days before scheduled time for the screening examination.
To more appropriately characterize the impact of implementing a national screening program, we use invitations to the screening program as a denominator. The use of such a denominator ensures comparability among all Norwegian women in the target age population for screening. By noting the time of cancer diagnosis in the same invitation calendar period, we ensure similar diagnostic techniques and identical procedures for the reporting of breast cancer cases across all three possible subpopulations.
We define stage as created and registered in the Cancer Registry of Norway: Stage 0: DCIS; Stage I: localized breast cancer; Stage II: regional breast cancer; Stage III: breast cancer fixed to either skin or chest wall; and Stage IV: breast cancer with distant metastases. We categorize histopathological tumor size into four groups: ≤20 mm, >20 and ≤50 mm, >50 mm, and missing/not available, according to current International Union Against Cancer guidelines [26]. We categorize lymph node involvement and distant metastasis as yes, no, and missing/not available. The primary treatment is assumed to be the type of surgical procedure performed (e.g., mastectomy and breast conserving treatment). Data regarding primary adjuvant hormone therapy, chemotherapy, and radiation therapy are available only as “given” or “not given” by the treating surgeon.
We provide incidence rates of all breast tumors by stage (0–IV) per 100,000 invitations for participants and non-participants. Stage distribution was given for the two groups of invited women and for group of not yet invited women. We also provide distributions for the three study groups by age, tumor size, lymph node involvement, and distant metastases. We further provide rates and distribution of surgical treatment, radiation therapy, and hormonal therapy among participants and non-participants.
Results
Overall incidence rates
In total, 11,569 breast tumors (1,670 DCIS and 9,899 invasive cancer) were diagnosed among 640,347 women who were invited into the screening program during the study period (1996–2007). Participants in the screening program accounted for 9,726 breast tumors (1,517 DCIS and 8,209 invasive cancer) and non-participants for 1,843 breast tumors (153 DCIS and 1,690 invasive cancer) (Fig. 1; Table 1). Women not yet invited accounted for an additional 1,594 breast tumors (126 DCIS and 1,468 invasive cancer; Table 2). On average, participants with a breast tumor were 59.1 years of age at diagnosis (median 59, SD 5.4), non-participants were 59.3 years of age (median 59, SD 5.4), and women not yet invited were 56.0 years of age (median 54, SD 5.9) at the time of diagnosis.
Table 1.
Stage-specific incidence rate, tumor size, lymph node involvements, and distant metastasis of breast tumors diagnosed in invited participants and invited non-participants in the Norwegian Breast Cancer Screening Program 1996–2007
| All tumors | 1,925,725 biennial invitations sent to 640,347 women between 1996 and 2007 |
||||||
|---|---|---|---|---|---|---|---|
| All invitations n = 1,925,725 |
Invited participants n = 1,475,978 |
Invited non-participants n = 449,747 |
Rate ratio |
||||
| N | Rate per 100,000 invitations |
N | Rate per 100,000 invitations |
N | Rate per 100,000 invitations |
Participants/non- participants |
|
| Stage | |||||||
| 0 (DCIS) | 1,670 | 86.7 | 1,517 | 102.8 | 153 | 34.0a | 3.0 |
| I | 5,885 | 305.5 | 5,105 | 345.9 | 780 | 173.4a | 2.0 |
| II | 3,552 | 184.4 | 2,845 | 192.8 | 707 | 157.2a | 1.2 |
| III | 173 | 9.0 | 108 | 7.3 | 65 | 14.5a | 0.5 |
| IV | 272 | 14.1 | 134 | 9.1 | 138 | 30.7a | 0.3 |
| Missing/not available | 17 | 0.9 | 17 | 1.1 | – | – | – |
| All | 11,569 | 600.6 | 9,726 | 659.0 | 1,843 | 409.8a | 1.6 |
|
| |||||||
| Invasive tumors | n = 9,899 | n = 8,209 | n = 1,690 | ||||
|
| |||||||
| Tumor size | |||||||
| ≤20 mm | 6,364 | 330.5 | 5,573 | 377.6 | 791 | 175.9a | 2.1 |
| >20 and ≤50 mm | 1,854 | 96.3 | 1,431 | 97.0 | 423 | 94.1 | 1.0 |
| >50 mm | 334 | 17.4 | 198 | 13.4 | 136 | 30.2a | 0.7 |
| Missing/ not available |
1,347 | 69.9 | 1,007 | 68.2 | 340 | 75.6 | – |
| Lymph node involvements | |||||||
| No | 6,158 | 319.8 | 5,331 | 362.2 | 827 | 183.9a | 2.0 |
| Yes | 3,013 | 156.5 | 2,376 | 161.0 | 637 | 141.6a | 1.1 |
| Missing/not available | 728 | 37.8 | 502 | 34.0 | 226 | 50.3 | – |
| Distant metastasis | |||||||
| No | 7,465 | 387.6 | 6,370 | 431.6 | 1,095 | 243.2a | 1.8 |
| Yes | 267 | 13.9 | 132 | 8.9 | 135 | 30.0a | 0.3 |
| Missing/ not available |
2,167 | 112.5 | 1,707 | 115.7 | 460 | 102.3 | – |
p value <0.05 for Chi-squared between the groups “Invited Participants” and “Invited Non-participants”
Table 2.
Stage-specific distribution, tumor size, lymph node involvement, and metastases in invasive breast tumors diagnosed in invited participants and invited non-participants in the Norwegian Breast Cancer Screening Program 1996–2007, and in women not yet invited (before invitation)
| All tumors | Invited participants n = 9,726 |
Invited non-participants n = 1,843 |
Before invitation n = 1,594 |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Stage | ||||||
| 0 (DCIS) | 1,517 | 15.6 | 153 | 7.9a | 126 | 7.9a |
| I | 5,105 | 52.5 | 780 | 42.3a | 696 | 43.7a |
| II | 2,845 | 29.3 | 707 | 38.4a | 655 | 41.1a |
| III | 108 | 1.1 | 65 | 3.5a | 43 | 2.7a |
| IV | 134 | 1.4 | 138 | 7.5* | 74 | 4.6a,b |
| Missing/not available | 17 | 0.2 | 0 | 0 | ||
|
| ||||||
| Invasive tumors | n = 8,209 | n = 1,690 | n = 1,468 | |||
|
| ||||||
| Tumor size | ||||||
| ≤20 mm | 5,573 | 67.9 | 791 | 46.8a | 715 | 48.7a |
| >20 and ≤50 mm | 1,431 | 17.4 | 423 | 25.0a | 377 | 25.7a |
| >50 mm | 197 | 2.4 | 136 | 8.0a | 93 | 6.3a |
| Missing/not available | 1,009 | 12.3 | 340 | 20.2 | 283 | 19.3 |
| Lymph node involvement | ||||||
| No | 5,331 | 65.0 | 827 | 48.9a | 742 | 50.5a |
| Yes | 2,376 | 28.9 | 637 | 37.7a | 590 | 40.2a |
| Missing/not available | 502 | 6.1 | 226 | 13.4 | 136 | 9.3 |
| Distant metastasis | ||||||
| No | 6,370 | 77.7 | 1,095 | 64.8a | 943 | 64.3a |
| Yes | 132 | 1.6 | 135 | 8.0a | 74 | 5.0a,b |
| Missing/not available | 1,707 | 20.7 | 460 | 27.2 | 451 | 30.7 |
p value <0.05 for Chi-squared between the groups “Invited Participants” and “Invited Non-participants” and the groups “Invited Participants” and “Before invited”
p value <0.05 for Chi-squared between the groups “Invited Non-participants” and “Before invited”
Stage-specific incidence rates
The incidence rate of DCIS (Stage 0) was three times higher for participants compared to non-participants, while the incidence rate of Stage I cancer was two times higher for participants compared to non-participants (Table 1). The incidence rate for all invasive cancers (Stage I–IV) was 1.5 times higher among participants. However, the incidence rate for advanced invasive cancer (Stage III and IV) was lower among participants (16.4 per 100,000) compared to non-participants (45.1 per 100,000) (p < 0.001).
When stratified by 5-year age groups, the incidence rate of Stage I cancers increased by age among participants, from 161.7 per 100,000 invitations in women aged 50–54 years to 426.4 per 100,000 invitations in women aged 65–69 years. For non-participants, the lowest Stage I cancer incidence rate (157.4 per 100,000 invitations) was found among the oldest women (65–69 years) and the highest Stage I cancer incidence rate was found among women aged 55–59 and 60–64 years (187.0 per 100,000 invitations and 186.8 per 100,000 invitations, respectively). The rate of advanced invasive cancer (Stages III and IV) was 12.2, 18.2, 20.2, and 14.9 per 100,000 invitations among participants aged 50–54, 55–59, 60–64, and 65–69 years, respectively. Corresponding incidence rates of advanced breast cancer stratified by age for non-participants were 33.1, 43.3, 49.8, and 48.2 per 100,000 invitations, respectively.
The stage-specific distribution, tumor size, lymph node involvement, and metastases differed significantly between the cancers diagnosed in participants compared with both non-participants and women diagnosed with breast cancer before invitation, for almost all variables (Tables 1, 2). The incidence rate of invasive cancers with large tumor size was lower in participants compared with non-participants while the incidence rate of breast tumors without lymph node involvement was two times higher in participants compared to non-participants. Finally, the incidence rate of distant metastases was three to four times lower in screening participants compared to non-participants.
In contrast, stage-specific distribution, tumor size, lymph node involvement, and metastasis did not differ significantly between non-participants and women not yet invited, except for stage IV cancer and distant metastases rates which were higher among non-participants (Stage IV: 7.5 vs. 4.6 %; distant metastases: 8.0 vs. 5.0 %; p = 0.001 for both; Table 2). While there was a trend of less prognostically favorable tumor characteristics in non-participants compared with women not yet invited, these differences were not statistically significant.
Surgery and treatment utilization rates
When considering women with any type of breast tumor (e.g., DCIS or invasive breast cancer), mastectomy was performed in 38 % (3,698/9,726) of the participants, in 46 % (845/1,843) of the non-participants and in 58 % (806/1,594) of the women not yet invited. A total of 58 % (5,628/9,726) of the participants with breast tumors received radiation treatment, compared with 54 % (1,000/1,843) of the non-participants, and 51 % (806/1,594) of the women not yet invited. The rates of mastectomy per 100,000 invitations to women were 250.5 per 100,000 invitations for participants compared to 187.9 per 100,000 invitations for non-participants (p < 0.001).
The treatments received by women with invasive breast cancer alone are shown in Table 3. The rate of mastectomy for women with invasive breast cancer was 211.4 per 100,000 invitations for participants compared to 174.5 per 100,000 invitations for non-participants (p < 0.001) (Table 3). The rate of breast conservation treatment for invasive cancers was 334.4 per 100,000 for participants compared to 167.6 per 100,000 for non-participants (p < 0.001). The rate of radiation treatment for invasive breast cancer among participating women was 358.7 per 100,000 invitations compared to 215.5 per 100,000 invitations among non-participants (p < 0.001). Finally, the rate of hormonal treatment was 189.6 per 100,000 invitations among participants compared with 151.0 per 100,000 invitations among non-participants (p < 0.001).
Table 3.
Rates of surgery, radiation therapy, hormonal therapy, and chemotherapy in invited participants and invited non-participants of the Norwegian Breast Cancer Screening Program 1996–2007b who were diagnosed with invasive breast tumors
| 1,925,725 invitations sent to 640,347 women |
|||
|---|---|---|---|
| All invitations n = 1,925,725 |
Invited participants n = 1,475,978 |
Invited non-participants n = 449,747 |
|
|
|
|||
| Rate per 100,000 invitations |
|||
| N = 9,899 | N = 8,209 | N = 1,690 | |
| Surgery | |||
| Mastectomy | 202.8 | 211.4 | 174.5a |
| Breast conserving treatment | 295.4 | 334.4 | 167.6a |
| Missing | 15.8 | 10.4 | 33.7 |
| Radiation therapy | |||
| Yes | 325.2 | 358.7 | 215.5a |
| No | 111.9 | 119.2 | 87.8a |
| Missing | 76.9 | 78.3 | 72.5 |
| Hormonal therapy | |||
| Yes | 180.6 | 189.6 | 151.0a |
| No | 216.4 | 245.2 | 122.1a |
| Missing | 117.0 | 121.4 | 102.7 |
| Chemotherapy | |||
| Yes | 108.6 | 107.9 | 111.4 |
| No | 236.6 | 268.2 | 133.0a |
| Missing | 168.8 | 180.1 | 131.4 |
p value <0.001 for Chisquared between the groups “Invited Participants” and “Invited Non-participants”
Only invasive breast tumors included in the table
Discussion
Women invited and participating in the Norwegian Breast Cancer Screening Program are three times more likely to be diagnosed with DCIS and 1.5 times more likely to be diagnosed with invasive breast cancer compared to women who were invited but did not participate. The prognostic tumor characteristics were better in women who participated in the screening program compared to those who did not and those who were not yet invited. The favorable tumor characteristics suggest, but do not prove a future mortality reduction, as a benefit of screening.
Prior studies have suggested minimal mortality reduction at the cost of over-diagnosis after implementation of a national screening program [5, 6]. Most randomized clinical trials of breast cancer screening are analyzed using an intention to treat method to reduce bias. Intention to treat means that the women should have been invited to be included in the “exposed” group. Thus, the intention to treat analysis is problematic for evaluating the Norwegian Breast Cancer Screening Program described here, as the screening invitations have been rolled out county by county over several years.
Our analysis is distinctive in two ways. First, we include all women not yet invited to participate in screening for one of the comparator groups. Second, we use invitations to screen (received by the women every 2 years) as a denominator. By incorporating these into our analysis, we believe our descriptive evaluation of the impact of implementing a population-based breast cancer screening program in Norway provides an important different perspective than previous reports [20]. Specifically, our analytic technique allows for descriptive statistics regarding stage-specific incidence of breast cancer differences in prognostic tumor characteristics among all women—those who participate, those who do not participate, and those not yet invited.
We found that the incidence rate of DCIS and invasive cancer combined was higher among women who participate in the screening program compared to those who do not. Such an increase in incidence of breast cancer is expected among screening participants due to lead-time [27]. Some would argue that the increased incidence represents over-diagnosis [6]. Over-diagnosis is a complex issue and estimating rates of such an event require advanced statistical analyses and long follow-up time [28]. The incidence of DCIS, however, is nearly three times higher for participants compared to non-participants. While some of this may represent true early detection of a preinvasive lesion with strong malignant potential, some may represent diagnosis of slow-growing pre-invasive lesions without any true mortality benefit from early diagnosis. In our study, the number of women diagnosed with DCIS in the non-screened group accounted for 8 % (153/1,843) of cases, which is somewhat higher than expected in a non-screened population, suggesting extensive use of opportunistic screening in these women [29].
However, we did find that the incidence rate of cancer with distant metastasis is higher in non-participants compared to participants. Our analysis also showed less prognostically favorable tumor characteristics in women diagnosed with breast cancer in the time period between the start of screening and the time they received an invitation to participate, compared to participants diagnosed with breast cancer. In other words, the prognostic tumor characteristics were more favorable in invited women who actually participate in screening compared to invited women who choose not to participate. As the tumor characteristics in non-participants trend toward being less favorable compared to tumors detected in women who were not yet invited, a greater risk of morbidity and mortality is implied by declining participation in the screening program. A recent paper by Kalager et al. [7] showed a 10 % reduction in mortality due to the organized screening program in Norway. The study had no information at the level of the individual woman about their invitation or participation in the screening program and the estimates were based on an average of 2.2 years of follow-up. Our study is based on individual screening data.
Stage distribution was more favorable in women invited who participated versus in women invited who did not participate. Stage migration (down-staging) is an expected effect of screening. However, stage is a rough estimate for measuring prognostic factors since the range for each factor (tumor size, lymph node involvements, and metastasis) is wide. The significantly higher rates of metastases and lymph node involvement among those who decline invitations to breast screening indicate poorer prognoses for women with cancer in this subpopulation. Further studies are required to examine why this cohort of women decline the invitations, and if this cohort represents a socioeconomically disadvantaged group that is experiencing an unidentified barrier to access that can be addressed at a policy level in Norway. Some of these women may make use of mammography at private clinics (opportunistic screening) [29], but the extent, age distribution and county of residence is not known.
We also found that the rates of mastectomy, breast conservation therapy, radiation treatment, and hormonal treatment were all significantly higher for the participating population versus the non-participating population. While not unexpected, these findings highlight the large amount of resources directed at treating breast tumors once they are detected by screening. These figures are based on the total population, and, as screening detects more breast tumors this leads to a higher percentage of the population being treated. However, when women with invasive cancer are compared between groups, the women undergoing mastectomy was more than 20 % lower for women who participated versus those not yet invited and 10 % lower for participants compared with non-participants. Furthermore, the percentage of women that underwent hormonal therapy and/or chemotherapy was lower among participants than in the other two groups. Given the lack of mortality data, whether a large expenditure of resources is warranted is currently unknown. However, as long-term mortality data become available in the coming years, further analyses can be conducted to determine whether there is over-treatment of non-aggressive breast cancers.
There are several limitations to our study. First, after 10 years of a national screening program, mortality analyses would be valuable in evaluating the program’s effectiveness. However, all data collected as a part of the screening program were not publicly available between 2007 and 2011 due to issues related to the informed consent signed by women with negative screening results. This issue has recently been resolved and individual screening information linked with mortality will be used for subsequent evaluations of the screening program. As a consequence, our methods for analysis in this study were limited to the use of aggregate data without the ability to link individual screening information with mortality data.
Our descriptive analysis of stage distribution and histological tumor size, lymph node involvement, and distant metastases lack some coherence due to the Cancer Registry’s categorization of stage for cases with missing information. Therefore, the Cancer Registry’s stage categorization may differ from the stage categorization in other published studies. In addition, information about use of opportunistic screening instead or in addition to the organized screening is not available. Some of the women in the invited but not-screened group might thus be screened regularly at private clinics. Furthermore, there are missing data in the registries, particularly among non-participants, which cannot be addressed at this time. There may be a general underestimation of mastectomy rates given that any surgeries performed after the initial therapies are not captured by the available databases. Finally, at the time of this study, long-term mortality data are unavailable. Nevertheless, we believe that a descriptive analysis of stage-specific incidence and utilization of surgery and treatment are important surrogates prior to mortality data becoming available.
Conclusion
We provide a comprehensive description of stage-specific breast cancer incidence in Norway between 1996 and 2007 among screening participants, invited non-participants, and those not yet invited to the national screening program. We found that both the overall and lower stage breast cancer incidence is greater among women actively participating in a breast cancer screening program compared to women invited, but who are not participating. It is not clear if part of these differences represents diagnosis of slow-growing DCIS and invasive breast cancer or if the differences noted are commensurate with what is expected due to lead time according to European guidelines [27]. We also found that advanced stage breast cancer is less common in women participating compared to non-participants, possibly suggesting an overall mortality benefit from screening. Future research using the Norwegian screening and cancer registry data currently being collected will help in determining whether these differences in stage-specific incidences between screening participants and non-participants actually lead to a clinically significant mortality benefit.
Acknowledgments
This work was supported in part by the US National Cancer Institute at the National Institutes of Health (KO5 CA 104699 to J.G.E).
Abbreviations
- NBCSP
Norwegian Breast Cancer Screening Program
- CRN
Cancer Registry of Norway
- DCIS
Ductal carcinoma in situ
Footnotes
Competing interests The authors report no financial or non-financial competing interests.
Contributor Information
Solveig Hofvind, Cancer Registry of Norway, Research Department, P.O. Box 5313, 0304 Oslo, Norway; Oslo and Akershus University College of Applied Sciences, Faculty of Health Sciences, P.O. Box 4, St. Olavs plass, 0130 Oslo, Norway.
Christoph I. Lee, Robert Wood Johnson Clinical Scholars Program, Departments of Radiology and Medicine, UCLA David Geffen School of Medicine, 911 Broxton Avenue #303, Los Angeles, CA 90024, USA stophlee@gmail.com
Joann G. Elmore, University of Washington, Department of Medicine, School of Medicine, 325 Ninth Avenue, P.O. Box 359780, Seattle, WA 98104-2499, USA jelmore@u.washington.edu; Department of Epidemiology, School of Public Health Seattle, University of Washington, 325 Ninth Avenue, P.O. Box 359780, Seattle, WA 98104-2499, USA
References
- 1.Vainio H, Bianchini F, editors. IARC handbook of cancer prevention Volume 7 Breast Cancer Screening. IARCPress; Lyon: [Accessed December 7, 2011]. 2002. http://www.iarc.fr. [Google Scholar]
- 2.Food and Drug Administration . Quality Mammography Standards. Final Rules-21 CRF Parts 16 and 900. RIN 0910-AA24 edition. 2011. Department of Health and Human Services; Washington, DC: 1997. pp. 55925–55926. (docket No. 95N-0192) [Google Scholar]
- 3.Screening for Breast Cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;151:716–236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 4.Duffy SW, Tabar L, Olsen AH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen. 2010;17:25–30. doi: 10.1258/jms.2009.009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen KJ, Klahn A, Gotzsche PC. Are benefits and harms in mammography screening given equal attention in scientific articles? A cross-sectional study. BMC Med. 2007;5:12. doi: 10.1186/1741-7015-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahl PH, Gotzsche PC, Maehlen J. Natural history of breast cancers detected in the Swedish mammography screening programme: a cohort study. Lancet Oncol. 2011;12:1118–1124. doi: 10.1016/S1470-2045(11)70250-9. [DOI] [PubMed] [Google Scholar]
- 7.Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–1210. doi: 10.1056/NEJMoa1000727. [DOI] [PubMed] [Google Scholar]
- 8.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260:658–663. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 9.Autier P, Boniol M, Gavin A, Vatten L. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. doi:10.1136/bmj.d441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen AH, Njor SH, Lynge E. Estimating the benefits of mammography screening: the impact of study design. Epidemiology. 2007;18:487–492. doi: 10.1097/EDE.0b013e318060cbbd. [DOI] [PubMed] [Google Scholar]
- 11.Paap E, Holland R, den Heeten GJ, et al. A remarkable reduction of breast cancer deaths in screened versus unscreened women: a case-referent study. Cancer Causes Control. 2010;21:1569–1573. doi: 10.1007/s10552-010-9585-7. [DOI] [PubMed] [Google Scholar]
- 12.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 13.Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–1410. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 14.Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011:CD001877. doi: 10.1002/14651858.CD001877.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Swedish Organised Service Screening Evaluation Group Reduction in breast cancer mortality from the organised service screening with mammography: 2. Validation with alternative analytic methods. Cancer Epidemiol Biomarkers Prev. 2006;15:52–56. doi: 10.1158/1055-9965.EPI-05-0953. [DOI] [PubMed] [Google Scholar]
- 16.Swedish Organised Service Screening Evaluation Group Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol Biomarkers Prev. 2006;15:45–51. doi: 10.1158/1055-9965.EPI-05-0349. [DOI] [PubMed] [Google Scholar]
- 17.Kalager M, Haldorsen T, Bretthauer M, Hoff G, Thoresen SO, Adami HO. Improved breast cancer survival following introduction of an organized mammography screening program among both screened and unscreened women: a population-based cohort study. Breast Cancer Res. 2009;11:R44. doi: 10.1186/bcr2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmore JG, Nakano CY, Koepsell TD, Desnick LM, D’Orsi CJ, Ransohoff DF. International variation in screening mammography interpretations in community-based programs. J Natl Cancer Inst. 2003;95(18):1384–1393. doi: 10.1093/jnci/djg048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Bindman R, Chu PW, Miglioretti DL, et al. Comparison of screening mammography in the United States and the United Kingdom. JAMA. 2003;290(16):2129–2137. doi: 10.1001/jama.290.16.2129. [DOI] [PubMed] [Google Scholar]
- 20.Hofvind S, Geller B, Vacek PM, Thoresen S, Skaane P. Using the European guidelines to evaluate the Norwegian Breast Cancer Screening Program. Eur J Epidemiol. 2007;22:447–455. doi: 10.1007/s10654-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 21.Buiatti E, Barchielli A, Bartolacci S, et al. The impact of organised screening programmes on the stage-specific incidence of breast cancer in some Italian areas. Eur J Cancer. 2003;39:1776–1782. doi: 10.1016/s0959-8049(03)00322-8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson WF, Jatoi I, Devesa SS. Assessing the impact of screening mammography: breast cancer incidence and mortality rates in Connecticut (1943–2002) Breast Cancer Res Treat. 2006;99:333–340. doi: 10.1007/s10549-006-9214-z. [DOI] [PubMed] [Google Scholar]
- 23.Norman SA, Localio AR, Zhou L, et al. Benefit of screening mammography in reducing the rate of late-stage breast cancer diagnoses (United States) Cancer Causes Control. 2006;17:921–929. doi: 10.1007/s10552-006-0029-3. [DOI] [PubMed] [Google Scholar]
- 24.Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1219. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed January 24, 2011];Regulations on the collection and processing of personal health data in the Cancer Registry of Norway (Cancer Registry Regulations) 2001 www.ub.uio.no/ujur/ulovdata/for-20011221-1477-eng.doc.
- 26.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (UICC International Union Against Cancer) 7th edn Wiley; Chichester: 2010. [Google Scholar]
- 27.Perry N, Broeders MJ, de Wolf C, Holland R, von Karsa L. Office for Official Publication of the European Communities. European Communities; Luxembourg: 2006. European guidelines for quality assurance in breast cancer screening and diagnosis. 2006. ISBN 92-79-01258-4. [Google Scholar]
- 28.de Gelder R, Heijnsdijk EA, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev. 2011;33:111–121. doi: 10.1093/epirev/mxr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynge E, Braaten T, Njor SH, et al. Mammography activity in Norway 1983 to 2008. Acta Oncol. 2011;50:1062–1067. doi: 10.3109/0284186X.2011.599339. [DOI] [PubMed] [Google Scholar]