Abstract
The animal pathogen Brucella abortus contains a gene cgt, which complemented Sinorhizobium meliloti nodule development (ndvA) and Agrobacterium tumefaciens chromosomal virulence (chvA) mutants. Complemented strains recovered the presence of anionic cyclic β-1,2-glucan, motility, tumor induction in A. tumefaciens, and nodule occupancy in S. meliloti, all traits strictly associated with the presence of cyclic β-1,2-glucan in the periplasm. Nucleotide sequencing revealed that B. abortus cgt contains a 1,797-bp open reading frame coding for a predicted membrane protein of 599 amino acids (65.9 kDa) that is 58.5 and 59.9% identical to S. meliloti NdvA and A. tumefaciens ChvA, respectively. Additionally, B. abortus cgt, like S. meliloti ndvA and A. tumefaciens chvA possesses ATP-binding motifs and the ABC signature domain features of a typical ABC transporter. Characterization of Cgt was carried out by the construction of null mutants in B. abortus 2308 and S19 backgrounds. Both mutants do not transport cyclic β-1,2-glucan to the periplasm, as shown by the absence of anionic cyclic glucan, and they display reduced virulence in mice and defective intracellular multiplication in HeLa cells. These results suggest that cyclic β-1,2-glucan must be transported into the periplasmatic space to exert its action as a virulence factor.
Brucella spp. are facultative intracellular gram-negative bacteria pathogenic for a variety of mammalian species including humans. They cause a chronic infectious disease known as brucellosis, a major zoonosis in several countries (12). Six Brucella spp. with different host specificities have been described previously (19, 46). Brucella abortus is the etiological agent of bovine brucellosis and can also infect humans, causing undulant fever.
Brucella, Agrobacterium, and Rhizobium belong, according to 16S rRNA sequences, to the α-2 subgroup of the Proteobacteria (28). Complete-genome sequencing reveals similarities in transport, metabolic capabilities, and genome structure with these soil- and plant-associated bacteria. Extensive gene synteny between chromosome 1 and the genome of the plant symbiont Mesorhizobium loti emphasizes the similarity between this animal pathogen with plant pathogens and symbionts. A limited repertoire of genes homologous to known bacterial virulence factors were identified (13, 31, 44). Several studies revealed common themes between animal and plant pathogens and endosymbionts. For example the Brucella two-component regulatory system BvrS-BvrR (39) is highly similar to the two-component regulatory systems ChvG-ChvI of Agrobacterium tumefaciens (9) and ExoS-ChvI of Sinorhizobium meliloti (10). These two-component regulatory systems are equivalent to the Salmonella PhoP-PhoQ (40) and the Bordetella bronchiseptica BvgA-BvgS systems (42). These two-component sensory systems are involved in controlling virulence or, in the case of Rhizobium, nodule invasion; in B. abortus bvrS-bvrR, mutants also display reduced invasiveness and virulence (32, 39).
The Brucella virB operon was recently identified (29, 38); it is highly homologous to the A. tumefaciens virB operon. The B. abortus virB10 mutant lost the ability to multiply in HeLa cells and was not recovered from the spleens of infected BALB/c mice (38). The same results were obtained with a Brucella suis virB10 mutant (29), thus demonstrating that in Brucella, as in Agrobacterium, the virB operon is involved in virulence.
A highly conserved B. abortus homologue of the S. meliloti bacA gene, which encodes a putative cytoplasmic membrane transport protein required for symbiosis, was identified (26). The B. abortus bacA mutant shows decreased survival in macrophages and reduced virulence in BALB/c mice (26).
Brucella, like Agrobacterium and Rhizobium, produces cyclic β-1,2-glucans (45). chvB in A. tumefaciens and ndvB in S. meliloti are the genes coding for the cyclic β-1,2-glucan synthetase (cgs) (50). The biosynthesis of cyclic β-1,2-glucan proceeds in Brucella by the same mechanism as in Rhizobium and Agrobacterium (7). Cgs acts as an intermediate during the synthesis of the cyclic β-1,2-glucan (21). Cyclic glucan is required for effective nodule invasion and symbiotic nitrogen fixation in S. meliloti and for crown gall tumor induction in A. tumefaciens (4). Agrobacterium cyclic β-1,2-glucan mutants have several altered cell surface properties, including loss of motility due to a defective assembly of flagella and increased sensitivity to certain antibiotics and detergents (4). B. abortus cgs codes for the cyclic β-1,2-glucan synthetase (21). B. abortus cgs mutants showed reduced survival in BALB/c mouse spleen tissues and impeded intracellular multiplication, indicating that, as in Rhizobium and Agrobacterium, cyclic glucan is required for effective host interaction (6). Moreover, Agrobacterium or Rhizobium cyclic β-1,2-glucan mutants can be complemented by the Brucella cgs gene, indicating that their functions are highly conserved (6, 21).
The presence of cyclic β-1,2-glucan in the periplasmic space is also required for effective S. meliloti nodule invasion (17, 41) and A. tumefaciens crown gall tumor induction (15, 22). Two chromosomal homologue loci, ndvA in S. meliloti and chvA in A. tumefaciens, code for cyclic β-1,2-glucan transport genes (cgt). ndvA and chvA genes are interchangeable, and mutations in one gene can be complemented by the other, indicating that their functions are highly conserved (15). S. meliloti NdvA and A. tumefaciens ChvA are membrane proteins with homology to bacterial ATP-binding transporters of the ABC transporter superfamily (22, 41). ABC transporters utilize the energy of ATP hydrolysis to transport a wide variety of molecules across cellular membranes. These molecular pumps are found in all phyla and form a large protein family (37). The amino acid sequence of the ATPase domain contains the characteristic Walker A and B motifs involved in ATP binding (48). However, the intervening sequence between these two motifs is usually longer than in other ATP-binding enzymes, and the unique signature motif LSGGQ absent in other ATPases is always present.
Once cyclic β-1,2-glucan is transported into the periplasm, a variety of nonglycosidic substituents (glycerol phosphate, succinate, and/or methyl malonate) are added, leading to the accumulation of periplasmic anionic cyclic β-1,2-glucans (5, 8, 22). S. meliloti ndvA and A. tumefaciens chvA mutants have less than 15% of the wild-type levels of anionic periplasmic cyclic β-1,2-glucan (41). The arrangement of ndvA and ndvB in the S. meliloti chromosome is similar to that of chvA and chvB in A. tumefaciens, with the two loci being adjacent to each other and transcribed in a convergent fashion (15, 17).
In this report we describe the isolation of the B. abortus cyclic β-1,2-glucan transporter gene (cgt). B. abortus cgt complemented the phenotypes associated with A. tumefaciens chvA and S. meliloti ndvA mutations. On the other hand, B. abortus cgt mutants do not accumulate anionic cyclic β-1,2-glucan and have reduced virulence in mice and defective intracellular multiplication in HeLa and J774 cells. These results suggest that, as in Agrobacterium and Rhizobium, B. abortus cyclic β-1,2-glucan must be transported into the periplasm to exert its action.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. A. tumefaciens, Escherichia coli, and S. meliloti strains were grown on tryptone-yeast extract (50), Luria broth (35), and yeast extract-mannitol medium (23), respectively. B. abortus strains were grown in brucella broth (BB) (Difco Laboratories, Detroit, Mich.). If necessary, media were supplemented with the appropriate antibiotics at the following concentrations: carbenicillin, 100 μg/ml; kanamycin, 50 μg/ml; ampicillin, 100 μg/ml. Merodiploid strains for complementation analysis were obtained by tri- and biparental mating as described previously (14). Motility assays were carried out in GYM medium (16) (0.35% agar) for 3 days at 28°C. The absence of smooth-to-rough dissociation was checked by testing the sensitivity of smooth-specific phages (Tb, Wb, and Iz) (1)
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Phenotype or genotypea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | F′φ80dlaczΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK+ mK+) phoA supF44 λ−thi-1 gyrA96 relA1/F′ proAB+ LacIqZΔM15 zzf:: Tn5(Kmr) | 49 |
| K-12 | ||
| DH5αF′IQ | ||
| S. meliloti | ||
| 102 F34 | Wild type | 14 |
| LI1 | ndvA, deficient in the secretion and modification of cyclic β-1,2-glucan, Kmr | 41 |
| LI1(PBB4cgt) | ndvA with plasmid PBB4cgt; Kmr Ampr | This study |
| A. tumefaciens | ||
| A348 | Wild type, Vir+, pTiA6NC | 18 |
| A1011 | Vir−, Kmr, Tn5 chromosomal in chvB region | 15 |
| ME104 | Vir−, Cbr, Tn3::HoHo1 in chvA region | 15, 22 |
| ME104(pBB2cgt) | Vir+, Cbr, Kmr, ME104(pBB2cgt) | This study |
| ME104(pCD522) | Vir+, Cbr, Kmr, ME104(pCD522) | This study |
| B. abortus | ||
| 2308 | Virulent, field isolated; wild type, Nalr, erythritol resistant | 36 |
| Cgs08 | B. abortus 2308 cgs mutant, Tn3-HoHo1 chromosome; Ampr | 6 |
| Cgt08 | B. abortus 2308 cgt mutant, cgt::Kmr | This study |
| Cgt08(pBB4cgt) | B. abortus 2308 cgt mutant with plasmid PBB4cgt, Kmr Ampr | This study |
| Cgt08(pBB4522) | B. abortus 2308 cgt mutant with plasmid pBB4522, Kmr Ampr | This study |
| S19 | Vaccine strain, Nalr, erythritol sensitive, naturally occuring derivate of B. abortus 2308 | 36 |
| Cgs19 | B. abortus S19 cgs mutant, Tn3-HoHo1 chromosome, Ampr | 21 |
| Cgt19 | B. abortus S19 cgt mutant, cgt::Kmr | This study |
| Cgt19(pBB4cgt) | B. abortus S19 cgt mutant with plasmid PBB4cgt, Kmr Ampr | This study |
| Cgt19(pBB4522) | B. abortus S19 cgt mutant with plasmid pBB4522, Kmr Ampr | This study |
| Plasmids | ||
| pBBR1MCS-4 | Broad-host-range cloning vector (Ampr) | 25 |
| pBBR1MCS-2 | Broad-host-range cloning vector (Kmr) | 25 |
| pBB4cgt | pBBR1MCS-4 containing B. abortus cgt gene, Ampr | This study |
| pBB2cgt | pBBR1MCS-2 containing B. abortus cgt gene, Kmr | This study |
| pBB4522 | pBBR1MCS-4 containing A. tumefaciens SalI chvA gene, Ampr | This study |
| pCD522 | pVK102 A. tumefaciens chvA Kmr | 15 |
| pBKcgt | pbluescript KS II(+) containing B. abortus cgt gene, Ampr | This study |
Ampr, ampicillin resistance; Cbr, carbenicillin resistance; Kmr, kanamycin resistance; Nalr, nalidixic acid resistance.
All the protocols which used live brucellae were performed in a biosafety level 3 laboratory facility.
Cloning and DNA sequencing.
The putative B. abortus cgt gene was amplified from B. abortus S2308 genomic DNA by PCR with primers cgt-fw (5′-CTCGCCCGCATCCACAATCT-3′) and cgt-rev (5′-CCGCACCCAAGCCATTTTTC-3′), designed according to the sequence of a B. abortus gene highly homologue to A. tumefaciens chvA (complete genome of B. abortus strain 2308) (unpublished data).
The amplified products were ligated to pBluescript II KS(+) (Stratagene, La Jolla, Calif.) and digested with EcoRV by following manufacturer's instructions. The resulting plasmid, containing a 2.3-kb fragment, was named pBKcgt. DNA sequencing was carried out by the dideoxy method with an automated model 373 DNA sequencer (Perkin-Elmer Applied Biosystems Division, Foster City, Calif.) according to the manufacturer's instructions.
TLC of cyclic β-1,2-glucan.
Cells from cultures grown for 48 h were harvested by centrifugation at 10,000 × g for 10 min. Cyclic β-1,2-glucans were extracted from cell pellets with ethanol (70% ethanol, 1 h at 37°C). Ethanolic extracts were centrifuged in an Eppendorf centrifuge, and supernatants were dried in a speed-vac centrifuge. Extracted glucans were dissolved in 70% ethanol and submitted to thin-layer chromatography (TLC) as described previously (7). TLC plates were developed by spraying with 5% sulfuric acid in ethanol and heating for 5 min at 120°C.
Nodulation and virulence test.
Alfalfa seeds were surface sterilized with concentrated sulfuric acid for 30 s and washed several times with sterile distilled water until total removal of the acid. Seeds were germinated on wet filter paper in petri dishes. Two-day-old seedlings were planted in autoclaved modified Leonard jars filled with Jensen's N-free solution (47). Seedlings were dipped into 2-day-old cultures immediately before planting. After 4 weeks, plants were removed and strains were isolated from nodules as described previously (20). Virulence assays were carried out on Kalanchoe leaves as previously described (18).
Construction of B. abortus β-1,2-glucan transporter mutant.
An NcoI fragment (300 bp) of cgt was digested from pBKcgt. The deleted plasmid was blunt ended with T4 DNA polymerase (New England BioLabs) and ligated to a 1.3-kb HincII fragment containing a kanamycin resistance cassette (30). The recombinant plasmid was electroporated into B. abortus S19 and B. abortus S2308 strains, where it is incapable of autonomous replication. Transconjugants were selected in BB agar with kanamycin (50 μg/ml). Double crossover events were selected by streaking colonies in duplicate in BB agar with kanamycin (50 μg/ml) and BB agar with ampicillin (100 μg/ml). Kanamycin-resistant, ampicillin-sensitive clones were selected as possible double recombinants. Putative double recombinants were confirmed by colony PCR with primers 5′-TCAGCAATGTTTCGGTGG-3′ and 5′-GAACGGCGGCTGACGGTG-3′. Genetic complementation of cgt mutants was carried out with plasmids pBB4cgt and pBB4522, containing a wild-type copy of the B. abortus and A. tumefaciens cgt genes, respectively. Plasmids pBB4cgt and pBB4522 were introduced in the mutants by biparental mating with E. coli S17.1 as the donor strain (6, 11).
Cell culture and infection assay.
HeLa cells were cultured at 37°C in 5% CO2 atmosphere in minimal essential medium (Gibco, Paisley, Scotland), supplemented with 2 mM glutamine and 5% fetal calf serum without antibiotic (cell culture medium). Infection of cells with different Brucella strains was performed at a multiplicity of infection of 100 as previously described (32, 38). The murine macrophage-like J774 cell line was used to test phagocytic cells. Cells at 105/well were infected with a bacterial suspension prepared as described above for HeLa cells except for the following changes: the culture medium was RPMI 1640 (Gibco) supplemented with 5% fetal bovine serum (Gibco) and the multiplicity of infection was 50.
Virulence in mice.
Nine-week-old female BALB/c mice were injected intraperitoneally with 0.2 ml of a suspension containing the appropriate number of viable Brucella organisms. Stock cultures were grown for 48 h on BB plates, and cells were suspended in sterile phosphate-buffered saline and adjusted turbidimetrically to the selected concentration. The exact bacterial concentration was calculated retrospectively by viable count. At selected postinfection times, groups of 5 mice were killed by cervical dislocation and spleens were homogenized in 1 ml of phosphate-buffered saline, serially diluted, and plated in triplicate on BB plates with the appropriate antibiotic (27).
Statistical analysis.
Differences between the means of experimental groups were analyzed by using the Student t test. Differences were considered significant at P values of <0.05.
Nucleotide sequence accession number.
The sequence of the B. abortus cyclic β-1,2-glucan transporter gene (cgt) has been assigned GenBank accession number AY237159.
RESULTS
Identification and cloning of B. abortus ABC transporter gene.
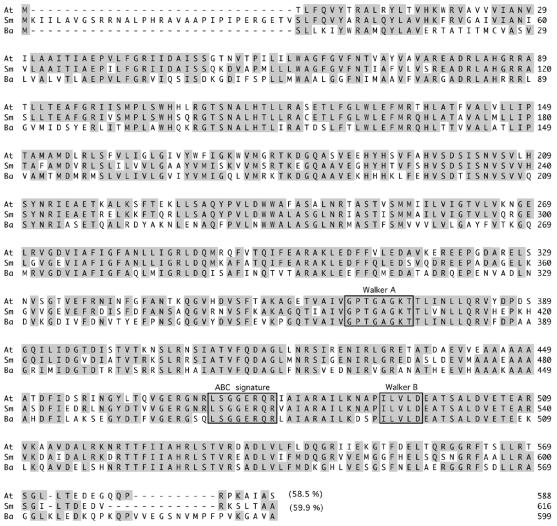
A B. abortus 1,797-bp open reading frame highly similar to A. tumefaciens chvA was identified by genome sequencing of B. abortus strain 2308 (unpublished data). The gene is highly conserved in Brucella melitensis and B. suis (13, 31). A set of two primers (5′-CTCGCCCGCATCCACAATCT-3′ and 5′-CCGCACCCAAGCCATTTTTC-3′) was used for PCR amplification of the complete B. abortus ABC transporter gene. Amplified products were cloned in pBluescript II KS(+) and sequenced as described in Materials and Methods. Analysis of the sequence revealed that the gene codes for a predicted protein with 599 amino acid residues that is 58.5 and 59.9% identical to S. meliloti NdvA and A. tumefaciens ChvA, respectively (Fig. 1). Accordingly, the gene was named B. abortus cgt, for cyclic glucan transporter.
FIG. 1.
Comparison of the B. abortus (Ba) Cgt cyclic β-1,2-glucan transporter protein with the A. tumefaciens (At) ChvA and S. meliloti (Sm) NdvA proteins. Conserved amino acids are indicated by gray boxes. Three typical ABC transporter motifs, Walker A and B and the SGG(Q) ABC signature, are indicated by boxes. The percentages of identity with the amino acid sequence of the ABC transporter protein (Cgt) from B. abortus are indicated in parentheses. The alignment was performed with the MegAlign program.
The putative B. abortus Cgt has all the conserved features of a typical ABC transporter, such as the Walker site A (GXXGXGKS/T), the Walker site B (hhhhD), (where h is a hydrophobic amino acid residue), and the ABC signature (LSGGERQR) (Fig. 1). Kyte-Doolittle analysis predicts that Brucella Cgt may be a membrane protein with 6 membrane-spanning segments very similar at the N-terminal region to those of ChvA and NdvA. Sequence analysis of the regions upstream and downstream of the B. abortus cgt gene revealed no significant homology to the B. abortus cgs gene, which was surprising since, in A. tumefaciens and S. meliloti, the two loci chvA/chvB and ndvA/ndvB, respectively, are adjacent to each other. Complete genome sequencing of the B. abortus, B. melitensis, and B. suis genomes revealed that the cgs and cgt genes are both located in chromosome 1 but separated by 857 kb, thus suggesting that cgs and cgt were either acquired independently or have suffered a severe rearrangement in the Brucella genome.
Functional characterization of B. abortus cgt.
S. meliloti ndvA and A. tumefaciens chvA mutants were used as heterologous backgrounds to study function and expression of B. abortus cgt. Two plasmids, pBB4cgt and pBB2cgt (see Materials and Methods and Table 2), were introduced by mating in S. meliloti LI1 ndvA (41) and A. tumefaciens ME104 chvA mutants (15, 22), respectively.
TABLE 2.
Complementation of Sinorhizobium and Agrobacterium mutants
| Strain | Motilitya | Virulenceb | Nitrogen fixationc | Anionic glucand |
|---|---|---|---|---|
| S. meliloti | ||||
| F34 (wild type) | ++ | ND | ++ | ++ |
| LI1 (ndvA) | − | ND | − | − |
| LI1(pBB4cgt) | ++ | ND | ++ | ++ |
| A. tumefaciens | ||||
| A348 (wild type) | ++ | ++ | ND | ++ |
| ME104 (chvA) | + | + | ND | + |
| ME104(pBB2cgt) | ++ | ++ | ND | ++ |
Motility on 0.35% agar medium was studied as described in Materials and Methods. ++, motile; +, decreased motility; −, nonmotile.
Virulence was determined on Kalanchoe leaves as described previously (18). ++, wild-type tumor size; +, smaller tumor size (Fig. 2B); ND, not determined.
Nitrogen fixation was assessed visually by plant color, nodulation, production of leghaemoglobin in nodules, and eventual death of plants as a result of chlorosis. −, yellowish plant color, round white nodules; ++, green plant color, pink cylindrical nodules.
The presence of anionic glucan was determined by glucan extraction and characterization by TLC (Fig. 3). ++, accumulation of anionic glucans in the wild-type strain; +, less accumulation of anionic glucans than the wild-type strain; −, no accumulation of anionic glucans.
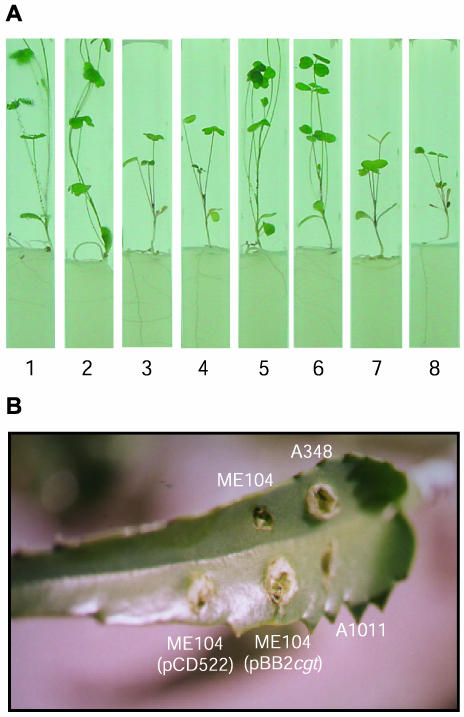
As shown in Table 2 and Fig. 2A, B. abortus cgt restored the formation of normal nodules by the S. meliloti LI1 ndvA mutant as judged by the induction of pink cylindrical nodules and the healthy aspect of plants inoculated with the S. meliloti LI1(pBB4cgt) strain after 1 month. In contrast, nodules formed by the S. meliloti LI1 ndvA mutant were round and white and plants remained blanched and stunted, indicating lack of nitrogen fixation. Moreover, wild-type S. meliloti 102F34 and LI1(pBB4cgt) strains were recovered from 4-week-old nodules, whereas no bacteria were recovered from nodules induced by the LI1 mutant.
FIG. 2.
Virulence and nodulation complementation assays. (A) Alfalfa plants inoculated with different strains of S. meliloti. (1 and 2) F34; (3 and 4) LI1; (5 and 6) LI1 (pBB4cgt); (7 and 8) noninoculated control. (B) Kalanchoe leaf inoculated with different strains of A. tumefaciens. The virulence and nodulation assays were carried out as described in Materials and Methods. Photos were taken 4 weeks postinoculation in both assays.
On the other hand, the resulting recombinant strain A. tumefaciens ME104(pBB2cgt) recovered the capacity to form tumors on Kalanchoe leaves at wild-type levels (Table 2 and Fig. 2B). In contrast, tumors formed by the A. tumefaciens ME104 chvA mutant were smaller than those formed by the wild-type strain, indicating reduced virulence. No tumor was formed by the A. tumefaciens A1011 chvB mutant (Fig. 2B). These results demonstrated that B. abortus cgt restores the normal plant interaction of S. meliloti ndvA and A. tumefaciens chvA mutants.
A variety of pleiotropic phenotypes linked to the synthesis and secretion of cyclic β-1,2-glucan were described previously (4) A. tumefaciens ME104 chvA and S. meliloti LI1 ndvA mutants are nonmotile due to defective flagellum assembly (4). Plasmids pBB2cgt and pBB4cgt restored to wild-type level the motility of chvA and ndvA mutants, respectively, thus indicating that B. abortus Cgt restored the correct assembly of flagella in these backgrounds.
Cyclic β-1,2-glucan transport.
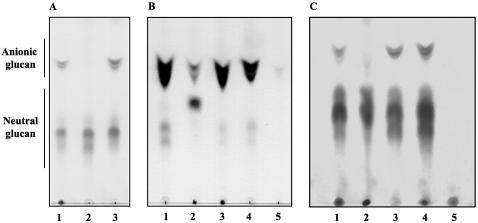
It has been described that once cyclic β-1,2-glucans are transported into the periplasm, they are replaced by nonglycosidic anionic substituents (4, 5). To study whether B. abortus cgt restores in A. tumefaciens and S. meliloti the transport of cyclic β-1,2-glucan into the periplasmic space, cyclic β-1,2-glucans were extracted from strains A. tumefaciens ME104(pBB2cgt) and S. meliloti LI1(pBB4cgt) and the presence of anionic glucans was analyzed by TLC as described in Materials and Methods. As shown in Fig. 3A and B and Table 2, the B. abortus cgt gene restored the accumulation of anionic glucan to wild-type levels in both mutants, thus indicating that Cgt is indeed a cyclic β-1,2-glucan transporter. As shown in Fig. 3B, the A. tumefaciens chvA mutant has strongly reduced the amount of anionic glucans as well as the degree of polymerization of neutral glucans. Both effects where reverted to the wild-type phenotype by pBB2cgt and pCD522 plasmids.
FIG. 3.
TLC of cyclic β-1,2-glucans formed by S. meliloti, A. tumefaciens, and B. abortus strains. Total cellular glucans of Sinorhizobium, Agrobacterium, and B. abortus strains were extracted and subjected to TLC as described in Materials and Methods. (A) S. meliloti. Lane 1, wild-type 102F34; lane 2, ndvA LI1; lane 3, LI1(pBB4cgt). (B) A. tumefaciens. Lane 1, wild-type A348; lane 2, ME104 mutant; lane 3, ME104(pBB2cgt); lane 4, ME104(pCD522); lane 5, chvB mutant. (C) B. abortus. Lane 1, wild-type 2308; lane 2, Cgt08 mutant; lane 3, Cgt08(pBB4cgt); lane 4, Cgt08(pBB4522); lane 5, Cgs08 mutant.
Characterization of B. abortus cgt mutants.
B. abortus cgt null mutant strains, B. abortus 2308 cgt::Km and B. abortus S19 cgt::Km, were obtained as described in Materials and Methods. The absence of smooth-to-rough dissociation of the mutants was demonstrated by studying sensitivity to three different phages that are known to lyse smooth strains (phages Tb, Wb, and Iq) and resistance to one phage that lyses rough strains (phage Rc) (data not shown).
Glucan extraction and characterization by TLC.
Localization of cyclic β-1,2-glucan in the periplasm was studied indirectly by TLC determination of the presence of anionic glucans. As shown in Fig. 3C, B. abortus 2308 cgt::Km does not accumulate anionic glucans; the same result was obtained with B. abortus S19 cgt::Km (data not shown). It is shown that plasmid pBB4cgt, containing the B. abortus cgt, and plasmid pBB4522, containing A. tumefaciens chvA, restored the presence of anionic glucans to the wild-type level. These results demonstrate that cgt is the B. abortus cyclic glucan transporter gene and that the phenotype can also be complemented by the A. tumefaciens gene, thus cgt and chvA are fully interchangeable.
Intracellular multiplication of B. abortus cgt mutants.
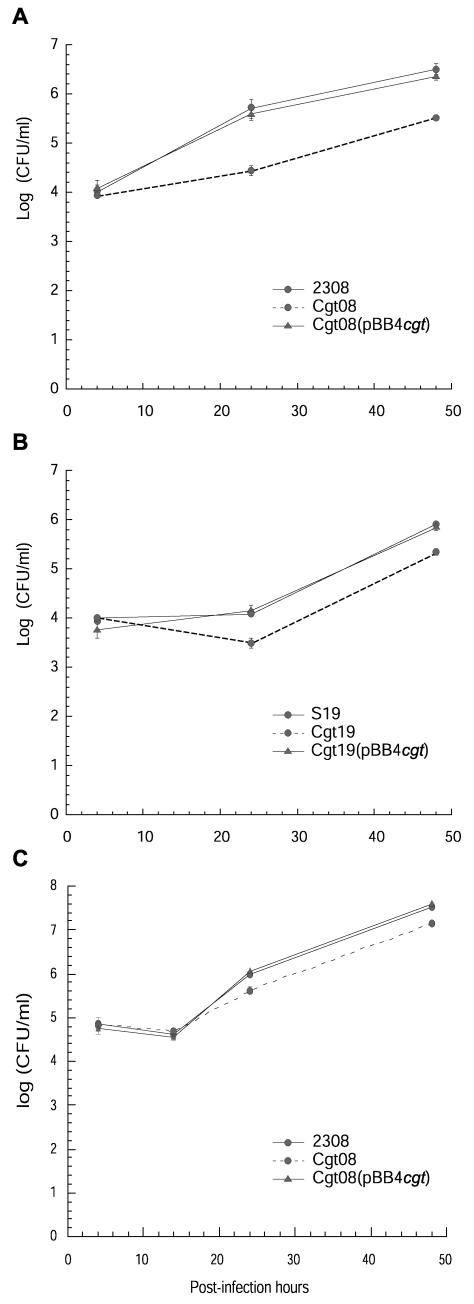
The importance of B. abortus cgt in invasion and intracellular survival was evaluated in HeLa and J774 cells as described in Materials and Methods. In HeLa cells, as shown in Fig. 4A and B, the number of intracellular bacteria recovered 4 h postinfection displayed no significant difference between the wild-type and cgt mutants, thus indicating that cgt does not have any apparent role during invasion. However, both B. abortus 2308 cgt::Km and B. abortus S19 cgt::Km mutants showed reduce intracellular multiplication at 24 and 48 h postinfection. J774 cells were infected with 2308 and the 2308 cgt mutant (Fig. 4C). Both strains invade the cells to the same extent and were equally recovered at 14 h postinfection. However, intracellular multiplication at 24 and 48 h were significantly reduced. Intracellular multiplication was restored to wild-type levels after complementation of the mutants with plasmid pBB4cgt. The intracellular multiplication of cgt mutants is similar to that described for cgs mutants (6), thus suggesting that cyclic glucan must be transported into the periplasm to exert its action.
FIG.4.
Intracellular replication of different strains of B. abortus. Monolayers of HeLa (A and B) and J774 (C) cells were inoculated with 107 and 5.106 CFU of bacteria, respectively. After 1 h of incubation at 37°C, cells were washed as described in Materials and Methods and streptomycin and gentamicin were added. Numbers of CFU were determined at the indicated times. Values are the means ± standard deviations of the results from one independent experiment out of two performed in duplicate; the P value is <0.05). Statistical analysis was performed with a t test.
Persistence of the B. abortus cgt mutants in mice.
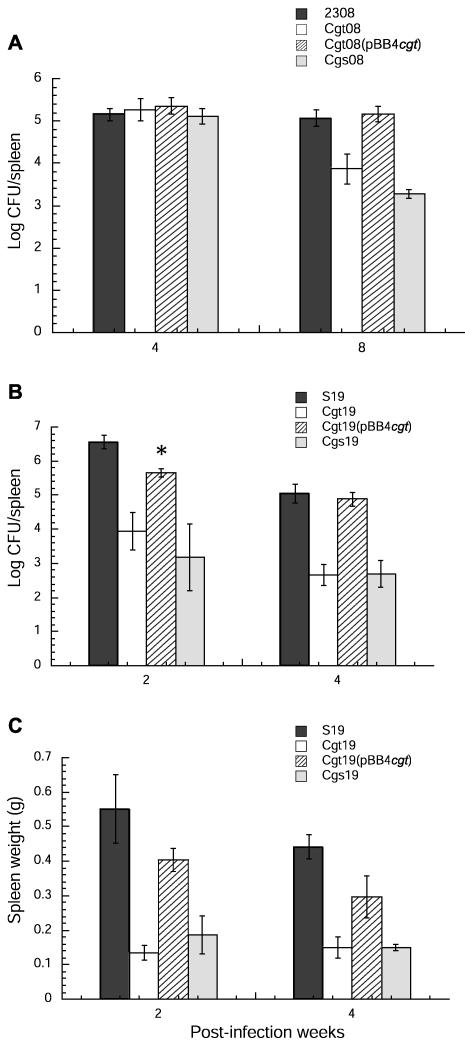
To assess the possible role of cyclic β-1,2-glucan transporter in the virulence of B. abortus, experimental infections in mice were carried out as described in Materials and Methods. Groups of 5 mice were injected intraperitoneally with 104 CFU of B. abortus wild type and cgt mutants. B. abortus 2308 and S19 cgs mutants were also used for comparison. At different postinoculation times, mice were sacrificed, spleens were weighed, and the numbers of CFU/spleen were determined. As shown in Fig. 5A, no significant difference was observed at 4 weeks postinfection between the wild-type 2308 strain and the corresponding cgt and cgs mutants. However, at 8 weeks postinfection, the numbers of CFU recovered from the spleens of mice infected with B. abortus 2308 cgt and B. abortus 2308 cgs mutants were 1.3 log and 1.5 log lower than those of the wild-type parental strain, respectively. Plasmid pBB4cgt restored spleen recovery of cgt mutants to the wild-type level, thus suggesting that cgt is required for full virulence. The similar behavior observed between cgt and cgs mutants suggests that cyclic glucans must be located in the periplasm to exert its action.
FIG. 5.
Brucella persistence in spleens of mice inoculated with different strains of B. abortus 2308 and S19. Mice were inoculated intraperitoneally with 104 CFU of B. abortus. At the indicated times postinfection, 5 mice per group were killed and their spleens were removed. The numbers of CFU in spleen tissues were determined as indicated in Materials and Methods. (A) B. abortus 2308 strains: recovery of viable bacteria from spleens at 4 or 8 weeks postinfection. (B) B. abortus S19 strains: recovery of viable bacteria from spleens at 2 or 4 weeks postinfection. (C) B. abortus S19 strains: weight of spleens of infected mice at 2 or 4 weeks postinfection. Values are expressed as means ± standard errors of the means (n = 5); the P value is <0.05. Statistical analysis was performed with a t test. *, mice inoculated with the B. abortus cgt mutant complemented with plasmid pBB4522 showed values not statistically different from those observed with the same strain complemented with pBB4cgt.
A more drastic effect on virulence reduction was observed with a cgt mutant of the partially attenuated strain S19. As shown in Fig. 5B, 2 and 4 weeks after infection the numbers of CFU recovered from the spleens of mice infected with the B. abortus S19 cgt mutant were 2.6 log and 2.4 log lower than that of the wild-type parental S19 strain, respectively. Plasmid pBB4cgt restored spleen recovery of the cgt mutant to wild-type levels. A similar result was observed with plasmid pBB4522 harboring the A. tumefaciens chvA gene at 2 weeks postinfection (Fig. 5B). A significant reduction of spleen weight was also observed with the B. abortus S19 cgt mutant (Fig. 5C), thus suggesting a strong inhibition of the inflammatory response of strain S19. This effect was also abolished by plasmid pBB4cgt. These results were similar to those obtained with cgs mutants that do not produce cyclic β-1,2-glucan (6), thus supporting the hypothesis that cyclic β-1,2-glucan plays a role during Brucella host interaction and that to exert its action it must be exported into the periplasm.
DISCUSSION
The role of cyclic glucans in bacterium-host interaction was extensively studied in Agrobacterium and Rhizobium. Synthesis and transport mutants have some associated pleiotropic phenotypes, for example, defective flagellum assembly (4), defective plant attachment (33), decreased temperature stability of the virB10 protein (2), all traits that may affect bacterium-host interaction. It was also described that the accumulation of cyclic glucan changes with the osmolarity of the media (4), thus suggesting that cyclic glucan may be involved in sensing environmental changes, and thus, its localization in the periplasm is predictable. However, it must be remarked that although B. abortus cyclic glucan is located in the periplasmic space (7), the protein involved in its transport has not been characterized.
ABC transporters are a major class of cellular translocation machinery in all bacterial species (37, 43). In this study, we have identified, sequenced, and disrupted a B. abortus ABC transporter that exhibits high homology to the cyclic β-1,2-glucan transporters of A. tumefaciens ChvA (60% identity) (15) and S. meliloti NdvA (59% identity) (17). Accordingly, the gene was name B. abortus cgt, for cyclic glucan transporter.
Computer analysis of the predicted amino acid sequence revealed the presence of Walker and ABC signatures found in all ABC transporters in the C-terminal domain of Brucella Cgt.
Contrary to what happens in S. meliloti and A. tumefaciens, in which cyclic β-1,2-glucan transporter (cgt) and cyclic β-1,2-glucan synthase (cgs) are contiguous and convergently transcribed, in Brucella, cgs and cgt genes are separated by 857 kb. This suggests that Brucella cgs and cgt were either acquired independently or have suffered a severe genome rearrangement during evolution.
Cross-complementation studies revealed that this B. abortus ABC transporter gene restores cyclic β-1,2-glucan transport of S. meliloti ndvA and A. tumefaciens chvA mutants. Conversely, the A. tumefaciens chvA gene complemented glucan transport of the B. abortus ABC transporter mutant. Thus, we concluded that this gene codes for the Brucella cyclic β-1,2-glucan transporter.
B. abortus cgt mutants accumulate nonsubstituted neutral forms of cellular cyclic β-1,2-glucan. The accumulation of nonsubstituted cyclic β-1,2-glucan may be due either to a defect of the enzymes or substrates required for the modifying reaction or to a defect in the transport of cyclic β-1,2-glucan to the periplasmic space. It was well established that cyclic β-1,2-glucan modifying reactions take place in the periplasmatic space of the bacteria (3-5). Cross-complementation of Brucella cgt mutants with the A. tumefaciens cyclic β-1,2-glucan transporter chvA gene demonstrated that the absence of anionic cyclic β-1,2-glucan in B. abortus cgt mutants is due to the absence of cyclic β-1,2-glucan transport into the periplasm.
A large number of genes with high homology to ABC transporters were identified in the genome of brucellae; however, this is the first report in which the role of a B. abortus ABC transporter was assigned. Recently, an ABC transporter homologue to S. meliloti ExsA was identified and characterized in B. abortus. exsA is critical for full B. abortus virulence (34); however, the substrate transported by ExsA was not identified. Mutants in the Brucella ATP-binding genes bapA and bapB (24) and in a B. abortus ABC transporter mapping in chromosome 1 have no effect on cyclic β-1,2-glucan transport (M. Roset, unpublished data), thus indicating the high specificity of ABC transporters.
In a previous work, it was described that the absence of cyclic β-1,2-glucan reduced the virulence of B. abortus in mice and impeded normal intracellular multiplication in HeLa cells (6). Cgs, the enzyme responsible for cyclic β-1,2-glucan synthesis, is a 300-kDa inner membrane protein. All the cgs mutants studied so far were obtained by transposon insertion; thus, the lack of cyclic glucan was always accompanied by the lack of the 300-kDa inner membrane protein. Accordingly, the observed phenotype may be due to the lack of cyclic glucan, the lack of the 300-kDa inner membrane protein, or the lack of both.
In this study, we have obtained a B. abortus cyclic β-1,2-glucan transport mutant. The mutant lacks the ABC-type transport protein Cgt but contains an intact Cgs and accumulates cytoplasmic nonsubstituted neutral cyclic β-1,2-glucan. Interestingly, cgt mutants have reduced virulence in mice and defective intracellular multiplication in HeLa and J774 cells, a phenotype identical to that described for cgs null mutants. These results suggest that the presence of cyclic glucan in the periplasmic space is required in B. abortus for appropriate host interaction and full expression of virulence.
Further work is required to determine whether cyclic β-1,2-glucan is an extracellular signal recognized by the host or whether the presence of the glucan in the periplasmic space stabilizes and/or promotes the correct folding of other membrane proteins required for successful bacterium-host interaction.
Acknowledgments
This work was supported by a grant from the Agencia Nacional de Promoción Científica y Técnica, Buenos Aires, Argentina, PICT 2000 no. 01-09194. M.S.R. is a fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas, CONICET, Buenos Aires, Argentina. N.I.D.I. and R.A.U. are members of the research carrier of the CONICET.
We thank J. J. Cazzulo for critical reading of the manuscript. We acknowledge Fabio Fraga and Pablo Briones, University of General San Martín, for technical assistance and L. Ielpi for providing the S. meliloti LI1 strain.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. INRA, Paris, France.
- 2.Banta, L. M., J. Bohne, S. D. Lovejoy, and K. Dostal. 1998. Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J. Bacteriol. 180:6597-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohin, J. P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 4.Breedveld, M. W., and K. J. Miller. 1994. Cyclic beta-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 58:145-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedveld, M. W., and K. J. Miller. 1995. Synthesis of glycerophosphorylated cyclic (1,2)-beta-glucans in Rhizobium meliloti strain 1021 after osmotic shock. Microbiology 141(Pt 3):583-588. [DOI] [PubMed] [Google Scholar]
- 6.Briones, G., N. Iñón de Iannino, M. Roset, A. Vigliocco, P. S. Paulo, and R. A. Ugalde. 2001. Brucella abortus cyclic beta-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect. Immun. 69:4528-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briones, G., N. Iñón de Iannino, M. Steinberg, and R. A. Ugalde. 1997. Periplasmic cyclic 1,2-beta-glucan in Brucella spp. is not osmoregulated. Microbiology 143(Pt 4):1115-1124. [DOI] [PubMed] [Google Scholar]
- 8.Cangelosi, G. A., G. Martinetti, J. A. Leigh, C. C. Lee, C. Theines, and E. W. Nester. 1989. Role for Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J. Bacteriol. 171:1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles, T. C., and E. W. Nester. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 175:6614-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comerci, D. J., G. D. Pollevick, A. M. Vigliocco, A. C. Frasch, and R. A. Ugalde. 1998. Vector development for the expression of foreign proteins in the vaccine strain Brucella abortus S19. Infect. Immun. 66:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbel, J. M. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 2:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA. 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas, C. J., R. J. Staneloni, R. A. Rubin, and E. W. Nester. 1985. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J. Bacteriol. 161:850-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dylan, T., D. R. Helinski, and G. S. Ditta. 1990. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1,2)-glucan. J. Bacteriol. 172:1400-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dylan, T., L. Ielpi, S. Stanfield, L. Kashyap, C. Douglas, M. Yanofsky, E. Nester, D. R. Helinski, and G. Ditta. 1986. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 83:4403-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garfinkel, D. J., and E. W. Nester. 1980. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J. Bacteriol. 144:732-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer, B. H., and N. B. McCullough. 1968. Homologies of deoxyribonucleic acids from Brucella ovis, canine abortion organisms, and other Brucella species. J. Bacteriol. 96:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iñón de Iannino, N., G. Briones, G. Kreiman, and R. Ugalde. 1996. Characterization of the biosynthesis of beta(1-2) cyclic glucan in R. Fredii. Beta(1-2) glucan has no apparent role in nodule invasion of Mc Call and Peking soybean cultivars. Cell. Mol. Biol. 42:617-629. [PubMed] [Google Scholar]
- 21.Iñón de Iannino, N., G. Briones, M. Tolmasky, and R. A. Ugalde. 1998. Molecular cloning and characterization of cgs, the Brucella abortus cyclic β(1-2) glucan synthetase gene: genetic complementation of Rhizobium meliloti ndvB and Agrobacterium tumefaciens chvB mutants. J. Bacteriol. 180:4392-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iñón de Iannino, N., and R. A. Ugalde. 1989. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1-2)glucan. J. Bacteriol. 171:2842-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iñón de Iannino, N., and R. A. Ugalde. 1993. Biosynthesis of cyclic beta-(1-3), beta-(1-6) glucan in Bradyrhizobium spp. Arch. Microbiol. 159:30-38. [DOI] [PubMed] [Google Scholar]
- 24.Ko, J., and G. A. Splitter. 2000. Brucella abortus tandem repeated ATP-binding proteins, BapA and BapB, homologs of Haemophilus influenzae LktB, are not necessary for intracellular survival. Microb. Pathog. 29:245-253. [DOI] [PubMed] [Google Scholar]
- 25.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 26.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop II, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 27.Montaraz, J. A., and A. J. Winter. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno, E., E. Stackebrandt, M. Dorsch, J. Wolters, M. Busch, and H. Mayer. 1990. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J. Bacteriol. 172:3569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Pti type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 30.Oka, A., H. Sugisaki, and M. Takanami. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147:217-226. [DOI] [PubMed] [Google Scholar]
- 31.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:13148-13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puvanesarajah, V., F. M. Schell, G. Stacey, C. J. Douglas, and E. W. Nester. 1985. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J. Bacteriol. 164:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosinha, G. M., D. A. Freitas, A. Miyoshi, V. Azevedo, E. Campos, S. L. Cravero, O. Rossetti, G. Splitter, and S. C. Oliveira. 2002. Identification and characterization of a Brucella abortus ATP-binding cassette transporter homolog to Rhizobium meliloti ExsA and its role in virulence and protection in mice. Infect. Immun. 70:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sangari, F. J., J. M. Garcia-Lobo, and J. Aguero. 1994. The Brucella abortus vaccine strain B19 carries a deletion in the erythritol catabolic genes. FEMS Microbiol. Lett. 121:337-342. [DOI] [PubMed] [Google Scholar]
- 37.Schneider, E., and S. Hunke. 1998. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol. Rev. 22:1-20. [DOI] [PubMed] [Google Scholar]
- 38.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sola-Landa, A., J. Pizarro-Cerda, M. J. Grillo, E. Moreno, I. Moriyon, J. M. Blasco, J. P. Gorvel, and I. Lopez-Goñi. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 29:125-138. [DOI] [PubMed] [Google Scholar]
- 40.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanfield, S. W., L. Ielpi, D. O'Brochta, D. R. Helinski, and G. S. Ditta. 1988. The ndvA gene product of Rhizobium meliloti is required for beta-(1,2)glucan production and has homology to the ATP-binding export protein HlyB. J. Bacteriol. 170:3523-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stibitz, S., and M.-S. Yang. 1991. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J. Bacteriol. 173:4288-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomii, K., and M. Kanehisa. 1998. A comparative analysis of ABC transporters in complete microbial genomes. Genome Res. 8:1048-1059. [DOI] [PubMed] [Google Scholar]
- 44.Tsolis, R. M. 2002. Comparative genome analysis of the alpha-Proteobacteria: relationships between plant and animal pathogens and host specificity. Proc. Natl. Acad. Sci. USA 99:12503-12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ugalde, R. A. 1999. Intracellular lifestyle of Brucella spp. Common genes with other animal pathogens, plant pathogens, and endosymbionts. Microbes Infect. 1:1211-1219. [DOI] [PubMed] [Google Scholar]
- 46.Verger, J. M., F. Grimont, P. A. D. Grimont, and M. Crayon. 1985. Brucella, a monospecific genus as shown by deoxirribonucleotic acid hybridization. Int. J. Syst. Bacteriol. 35:292-295. [Google Scholar]
- 47.Vincent, M. 1970. A manual for practical study of root nodule bacteria. Blackwell Scientific Publication, Ltd., Oxford, United Kingdom.
- 48.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zorreguieta, A., and R. A. Ugalde. 1986. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in beta-D(1-2) glucan synthesis. J. Bacteriol. 167:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]