Abstract
Chagas’ disease is a chronic infection caused by Trypanosoma cruzi and represents an important public health burden in Latin America. Frequently the disease evolves undetectable for decades, while in a significant fraction of the affected individuals it culminates in death by heart failure. Here, we describe a novel murine model of the chronic infection with T. cruzi using a stable clone isolated from a human patient (Sylvio X10/4). The infection in the C3H/HePAS mouse strain progresses chronically and is mainly characterized by intense cardiac inflammatory lesions that recapitulate the chronic cardiac pathology observed in the human disease. Moderate striated muscle lesions are also present in C3H/HePAS mice. Viable parasites are detected and recovered from the chronic heart lesions of C3H/HePAS mice, supporting the current notion that development of heart pathology in Chagas’ disease is related to parasite persistence in the inflamed tissue. By contrast, in infected A/J mice, chronic inflammatory lesions are targeted to the liver and the skeletal muscle, while pathology and parasites are undetectable in the heart. The phenotypic analysis of F1 (A/J × C3H/HePAS) and F2 (A/J × C3H/HePAS) mice suggests that the genetic predisposition to develop the inflammatory lesions caused by T. cruzi (Sylvio X10/4 clone) is heterogeneous because the heart and liver pathology segregate in the F2 generation. These findings raise the hypothesis that the pathology heterogeneity observed in humans with Chagas’ disease (absence and presence of cardiac or digestive chronic lesions) may be attributable to host genetic factors.
Chagas’ disease (American trypanosomiasis) is an infection with protozoan Trypanosoma cruzi that represents an important health problem in Latin America (29). After an acute phase with parasitemia, which often proceeds unnoticed, the disease progresses to an asymptomatic indeterminate phase with virtually undetectable parasitemias, where a strong humoral and cellular anti-T. cruzi response guarantees a nonsterile control of the parasite. Years after the infection, a significant proportion (20 to 30%) of the infected individuals develops the severe cardiac or digestive manifestations of chronic disease: cardiomyopathy and/or arrythmias that may lead to congestive heart failure and esophageal or colonic digestive megasyndromes. In the murine experimental model of the disease, mice surviving long-term infection with certain stocks of T. cruzi develop myocardial lesions (2) and electrocardiographical changes (10) resembling those found in human chagasic myocarditis.
Chagas’ disease pathogenesis has been attributed to autoimmunity (5, 6, 15, 17-19), but this issue still remains controversial (22, 31). Several pieces of evidence suggest that, independently of the eventual contribution of an autoimmune component, pathology relates to persistence of T. cruzi parasites at the affected organs where they evoke a chronic inflammatory process (3, 13, 22, 27, 31). According to this view, the level of chronic commitment of a particular organ reflects to a certain extent its local parasite load. This parameter depends on parasite elements, such as virulence, inoculum size (11), tropism (1, 12, 26, 28), immunogenicity, and systemic and local host factors, such as those controlling the magnitude and quality of the anti-T. cruzi immune response (24, 25, 30).
Characterization of the host elements contributing to the occurrence of chronic lesions is an important goal, which may have inestimable prognostic value for chronic patients. Such characterization can be approached through the study of genetic crosses derived from parental strains with high and low susceptibilities to developing T. cruzi-induced pathology. With the above objective in mind, in this study we have approached the identification of such polar mouse strains during the chronic infection with the Sylvio X10/4 clone. This parasite was chosen both because it is a clone with stable biological characteristics (16) and because it does not cause a symptomatic acute disease but does induce chronic cardiac lesions incorporating several pathological features of the human chagasic cardiomyopathy.
MATERIALS AND METHODS
Mice, parasites, and infection.
Six- to eight-week-old A/J, C3H/HePAS, BALB/c, DBA/2, C57BL/6, F1 (A/J × C3H/HePAS) mice, as well as F2 (A/J × C3H/HePAS) mice, were obtained from our animal facilities (Biotério de Camundongos Isogênicos, ICB/USP, São Paulo, Brazil). C3H/HePAS is a lipopolysaccharide-responsive variant of the C3H strain (21) and was originally obtained from the Pasteur Institute, Paris, France. T. cruzi trypomastigotes of the Sylvio X10/4 clone were obtained by infection of LLCMK2 cells. Mice infected intraperitoneally (i.p.) with 106 parasites were bled and sacrificed at the chronic phase of the disease, from day 120 to 600 postinfection, for histopathological analysis of the heart, liver, and striated muscle.
Parasite screening.
In the acute phase of infection, parasitemias were determined by microscopic examination of 5-μl blood samples collected from the tail vein with a heparinized capillary tube. In the chronic phase of infection, the presence of T. cruzi parasites in the blood, liver, or heart was detected by searching for trypomastigotes in aliquots of blood (0.1 ml) or tissue homogenates (approximately 10 mg of tissue) cultured in triplicate, at 28°C, for a month, in axenic liver infusion tryptose (LIT) medium. To avoid blood contamination of heart and liver tissue samples, the inferior cava vein of chronically infected mice was sectioned above the diaphragm and connected through the left ventricle to a KDS 200 Two-Syringe Infusion Pump (KD Scientific, New Hope, Pa.), which delivered sterile phosphate-buffered saline for 5 min, at a flow rate of 2 ml/min.
ELISA for parasite specific antibodies.
Anti-T. cruzi serum antibodies were quantified by enzyme-linked immunosorbent assay (ELISA). In brief, 96-well flat-bottom microtest plates were coated overnight (4°C) with a T. cruzi extract (50 μg/ml) obtained from the supernatant of tissue culture trypomastigotes of the Sylvio X10/4 clone subjected to several freeze-thawing cycles. Plates were saturated with 1% gelatin for 1 h. After washing, 50 μl of mouse serum samples (diluted 1/100 to 1/400 for immunoglobulin G1 [IgG1] and 1/1,000 to 1/4,000 for IgG2a) were added and left for 1 h at room temperature. The assays were developed by adding goat anti-mouse IgG1 or IgG2a biotinylated antibodies (Southern Biotechnology Associates, Birmingham, Ala.) followed by a peroxidase-avidin conjugate and O-phenylenediamine (Sigma Chemical Co., St. Louis, Mo.). Enzyme reaction mixtures were developed for 10 min and blocked with 3 N HCl (50 μl/well). A Dynatech reader (Dynatech Laboratories Inc., Chantilly, Va.) using a 450-nm-wavelength filter quantified the absorbance values.
Histopathological analysis.
Tissue specimens from chronically infected mice were collected and fixed in paraformaldehyde for further processing. Paraffin-embedded tissue sections were stained with hematoxylin-eosin and examined in a light microscope. Six nonconsecutive slides from the heart, liver, and quadriceps muscle of each mouse were examined in a blind fashion. Areas of inflammatory infiltrates in the myocardium, pericardium, endocardium, liver, or striated muscle were quantified using an image analysis system (Image Pro Plus Media Cybernetics, Silver Spring, Md.). The sum of infiltrated areas in the six slides was calculated for each mouse. The final individual score was expressed in square micrometers of inflammatory infiltrates per square millimeter of examined tissue.
Statistical analysis.
The differences between the groups of mice used in this study were determined by the Kruskal-Wallis test.
RESULTS
Histopathology of the heart, liver, and striated muscle in Sylvio X10/4 chronically infected mice.
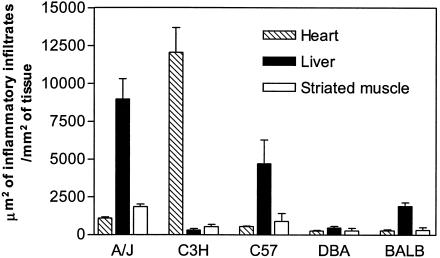
Considering the presence of inflammatory infiltrates in the heart, liver, and striated muscle, three distinct patterns of chronic Chagas’ pathology were identified among the different inbred strains of mice infected with Sylvio X10/4 parasites (Fig. 1). The most common pattern, observed in A/J, C57BL/6, and BALB/c mice, included various degrees of hepatitis and striated muscle inflammatory lesions, but no heart pathology. Chronically infected C3H/HePAS mice presented the opposite pattern of the disease, characterized by inflammatory lesions that were intense in the heart, moderate in the striated muscle and absent in the liver. A third pattern of pathology was observed in chronically infected DBA/2 mice, which displayed minimal lesions in the three organs studied.
FIG. 1.
Intensity of inflammation in the heart, liver, and striated muscle of chronically infected mice from different strains. Mice were infected i.p. with 106 Sylvio X10/4 trypomastigotes, and the infiltrated areas were evaluated at day 200 postinfection, as described in Materials and Methods. Each bar represents the mean ± SE (error bar) of individual values (n = 5).
Examination of heart sections from chronically infected C3H/HePAS mice revealed increased numbers of focal inflammatory foci, considerably higher than those observed in mice of the other strains in which lesions were minimal or undetectable (Fig. 1). Heart inflammatory lesions in C3H/HePAS mice included intense focal myocarditis, pericarditis and endocarditis in both ventricles and atria. Focal infiltrates containing predominantly mononuclear cells were observed, with vacuolar degeneration of myocardial fibers (Fig. 2A and B). Cumulative data from 23 C3H/HePAS mice and 24 A/J mice, sacrificed from day 120 to 600 of infection, revealed that the mean heart areas occupied by inflammatory infiltrates were, respectively, 2,981 ± 639 and 289 ± 84 μm2/mm2 of cardiac tissue for the myocardium, 7,175 ± 3,127 and 109 ± 53 μm2/mm2 of cardiac tissue for the pericardium, and 616 ± 199 and 85 ± 33 μm2/mm2 of cardiac tissue for the endocardium (means ± standard deviations). Amastigote nests were observed in 13.04% of heart sections from chronically infected C3H/HePAS mice (3 out of 23 mice [Table 1]), but they were undetectable in the heart tissue from A/J (0 out of 24 mice [Table 1]), or C57BL/6, BALB/c, and DBA/2 chronically infected mice (data not shown). Parasite nests in the hearts of C3H/HePAS mice were apparently viable, showing no degeneration signs. Interestingly, parasites were not only found in areas displaying inflammatory foci but also in histologically normal areas where they seemed to be ignored by the immune system (Fig. 3).
FIG. 2.
Examples of lesions in the heart, liver, and striated muscle of C3H/HePAS and A/J mice infected for 200 days with 106 Sylvio X10/4 trypomastigotes. (A) Heart section of a C3H/HePAS infected mouse displaying intense cardiac pathology with pronounced mononuclear infiltration at the pericadium and myocardium. (B) Heart section of an A/J infected mouse showing no inflammation. (C) Absence of liver lesions in a C3H/HePAS infected mouse. (D) Intense hepatitis in an A/J infected mouse. Inflammatory infiltrates of different intensities in the striated muscle of C3H/HePAS (E)- and A/J (F)-infected mice. Bar, 50 μm; magnification, ×17.
TABLE 1.
Tissue parasitism in the chronic phase of T. cruzi infectiona
| Mouse group | % Parasitized samples (no. parasitized/total no. examined)
|
|||
|---|---|---|---|---|
| Heart | Liver | Striated muscle | Blood | |
| With tissue nestsb | ||||
| A/J | 0 (0/24) | 0 (0/24) | 4.8 (1/21) | |
| C3H | 13.0 (3/23) | 0 (0/23) | 0 (0/20) | |
| With positive LIT culturesc | ||||
| A/J | 0 (0/14) | 0 (0/14) | NDd | 28.6 (4/14) |
| C3H | 38.5 (5/13) | 0 (0/13) | ND | 38.5 (5/13) |
At the time of sacrifice, all C3H/HePAS and A/J chronically infected mice displayed positive serology for Sylvio X10/4 parasite antigen.
Parasite nests were observed by optical microscopy in tissue sections stained with hematoxylin-eosin.
Tissue parasites were evaluated by culture of tissue homogenate aliquots in LIT medium, as described in Materials and Methods.
ND, not done.
FIG. 3.
Parasite nest in the myocardium of C3H/HePAS mice infected for 200 days with 106 Sylvio X10/4 trypomastigotes. A magnified picture of the pseudocyst can be seen in the insert. Bar, 50 μm; magnification, ×17.
Multifocal mononuclear inflammation was observed all over the hepatic tissue from chronically infected A/J mice (and with less intensity in the liver of C57BL/6 and BALB/c mice), with occasional periportal mononuclear infiltrates (Fig. 2C and D). No lesion was observed in the hepatic tissue from chronically infected C3H/HePAS or DBA/2 mice. Interestingly, irrespective of the existence of hepatitis, we did not detect any T. cruzi nest in the liver of chronically infected A/J mice (Table 1) or the other mouse strains (data not shown).
The skeletal muscle of chronically infected A/J, C3H/HePAS, and C57BL/6 mice showed moderate degrees of myositis and degenerating fibers (Fig. 1 and 2E and F). Among these mouse strains, A/J mice displayed the higher number of inflammatory foci. In the striated muscle, parasite nests were observed in only 1 out of 21 chronically infected A/J mice (4.8%), while no amastigote could be detected in chronically infected C3H/HePAS mice (Table 1) or mice from the other strains (data not shown).
Most importantly, regarding the predominance of heart and liver inflammatory lesions, chronically infected C3H/HePAS and A/J mice exhibited a clearly distinct pathology, as illustrated in Fig. 4. Pathology in C3H/HePAS mice was focused on the heart, whereas the disease affected the liver of A/J mice. Moreover, no difference was observed in the intensity of heart or liver infiltrates between male and female chronically infected C3H/HePAS or A/J mice (data not shown).
FIG. 4.
Analysis of inflammatory infiltrates in the heart, liver, and striated muscle of chronically infected C3H/HePAS and A/J mice. Cumulative data of various experiments done at various times from day 120 to 600 after infection with 106 Sylvio X10/4 trypomastigotes are shown. Each dot represents one mouse and mean values are indicated by horizontal lines. Infiltrated areas were evaluated as described in Materials and Methods.
Mortality rates in C3H/HePAS and A/J mice at the chronic phase of the disease.
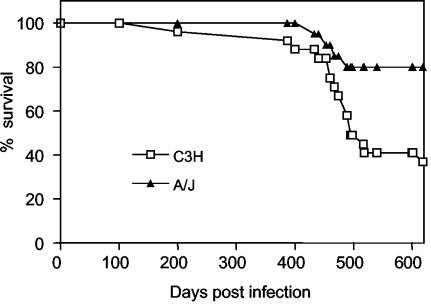
The polarity observed in the distribution of organ lesions in chronically infected C3H/HePAS and A/J mice prompted us to concentrate our studies on these two mouse strains. To evaluate if an increased mortality rate would be observed in any of the two strains as a consequence of their respective pathologies, C3H/HePAS and A/J mice infected with Sylvio X10/4 parasites were monitored for up to 20 months. As shown in Fig. 5, chronically infected C3H/HePAS mice exhibited higher cumulative mortality, which represented 63% of the animals at day 600 postinfection. At this time point, only 20% of chronically infected A/J mice were dead, a frequency similar to that of noninfected controls of the two strains (data not shown). Most deaths occurred after day 350 postinfection. The higher mortality rate observed in chronically infected C3H/HePAS mice could be a consequence of their intense heart pathology.
FIG. 5.
Cumulative mortality curves of C3H/HePAS and A/J mice infected i.p. with 106 Sylvio X10/4 trypomastigotes/mouse (20 mice/group).
Analysis of the parasite load at the early and late phases of the infection.
The murine infection with the Sylvio X10/4 clone of T. cruzi occurs in the absence of a patent acute phase. As determined by direct analysis of blood smears, parasitemias were negative in the weeks following infection, except for the very occasional observation of a single parasite in one mouse or another, irrespective of the strain (data not shown). Searching for differences in chronic-phase parasite loads that might be associated with the polar pathology observed in C3H/HePAS and A/J mice, we complemented the histopathological screening of parasite nests cited above with tissue culture in LIT medium, a parasite amplification method that reveals the presence of very low numbers of live T. cruzi organisms. Blood aliquots or fragments of perfused heart or liver tissue obtained from chronically infected mice were cultured for up to a month and were subjected to periodic microscope screening. Our results showed that 38.5% of heart samples from C3H/HePAS mice contained live T. cruzi parasites, whereas none of the samples from A/J mice were positive (Table 1). Interestingly, blood samples of chronically infected C3H/HePAS and A/J mice yielded similar frequencies of positive cultures (28.6 and 38.5%), an indication that the systemic parasite load was not very different in the two mouse strains. In contrast, concordant with the absence of T. cruzi nests in the hepatic tissue of chronically infected mice, none of the liver samples of A/J or C3H/HePAS mice resulted in parasite growth after in vitro culture. This observation was not a technical artifact due to interference of liver enzymatic content over T. cruzi expansion in culture, since parasite growth was always obtained when liver samples were cultured together with 50 LLCMK2-derived culture trypomastigote forms (positive controls). Moreover, the sensitivity of the LIT culture method for graded numbers of culture trypomastigotes was not diminished by addition of liver tissue (data not shown).
Histopathology of F1 (A/J × C3H/HePAS) and F2 (A/J × C3H/HePAS) chronically infected mice.
To investigate the inheritance patterns of T. cruzi-induced inflammatory lesions observed in the heart of C3H/HePAS mice and in the liver of A/J mice, we analyzed F1 (A/J × C3H/HePAS) mice and 71 cohorts of F2 (A/J × C3H/HePAS) mice.
Analysis of the F1 generation revealed that both heart and liver pathologies were recessive traits with reduced penetrance in the heterozygous mice (Fig. 6). In the F2 (A/J × C3H/HePAS) generation, it was possible to identify animals that displayed heart and liver pathology, animals that had only liver pathology, and animals without pathology (Fig. 7). Despite the absence of pathology in some chronically infected F1 and F2 mice, these animals were not cured as they kept titers of anti-T. cruzi IgG2a antibodies in serum as high as those observed in parental chronically infected A/J and C3H/HePAS mice (Fig. 8). Moreover, in all these mice, IgG2a-specific antibodies predominated over those of the IgG1 subclass, suggesting a similar Th1/Th2 imbalance.
FIG. 6.
Analysis of inflammatory infiltrates in the heart and liver of chronically infected F1 (A/J × C3H/HePAS) and F2 (A/J × C3H/HePAS) mice. Mice from the F1 and F2 generations, as well as from parental controls, were infected for 200 days with 106 Sylvio X10/4 trypomastigotes. Each dot represents one mouse, and median valuesare indicated by horizontal lines. Infiltrated areas were evaluated as described in Materials and Methods.
FIG. 7.
Genetic heterogeneity in heart and liver pathology of F2 (A/J × C3H/HePAS) chronically infected mice. The figure represents the correlation of heart and liver pathologies in 71 mice at day 200 of infection. Tissue inflammation is represented on each axis as the log10 of the infiltrated area.
FIG. 8.
Parasite-specific antibodies in the serum of C3H/HePAS, A/J, F1 (A/J × C3H/HePAS), and F2 (A/J × C3H/HePAS) chronically infected mice and in noninfected age-matched C3H/HePAS and A/J controls. Mice from the F1 and F2 generations, as well as from parental controls, were infected with 106 Sylvio X10/4 trypomastigotes. At day 200 after infection, mice were bled and their sera were analyzed by ELISA with T. cruzi antigen to determine the level of specific IgG2a and IgG1 antibodies.
DISCUSSION
T. cruzi infection in humans results in a lifelong host-parasite interaction with a large variety of outcomes, which range from the absence of symptoms to a selective commitment of the heart or esophagus and/or the colon in individuals suffering from cardiac or digestive forms of the disease. These distinct patterns of T. cruzi infection are thought to be due to the heterogeneity of both parasite and host. A major role of T. cruzi heterogeneity in the development of pathology was confirmed by several research groups that analyzed the disease in mice infected with different parasite isolates (1, 12, 28). However, the contribution of host heterogeneity in T. cruzi-induced pathology is still an open field for scientific investigation, particularly with respect to its genetic basis. Infections with T. cruzi clones displaying stable biological characteristics should limit the spectrum of chronic pathology patterns to those arising from host heterogeneity. Therefore, evaluating the chronic phase of the disease in different inbred mouse strains infected with a cloned T. cruzi parasite may help us understand the role of the genetic background of the host for the development of the distinct patterns of Chagas’ pathology.
In this study, we evaluated the ability of different individuals (different inbred mouse strains) to develop heart, liver, and striated muscle pathology, during the chronic phase of infection with Sylvio X10/4 T. cruzi clone. A notable observation was the finding that different mouse strains chronically infected with a single parasite clone displayed inflammatory lesions in distinct organs, with the heart and the liver being selectively affected in chronically infected C3H/HePAS and A/J mice. Thus, while the majority of chronically infected C3H/HePAS mice showed intense heart pathology, these animals exhibited very low levels of or no liver inflammatory lesions. By contrast, all chronically infected A/J mice presented intense infiltrates in the liver but no heart pathology. A/J mice also showed higher levels of inflammation in the skeletal muscles compared with chronically infected C3H/HePAS mice, but these differences were not as evident as those found for the liver. Chronically infected BALB/c and C57BL/6 mice showed a pathology pattern similar to that of A/J mice, and chronically infected DBA/2 mice showed no signs of pathology. To our knowledge, this is the first description showing that the genetic background of mice determines a clear-cut polarity in the organ targeted by T. cruzi-induced chronic pathology. The fact that Sylvio X10/4 infection in C3H/HePAS mice partially recapitulates the cardiac lesions observed in the human disease, while in the other mouse strains it determines no lesions or distinct pathology phenotypes, reinforces the convenience of this model for determining the genes involved in Chagas’ disease pathology.
Histopathological analysis of chronic infected mice also revealed the presence of amastigote nests in the hearts of C3H/HePAS mice, but not in those of A/J mice. Differences in heart parasitism in these strains were confirmed by culturing heart homogenate samples in LIT medium, a procedure that detected parasites in 38.5 and 0% of C3H/HePAS and A/J hearts, respectively. The correlation between heart parasitism and development of cardiac lesions support the current hypothesis for the pathogenesis of Chagas’ disease, according to which pathology mainly results from the immune response to locally persisting parasites (3, 13, 22, 27). This hypothesis, however, was based on studies where tissue parasitism was evaluated by immunohistochemistry or PCR, two approaches that can be criticized for the fact that parasite antigens or DNA may remain in the lesions for extended periods of time. Such an argument cannot be applied to our study, since live parasites were grown from the heart tissue or observed in the myocardium as amastigote nests with no degeneration signs. Therefore, our results reveal that the occurrence of cardiac infiltrates correlates with the presence of live T. cruzi at the heart, corroborating the notion that local parasite persistence is the primary cause of Chagas’ disease.
The intensity of heart parasitism could reflect the level of systemic parasitism or the existence of local factors that control parasite replication in the cardiac tissue. In our study, systemic parasitism was apparently similar in chronically infected C3H/HePAS and A/J mice, as positive LIT cultures were obtained from their blood at comparable frequencies. Therefore, we suggest that these mice could vary in terms of local tissue factors that operate either by promoting the colonization of the heart (8, 14, 20) or by providing innate or adaptive local effector mechanisms that result in the in situ destruction of parasites. Candidates for these hypothetical effector mechanisms could be nitric oxide production by myocytes (9), expression of class I major histocompatibility complex molecules, and presentation of parasite peptides by endothelial cells and myocytes (32) or local production of chemokines (23), among other possibilities.
The notion that local persistence of T. cruzi parasites, in the long run, propitiates a higher incidence of inflammatory infiltrates fits with our observations in the heart (and probably in the striated muscle), but it does not totally match our findings in the liver. Parasites were not detected in the hepatic tissue of chronically infected mice—neither by direct histological examination nor by culture of liver tissue in LIT medium. Most importantly, chronically infected A/J mice exhibited an intense inflammatory response in the liver that could not be associated with the presence of live parasites. A possible explanation for this discrepancy is that liver inflammation observed in these mice, instead of being the consequence of a higher local parasitism, is determined by the immune response to antigens from blood trypomastigotes that have been removed and destroyed by liver phagocytes.
To study the inheritance patterns of T. cruzi-induced chronic lesions we analyzed first- and second-generation intercrosses of the A/J × C3H/HePAS mouse strains. We observed that resistance to pathology in both heart and liver are reduced-penetrance phenotypes in the first-generation intercross. In the second generation, the lack of a strict correlation between the pathology in the two organs suggests that the genetic control of the two pathologies is not identical. In fact, the phenotype spread of the two phenotypes across the F2 generation strongly suggests that both these traits are polygenic in nature. It is therefore possible some genetic factors segregating in the cross could be common to both organ pathologies while others would be organ-pathology specific.
Our data show that development of chronic lesions after infection with the Sylvio X10/4 T. cruzi clone may depend on the genetic background of the host. Therefore, these genetic crosses will be used to perform genetic analysis and mapping the T. cruzi pathology phenotypes. This approach has been successfully followed in the identification of susceptibility genetic factors in murine models of other infectious diseases, as is the case for malaria (4).
Studies of genetic susceptibility to Chagas’ disease are scarce. Recently published work with a murine model focused on the resistance to acute T. cruzi infection, not addressing the development of chronic pathology that is the hallmark of the human disease (7). By contrast, the animal models described here may permit the identification of the genetic factors controlling resistance and susceptibility to the development of chronic-phase pathology that may bring important contributions to understand the pathogenesis of the Chagas’ disease chronic lesions in humans.
Acknowledgments
We acknowledge Rogério Silva do Nascimento and Bernardo Paulo Albe for technical support and Silvia Massironi and Thais Marques from our animal facilities for helping us with breeding of F1 and F2 mice.
Financial support was provided by FAPESP (02/03133-7) CAPES/GRICES (104.03) and CNPq.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andrade, L. O., C. R. Machado, E. Chiari, S. D. Pena, and A. M. Macedo. 1999. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol. Biochem. Parasitol. 100:163-172. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, S. G., and Z. A. Andrade. 1968. Pathology of prolonged experimental Chagas’ disease. Rev. Inst. Med. Trop. Sao Paulo 10:180-187. [PubMed] [Google Scholar]
- 3.Anez, N., H. Carrasco, H. Parada, G. Crisante, A. Rojas, C. Fuenmayor, N. Gonzalez, G. Percoco, R. Borges, P. Guevara, and J. L. Ramirez. 1999. Myocardial parasite persistence in chronic chagasic patients. Am. J. Trop. Med. Hyg. 60:726-732. [DOI] [PubMed] [Google Scholar]
- 4.Bagot, S., S. Campino, C. Penha-Goncalves, S. Pied, P. A. Cazenave, and D. Holmberg. 2002. Identification of two cerebral malaria resistance loci using an inbred wild-derived mouse strain. Proc. Natl. Acad. Sci. USA 99:9919-9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunha-Neto, E., V. Coelho, L. Guilherme, A. Fiorelli, N. Stolf, and J. Kalil. 1996. Autoimmunity in Chagas’ disease. Identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas’ cardiomyopathy patient. J. Clin. Investig. 98:1709-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engman, D. M., and J. S. Leon. 2002. Pathogenesis of Chagas heart disease: role of autoimmunity. Acta Trop. 81:123-132. [DOI] [PubMed] [Google Scholar]
- 7.Graefe, S. E., B. S. Meyer, B. Muller-Myhsok, F. Ruschendorf, C. Drosten, T. Laue, C. Steeg, P. Nurnberg, and B. Fleischer. 2003. Murine susceptibility to Chagas’ disease maps to chromosomes 5 and 17. Genes Immun. 4:321-325. [DOI] [PubMed] [Google Scholar]
- 8.Hall, B. S., and M. A. Pereira. 2000. Dual role for transforming growth factor beta-dependent signaling in Trypanosoma cruzi infection of mammalian cells. Infect. Immun. 68:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, H., J. Chan, M. Wittner, L. A. Jelicks, S. A. Morris, S. M. Factor, L. M. Weiss, V. L. Braunstein, C. J. Bacchi, N. Yarlett, M. Chandra, J. Shirani, and H. B. Tanowitz. 1999. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol. Cell. Cardiol. 31:75-88. [DOI] [PubMed] [Google Scholar]
- 10.Laguens, R. P., P. C. Meckert, and R. J. Gelpi. 1981. Chronic Chagas disease in the mouse. I. Electrocardiographic and morphological patterns of the cardiopathy. Medicina (Buenos Aires) 41:35-39. [PubMed] [Google Scholar]
- 11.Marinho, C. R., M. R. D’Imperio Lima, M. G. Grisotto, and J. M. Alvarez. 1999. Influence of acute-phase parasite load on pathology, parasitism, and activation of the immune system at the late chronic phase of Chagas’ disease. Infect. Immun. 67:308-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo, R. C., and Z. Brener. 1978. Tissue tropism of different Trypanosoma cruzi strains. J. Parasitol. 64:475-482. [PubMed] [Google Scholar]
- 13.Palomino, S. A., V. D. Aiello, and M. L. Higuchi. 2000. Systematic mapping of hearts from chronic chagasic patients: the association between the occurrence of histopathological lesions and Trypanosoma cruzi antigens. Ann. Trop. Med. Parasitol. 94:571-579. [DOI] [PubMed] [Google Scholar]
- 14.Petkova, S. B., H. B. Tanowitz, H. I. Magazine, S. M. Factor, J. Chan, R. G. Pestell, B. Bouzahzah, S. A. Douglas, V. Shtutin, S. A. Morris, E. Tsang, L. M. Weiss, G. J. Christ, M. Wittner, and H. Huang. 2000. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc. Pathol. 9:257-265. [DOI] [PubMed] [Google Scholar]
- 15.Pontes-de-Carvalho, L., C. C. Santana, M. B. Soares, G. G. Oliveira, E. Cunha-Neto, and R. Ribeiro-dos-Santos. 2002. Experimental chronic Chagas’ disease myocarditis is an autoimmune disease preventable by induction of immunological tolerance to myocardial antigens. J Autoimmun. 18:131-138. [DOI] [PubMed] [Google Scholar]
- 16.Postan, M., J. P. McDaniel, and J. A. Dvorak. 1986. Trypanosoma cruzi: constancy of clone pathogenicity for inbred mice during long-term in vitro maintenance. Trans. R. Soc. Trop. Med. Hyg. 80:659-662. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro Dos Santos, R., and L. Hudson. 1980. Trypanosoma cruzi: immunological consequences of parasite modification of host cells. Clin. Exp. Immunol. 40:36-41. [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro-Dos-Santos, R., J. O. Mengel, E. Postol, R. A. Soares, E. Ferreira-Fernandez, M. B. Soares, and L. C. Pontes-De-Carvalho. 2001. A heart-specific CD4+ T-cell line obtained from a chronic chagasic mouse induces carditis in heart-immunized mice and rejection of normal heart transplants in the absence of Trypanosoma cruzi. Parasite Immunol. 23:93-101. [DOI] [PubMed] [Google Scholar]
- 19.Said, G., M. Joskowicz, A. A. Barreira, and H. Eisen. 1985. Neuropathy associated with experimental Chagas’ disease. Ann. Neurol. 18:676-683. [DOI] [PubMed] [Google Scholar]
- 20.Scharfstein, J., V. Schmitz, V. Morandi, M. M. Capella, A. P. Lima, A. Morrot, L. Juliano, and W. Muller-Esterl. 2000. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors. J. Exp. Med. 192:1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starobinas, N., M. Russo, P. Minoprio, and M. Hontebeyrie-Joskowicz. 1991. Is TNF alpha involved in early susceptibility of Trypanosoma cruzi-infected C3H/He mice? Res. Immunol. 142:117-122. [DOI] [PubMed] [Google Scholar]
- 22.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira, M. M., R. T. Gazzinelli, and J. S. Silva. 2002. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 18:262-265. [DOI] [PubMed] [Google Scholar]
- 24.Trischmann, T., H. Tanowitz, M. Wittner, and B. Bloom. 1978. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp. Parasitol. 45:160-168. [DOI] [PubMed] [Google Scholar]
- 25.Trischmann, T. M., and B. R. Bloom. 1982. Genetics of murine resistance to Trypanosoma cruzi. Infect. Immun. 35:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vago, A. R., L. O. Andrade, A. A. Leite, D. d’Avila Reis, A. M. Macedo, S. J. Adad, S. Tostes, Jr., M. C. Moreira, G. B. Filho, and S. D. Pena. 2000. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am. J. Pathol. 156:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vago, A. R., A. M. Macedo, S. J. Adad, D. D. Reis, and R. Correa-Oliveira. 1996. PCR detection of Trypanosoma cruzi DNA in oesophageal tissues of patients with chronic digestive Chagas’ disease. Lancet 348:891-892. [DOI] [PubMed] [Google Scholar]
- 28.Vera-Cruz, J. M., E. Magallon-Gastelum, G. Grijalva, A. R. Rincon, C. Ramos-Garcia, and J. Armendariz-Borunda. 2003. Molecular diagnosis of Chagas’ disease and use of an animal model to study parasite tropism. Parasitol. Res. 89:480-486. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. 1997. Tropical disease research. UNDP/World Bank/W. H. O. special programme for research and training in tropical diseases. Thirteenth programme report, p. 113-123. World Health Organization, Geneva, Switzerland.
- 30.Wrightsman, R., S. Krassner, and J. Watson. 1982. Genetic control of responses to Trypanosoma cruzi in mice: multiple genes influencing parasitemia and survival. Infect. Immun. 36:637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, L., and R. L. Tarleton. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect. Dis. 180:480-486. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, L., and R. L. Tarleton. 1996. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp. Parasitol. 84:203-213. [DOI] [PubMed] [Google Scholar]