Abstract
In the development of vaccines capable of providing immunity against brucellosis, Cu-Zn superoxide dismutase (SOD) has been demonstrated to be one of the protective immunogens of Brucella abortus. In an earlier study, we provided strong evidence that intramuscular injection with a plasmid DNA carrying the SOD gene (pcDNA-SOD) was able to induce a protective immune response. The present study was designed to characterize T-cell immune responses after an intraspleen (i.s.) vaccination of BALB/c mice with pcDNA-SOD. Animals vaccinated with pcDNA-SOD did not develop SOD-specific antibodies, at least until week 4 after immunization (the end of the experiment), and in vitro stimulation of their splenocytes with either recombinant Cu-Zn SOD or crude Brucella protein induced the secretion of gamma interferon (IFN-γ), but not interleukin-4, and elicited the induction of cytotoxic-T-lymphocyte activity. Upon analyzing the SOD-specific T-cell responses, the pcDNA-SOD vaccination was found to be stimulating both CD4+- and CD8+-T-cell populations. However, only the CD4+ population was able to produce IFN-γ and only the CD8+ population was able to induce cytotoxic activity. Nevertheless, although i.s. route vaccination induces a significant level of protection in BALB/c mice against challenge with the virulent B. abortus strain 2308, vaccination by the intramuscular route with a similar amount of plasmid DNA does not protect. Based on these results, we conclude that i.s. immunization with pcDNA-SOD vaccine efficiently induced a Th1 type of immune response and a protective response that could be related to IFN-γ production and cytotoxic activity against infected cells by SOD-specific CD4+ and CD8+ T cells, respectively.
Brucellosis is a zoonotic disease that is endemic in some regions of the world. In human populations, the major cause of the disease is Brucella melitensis, but several cases have also been attributed to Brucella abortus, which otherwise primarily affects bovines. Because of the economic losses to the cattle industry caused by B. abortus, as well as because of the zoonotic infections by these bacterial species (8), great efforts are being made to eradicate bovine brucellosis all over the world. In order to achieve this objective, vaccine strains of B. abortus S-19 and RB51 (19, 32) have been used with relatively good results. However, even these vaccine strains are far from ideal, since they present some disadvantages, e.g., causing reactions in humans, inducing abortion in pregnant cattle, and showing a likelihood of changing to a virulent form (33).
Brucella is an intracellular pathogen; therefore, cellular immune response is critical in generating protection against infection (42). It is well documented that gamma interferon (IFN-γ) production by CD4+ T cells is essential to the protective response; IFN-γ activates macrophages by enhancing their ability to kill bacteria (18, 20, 34, 43). It is still unknown if there is a correlation between the degree of in vitro cytotoxic-T-lymphocyte (CTL) activity and in vivo levels of protection against brucellosis. However, is expected that vaccination against Brucella should elicit a CTL response, since it is related to the development of a Th1-type immune response (16).
Several studies of murine models have been carried out to test the abilities of different proteins of B. abortus to induce a protective immune response. Bacterioferritine and the P39 protein have been reported to be T-cell immunodominant Brucella antigens (10) that induce a Th1-type immune response (2). Among the other recombinant antigens that have been tested so far, HtrA (31), GroEL, GroES, UvrA (25), and YajC (38) induced cellular and humoral immune responses in mice, but only the L7/L12 (,23, 24) and Cu-Zn superoxide dismutase (SOD) proteins (27, 35, 39) elicited some level of protection. On the other hand, DNA vaccination is a relatively novel and powerful method of immunization that induces both humoral and cellular immune responses to a wide range of pathogens in many animal models for different diseases (12). Based on the results obtained with DNA vaccines against other pathogenic intracellular bacteria, many studies are being developed using immunizations with DNA vectors that code for proteins with immunogenic properties for brucellosis. The results of these experiments show that these vaccines induce an immune response and some level of protection against challenge with a pathogenic strain of B. abortus (3, 28). Previous reports have demonstrated that intramuscular (i.m.) inoculation with a DNA vaccine that codes for SOD elicits a strong protective response (28).
The effectiveness of DNA vaccination depends on the method and site of vaccine application. Different methods of DNA delivery have been used, from simple plasmid inoculation to gene gun technology (29) or in vivo electroporation (40). The route of immunization most commonly used is i.m. injection (13, 36), but intradermal immunization (30) and oral administration (7) have also been tested with good results. Intraspleen (i.s.) inoculation with DNA vaccines has been reported to be capable of eliciting a strong proliferative response and providing a protective cellular immune response. When similar doses of DNA vaccine were injected by the i.m. and i.s. routes, the results showed that the magnitude of the response and the level of protection obtained by i.s. inoculation were far better than those induced by the i.m. route (4, 22). Based on these results, we decided to study the effects of i.s. inoculation of pcDNA-SOD on T-cell populations and to determine whether this pathway of DNA vaccination is capable of eliciting T-cell responses and providing a protective response to Brucella.
MATERIALS AND METHODS
Animals.
Seven- to 8-week-old female BALB/c mice (obtained from the Instituto de Salud Publica, Santiago, Chile) were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum.
Bacterial strains and cell line.
The virulent B. abortus strain 2308, the attenuated strain RB51, and RB51-SOD, a strain that overexpresses SOD, were obtained from our own culture collection; strains RB51 (32) and RB51-SOD (39) were originally obtained from the Virginia-Maryland Regional College of Veterinary Medicine (Virginia Polytechnic Institute and State University, Blacksburg, Va.). The bacterial cells were grown under aerobic conditions in tryptose-soy broth (Difco Laboratories, Detroit, Mich.) for 72 h at 37°C. For inoculation, the bacterial suspensions were adjusted spectrophotometrically to an optical density at 600 nm (OD600) corresponding to 104 CFU of B. abortus strain 2308. All experiments with live brucellae were performed in a biosafety level 2 facility. Escherichia coli strain DH5α (Life Technology, Gaithersburg, Md.) was used for producing the necessary plasmid constructs. The E. coli bacteria were routinely grown at 37°C in Luria-Bertani broth or in agar supplemented when required with 100 μg of ampicillin per ml. Strain RB51-SOD was cultured in the presence of 30 μg of chloramphenicol/ml. The murine macrophage cell line J774.A1 (H-2d; ATCC TIB 67) was purchased from the American Type Culture Collection (Manassas, Va). The cells were cultured in complete tissue culture medium (c-RPMI) consisting of RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (GIBCO BRL), 2 mM l-glutamine, 100 μg of streptomycin/ml, and 100 IU of penicillin/ml.
Construction of Cu-Zn SOD DNA vaccine.
The recombinant plasmid pBAII-3, containing the gene for B. abortus Cu-Zn SOD (sodC), along with its own promoter, was initially obtained from a pUC9 genomic library of B. abortus strain 2308 (27). A 1.1-kb fragment containing the sodC gene and its promoter sequences was excised from the insert of pBAII-3 by EcoRI and XhoI restriction enzyme digestion and ligated into the expression vector pcDNA3 downstream of the cytomegalovirus promoter (Invitrogen, San Diego, Calif.). The resulting plasmid was designated pcDNA-SOD. A colony of E. coli containing pcDNA-SOD was cultured in Luria-Bertani broth containing 100 μg of ampicillin/ml. Large-scale plasmid DNA isolation was performed using an EndoFree Plasmid Giga kit (Qiagen, Valencia, Calif.) according to the manufacturer's directions. The DNA was finally resuspended in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml. The DNA concentration and purity were determined by the OD, and the A260/A280 ratio was typically >1.8. The pcDNA-SOD plasmid construct was verified by restriction digestion and by sequencing of the complete insert at the Universidad de Concepción Sequencing Facility.
Immunization.
Mice premedicated with atropine (16 μg/mouse; Biosano, Santiago, Chile) were anesthetized with ketamine (2 μg/mouse; Rhodia Merieux, Santiago, Chile), and their abdominal regions were shaved. After a 3-mm-long incision was made through the skin, the mice were injected in the spleen with 10 μg of plasmid DNA in 30 μl of PBS by using an insulin syringe with a 28-gauge needle. For i.m. immunization, mice were injected in the right tibialis anterior muscle with 10 μg of plasmid DNA diluted in 50 μl of PBS (28). The mice were vaccinated once with pcDNA-SOD construct or with pcDNA3 alone as a negative control. For protection assays, an additional group was inoculated with PBS as a negative control.
Splenocyte cultures and lymphocyte proliferation.
Four weeks after immunization, the mice were sacrificed and their spleens were removed under aseptic conditions. Single-cell suspensions were prepared from the spleens according to a standard procedure (27), and erythrocytes were eliminated with ACK lysis solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]). Splenocytes were cultured at 37°C with 5% CO2 in a 96-well flat-bottom plate at a concentration of 4 × 105 viable cells/well for total T-cell populations and at a concentration of 5 × 104 cells/well for T-cell populations separated by flow cytometry in the presence of 0.4 μg of crude B. abortus RB51 proteins (CBPs) per well, an extract obtained from bacteria submitted to hypertonic salt solution (1 M NaCl with 0.1 M sodium citrate) and sonication (26); 0.04 μg of purified recombinant Cu-Zn SOD (rSOD) per well, obtained by a chromatography affinity procedure (26); 0.25 μg of concanavalin A (ConA) per well; or no additives (unstimulated control). Separated T cells were also cultivated in the presence of mitomycin C-treated (50 μg/ml) splenocytes from syngeneic mice as feeder cells. Splenocytes and separated T cells were cultured in c-RPMI medium for 3 days and pulsed for 8 h with 0.4 μCi of thymidine (40 Ci/mmol; Amersham, London, United Kingdom) per well, and the radioactivity incorporated in the DNA was measured in a liquid scintillation counter. Cell proliferation was expressed as mean counts per minute from triplicate cultures obtained from a cell pool of each experimental group (five mice per group).
Cytotoxicity assay.
The cytotoxicity assay was carried out as described by He et al. (16). Briefly, stimulator cells were prepared by infecting J774.A1 macrophages at confluent growth with live B. abortus strain RB51-SOD at a ratio of 1:100 (cells to RB51-SOD) for 5 h. Extracellular bacteria were rinsed away with c-RPMI containing 50 μg of gentamicin per ml. Macrophages were scraped off with a sterile rubber policeman and centrifuged at 200 × g for 5 min. The pulsed macrophages were suspended in 5 ml of c-RPMI with 35 μg of mitomycin C per ml in a 37°C water bath for 45 min and then washed by centrifugation with RPMI supplemented with 5% heat-inactivated fetal bovine serum.
The immunized mice were killed by cervical dislocation 4 weeks after DNA inoculation. The spleens were removed, and erythrocytes were eliminated with ACK lysis solution. Adherent cells were removed by adherence to plastic, and then the enriched T cells were distributed to wells of 24-well cell culture plates (Corning, Corning, N.Y.) at a concentration of 4 × 106 viable cells/well. Stimulator cells were also added to the wells at a concentration of 0.4 × 106/well, and the mix of enriched T cells and stimulator cells was then incubated for 5 days at 37°C with 5% CO2. After 5 days of incubation, the live effector cells were obtained by removing dead cells with Histopaque (density, 1.083 g/ml). Effectors and target cells (B. abortus RB51-infected J774.A1 macrophages which were not treated with mitomycin C) were mixed at different ratios and incubated for 16 h at 37°C; 200 μl of 0.036% neutral red solution in PBS was added to stain unlysed target cells. After 30 min, the cells were washed and then lysed with 0.22 ml of 0.05 M acetic acid- 0.05% sodium dodecyl sulfate solution. The amount of dye released was measured by taking OD readings at 570 nm. As a control for nonlysis and maximal uptake of neutral red stain, target cells were cultured alone without effector cells. The percentage of specific lysis was established by applying the following formula: specific lysis = (OD of control − OD of experimental group)/OD of control × 100.
Cell sorting.
Splenocytes derived from pcDNA-SOD- or pcDNA3-inoculated mice were sorted by flow cytometry or by immunomagnetic methods to separate the CD4+- and CD8+-T-cell populations. For lymphocyte proliferation assays, 107 spleen cells treated as described above were stained with 1 μg of fluorescein isothiocyanate-conjugated anti-CD4 (clone RM4-5; isotype, rat immunoglobulin G2a [IgG2a], κ) or phycoerythrin-conjugated anti-CD8 (clone 53-6.7; isotype, rat IgG2a, κ) monoclonal antibody (BD Biosciences) per 106 cells on ice in the dark for 30 min. The cells were separated by a Becton Dickinson flow cytometer (Hospital del Trabajador, Concepción, Chile) with Cell Quest version 3.3 software (Becton Dickinson). The positive CD4+ or CD8+ T cells were used for lymphocyte proliferation assays. The purities of the selected CD4+- or CD8+-T-cell populations were >92% as determined by flow cytometry.
To obtain pure T-cell populations for cytotoxic assays and detection of cytokines in cultures, T cells were purified by immunomagnetic methods. Single-cell suspensions from spleens of inoculated mice, obtained as described above, were cocultivated with J744.A1 macrophages infected with B. abortus strain RB51-SOD. After 5 days of culture, the cells were removed from the plates and live T cells were recovered by Histopaque (1083) column purification. The T cells were stained with monoclonal antibodies against the CD4 (clone GK1.5; isotype, rat IgG2b) or CD8 (clone 53-6.7; isotype, rat IgG2a) molecule coupled with microbeads (Miltenyi Biotec, Auburn, Calif.) at a concentration of 10 μl of microbeads per 107 total cells for 15 min at 4°C. The cells were then washed with PBS supplemented with 0.5% bovine serum albumin. Following passage of the cells through an MS column in a magnetic field (VarioMACS separator system; Miltenyi Biotec), the selected CD4+ or CD8+ T cells were eluted out. The purities of the selected CD4+- or CD8+-T-cell populations were >90% as determined by flow cytometry or staining with 1 μg of fluorescein isothiocyanate-conjugated anti-CD3 (clone 145-2C11; isotype, Armenian hamster IgG, κ) per 106 cells and 1 μg of phycoerythrin-conjugated anti-CD4 (clone GK1.5; isotype, rat IgG2b, κ) or anti-CD8 (clone 53-6.7; isotype, rat IgG2a, κ) monoclonal antibody (BD Biosciences) per 106 cells on ice in the dark for 30 min.
Detection of cytokines.
Cytokines were quantified by antigen capture enzyme-linked immunosorbent assay (ELISA) using OptEIA set mouse IFN-γ and interleukin 4 (IL-4) (BD Biosciences) from 48-h T-cell culture supernatants or from culture supernatants of stimulated T-cell populations cocultured with infected J744.A1 macrophages. All assays were performed in triplicate. The concentrations of IFN-γ and IL-4 in the culture supernatants were calculated by using a linear regression equation obtained from the absorbance values of the standards.
Protection experiments.
Protection experiments were performed as described previously (26). Briefly, 4 weeks after vaccination, six mice from each group were challenged by intraperitoneal injection of 104 CFU of B. abortus 2308. Two weeks later, the infected mice were sacrificed, their spleens were homogenized, and dilutions were plated to determine the number of Brucella CFU per spleen. Log10 units of protection were obtained by subtracting the mean log10 CFU for the experimental group from the mean log10 CFU of the corresponding control group.
Statistical analysis.
The data for lymphocyte proliferation, detection of cytokines, and protection experiments were analyzed by Student's paired t test. The data for CTL lysis were subjected to analysis of variance, and the means were compared by using Tukey's honest significant difference procedure (SAS system for mixed models; SAS Institute Inc., Cary, N.C.).
RESULTS
Immune response of mice vaccinated i.s. with pcDNA-SOD.
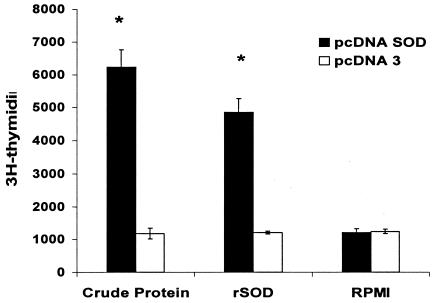
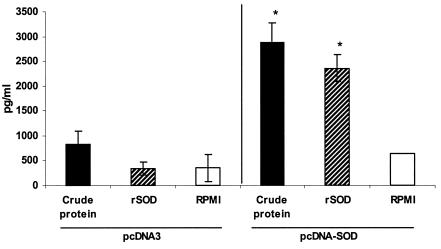
To examine the cell-mediated immunity response to Brucella rSOD protein and CBPs, the proliferative response and cytokine profile of spleen cells from mice immunized i.s. with pcDNA-SOD and pcDNA3 were determined. As demonstrated in Fig. 1, splenocytes from mice immunized with pcDNA-SOD had an increased and significant proliferative response to rSOD and CBPs (P < 0.005) 4 weeks after immunization. Only low levels of spontaneous proliferation occurred in cultures without antigen (medium control). The splenocytes from the two experimental groups had very similar proliferative responses to the mitogen ConA throughout the study (data not shown). With respect to cytokine profiles, at week 4 after immunization, the supernatants from cultures of spleen cells from pcDNA-SOD-vaccinated mice stimulated with rSOD protein or CBPs contained a significantly greater quantity of IFN-γ (P < 0.07 in both groups) than those from pcDNA3-vaccinated mice (Fig. 2). In addition, no IL-4 was detected in any of the culture supernatants of splenocytes stimulated with specific antigens (data not shown). Splenocytes from all groups of mice produced similar levels of IFN-γ and IL-4 upon stimulation with ConA (data not shown). Specific anti-SOD antibody was not detected by ELISA in sera from mice immunized with pcDNA-SOD or pcDNA3 (data not shown). Taken together, our results indicate that i.s. immunization with pcDNA-SOD induces a specific Th1-type immune response in mice.
FIG. 1.
Lymphocyte proliferation assay. BALB/c mice were immunized with pcDNA-SOD and the parental plasmid pcDNA3. The T-cell proliferative response was measured at week 4 after immunization. Splenocytes from each group (4 × 105 per well) were prepared and stimulated in vitro with CBPs (4 μg/ml) and purified rSOD (0.4 μg/ml) as antigens. Each value is the average number of counts per minute of triplicate cultures of cells (± standard deviation) obtained from a pool of five mice in each group. *, P < 0.005 compared with the value for pcDNA3-immunized control mice.
FIG. 2.
Quantitative ELISA analysis of IFN-γ secreted by lymphocytes upon stimulation with different antigens. Spleen cells (4 × 106/ml) from mice inoculated with pcDNA3 or pcDNA-SOD were stimulated with CBPs (4 μg/ml), rSOD (0.4 μg/ml), or RPMI 1640 (negative control) for 48 h. Each bar represents the geometric mean ± standard deviation (error bars) of the responses in spleen cells from five individual mice. *, statistically significant differences compared to RPMI 1640 (P ≤ 0.07).
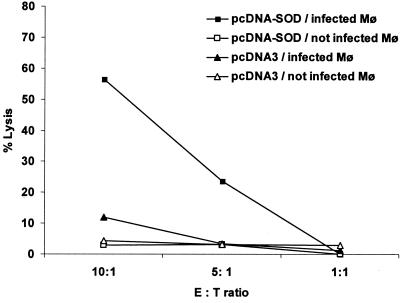
CTL response in mice vaccinated i.s. with pcDNA-SOD.
Antigen-specific CTLs can be evaluated by quantifying their capacity to lyse Brucella-infected macrophages. As depicted in Fig. 3, purified T cells derived from mice inoculated i.s. with pcDNA-SOD specifically lysed strain RB51-SOD-infected J774.A1 macrophages. The lysis level reached ∼60% when the effector/target ratio was 10:1, 4 weeks after immunization. The effector T cells derived from pcDNA3-injected mice produce very low lysis levels (∼10%), suggesting that the cytotoxic-T-cell activity was specific and was induced by pcDNA-SOD vaccination of the mice.
FIG. 3.
Specific cytotoxic activity of total T cells. Four weeks after immunization, splenocytes from mice immunized with pcDNA-SOD or the parental plasmid pcDNA3 were stimulated for 5 days with J774.A1 macrophages (Mφ) infected with B. abortus RB51-SOD at a ratio of 1:100 (cells to RB51-SOD). These effector cells were incubated with J774.A1 cells alone or J744.A1 cells infected with RB51-SOD. A neutral red uptake assay was used to measure target lysis. The data are means for triplicate estimations, and standard deviations did not exceed 20% of the means. E, effector; T, target.
Protection efficacy of i.s. SOD gene immunization.
To evaluate the protective capacity of i.s. and i.m. DNA vaccination, 4 weeks after vaccination, mice were challenged by intraperitoneal injection of virulent B. abortus 2308 and were sacrificed 14 days later to determine the number of CFU in their spleens. The results indicated that i.s. immunization with pcDNA-SOD induced a significant degree of protection (a 1.52-log-unit increase in protection) compared to the control group (P < 0.008). The mice that were i.m. immunized with pcDNA-SOD showed protection, but it was not significant (P < 0.2) (Table 1). No significant difference was seen between the numbers of CFU in groups injected with pcDNA3 (control group) and PBS (data not shown). These results indicate that pcDNA-SOD i.s. vaccination provided a significant degree of protection against Brucella infection.
TABLE 1.
Protection of mice against challenge with B. abortus 2308 after immunization with DNA vaccine coding for Cu-Zn SODa
| Vaccine injection route | Vaccine (dose [μg]) | Log10 CFU of B. abortus 2308 in spleen (mean ± SD) | Log10 units of protection |
|---|---|---|---|
| I.s. | pcDNA-SOD (10) | 4.57 ± 0.94b | 1.52 |
| I.s. | pcDNA3 (10) | 6.09 ± 0.04 | |
| I.m. | pcDNA-SOD (10) | 4.91 ± 1.50c | 1.15 |
| I.m. | pcDNA3 (10) | 6.06 ± 0.26 |
Mice were challenged intraperitoneally with 104 CFU of strain 2308 2 weeks prior to sacrifice.
P < 0.008 (significant) compared with value for control pcDNA3-treated mice.
P < 0.2 compared to the control group.
CD4+- and CD8+-T-cell immune responses to i.s. DNA vaccination.
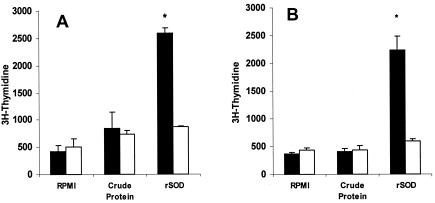
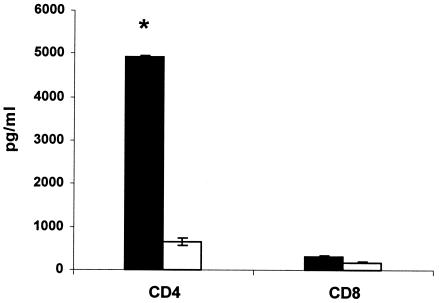
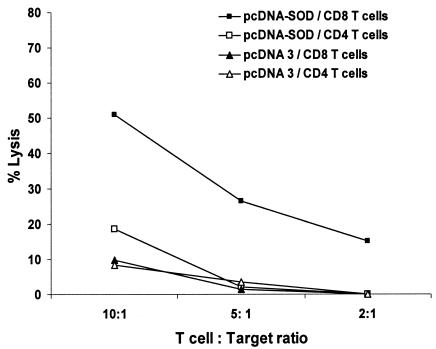
From the results reported above, we observed that i.s. pcDNA-SOD vaccination was able to induce T-cell-proliferative response, IFN-γ secretion by spleen cells, cytolytic-T-cell activity, and protection. To evaluate the roles of the CD4+ and CD8+ lymphocytes in this specific response, 4 weeks after DNA immunization, single-cell suspensions were obtained from spleens of vaccinated mice and the cells were sorted by flow cytometry. To detect a proliferative response, the separated CD4+ and CD8+ populations were challenged with either CBPs or rSOD for 3 days in cRPMI. As shown in Fig. 4, there was a significant proliferative response against rSOD protein by both cell subsets (P < 0.02 and P < 0.05, respectively), but no significant proliferation was observed when the cells were stimulated with CBP (Fig. 4). Low levels of spontaneous proliferation occurred in cultures without antigen (medium control). The T-cell subsets from both groups had very similar proliferative responses to the mitogen ConA throughout the study (data not shown). With respect to the cytokine profile, IFN-γ and IL-4 were measured in supernatants after 48 h of coculture of T-cell subsets with target cells. As shown in Fig. 5, significant IFN-γ production was detected only in CD4+ T cells (P < 0.005); meanwhile, no significant differences were found between CD8+ T cells derived from experimental and control mice. No IL-4 was detected in any of the culture supernatants of T-cell populations costimulated with target cells (data not shown). In order to determine the contributions of specific T-cell populations to the observed CTL activity, T-cell populations were sorted after 5 days of coculture of enriched T cells from spleens with B. abortus-infected J774A.1 macrophages by immunomagnetic methods. The separated CD4+- or CD8+-T-cell populations were cocultivated with target cells for 16 h, and the cytotoxic activity over target cells was measured by neutral red cytotoxic assay. The CD8+ T cells sorted from mice immunized with pcDNA-SOD achieved high specific levels of lytic ability against strain RB51-SOD-pulsed macrophages compared to the pcDNA3-immunized control group. On the other hand, when the CD4+ T cells from mice immunized with pcDNA-SOD were cocultured with strain RB51-SOD-pulsed target cells, they showed lytic activity, but it was not significantly different from that of the control group (Fig. 6).
FIG. 4.
Lymphocyte proliferation assay for T-cell populations. BALB/c mice were immunized with pcDNA-SOD (solid bars) or the parental plasmid pcDNA3 (open bars). At week 4 after immunization, splenocytes from each mouse were separated by flow cytometry into CD4+ (A) and CD8+ (B) T cells and stimulated in vitro with CBPs (4 μg/ml) and purified rSOD (0.4 μg/ml) as antigens. Each value is the average number of counts per minute of triplicate cultures of cells plus standard deviation (error bars) obtained from a pool of five mice in each group. *, P ≤ 0.05 compared with the value for c-RPMI medium.
FIG. 5.
IFN-γ release in CD4+- and CD8+-T-cell cultures exposed to target cells. Four weeks after immunization, splenocytes from immunized mice were collected and cocultivated with stimulator cells for 5 days as described in Material and Methods. T cells were recovered and sorted into CD4+ and CD8+ T cells. The separated cells were cultivated with J744.A1 cells infected with RB51-SOD (target cells), and IFN-γ release was measured in the supernatants after 48 h. The solid bars represent pcDNA-SOD-immunized mice, and the open bars represent pcDNA3-immunized mice. *, statistically significant difference compared to CD4+ T cells obtained from mice immunized with pcDNA3 (P < 0.005). The error bars indicate standard deviations.
FIG. 6.
Specific cytotoxic activity of CD4+ or CD8+ T cells. Four weeks after immunization, splenocytes from mice immunized with pcDNA-SOD or the parental plasmid pcDNA3 were stimulated for 5 days with J774.A1 macrophages infected with B. abortus RB51-SOD. These effector cells were recovered and sorted into CD4+ and CD8+ T cells. The separated T-cell subsets were incubated with target cells (J774.A1 cells infected with RB51-SOD), and cytotoxic activity was measured 16 h later by neutral red assay. The data are means for triplicate estimations, and standard deviations did not exceed 20% of the means.
DISCUSSION
DNA vaccines have become one of the most successful methods of vaccination since the discovery that direct in vitro or in vivo gene transfer of recombinant DNA by a variety of techniques resulted in the expression of protein in mammalian cells (12, 41) and that antigen production by these means resulted in an enhanced immune response capable of providing protection against challenge from a variety of infections (12, 36). It is well documented that different methods of DNA or antigen inoculation influence immune response development. The i.m. pathway is the most common route of DNA administration. In DNA vaccination against brucellosis, injection of vector encoding either P39 (3) or SOD (28) by this route elicits protection against pathogen infection. Recently, it has been reported for other pathogen models that direct administration of DNA into secondary lymphoid organs, such as the spleen or lymph nodes, induces a higher protective immune response than the usual i.m. or intradermal DNA inoculation pathway (22). In this study, we showed that i.s. injection of a DNA vector containing the DNA insert of Brucella SOD (28) was able to generate a protective immune response. I.m. administration of this vector in the same doses and under the same conditions as the i.s. inoculation did not lead to a significant protective immune response (28). Therefore, this difference in protection might be attributed to the amount of vector and/or the number of doses. These results demonstrated a higher efficacy of the i.s. route of administration, and they are in agreement with the results obtained by Cano et al. (4) and Maloy et al. (22), who reported that the use of direct administration to secondary lymphoid organs enhances the immune response.
The mechanism by which an immune response is induced after DNA vaccination has been widely discussed (15). Recently, it has been proposed that dendritic cells, professional antigen-presenting cells, play a major role in the initiation of an immune response in DNA vaccination by efficiently priming T cells with exogenous or endogenous antigens (1, 6, 9, 11, 17, 29). It seems that the higher percentage of transfected dendritic cells in direct immunization of secondary lymphoid organs is related to the higher efficiency of the i.s. pathway of inoculation.
After concluding that i.s. inoculation with DNA is able to provide protection against challenge, we wanted to investigate the effect of this inoculation on the behavior of T-cell subpopulations. It is well documented that cellular immune response plays a major role in the establishment of a protective response against Brucella (18, 20, 34, 43), and for that reason the design of a preventive vaccine against brucellosis must be based on its capacity to generate a strong Th1-type immune response, with high levels of IFN-γ and T-cytotoxic activity involved in the immune process. Previous reports of i.m. pcDNA-SOD administration have demonstrated that inoculation with this vector elicits cellular immune response, as characterized by high levels of IFN-γ production by T cells and high production of IgG2a in immunized animals (28). Our present results indicate that in our i.s. inoculation model pcDNA-SOD inoculation was also able to induce a strong TH-1-type response, with high levels of IFN-γ and no detected levels of IL-4 in T-cell cultures from vaccinated mice. After separating the T cells into CD4+ and CD8+ T cells, we noticed that IFN-γ secretion was observed only in CD4+-T-cell cultures and not in CD8+-T-cell cultures. This finding is rather surprising, since there is general agreement that in other experimental situations CD8+ T cells produce a significant amount of IFN-γ; moreover, the great majority of cytolitic-CD8+-T-cell clones produce this cytokine (14, 21). However, a similar finding was reported by He et al. (16) using a different experimental model. The fact that no CD8 production was shown in our case might be explained by the work of Van Pinxteren et al. (37) and Caruso et al. (5), in which the authors demonstrated that CD4+ T cells readily produced IFN-γ after infection with Mycobacterium tuberculosis while CD8+ T cells secreted the cytokine later in the response. It is possible that in our model system also, CD8+ T cells may secrete IFN-γ when tested at a later time. However, our results demonstrate that 4 weeks after vaccination, CD8+ T cells possessed significant levels of specific lytic activity against Brucella-infected cells. This cytotoxic response was not previously studied for i.m. inoculation of this vector expressing SOD. Probably, the types of response generated by spleen and muscle inoculations do not differ, since our previous results with i.m. injection also showed a type of immunity similar to the results obtained in this experiment. The protective response elicited by pcDNA-SOD inoculation could be related to IFN-γ production by CD4+ T cells and to the cytolytic activity of CD8+ T cells, but further studies are needed to asses these issues. Our failure to detect any antibody in serum suggests that antibody to SOD does not play a significant role in protective immunity against brucellosis.
In conclusion, the present study has shown that i.s. inoculation with pcDNA-SOD elicits a strong cellular protective immune response, stimulating both Th cells and CTLs. Th cells from immunized mice produced high levels of IFN-γ, which have been reported to be essential for protective immunity against Brucella, and CD8+ T cells exhibited a strong specific cytotoxic activity. Although the i.s. route per se is unattractive for human and veterinary clinical use, it represents another possibility in the exploration of alternative techniques to deliver plasmid DNA to antigen-presenting cells of the spleen.
Acknowledgments
This work was supported by grant 1010851 from the Fondo Nacional de Investigación Científica y Tecnologica (FONDECYT), Santiago, Chile.
We are grateful to Ramesh Vemulapalli, Purdue University, for comments on the manuscript.
Editor: J. B. Bliska
REFERENCES
- 1.Akbari, O., N. Panjwani, S. Garcia, R. Tascon, D. Lowrie, and B. Stockinger. 1999. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J. Exp. Med. 189:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godefroid, K. Walravens, and J. Letesson. 2001. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun. 69:4816-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bolle, P. Michel, J. Godfroid, K. Walravens, and J. J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cano, A., G. Fragoso, G. Gevorkian, L. I. Terrazas, P. Petrossian, T. Govezensky, E. Sciutto, and K. Manoutcharian. 2001. Intraspleen DNA inoculation elicits a protective cellular immune response. DNA Cell Biol. 20:215-221. [DOI] [PubMed] [Google Scholar]
- 5.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 6.Casares, S., K. Inaba, T. D. Brumeanu, R. M. Steinman, and C. A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S. C., D. H. Jones, E. F. Fynan, G. H. Farrar, J. C. S. Clegg, H. B. Greenberg, and J. E. Herrmann. 1998. Protective immunity induced by oral immunization with rotavirus DNA vaccine encapsulated in microparticles. J. Virol. 72:5757-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corr, M., D. J. Lee, D. A. Carson, and H. Tighe. 1996. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J. Exp. Med. 184:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denoël, P. A., T. K. Vo, V. E. Weynants, A. Tibor, D. Gilson, M. S. Zygmunt, J. N. Limet, and J. J. Letesson. 1997. Identification of the major T-cell antigens present in the Brucella melitensis B115 protein preparation, Brucellergene OCB. J. Med. Microbiol. 46:801-806. [DOI] [PubMed] [Google Scholar]
- 11.Doe, B., M. Selby, S. Barnett, J. Baenziger, and C. M. Walker. 1996. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc. Natl. Acad. Sci. USA 93:8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis, M., K. Denis-Mize, C. Woo, C. Goldbeck, M. J. Selby, M. Chen, G. R. Otten, J. B. Ulmer, J. J. Donnelly, G. Ott, and D. M. McDonald. 2000. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 165:2850-2858. [DOI] [PubMed] [Google Scholar]
- 14.Fong, T. A., and T. R. Mosmann. 1990. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J. Immunol. 144:1744-1752. [PubMed] [Google Scholar]
- 15.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 16.He, J., R. Vemulapalli, A. Zeytun, and G. G. Schurig. 2001. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69:5502-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki, A., C. A. Torres, P. S. Ohashi, H. L. Robinson, and B. H. Barber. 1997. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 159:11-14. [PubMed] [Google Scholar]
- 18.Jiang, X., and C. L. Baldwin. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61:124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez de Bagues, M. P., P. H. Elzer, S. M. Jones, J. M. Blasco, F. M. Enright, G. G. Schurig, and A. J. Winter. 1994. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect. Immun. 62:4990-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, S., and A. J. Winter. 1992. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect. Immun. 60:3011-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelso, A., and A. L. Glasebrook. 1984. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J. Immunol. 132:2924-2931. [PubMed] [Google Scholar]
- 22.Maloy, K. J., I. Erdmann, V. Basch, S. Sierro, T. A. Kramps, R. M. Zinkernagel, S. Oehen, and T. M. Kündig. 2001. Intralymphatic immunization enhances DNA vaccination. Proc. Natl. Acad. Sci. USA 98:3299-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira, S. C., A. N. D. Zhu, and G. A. Splitter. 1994. Recombinant L7/L12 ribosomal protein and gamma-irradiated Brucella abortus induce a T-helper 1 subset response from murine CD4+ T cells. Immunology 83:659-666. [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira. S. C., J. S. Harms, M. Banai, and G. A. Splitter. 1996. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cell. Immunol. 172:262-268. [DOI] [PubMed] [Google Scholar]
- 26.Oñate, A., E. Andrews, A. Beltran, G. Eller, G. Schurig, and H. Folch. 2000. Frequent exposure of mice to crude Brucella abortus proteins down-regulates immune response. J. Vet. Med. B 47:677-682. [DOI] [PubMed] [Google Scholar]
- 27.Oñate, A. A., R. Vemulapalli, E. Andrews, G. G. Schuring, S. Boyle, and H. Folch. 1999. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect. Immun. 67:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oñate, A. A., S. Cespedes, A. Cabrera, R. Rivers, A. Gonzalez C. Munoz, H. Folch, and E. Andrews. 2003. A DNA vaccine encoding Cu-Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porgador, A., K. R. Irvine, A. Iwasaki, B. H. Barber, N. P. Restifo, and R. N. Germain. 1998. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 188:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raz, E., D. A. Carson, S. E. Parker, T. B. Parr, A. M. Abai, G. Aichinger, S. H. Gromkowski, M. Singh, D. Lew, and M. A. Yankauckas. 1994. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to virus. Proc. Natl. Acad. Sci. USA 91:9519-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roop, R. M., T. W. Fletcher, N. M. Sriranganathan, S. M. Boyle, and G. G. Schurig. 1994. Identification of an immunoreactive Brucella abortus HtrA stress response protein homolog. Infect. Immun. 62:1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schurig, G. G., R. M. Roop II, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 33.Smith, L. D., and T. A. Ficht. 1990. Pathogenesis of Brucella. Crit. Rev. Microbiol. 17:209-230. [DOI] [PubMed] [Google Scholar]
- 34.Stevens, M. G., G. W. Pugh, Jr., and L. B. Tabatabai. 1992. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect. Immun. 60:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabatabai, L. B., and G. W. Pugh, Jr. 1994. Modulation of immune response in BALB/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919-924. [DOI] [PubMed] [Google Scholar]
- 36.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, D. M. De Witt, A. Friedman, L. A. Hawe, K. R. Leander, D. Martínez, H. C. Perry, J. W. Shiver, D. C. Montgomery, and M. A. Liu. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1749. [DOI] [PubMed] [Google Scholar]
- 37.Van Pinxteren, L. A. H., J. P. Cassidy, B. H. C. Smedegaard, E. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]
- 38.Vemulapalli, R., S. Cravero, C. L. Calvert, T. E. Toth, N. Sriranganathan, S. M. Boyle, O. L. Rossetti, and G. G. Schurig. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab. Immunol. 7:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vemulapalli, R., Y. He, S. Cravero, N. Sriranganathan, S. M. Boyle, and G. G. Schurig. 2000. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun. 68:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 41.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 42.Zhan, Y., J. Yang, and C. Cheers. 1993. Cytokine response of T-cell subsets from Brucella abortus-infected mice to soluble Brucella proteins. Infect. Immun. 61:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]