Introduction
The spectrum of human immunodeficiency virus (HIV)-associated pulmonary diseases is broad and the lungs continue to be one of the most frequently affected organ systems in HIV-infected persons. Opportunistic infections and neoplasms are common even in the current combination antiretroviral therapy (ART) era. The extent of immunosuppression, use of ART and antimicrobial prophylaxis, prior history of illness, geographic setting, and life style choices influence the etiology and relative frequency of observed respiratory disease. In addition to pulmonary infections classically associated with advanced immunosuppression, such as Pneumocystis pneumonia (PCP), persons infected with HIV are also susceptible to community-acquired pathogens such as Streptococcus pneumoniae and to hospital-acquired pathogens such as methicillin resistant Staphylococcus aureus (MRSA). Worldwide, Mycobacterium tuberculosis is a dominant cause of pneumonia in HIV-infected persons. Furthermore, lung diseases commonly seen in individuals without HIV, including chronic obstructive pulmonary disease (COPD) and lung cancer are prevalent in those with HIV and there is growing evidence that HIV infection predisposes patients to develop COPD and lung cancer and to present with these diseases at a younger age. Risk factors for HIV infection, such as injection drug use, also make patients susceptible to a wide variety of pulmonary complications such as aspiration pneumonia, septic emboli from right sided endocarditis, and talc granulomatosis. Given the breadth of pulmonary illnesses faced by people living with HIV, clinicians must have systematic approach to evaluate this patient population. This review will present an overview of the evaluation of respiratory disease in persons with HIV/AIDS.
Evaluation of Respiratory Disease
The evaluation of respiratory disease in HIV-infected persons begins with a thorough history and physical examination. The information from the history and physical examination can then be used to determine whether additional testing (e.g., laboratory tests, chest radiograph) is indicated. Frequently, the clinical, laboratory, and chest radiographic presentation suggests a specific diagnosis or, at most, a few diagnoses, which then prompts specific diagnostic testing and treatment.
Symptoms and Signs
All HIV-associated respiratory diseases may present with similar respiratory symptoms including, cough, dyspnea, and occasionally pleuritic chest pain. However, the character and duration of these symptoms may be useful to guide clinicians toward a particular diagnosis. For example, most patients with bacterial bronchitis or pneumonia present with cough productive of purulent sputum, whereas most patients with PCP have a dry, nonproductive cough (1). Bacterial pneumonias due to S. pneumoniae and Haemophilus species characteristically present abruptly with patients reporting 3–5 days of symptoms; on the other hand, PCP often presents with an insidious onset with patients noting 2–4 weeks of symptoms (2).
Constitutional symptoms such as fever, night sweats, and weight loss may indicate the presence of a systemic or disseminated disease. Fever and weight loss may be the sole presenting complaints of disseminated mycobacterial or fungal disease or may be “B” symptoms associated with non-Hodgkin lymphoma (NHL).
HIV-infected patients are predisposed to systemic dissemination of infections that originated in the lungs and the symptoms and signs associated with disseminated disease may dominate the clinical presentation. Bacterial pneumonia due to S. pneumoniae is often accompanied by bacteremia and septicemia in HIV-infected patients with CD4 counts <200 cells/μl and thus the clinical presentation may be that of sepsis and multi-organ system dysfunction. Similarly, tuberculosis is often accompanied by mycobacteremia in HIV-infected patients with CD4 counts <200 cells/μl and HIV-infected patients with advanced HIV/AIDS are more likely to present with extrapulmonary tuberculosis and disseminated disease than HIV-uninfected patients (3). Meningitis is a well-described acute presentation of tuberculosis and 43% to 65% of these patients are HIV-infected with CD4 counts <200 cells/μl (4). In patients with tuberculous meningitis, clinical syndromes range from those mimicking typical bacterial meningitis to a nonspecific, subacute illness characterized by fever and headache.
Although the lungs are the portal of entry for Cryptococcus neoformans, patients are often minimally symptomatic until the infection has spread to the central nervous system (CNS). In a series of 106 patients with C. neoformans infection, 84% presented with meningitis; cough or dyspnea was present in less than a third (n=28, 31%) (5). The signs of extrapulmonary infection, including meningoencephalitis and cutaneous lesions (which resemble the lesions caused by molluscum contagiosum), should be recognized in attempting to establish a unifying diagnosis. Histoplasmosis may present with hypotension, hepatosplenomegaly, pancytopenia, hypoadrenalism, and necrotic skin and oral lesions (6). Dissemination of coccidioidomycosis to extrapulmonary sites may result in cutaneous and soft tissue infection, lymph node involvement, osteomyelitis, arthritis, and meningitis (7). Blastomycosis can disseminate into the skin, genitourinary tract, bone and, rarely, the central nervous system (CNS).
CNS presentations typically dominate the presentations of Toxoplasma gondii and Cytomegalovirus (CMV). For Toxoplasma, encephalitis and retinochoroiditis are frequent presentations while CMV most often presents with retinitis, esophagitis, and colitis. In both cases, pulmonary involvement occurs in only a subset of patients.
Patients with Human Herpes Virus (HHV)-8 and Epstein Barr Virus (EBV)-related malignancies tend to present with symptoms of systemic disease. Most commonly, patients with pulmonary Kaposi’s sarcoma (KS) have previously recognized cutaneous lesions or other visceral involvement; nonetheless, cutaneous involvement is absent in 5% to 23% of patients with symptomatic pulmonary KS (8, 9). The most common respiratory symptoms are progressive dyspnea, non-productive cough, and fever. Patients with NHL typically present with “B symptoms” including fever, night sweats, and weight loss. The majority of those with pulmonary involvement have respiratory symptoms which vary depending on extent of parenchymal and/or pleural involvement. Patients with multicentric Castleman disease typically have fever and lymphadenopathy, as well as splenomegaly, and hepatomegaly. Involvement of the respiratory system occurs in about one third of patients and is characterized by lymphocytic interstitial pneumonitis (LIP) (10). The symptoms associated with primary effusion lymphoma (PEL) depend on the serosal surface affected; commonly affected serosal surfaces include the pleura (60–90%), the pericardium (0–30%), the peritoneum (30–60%), joint spaces, and, rarely, the meninges (11, 12). Most affected patients present with symptoms related to fluid accumulation such as dyspnea (from pleural or pericardial effusions), abdominal distension (from ascites), or joint swelling.
Pulmonary manifestations of the immune reconstitution inflammatory syndrome (IRIS) depend on the underlying infectious, neoplastic, or autoimmune pathology (13). The risk of IRIS is associated with the CD4 count at the start of ART, with a higher risk in HIV-infected patients with CD4 <50 cells/ul (14). IRIS associated with tuberculosis (TB-IRIS) presents with fever, cough, dyspnea, lymph node enlargement, and new or worsening parenchymal opacities or new or enlarging pleural effusions on chest radiograph (13, 15). New or worsening tuberculous meningitis, granulomatous hepatitis, and subcutaneous or deep tissue abscesses are common extrapulmonary TB-IRIS presentations. Non-tuberculous mycobacteria (NTM, e.g., Mycobacterium avium complex, MAC) can also produce IRIS. In the lungs, these organisms commonly cause endobronchial disease; other manifestations include masses and cavitary lesions (13, 16). PCP-IRIS presents with symptoms seen in acute PCP including fever, dry non-productive cough, and dyspnea, although development of organizing pneumonia has also been described (16–18). Occasionally, PCP-IRIS is severe and patients develop acute respiratory failure (19). While paradoxical cryptococcal-IRIS predominantly affects the CNS, lung involvement is also well-described. Unlike TB-IRIS, which is rarely fatal, cryptococcal-IRIS has a mortality rate as high as 83% (20–24). KS-associated IRIS usually presents with inflammation or enlargement of existing skin lesions, new skin lesions, and mucosal involvement, however pulmonary deterioration is also common (25). Pulmonary sarcoidosis can worsen in the setting of immune reconstitution and result in clinical and radiographic findings similar to those seen in HIV-uninfected patients (26, 27).
As extent of immunosuppression decreases in the era of ART, it’s important to consider non-infectious pulmonary diseases in HIV-infected patients. Due to higher rates of tobacco and drug use in HIV-infected persons, and possibly to HIV infection itself, COPD, lung cancer, and pulmonary arterial hypertension (PAH) are more common in HIV-infected persons (28–31). Patients with COPD often complain of dyspnea and chronic cough; in the setting of an acute exacerbation these patients present with worsening shortness of breath, wheezing, and increased sputum production. The majority of patients with lung cancer complain of cough, especially those with endobronchial involvement; some also complain of hemoptysis, chest pain, and dyspnea due to parenchymal or pleural involvement. Less frequent presentations of underlying lung cancer include superior vena cava (SVC) syndrome and Pancoast syndrome. SVC syndrome typically causes a sensation of head fullness due to compression of the SVC, while Pancoast syndrome is characterized by shoulder pain, Horner syndrome, and local tissue destruction caused by tumor at the apex of the lung. Most patients with PAH initially experience dyspnea, decreased exercise tolerance, and fatigue due to an inability to increase cardiac output with exertion. As the PAH progresses and right ventricular failure develops, angina, syncope, and peripheral edema may develop.
CD4 Count
The CD4 count should be used to assess a patient’s susceptibility to opportunistic infections and neoplasms (Table 1). Ideally, the CD4 count will have been obtained prior to the onset of pneumonia as the CD4 count is often decreased in the setting of an acute illness. Although bacterial pneumonia can occur at any CD4 count, Hirschtick and colleagues in the Pulmonary Complications of HIV Infection Study (PCHIS) found that the risk of bacterial pneumonia was 5 times higher in HIV-infected subjects with CD4 counts <200 cells/μl compared to those with >500 cells/μl (32). Furthermore, the incidence of bacteremia is also more common in HIV-infected patients and correlates with the extent of immunosuppression; the lower the CD4 count, the higher the incidence of bacteremia. Redd and colleagues reviewed the incidence of pneumococcal bacteremia at 10 San Francisco hospitals during a 4-year period. They estimated that the rate of bacteremia in patients with pneumococcal pneumonia increased 100 fold since the onset of the HIV epidemic (33). Virulent, hospital-acquired, pathogens are also more common in HIV-infected adults; surveys have found that P. aeruginosa accounts for 16% to 67% of nosocomial pneumonias and CD4 counts <100 cells/μl predispose patients to this infection (34–36). Similar to bacterial pneumonia, TB can occur at any CD4 count. As with bacterial pneumonia accompanied by bacteremia, the incidence of TB accompanied by mycobacteremia also correlates with the extent of immunosuppression; the lower the CD4 count, the higher the incidence of mycobacteremia.
Table 1.
| CD4 count | Lower Respiratory Illness |
|---|---|
|
| |
| Any | Acute bronchitis |
| Bacterial pneumonia | |
| Tuberculosis | |
| Non-Hodgkin lymphoma | |
| Pulmonary embolus | |
| Bronchogenic carcinoma | |
| Chronic obstructive pulmonary disease | |
| Pulmonary arterial hypertension | |
|
| |
| ≤500 cells/μl | Recurrent bacterial pneumonia |
|
| |
| ≤200 cells/μl | Pneumocystis pneumonia |
| Cryptococcus neoformans pneumonia | |
| Sepsis secondary to bacterial pneumonia | |
| Disseminated or extrapulmonary tuberculosis | |
|
| |
| ≤100 cells/μl | Pulmonary Kaposi sarcoma |
| Toxoplasma pneumonitis | |
|
| |
| ≤50 cells/μl | Disseminated Histoplasma capsulatum |
| Disseminated Coccidioides immitis | |
| Aspergillus pneumonia | |
| Cytomegalovirus pneumonitis | |
| Disseminated non-tuberculous mycobacterial pneumonia | |
Once the CD4 count falls ≤200 cells/μl, PCP becomes a common cause of respiratory complaints. Stansell and the PCHIS showed that 95% of 145 cases of PCP occurred in patients with CD4 counts <200 cells/μl (37). Cryptococcus neoformans is also a frequent cause of pneumonia in patients with CD4 counts <200 cells/μl. In addition, at CD4 counts ≤100 cells/μl, T. gondii pneumonitis and pulmonary KS become more common. A study of 64 HIV-infected patients with pulmonary toxoplasmosis diagnosed via bronchoalveolar lavage (BAL) reported a mean CD4 count of 40 cells/μl, with 82% having a CD4 count ≤50 cells/μl and only 4% had counts >200 cells/μl (38). Another study of 168 HIV-infected patients with pulmonary KS diagnosed via bronchoscopy reported a median CD4 count of 19 cells/μl; 68% of patients had counts ≤50 cells/μl, and only 4% of patients had counts >200 cells/μl (8).
At CD4 counts ≤50 cells/μl, patients become susceptible to CMV, Aspergillus species, and disseminated endemic fungal diseases such as histoplasmosis and coccidioidomycosis. Although it’s important to use the CD4 count to assess a patient’s susceptibility to certain opportunistic infections, astute clinicians realize that these infections may still occur at CD4 counts above established guidelines.
Laboratory Tests
Arterial blood gas (ABG) analysis can be used to guide treatment decisions and is a useful prognostic tool. Patients with significant hypoxemia should probably be hospitalized. PCP patients with an arterial partial pressure of oxygen, PaO2, ≤70 mm Hg or an alveolar-arterial oxygen gradient, A-aO2, ≥35 mm Hg should be treated with adjunctive corticosteroids.
The serum lactate dehydrogenase (LDH) is often elevated in patients with PCP; however, this marker is non-specific and can also be elevated in other pneumonias including tuberculosis and bacterial pneumonia and in neoplasms such as NHL. The diagnostic accuracy of serum LDH was assessed in a retrospective analysis of 328 immunocompromised patients, 105 with and 193 without PCP (39). In HIV-uninfected patients with PCP, the sensitivity of an elevated LDH was 63% and the specificity was 43%. In HIV-infected patients, the sensitivity was 100% and the specificity was 47%. The overall accuracy of LDH for the diagnosis of PCP was 52%, 51% in HIV-negative and 58% in HIV-positive patients. The sensitivity of LDH in other published studies ranges from 83% to 100% with the lowest sensitivity seen in the outpatient setting (1).
The degree of LDH elevation correlates with prognosis and response to treatment (1, 40, 41). A high or rising serum LDH value despite PCP treatment correlates with a worse prognosis, a failure of therapy, and increased mortality. On the other hand, a low or declining serum LDH value on PCP treatment correlates with a better prognosis, a response to therapy, and decreased mortality. However, significant overlap in serum LDH levels between survivors and non-survivors precludes use of this lab value as an absolute predictor of mortality in individual patients (40).
The white blood cell count (WBC) is often elevated in patients with bacterial pneumonia. This WBC elevation may be relative to the patient’s baseline value in an individual whose baseline WBC is below the normal range. Laboratory evidence of hepatic and/or bone marrow involvement may be a sign of extrapulmonary involvement from disseminated opportunistic infection or malignancy (e.g., tuberculosis, NHL). The presence of these laboratory abnormalities may be important clues to the pulmonary diagnosis.
Chest Imaging
The chest radiographic appearance of pulmonary disease varies widely in HIV-infected patients and is dependent upon the etiology and severity of pulmonary disease and the degree of immunosuppression. Although chest radiographs are the foundation of the evaluation of respiratory disease in HIV-infected persons and are the appropriate initial test for suspected pneumonia, chest computed tomographic (CT) scans are often useful to identify and to more precisely define nodules, cavities, lymphadenopathy, and pleural fluid collections that may require drainage (42, 43).
Bacterial Pneumonia
Bacterial pneumonia most commonly presents with focal or multifocal areas of consolidation regardless of CD4 count (Figure 1) (32, 44). The typical chest radiographic appearance includes unilateral, lobar or segmental consolidation; however, as immune function deteriorates, multilobar and bilateral radiographic disease become more common. Pleural effusions are present in 12% to 47% of patients with bacterial pneumonia (45, 46) and empyemas develop in 2% to 6% (47, 48).
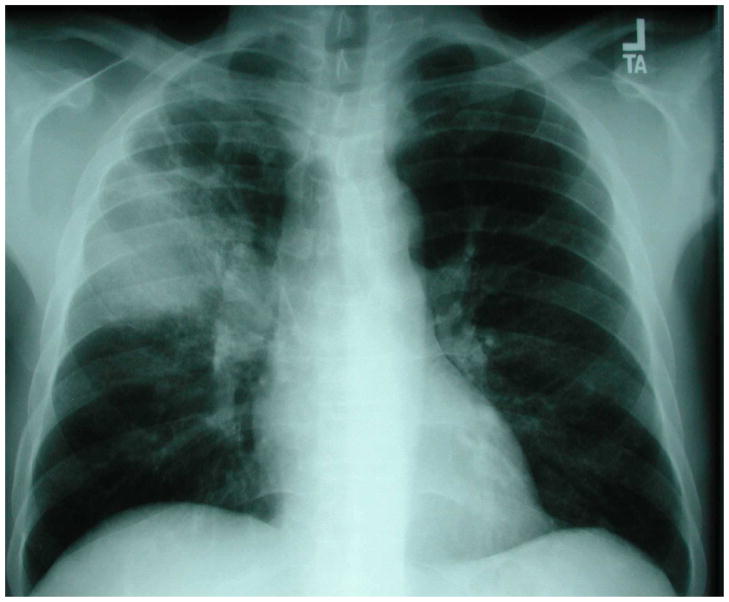
Figure 1.
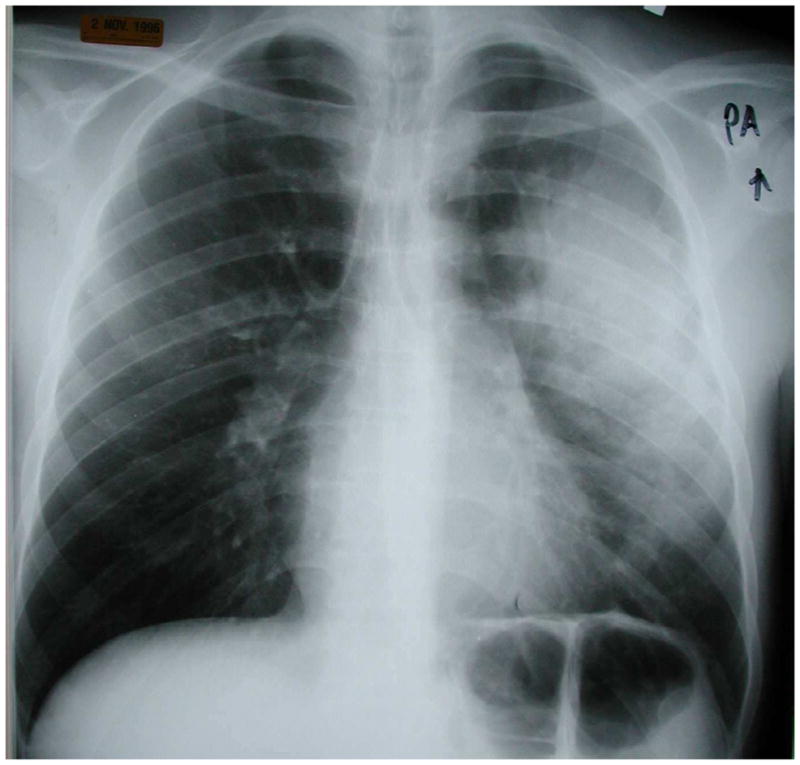
Chest radiograph of an HIV-infected person with multilobar consolidation due to Streptococcus pneumoniae detected in two blood cultures. Chest radiograph courtesy of Laurence Huang, MD.
Similarly to their HIV-uninfected counterparts, Streptococcus pneumoniae is the most common causative pathogen of bacterial pneumonia in patients with HIV. Rizzi, et al described the chest radiographic appearance of pneumococcal lung disease in 57 HIV-infected patients, in whom Streptococcus pneumoniae was the sole respiratory pathogen isolated (49). Lobar consolidation was found in 40% of patients, multifocal distribution in 25%, and interstitial pattern of disease in 17.5%; pleural effusions were identified in 23% of patients, 62% of whom had CD4 counts <200 cells/μl.
Bacterial infections may also present with pulmonary nodules. Franquet, et al reviewed 78 chest high-resolution CT (HRCT) scans of immunocompromised patients (25 of whom had AIDS) with infectious pulmonary nodules (50). A total of 20 patients had bacterial infection and 95% of them had multiple pulmonary nodules >10 mm in diameter. Nodules due to bacterial pneumonia can cavitate; this is especially common with pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus (51). Less common bacterial causes of cavitary nodules or cavitary consolidation include Rhodococcus equi and Nocardia asteroides. Rhodococcus equi typically manifests as an area of consolidation limited to one lobe, usually an upper lobe, which may undergo cavitation with an air fluid level (52). The most common radiographic presentation of Nocardia is similar to TB and Rhodococcus equi, a lobar or multilobar consolidation with an upper lobe predominance and frequent cavitation (53).
Tuberculosis
Pulmonary TB is commonly divided into primary infection and post-primary infection. Primary infection refers to the development of active tuberculosis within 5 years after initial exposure; while post-primary infection refers to reactivation of latent tuberculosis. The radiographic appearance of pulmonary tuberculosis is largely dependent upon the degree of immunosuppression (54). Similar to immunocompetent persons, tuberculosis in HIV-infected patients with relatively intact immunity appears radiographically as an opacity in the apical and posterior segments of the upper lobe and superior segment of the lower lobe. Cavitation is often present (Figure 2). As immune function deteriorates (CD4 count <200 cells/μl), middle and lower lung involvement and disseminated disease (including a military pattern) both become more common (Figure 3). Cavitation is less common at this point because the host lacks the ability to mount an effective immune response against M. tuberculosis. Mediastinal and/or hilar lymphadenopathy and pleural effusions are common in HIV-infected patients with low CD4 counts. Normal chest radiographic findings in HIV-infected patients with acid fast bacillus (AFB) smear-positive pulmonary tuberculosis have been reported in the range of 6% to 14%; the majority of these patients had CD4 cell counts <200 cells/μl (55, 56).
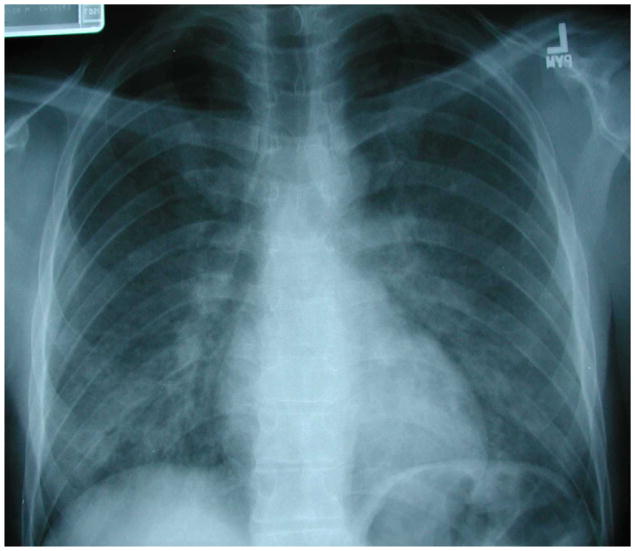
Figure 2.
Chest radiograph of an HIV-infected person, CD4 cell count greater than 200 cells/μL, revealing right upper lobe consolidation with areas of cavitation. Sputum acid-fast bacillus stain was positive and sputum cultures grew Mycobacterium tuberculosis. Chest radiograph courtesy of Laurence Huang, MD.
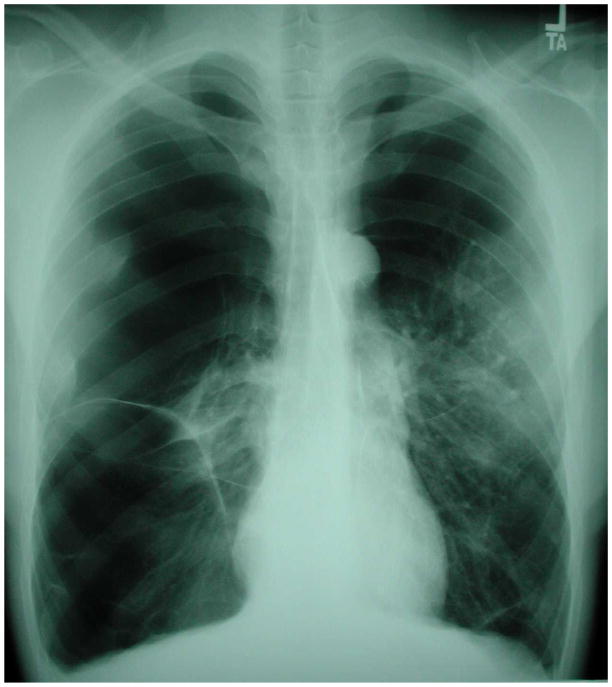
Figure 3.
Chest radiograph of an HIV-infected person, CD4 cell count less than 200 cells/μL, revealing right lower lung consolidation. Sputum acid-fast bacillus stains were negative but sputum cultures grew Mycobacterium tuberculosis that was mono-rifampin-resistant. In this case, the key to the diagnosis of tuberculosis was knowledge of the patient’s CD4 cell count and an understanding that tuberculosis can present with this pattern in HIV-infected individuals with advanced immunosuppression. Chest radiograph courtesy of Laurence Huang, MD.
Pneumocystis Pneumonia
Classically, PCP presents with a bilateral, symmetric, perihilar or diffuse interstitial, reticular, or granular pattern on chest radiograph (Figure 4) (57). These opacities typically begin in the perihilar region and then spread outward as the disease progresses. However, patients may also have minimal chest radiographic extent of disease or even normal chest radiographs (58). When the clinical suspicion of PCP is high and radiographic disease is minimal, a chest HRCT is often helpful to rule out the presence of disease (59). Infection with Pneumocystis jirovecii induces extensive ground glass attenuation on chest HRCT (Figure 5) and the absence of ground glass attenuation rules out PCP (59). The pattern of ground glass is geographic in appearance with relatively normal secondary lobules adjacent to affected ones.
Figure 4.
Chest radiograph of an HIV-infected person, CD4 cell count less than 200 cells/μL, with bilateral, symmetric granular opacities due to Pneumocystis pneumonia. Microscopic examination of bronchoscopy with bronchoalveolar lavage (BAL) fluid demonstrated characteristic Pneumocystis cystic and trophic forms. Chest radiograph courtesy of Laurence Huang, MD.
Figure 5.
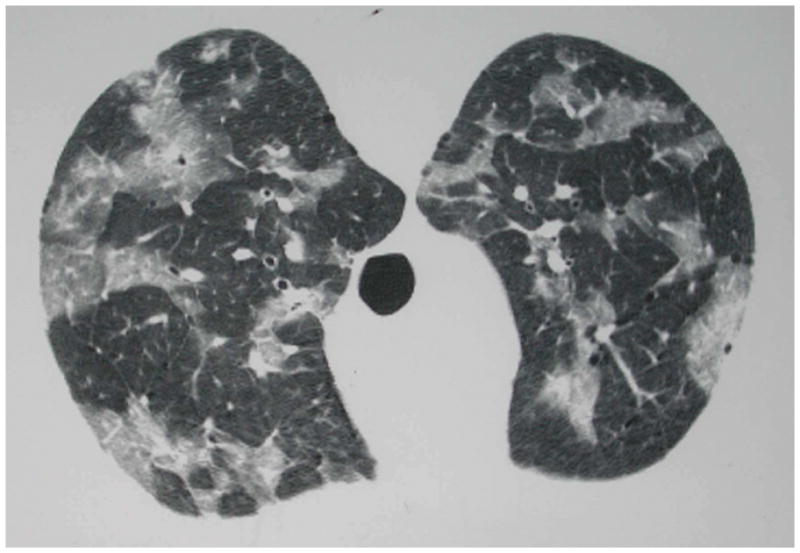
Chest high resolution computed tomographic (HRCT) scan of an HIV-infected person, CD4 cell count less than 200 cells/μL, with patchy ground-glass opacities due to Pneumocystis pneumonia. Microscopic examination of induced sputum demonstrated characteristic Pneumocystis cystic and trophic forms. This individual’s chest radiograph one day prior to chest HRCT was normal, demonstrating the increased sensitivity of chest HRCT compared to chest radiography for Pneumocystis pneumonia. Chest HRCT scan courtesy of Laurence Huang, MD.
Other features of PCP include consolidation when the disease is severe, pneumatoceles which may be single or multiple in number and small or large in size, and pneumothorax. Pneumatoceles occur in up to one-third of patients with PCP. The presence of these thin-walled cysts predisposes patients to spontaneous pneumothoraces. Pleural effusions and intrathoracic lymphadenopathy are extremely both uncommon chest radiographic presentations.
Cryptococcal Pneumonia
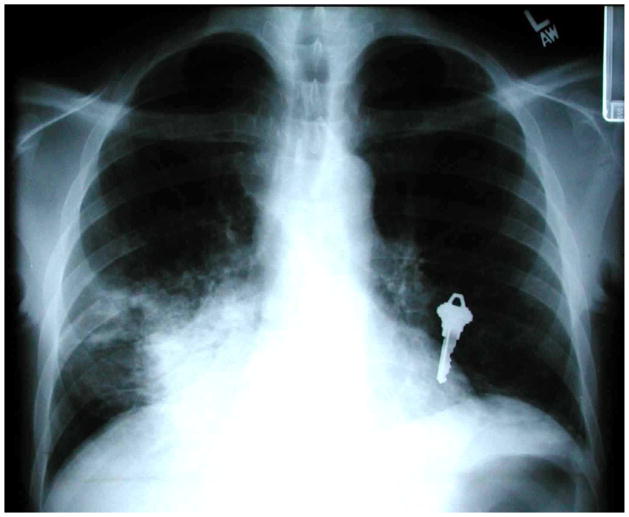
Cryptococcal pneumonia most often presents with a bilateral, reticular or interstitial pattern on chest radiograph (60). Other patterns of cryptococcal pneumonia include unilateral, reticular or interstitial infiltrates, consolidation, nodular opacities, cavitation, pleural effusion(s), and intrathoracic adenopathy. Occasionally, patients with pulmonary cryptococcosis present with a mass-like cryptococcoma (Figure 6).
Figure 6.
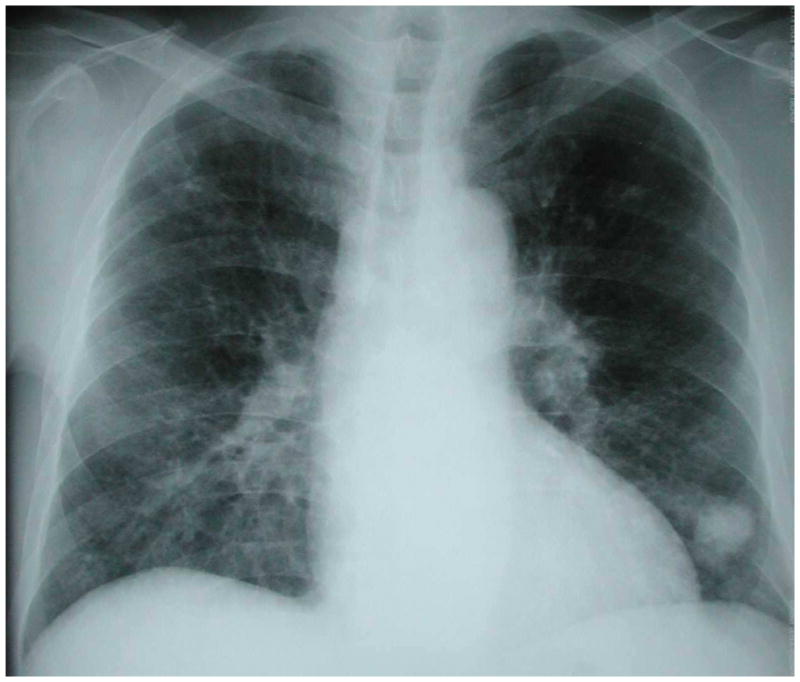
Chest radiograph of an HIV-infected person, CD4 cell count less than 200 cells/μL, with left lower lobe lung mass that was initially concerning for lung cancer. CT-guided fine needle aspiration revealed Cryptococcus neoformans. Chest radiograph courtesy of Laurence Huang, MD.
Toxoplasma Pneumonia
Toxoplasma pneumonia most frequently presents with bilateral, reticular or interstitial opacities on chest radiograph (61). Occasionally, a reticulonodular or coarse nodular pattern is seen. Rarely, patients present with a chest radiographic picture of ARDS.
Viral Pneumonia
Unfortunately, it is often difficult to differentiate between viral and bacterial pneumonia based on diagnostic imaging (62). In patients with viral pneumonia due to typical respiratory pathogens such as CMV, RSV, adenovirus, and influenza, chest CT scan findings can include bronchial wall thickening, ground glass opacities (Figure 7), multifocal consolidations, nodules, or nodular opacities, and diffuse airspace disease.
Figure 7.
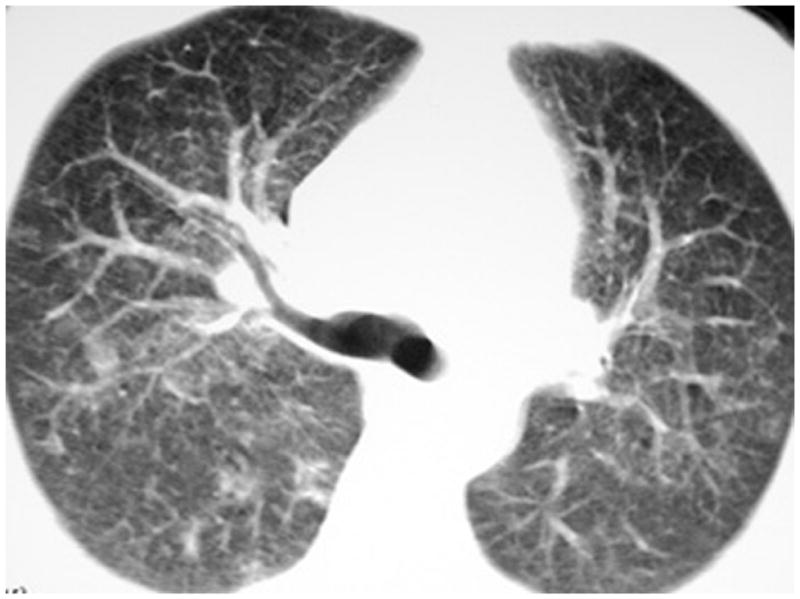
Chest high resolution computed tomographic (HRCT) scan of an HIV-infected person, CD4 cell count less than 50 cells/μL, with patchy ground-glass opacities due to Cytomegalovirus (CMV). The patient was initially thought to have Pneumocystis pneumonia but bronchoscopy with bronchoalveolar lavage was negative for Pneumocystis cystic and trophic forms. The patient then underwent video-assisted thoracoscopic surgical biopsy which established the diagnosis of CMV. Chest HRCT scan courtesy of Laurence Huang, MD.
Kaposi Sarcoma, Non-Hodgkin Lymphoma, Multicentric Castleman Disease and Primary Effusion Lymphoma
Although the gold standard for diagnosis of pulmonary KS remains direct visualization of typical endobronchial KS lesions via bronchoscopy (Figure 8), there are certain patterns on chest imaging that are very suggestive (63). On chest radiograph, bilateral, perihilar middle and lower lung zone infiltrates occur in the majority of patients and coalescent nodular opacities are also common (Figure 9). On CT, densities along the perivascular bundles (classically called “flame hemorrhages”) are commonly seen as are pleural effusions. Nuclear scans can also be helpful diagnostic tools; KS does not take up gallium on scans but does appear to accumulate thallium. The presence of an abnormal chest radiograph, a negative gallium scan, and a positive thallium scan is suggestive of KS (64). Thus, thallium and gallium scanning can be helpful in distinguishing KS from PCP and other infections.
Figure 8.
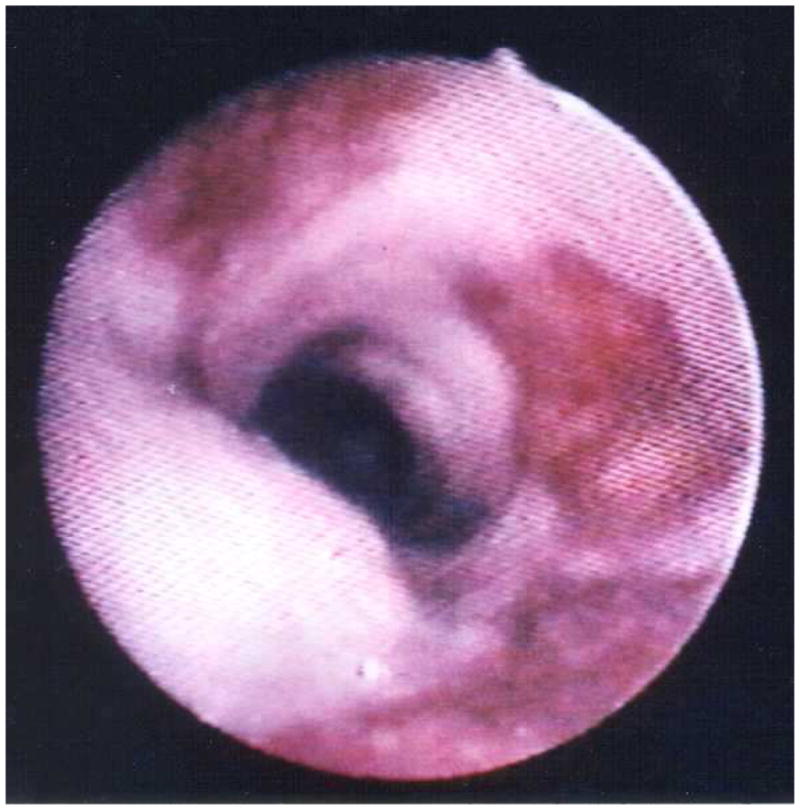
Characteristic violaceous Kaposi sarcoma lesions seen in the trachea of an HIV-infected person, CD4 cell count less than 100 cells/μL. Image courtesy of Laurence Huang, MD.
Figure 9.
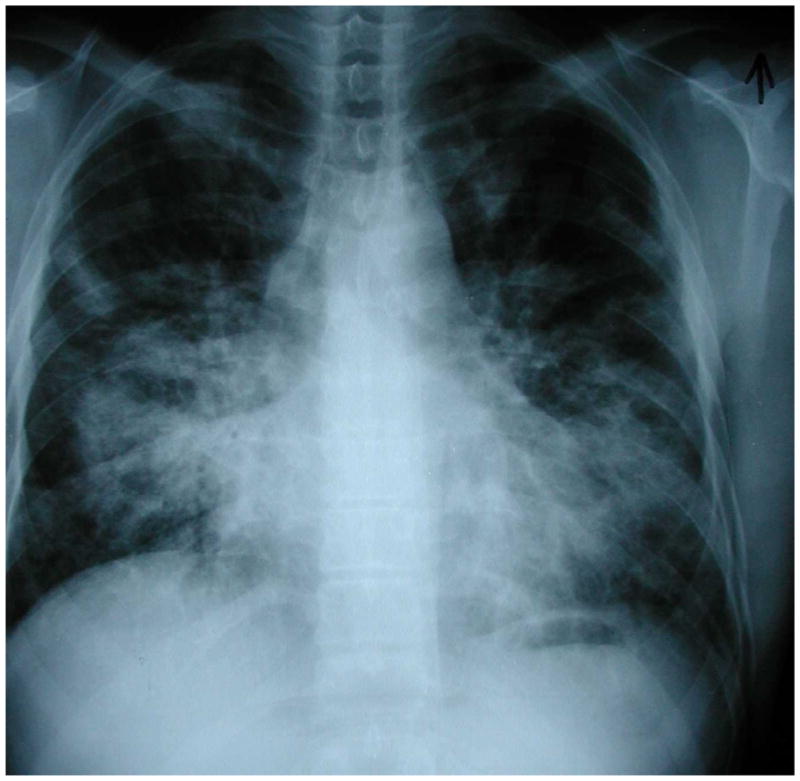
Chest radiograph of an HIV-infected person, CD4 cell count less than 100 cells/μL, demonstrating the characteristic bilateral, middle and lower lung zone, perihilar or central distribution of abnormalities of pulmonary Kaposi sarcoma. This individual had no evidence of mucocutaneous Kaposi sarcoma and the diagnosis of pulmonary Kaposi sarcoma was established by bronchoscopy with visualization of multiple, characteristic Kaposi sarcoma lesions (see Figure 7). Chest radiograph courtesy of Laurence Huang, MD.
Pulmonary NHL most often presents with single or multiple nodules, nodular opacities, or masses (65). Lobar infiltrates and diffuse interstitial infiltrates have also been described. In patients with MCD and resultant LIP, the main CT findings include poorly defined centrilobular nodules, thin-walled cysts, thickening of bronchovascular bundles, and interlobular septal thickening (66). Chest radiographs and chest CTs in patients with PEL show effusions (pleural and/or pericardial), slight serosal thickening, and the absence of parenchymal abnormalities, solid masses, or mediastinal enlargement (11).
Non-infectious pulmonary disease
Appearance on imaging of COPD (Figure 10), lung cancer, and pulmonary arterial hypertension in HIV-infected patients mirrors that of their HIV-uninfected counterparts (29–31).
Figure 10.
Chest radiograph of an HIV-infected person with hyperinflation, flattened diaphragms, increased radiolucency of the lungs, and multiple, large bullae from severe COPD. Chest radiograph courtesy of Laurence Huang, MD.
Diagnosis
In most cases, a confirmed microbiologic diagnosis is preferred to empiric therapy. A delayed or a missed diagnosis may have catastrophic consequences. That said, empiric therapy is usually indicated while a prompt diagnostic evaluation is undertaken.
Bacterial Pneumonia
Although bacteremia is 35 to 50 times more common in HIV-infected patients with bacterial pneumonia than HIV-uninfected patients, isolating the causative pathogen remains difficult. Hirschtick and colleagues in PCHIS isolated the responsible pathogen in 33% of HIV-infected patients diagnosed with bacterial pneumonia (32). Blood cultures were positive in only 6.8% of patients, thus the majority of bacterial isolates came from either sputum or BAL cultures. The three most commonly identified organisms were Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. A study by Boulware and colleagues suggests that urine pneumococcal C-polysaccharide antigen may be a useful diagnostic test in patients with pneumonia, especially in those with pneumonia caused by Streptococcus pneumoniae (67). Among patients with pneumococcal pneumonia, this antigen had a sensitivity of 81%, a specificity of 98%, a positive predictive value of 98%, and a negative predictive value of 82%, independent of the person’s HIV status. Despite low diagnostic yield of bacterial culture, blood and sputum cultures should be obtained in most HIV-infected patients because identifying the responsible pathogen can be used to guide antibiotic therapy.
Tuberculosis
Diagnosing pulmonary tuberculosis in HIV-infected subjects has been historically difficult due to the absence of rapid and sensitive diagnostic tools (68). Acid-fast bacillus smear microscopy of expectorated sputum, a tool commonly used for the rapid diagnosis of pulmonary tuberculosis, has poor sensitivity (20–50%) (69); isolation of the organism in culture, the diagnostic gold standard, can take up to 8 weeks. Nevertheless, sputum should be collected for AFB smear and mycobacterial culture and blood mycobacterial cultures should be performed in HIV-infected patients with CD4 count <200 cells/μl.
A polymerase chain reaction (PCR) assay has been developed to rapidly detect Mycobacterium tuberculosis DNA and presence rifampin resistance (Xpert MTB/RIF [Cepheid, Sunnyvale, CA]) (70, 71). In December 2010, the World Health Organization (WHO) recommended Xpert MTB/RIF to be the initial screening test in patients with HIV and suspected tuberculosis. Using a single assay on a single unprocessed sputum sample, M. tuberculosis complex-specific DNA was detected in 98% of smear-positive cases and in 72% of smear-negative cases using culture positivity as a reference standard (70).
Pneumocystis Pneumonia
Patients with PCP most often present with a non-productive cough and therefore diagnosis of PCP from sputum requires sputum induction (72). Analysis of induced sputum has a reported sensitivity ranging from 55% to 95%, with lower sensitivity reported in the earliest studies prior to routine centrifugation of sputum and higher sensitivity attributed to careful sample collection, concentration by centrifugation, and staining with fluorescent Pneumocystis antibodies (73, 74). In a San Francisco General Hospital and National Institutes of Health study, the use of sputum induction has led to definitive diagnoses in 80% to 95% of patients with PCP, thereby negating the need for bronchoscopy (73).
Bronchoscopy with bronchoalveolar lavage (BAL) is the gold standard for diagnosis of PCP with a sensitivity of up to 98% (74, 75). This procedure should be strongly considered in patients with a high suspicion of PCP and unrevealing induced sputum results or in medical facilities where induced sputum is unavailable or insensitive for diagnosis of PCP. Huang and colleagues examined 992 cases of PCP diagnosed over a 4 year period and found only 2 cases that required transbronchial biopsy for final diagnosis, the remainder were diagnosed via either sputum induction or BAL (n=800 and 190, accordingly) (74). It is important to note that there is institutional variation in the sensitivity of sputum induction and bronchoscopy with BAL, thus the diagnostic protocol should be tailored to individual institutions.
(1,3) Beta-D glucan (BG) is an element of the P. jirovecii cyst wall (and the cell wall of most other fungal organisms) that has been studied as a marker of PCP infection. A meta-analysis that included 357 cases of PCP and 1,723 controls (cases of invasive fungal infection were excluded) found that the average sensitivity and specificity of BG were 94.8% (95% Confidence Interval, CI, 90.8–97.1%) and 86.3% (95% CI 81.7–89.9%), respectively, and the area under the receiver operating characteristic (ROC) curve was 0.965 (0.945–0.978) (76). Another study by the AIDS Clinical Trials Group Study A5164 Team found that median BDG in patients with PCP was 408 pg/mL, compared with 37 pg/mL in patients without PCP (p <0.001); in this study the sensitivity of BG (dichotomized at 80 pg/mL) was 92% and the specificity was 65% (77). These findings suggest that BG may be a clinically useful tool to identify patients with PCP, especially in clinical settings where bronchoscopy is unavailable. In some clinical centers, it may be a more sensitive test than induced sputum examination and could reduce the need for both bronchoscopy and empirical therapy. In settings where other fungal pneumonias are common, however, the specificity of BG may be low and diagnostic utility may be limited.
Cryptococcal Pneumonia
HIV-infected patients are predisposed to dissemination of fungal infections that originate in the lungs, thus fungal culture and occasionally biopsy of involved sites may be necessary. Cultures of cerebrospinal fluid, blood, BAL, and skin biopsies often yield a diagnosis of cryptococcosis. Serum cryptococcal antigen (CrAg) is positive in virtually all patients with HIV and pulmonary cryptococcal infection (60). Since extra-pulmonary disease is extremely common among HIV-infected patients with cryptococcal pneumonia, a positive serum CrAg result should prompt investigation for disseminated disease with a work up including fungal blood cultures and lumbar puncture with cerebrospinal fluid (CSF) CrAg and fungal culture.
Histoplasmosis
Latent histoplasmosis can reactivate and disseminate in HIV-infected patients leading to involvement of extra-pulmonary sites such as the CNS, adrenal glands, mucocutaneous surfaces, and bone marrow. Tissue from these sites may be submitted for fungal culture in addition to blood, urine, and sputum/BAL cultures (6). Urine H. capsulatum carbohydrate antigen is a valuable tool in diagnosis and in therapeutic monitoring of disseminated histoplasmosis (78). Furthermore, a fourfold rise in antibody titer to H. capsulatum can also be diagnostic in patients with acute and chronic pulmonary histoplasmosis.
Aspergillus
Aspergillus infection is rare in patients with HIV, but has been reported. Bronchoscopy with BAL is commonly used to make a diagnosis, however, the diagnostic yield of culture is low and results often take days to come back. Galactomannan antigen detection and real-time PCR assays of BAL fluid have been studied as adjunctive tests for the detection of invasive aspergillosis (79). A study using a rabbit animal model of experimentally induced Aspergillus fumigatus infection confirmed the low sensitivity and high specificity of fungal culture (46% and 100%, respectively). In contrast, galactomannan antigen detection with enzyme immunoassay (EIA) and quantitative PCR assays demonstrated higher sensitivities, with EIA being superior to PCR (100% and 80% sensitivity, respectively) (79). These findings are consistent with those reported in studies of galactomannan in BAL fluid in HIV-uninfected patients with invasive pulmonary aspergillosis.
Viral Pneumonia
Historically, viral pneumonia was diagnosed via culture or immunofluorescence microscopy of upper respiratory specimens (e.g., nasopharyngeal aspirates) and lower respiratory samples (e.g., induced sputum, tracheal aspirate, BAL fluid). Introduction of PCR has increased the ability to detect respiratory viruses, including those that are difficult to culture. Nasopharyngeal swabs have a higher sensitivity than throat swabs, but may be less sensitive than nasopharyngeal washes (80). Transnasal nasopharyngeal flocked swabs also have high virus detection rates (81). In general, PCR-based methods are between two and five times more sensitive than conventional diagnostic methods (culture, antigen detection, and serological assays) for detection of respiratory viruses. This benefit applies particularly to adults and to the elderly, who might have a smaller nasopharyngeal viral load than children.
Establishing the diagnosis of CMV pneumonia in patients with AIDS is extremely difficult for several reasons (82). First, the clinical and radiographic findings overlap with other HIV-associated opportunistic pneumonias. Second, the virus may be cultured from pulmonary secretions in the absence of histologic evidence of disease. Third, CMV often coexists with other pulmonary pathogens. Miles, et al performed 120 BALs on 79 HIV-infected patients and detected CMV in 51.6% of cases; among 65 patients with PCP, CMV was detected in 40 (61.5%) (83). In this study, there was no association between CMV detection and hypoxemia, abnormal chest radiograph, or increased mortality. Thus, the diagnosis of CMV pneumonitis cannot be based solely on detection of the virus in BAL fluid. In order to facilitate diagnosis, Waxman et al proposed the following diagnostic criteria for CMV pneumonia: 1) positive CMV cultures from both BAL and transbronchial biopsy specimens, 2) characteristic cytomegalic inclusion bodies from BAL and transbronchial biopsy specimens, and 3) absence of any other pulmonary pathogens (84). Unfortunately, although these diagnostic criteria improve the specificity of diagnosis, given the patchy nature of CMV-related pulmonary disease, sensitivity may be compromised.
Human Herpes Virus-8-associated Neoplasms: Kaposi Sarcoma, Multicentric Castleman Disease and Primary Effusion Lymphoma
The diagnosis of pulmonary KS is considered clinically confirmed if the characteristic endobronchial lesions of KS are seen at bronchoscopy (8, 85). Lung biopsy may be necessary to diagnose pulmonary KS if clinical, radiographic, or bronchoscopic picture is atypical. The diagnosis of MCD is made on tissue examination of a resected lymph node. These nodes from are positive for HHV-8 and 40% contain concurrent KS (86). Pleural fluid cytology is almost always positive in primary effusion lymphoma.
Conclusion
The spectrum of HIV-associated pulmonary diseases is broad. Opportunistic infections, neoplasms and, increasingly, non-infectious complications such as COPD and lung cancer are all major considerations. Thus, clinicians caring for HIV-infected persons must have systematic approach. The approach begins with a thorough history and physical examination and often involves selected laboratory tests and a chest radiograph. Frequently, the clinical, laboratory, and chest radiographic presentation suggests a specific diagnosis or, at most, a few diagnoses, which then prompts specific diagnostic testing and treatment.
Key Points.
The spectrum of human immunodeficiency virus (HIV)-associated pulmonary diseases is broad.
Opportunistic infections and neoplasms remain important considerations in HIV-infected persons even in the current combination antiretroviral therapy (ART) era.
Lung diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer are prevalent in HIV-infected persons.
Clinicians caring for HIV-infected persons must have systematic approach. The approach begins with a thorough history and physical examination and often involves selected laboratory tests and a chest radiograph.
The goal of the diagnostic evaluation is to arrive at a specific diagnosis or, at most, a few diagnoses, which then prompts specific diagnostic testing and treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang L, Stansell JD. AIDS and the lung. Med Clin North Am. 1996;80:775–801. doi: 10.1016/s0025-7125(05)70467-3. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–671. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson RE, Schecter GF, Theuer CP, et al. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 4.Jacob JT, Mehta AK, Leonard MK. Acute forms of tuberculosis in adults. Am J Med. 2009;122:12–17. doi: 10.1016/j.amjmed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 6.Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990;69:384–391. doi: 10.1097/00005792-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Schnapp LM, Gruden JF, et al. Presentation of AIDS-related pulmonary Kaposi’s sarcoma diagnosed by bronchoscopy. Am J Respir Crit Care Med. 1996;153:1385–1390. doi: 10.1164/ajrccm.153.4.8616570. [DOI] [PubMed] [Google Scholar]
- 9.Cadranel J, Naccache J, Wislez M, et al. Pulmonary malignancies in the immunocompromised patient. Respiration. 1999;66:289–309. doi: 10.1159/000029397. [DOI] [PubMed] [Google Scholar]
- 10.Mylona EE, Baraboutis IG, Lekakis LJ, et al. Multicentric Castleman’s disease in HIV infection: a systematic review of the literature. AIDS Rev. 2008;10:25–35. [PubMed] [Google Scholar]
- 11.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 12.Simonelli C, Spina M, Cinelli R, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol. 2003;21:3948–3954. doi: 10.1200/JCO.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Crothers K, Huang L. Pulmonary complications of immune reconstitution inflammatory syndromes in HIV-infected patients. Respirology. 2009;14:486–494. doi: 10.1111/j.1440-1843.2008.01468.x. [DOI] [PubMed] [Google Scholar]
- 14.Muller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Wilkinson RJ, Lipman MC, et al. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calligaro G, Meintjes G, Mendelson M. Pulmonary manifestations of the immune reconstitution inflammatory syndrome. Curr Opin Pulm Med. 2011;17:180–188. doi: 10.1097/MCP.0b013e328344f692. [DOI] [PubMed] [Google Scholar]
- 17.Godoy MC, Silva CI, Ellis J, et al. Organizing pneumonia as a manifestation of Pneumocystis jiroveci immune reconstitution syndrome in HIV-positive patients: report of 2 cases. J Thorac Imaging. 2008;23:39–43. doi: 10.1097/RTI.0b013e318149e808. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Polatino S, Estrada YMRM. Pneumocystis-associated organizing pneumonia as a manifestation of immune reconstitution inflammatory syndrome in an HIV-infected individual with a normal CD4+ T-cell count following antiretroviral therapy. Int J STD AIDS. 2009;20:662–665. doi: 10.1258/ijsa.2008.008428. [DOI] [PubMed] [Google Scholar]
- 19.Jagannathan P, Davis E, Jacobson M, et al. Life-threatening immune reconstitution inflammatory syndrome after Pneumocystis pneumonia: a cautionary case series. AIDS. 2009;23:1794–1796. doi: 10.1097/QAD.0b013e32832d9b20. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Bekker LG, Myer L, et al. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 21.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murdoch DM, Venter WD, Feldman C, et al. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 23.Sungkanuparph S, Filler SG, Chetchotisakd P, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49:931–934. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 24.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. 2005;19:635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 26.Morris DG, Jasmer RM, Huang L, et al. Sarcoidosis following HIV infection: evidence for CD4+ lymphocyte dependence. Chest. 2003;124:929–935. doi: 10.1378/chest.124.3.929. [DOI] [PubMed] [Google Scholar]
- 27.Foulon G, Wislez M, Naccache JM, et al. Sarcoidosis in HIV-infected patients in the era of highly active antiretroviral therapy. Clin Infect Dis. 2004;38:418–425. doi: 10.1086/381094. [DOI] [PubMed] [Google Scholar]
- 28.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8:320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk GD, Merlo CA. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8:326–332. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almodovar S, Hsue PY, Morelli J, et al. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc. 2011;8:308–312. doi: 10.1513/pats.201006-046WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333:845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 33.Redd SC, Rutherford GW, 3rd, Sande MA, et al. The role of human immunodeficiency virus infection in pneumococcal bacteremia in San Francisco residents. J Infect Dis. 1990;162:1012–1017. doi: 10.1093/infdis/162.5.1012. [DOI] [PubMed] [Google Scholar]
- 34.Meynard JL, Barbut F, Guiguet M, et al. Pseudomonas aeruginosa infection in human immunodeficiency virus infected patients. J Infect. 1999;38:176–181. doi: 10.1016/s0163-4453(99)90247-5. [DOI] [PubMed] [Google Scholar]
- 35.Manfredi R, Nanetti A, Ferri M, et al. Pseudomonas spp complications in patients with HIV disease: an eight-year clinical and microbiological survey. Eur J Epidemiol. 2000;16:111–118. doi: 10.1023/a:1007626410724. [DOI] [PubMed] [Google Scholar]
- 36.Sorvillo F, Beall G, Turner PA, et al. Incidence and determinants of Pseudomonas aeruginosa infection among persons with HIV: association with hospital exposure. Am J Infect Control. 2001;29:79–84. doi: 10.1067/mic.2001.110367. [DOI] [PubMed] [Google Scholar]
- 37.Stansell JD, Osmond DH, Charlebois E, et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1997;155:60–66. doi: 10.1164/ajrccm.155.1.9001290. [DOI] [PubMed] [Google Scholar]
- 38.Rabaud C, May T, Lucet JC, et al. Pulmonary toxoplasmosis in patients infected with human immunodeficiency virus: a French National Survey. Clin Infect Dis. 1996;23:1249–1254. doi: 10.1093/clinids/23.6.1249. [DOI] [PubMed] [Google Scholar]
- 39.Vogel M, Weissgerber P, Goeppert B, et al. Accuracy of serum LDH elevation for the diagnosis of Pneumocystis jiroveci pneumonia. Swiss Medical Weekly. 2011;141:w13184. doi: 10.4414/smw.2011.13184. [DOI] [PubMed] [Google Scholar]
- 40.Zaman MK, White DA. Serum lactate dehydrogenase levels and Pneumocystis carinii pneumonia. Diagnostic and prognostic significance. Am Rev Respir Dis. 1988;137:796–800. doi: 10.1164/ajrccm/137.4.796. [DOI] [PubMed] [Google Scholar]
- 41.Garay SM, Greene J. Prognostic indicators in the initial presentation of Pneumocystis carinii pneumonia. Chest. 1989;95:769–772. doi: 10.1378/chest.95.4.769. [DOI] [PubMed] [Google Scholar]
- 42.Jasmer RM, Edinburgh KJ, Thompson A, et al. Clinical and radiographic predictors of the etiology of pulmonary nodules in HIV-infected patients. Chest. 2000;117:1023–1030. doi: 10.1378/chest.117.4.1023. [DOI] [PubMed] [Google Scholar]
- 43.Jasmer RM, Gotway MB, Creasman JM, et al. Clinical and radiographic predictors of the etiology of computed tomography-diagnosed intrathoracic lymphadenopathy in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;31:291–298. doi: 10.1097/00126334-200211010-00004. [DOI] [PubMed] [Google Scholar]
- 44.Selwyn PA, Pumerantz AS, Durante A, et al. Clinical predictors of Pneumocystis carinii pneumonia, bacterial pneumonia and tuberculosis in HIV-infected patients. AIDS. 1998;12:885–893. doi: 10.1097/00002030-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Gil Suay V, Cordero PJ, Martinez E, et al. Parapneumonic effusions secondary to community-acquired bacterial pneumonia in human immunodeficiency virus-infected patients. Eur Respir J. 1995;8:1934–1939. doi: 10.1183/09031936.95.08111934. [DOI] [PubMed] [Google Scholar]
- 46.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117:1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- 47.Afessa B. Pleural effusion and pneumothorax in hospitalized patients with HIV infection: the Pulmonary Complications, ICU support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117:1031–1037. doi: 10.1378/chest.117.4.1031. [DOI] [PubMed] [Google Scholar]
- 48.Miller RF, Howling SJ, Reid AJ, et al. Pleural effusions in patients with AIDS. Sex Trans Infect. 2000;76:122–125. doi: 10.1136/sti.76.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzi EB, Schinina V, Rovighi L, et al. HIV-related pneumococcal lung disease: does highly active antiretroviral therapy or bacteremia modify radiologic appearance? AIDS Patient Care STDS. 2008;22:105–111. doi: 10.1089/apc.2007.0028. [DOI] [PubMed] [Google Scholar]
- 50.Franquet T, Muller NL, Gimenez A, et al. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–468. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Aviram G, Fishman JE, Sagar M. Cavitary lung disease in AIDS: etiologies and correlation with immune status. AIDS Patient Care STDS. 2001;15:353–361. doi: 10.1089/108729101750301906. [DOI] [PubMed] [Google Scholar]
- 52.Muntaner L, Leyes M, Payeras A, et al. Radiologic features of Rhodococcus equi pneumonia in AIDS. Eur J Radiol. 1997;24:66–70. doi: 10.1016/s0720-048x(96)01022-4. [DOI] [PubMed] [Google Scholar]
- 53.Kramer MR, Uttamchandani RB. The radiographic appearance of pulmonary nocardiosis associated with AIDS. Chest. 1990;98:382–385. doi: 10.1378/chest.98.2.382. [DOI] [PubMed] [Google Scholar]
- 54.Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, et al. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis. 2010;14:1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberg SD, Frager D, Suster B, et al. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;193:115–119. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- 56.Yoo SD, Cattamanchi A, Den Boon S, et al. Clinical significance of normal chest radiographs among HIV-seropositive patients with suspected tuberculosis in Uganda. Respirology. 2011;16:836–841. doi: 10.1111/j.1440-1843.2011.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeLorenzo LJ, Huang CT, Maguire GP, et al. Roentgenographic patterns of Pneumocystis carinii pneumonia in 104 patients with AIDS. Chest. 1987;91:323–327. doi: 10.1378/chest.91.3.323. [DOI] [PubMed] [Google Scholar]
- 58.Opravil M, Marincek B, Fuchs WA, et al. Shortcomings of chest radiography in detecting Pneumocystis carinii pneumonia. J Acquir Immune Defic Syndr. 1994;7:39–45. [PubMed] [Google Scholar]
- 59.Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967–975. doi: 10.2214/ajr.169.4.9308446. [DOI] [PubMed] [Google Scholar]
- 60.Meyohas MC, Roux P, Bollens D, et al. Pulmonary cryptococcosis: localized and disseminated infections in 27 patients with AIDS. Clin Infect Dis. 1995;21:628–633. doi: 10.1093/clinids/21.3.628. [DOI] [PubMed] [Google Scholar]
- 61.Pomeroy C, Filice GA. Pulmonary toxoplasmosis: a review. Clin Infect Dis. 1992;14:863–870. doi: 10.1093/clinids/14.4.863. [DOI] [PubMed] [Google Scholar]
- 62.Miller WT, Jr, Mickus TJ, Barbosa E, Jr, et al. CT of viral lower respiratory tract infections in adults: comparison among viral organisms and between viral and bacterial infections. AJR Am J Roentgenol. 2011;197:1088–1095. doi: 10.2214/AJR.11.6501. [DOI] [PubMed] [Google Scholar]
- 63.Gruden JF, Huang L, Webb WR, et al. AIDS-related Kaposi sarcoma of the lung: radiographic findings and staging system with bronchoscopic correlation. Radiology. 1995;195:545–552. doi: 10.1148/radiology.195.2.7724781. [DOI] [PubMed] [Google Scholar]
- 64.Getz JM, Bekerman C. Diagnostic significance of Tl-201-Ga-67 discordant pattern of biodistribution in AIDS. Clin Nucl Med. 1994;19:1117–1118. doi: 10.1097/00003072-199419120-00023. [DOI] [PubMed] [Google Scholar]
- 65.Eisner MD, Kaplan LD, Herndier B, et al. The pulmonary manifestations of AIDS-related non-Hodgkin’s lymphoma. Chest. 1996;110:729–736. doi: 10.1378/chest.110.3.729. [DOI] [PubMed] [Google Scholar]
- 66.Do KH, Lee JS, Seo JB, et al. Pulmonary parenchymal involvement of low-grade lymphoproliferative disorders. J Comput Assist Tomogr. 2005;29:825–830. doi: 10.1097/01.rct.0000179597.93844.23. [DOI] [PubMed] [Google Scholar]
- 67.Boulware DR, Daley CL, Merrifield C, et al. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect. 2007;55:300–309. doi: 10.1016/j.jinf.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 69.Getahun H, Harrington M, O’Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 70.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang L, Cattamanchi A, Davis JL, et al. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8:294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988;318:589–593. doi: 10.1056/NEJM198803103181001. [DOI] [PubMed] [Google Scholar]
- 74.Huang L, Hecht FM, Stansell JD, et al. Suspected Pneumocystis carinii pneumonia with a negative induced sputum examination. Is early bronchoscopy useful? Am J Respir Crit Care Med. 1995;151:1866–1871. doi: 10.1164/ajrccm.151.6.7767533. [DOI] [PubMed] [Google Scholar]
- 75.Golden JA, Hollander H, Stulbarg MS, et al. Bronchoalveolar lavage as the exclusive diagnostic modality for Pneumocystis carinii pneumonia. A prospective study among patients with acquired immunodeficiency syndrome. Chest. 1986;90:18–22. doi: 10.1378/chest.90.1.18. [DOI] [PubMed] [Google Scholar]
- 76.Karageorgopoulos DE, Qu JM, Korbila IP, et al. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 77.Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wheat LJ, Kohler RB, Tewari RP. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N Engl J Med. 1986;314:83–88. doi: 10.1056/NEJM198601093140205. [DOI] [PubMed] [Google Scholar]
- 79.Francesconi A, Kasai M, Petraitiene R, et al. Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J Clin Microbiol. 2006;44:2475–2480. doi: 10.1128/JCM.02693-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lieberman D, Shimoni A, Keren-Naus A, et al. Identification of respiratory viruses in adults: nasopharyngeal versus oropharyngeal sampling. J Clin Microbiol. 2009;47:3439–3443. doi: 10.1128/JCM.00886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez-Barradas MC, Stool E, Musher DM, et al. Diagnosing and treating cytomegalovirus pneumonia in patients with AIDS. Clin Infect Dis. 1996;23:76–81. doi: 10.1093/clinids/23.1.76. [DOI] [PubMed] [Google Scholar]
- 83.Miles PR, Baughman RP, Linnemann CC., Jr Cytomegalovirus in the bronchoalveolar lavage fluid of patients with AIDS. Chest. 1990;97:1072–1076. doi: 10.1378/chest.97.5.1072. [DOI] [PubMed] [Google Scholar]
- 84.Waxman AB, Goldie SJ, Brett-Smith H, et al. Cytomegalovirus as a primary pulmonary pathogen in AIDS. Chest. 1997;111:128–134. doi: 10.1378/chest.111.1.128. [DOI] [PubMed] [Google Scholar]
- 85.Meduri GU, Stover DE, Lee M, et al. Pulmonary Kaposi’s sarcoma in the acquired immune deficiency syndrome. Clinical, radiographic, and pathologic manifestations. The Am J Med. 1986;81:11–18. doi: 10.1016/0002-9343(86)90175-0. [DOI] [PubMed] [Google Scholar]
- 86.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman’s disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10:61–67. [PubMed] [Google Scholar]