Abstract
Bifunctional monodispersed Fe3O4 particles coated with an ultrathin Y2O3:Tb3+ shell layer were fabricated using a facile urea-based homogeneous precipitation method. The obtained composite particles were characterized by powder X-ray diffraction, transmission electron microscopy (TEM), quantum design vibrating sample magnetometry, and photoluminescence (PL) spectroscopy. TEM revealed uniform spherical core-shell-structured composites ranging in size from 306 to 330 nm with a shell thickness of approximately 25 nm. PL spectroscopy confirmed that the synthesized composites displayed a strong eye-visible green light emission. Magnetic measurements indicated that the composite particles obtained also exhibited strong superparamagnetic behavior at room temperature. Therefore, the inner Fe3O4 core and outer Y2O3:Tb3+ shell layer endow the composites with both robust magnetic properties and strong eye-visible luminescent properties. These composite materials have potential use in magnetic targeting and bioseparation, simultaneously coupled with luminescent imaging.
Keywords: Nanocomposites, Bifunctional composite particles, Superparamagnetic behavior
Background
In modern materials science, considerable attention has been paid to the precise manipulation and development of new user-friendly methods for fabricating a range of inorganic systems in the nanoscale region. Among these inorganic systems, bifunctional magnetic-luminescent composites are particularly attractive because of their unique magnetic and luminescent properties in combination in a single particle. Each bifunctional particle normally has a paramagnetic core structural domain covered by a luminescent shell domain. Unique paramagnetic properties of iron oxides have been studied intensively for many technological applications, such as jet printing, magnetic storage media, MRI contrast enhancement, hyperthermia treatment, targeted drug delivery, and cell separation [1-8]. Therefore, iron oxides (such as γ-Fe2O3 or Fe3O4) have been considered ideal candidates for core-shell structures owing to their strong paramagnetic properties. The formation of core-shell structures is followed conventionally by an encapsulation process, where the paramagnetic core is encapsulated by the silica shell layer with embedded organic dyes [9,10] or quantum dots [11,12]. On the other hand, the direct linking of a fluorescent moiety to a magnetic core normally requires the use of a sufficiently long molecular linker to bypass any possible quenching by the ferro/paramagnetic core. Furthermore, the photobleaching and quenching of organic dyes and the instability and toxicity of QDs have seriously limited the broad applications of such core-shell structures, particularly in biomedicine. Another class of a luminescent material is lanthanide-doped inorganic composites. Lanthanide-doped composites are quite promising owing to their large Stokes shift, sharp emission spectra, high luminescence quantum yield, superior photostability, and low toxicity [13,14]. Therefore, lanthanide-doped composites have become a new generation of optical probes with great potential in biomedical imaging [13].
A combination of magnetic and luminescent properties of different ceramic materials into a single composite system might enhance their application range significantly. A unique magneto-optical composite composed of a magnetite core and coated phosphor material would have great potential in both nano- and biotechnology. Up to now, there are few reports on the preparation of multifunctional composites consisting of a magnetite core with a sol–gel-coated YVO4:Eu3+ shell layer and directly linked NaYF4:Yb3+, Er3+ nanoparticles [14,15]. Therefore, the development of a simple and reliable synthetic method for the fabrication of bimodal nanostructures with controlled morphologies and designed chemical components is still a challenge. Moreover, magneto-optical nanostructures can provide an all-in-one diagnostic and therapeutic tool, which can be used to visualize and treat various diseases simultaneously. Another exciting application of bimodal nanocomposites is in cytometry and magnetic separation, which can be controlled and monitored easily by fluorescent microscopy.
This paper proposes a facile strategy for the fabrication of bimodal nanocomposites using Fe3O4 spheres as a core and a thin Y2O3:Tb3+ layer phosphor coating as the shell structure. Morphological, structural, and chemical analyses of the synthesized nanocomposites were performed using a range of microscopy and energy-dispersive X-ray analysis techniques. As the main focus of this study, the magnetic and optical properties of synthesized nanocomposites are also discussed in detail. Moreover, the simple approach presented in this paper can be applied to the fabrication of Y2O3 thin layers doped with other rare-earth ions or even for different rare-earth host oxides. Therefore, the synthesized bimodal magneto-optical system appears to be promising for magnetic separation and the diagnostic targeting and tracking of drug delivery.
Methods
Synthesis of core-shell Fe3O4@Y2O3:Tb3+ particles
All chemical reagents used in this study were of analytical grade (Sigma-Aldrich, St. Louis, MO, USA) and used as received. Spherical magnetic Fe3O4 particles were prepared using a solvothermal method according to reported protocols [15,16]. Core-shell Fe3O4@Y2O3:Tb3+ particles were further prepared using a facile urea-based homogeneous precipitation method [17-19]. In a typical process, rare-earth nitrates (0.0005 mol, Y/Tb = 99:1 mol%) were added to 40 ml of deionized (DI) water. Subsequently, 0.3 g of urea was dissolved in the solution with vigorous stirring to form a clear solution. The as-prepared Fe3O4 particles (50 mg) were then added to the above solution under ultrasonic oscillation for 10 min. Finally, the mixture was transferred to a 50-ml flask, sealed and heated to 90°C for 1.5 h. The resulting colloidal precipitates were centrifuged at 4,000 rpm for 30 min. The precipitates were washed three times each with ethanol and DI water and dried at 70°C for 24 h under vacuum. The dried precipitates were calcined in air at 700°C for 1 h.
Physical characterization
The structure of the samples was examined by X-ray diffraction (XRD;D8 Discover, Bruker AXS GmbH, Karlsruhe, Germany) with Cu Kα radiation (λ = 0.15405 nm) and with a scan range of 20° to 60° 2θ. The morphology of the particles was characterized by field emission transmission electron microscopy (FETEM;JEM-2100 F, JEOL Ltd., Tokyo, Japan). The elemental properties of the samples were characterized by energy-dispersive X-ray spectroscopy (EDX;EMAX 6853-H, Horiba Ltd., Kyoto, Japan). Photoluminescence (PL;F-7000, Hitachi High-Tech, Tokyo, Japan) excitation and emission measurements were performed using a spectrophotometer equipped with a 150-W xenon lamp as the excitation source. Size measurements were performed using the Malvern Zetasizer Nano ZS machine (Malvern, UK). Magnetization measurements were performed using a quantum design vibrating sample magnetometer (QD-VSM option on a physical property measurement machine, PPMS 6000). All measurements were performed at room temperature.
Results and discussion
Morphology and structural properties
Figure 1 presents the overall synthesis procedure. First, magnetic Fe3O4 particles were prepared solvothermally as the cores. Second, a facile urea-based homogeneous precipitation method was used to form a thin uniform Y,Tb(OH)CO3·nH2O layer on the surface of the Fe3O4 particles. Third, bifunctional Fe3O4@Y2O3:Tb3+ composite particles with a core-shell structure were obtained after thermal treatment at 700°C for 1 h.
Figure 1.
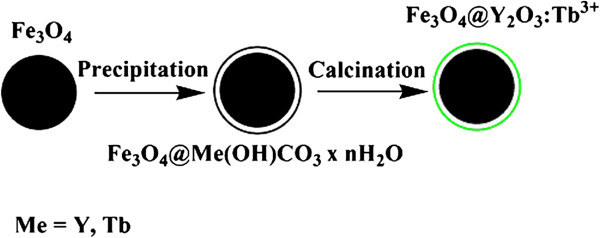
Schematic illustration of the formation of Fe3O4@Y2O3:Tb3+composite particles.
Figure 2 shows FETEM images of pure Fe3O4 microspheres with different magnifications together with the results of EDX analysis. The as-formed Fe3O4 consisted of well-separated microspheres with a mean particle size of 300 nm and a rough surface. EDX confirmed the presence of iron (Fe), oxygen (O), and carbon (C) (signal from the organic solvent).
Figure 2.
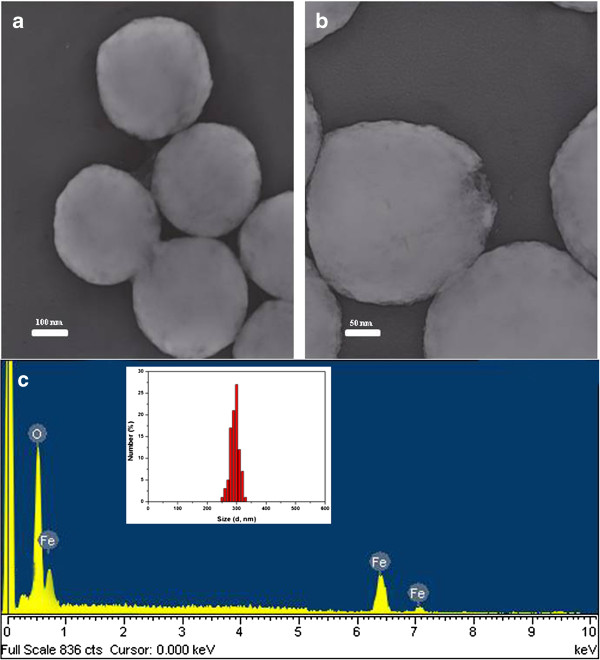
FETEM and EDX images of Fe3O4particles. (a) Low and (b) high magnifications of FETEM images and (c) EDX analysis and Fe3O4 size distribution (inset).
After coating with an ultrathin Y2O3:Tb3+ layer, the resulting core-shell Fe3O4@Y2O3:Tb3+ composite particles still maintained the spherical properties of the core Fe3O4 particles. On the other hand, the resulting Fe3O4@Y2O3:Tb3+ composite particles were slightly larger (approxi-mately 325 nm) than the bare Fe3O4 microspheres because of the additional coated layer of Y2O3:Tb3+, as shown in Figure 3. Moreover, the core-shell structure can also be observed clearly due to the small gap between the cores and shells. In addition, EDX analysis of the Fe3O4@Y2O3:Tb3+ composite particles revealed the presence of yttrium (Y), terbium (Tb), iron (Fe), and oxygen (O) in the final composite particles.
Figure 3.
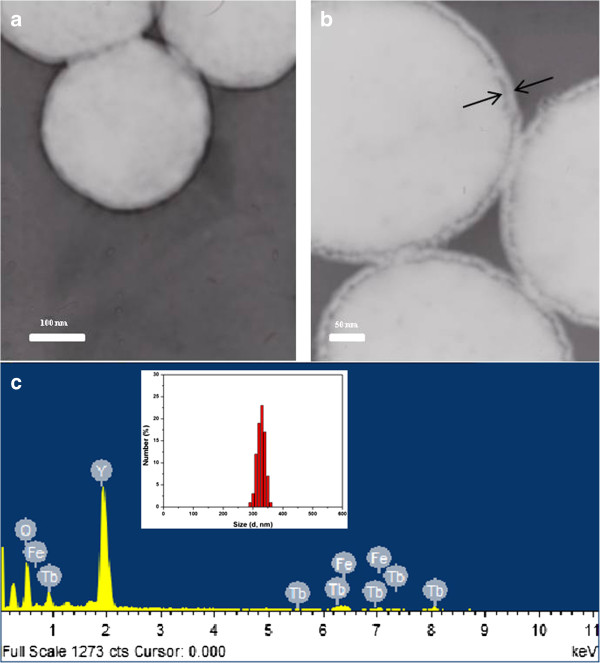
FETEM and EDX images of Fe3O4@Y2O3:Tb3+particles. (a) Low and (b) high magnifications of FETEM images and (c) EDX analysis and Fe3O4@Y2O3:Tb3+ size distribution (inset).
XRD was used to investigate the structure and composition of the synthesized particles. Figure 4 shows XRD patterns of the bare Fe3O4 and Fe3O4@Y2O3:Tb3+ composite particles. The bare magnetite cores were indexed to the face-centered cubic (Fd3m space group) magnetite structure (JCPDS no. 19–0629) [15,16]. In the case of Fe3O4@Y2O3:Tb3+ composite particles, in addition to the characteristic diffraction peaks of the cubic Fe3O4 structure, there were obvious diffraction peaks indexed to the cubic phase of Y2O3 (JCPDS no. 86–1107, marked with ●), which suggests the successful crystallization of a Y2O3:Tb3+ thin layer on the surface of Fe3O4 particles. In addition, no additional peaks for other phases were detected, indicating that no reaction had occurred between the core and shell during the annealing process.
Figure 4.
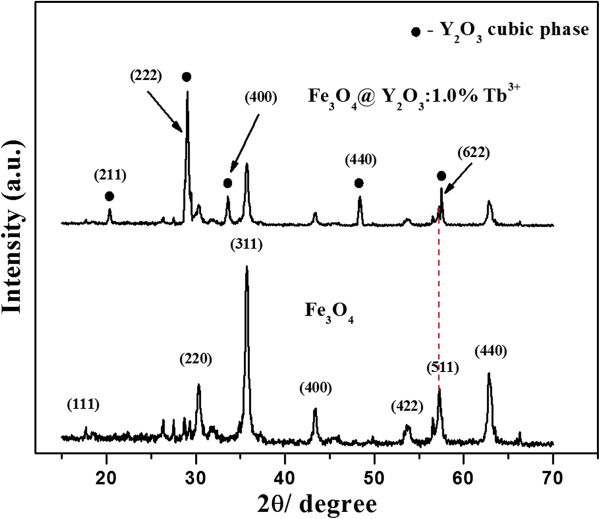
X-ray diffraction patterns of bare Fe3O4and Fe3O4@Y2O3:Tb3+particles.
Optical and magnetic properties of core-shell Fe3O4@Y2O3:Tb3+ particles
According to Li et al. [20] for the Y/Tb binary systems, homogeneous nucleation of Tb(OH)CO3 occurs in priority and then the precipitation of Y(OH)CO3 largely proceeds via heterogeneous nucleation on already-formed Tb(OH)CO3 layer. Therefore, it was assumed that Tb(OH)CO3 was firstly fully deposited (1 mol%) on a Fe3O4 surface and then doped into the Y2O3 structure (after the annealing process).
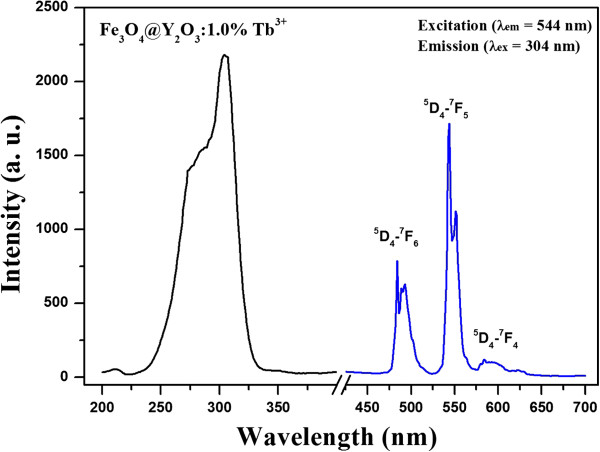
The PL properties of the core-shell Fe3O4@Y2O3:Tb3+ composite particles were characterized further by excitation and emission spectroscopy, as shown in Figure 5. The excitation peak of the Fe3O4@Y2O3:Tb3+ particles monitored at 544 nm (5D4 → 7F5 transition of Tb3+) consisted of a double-charge-transfer band, which was assigned to charge transfer from the 2p orbital of oxygen to the 4f orbital of Tb3+ between 250 and 320 nm [21,22]. The emission spectra of the Fe3O4@Y2O3:Tb3+ composite particles consisted of three easily distinguishable f-f transitions within the terbium ions. The strong green emission band with a maximum at 544 nm corresponds to the 5D4 → 7F5 transition. The blue emission at 480 to 510 nm is another characteristic of the 5D4 → 7F6 transition in Tb ions. The feeble yellow-near-red band in the range of 577 to 600 nm was assigned to the 5D4 → 7F4 transition. The characteristic emission and excitation peaks were similar to those observed in previous studies for pure Y2O3:Tb3+ nanocrystals, which suggest that the luminescent properties are maintained in the final composite particles [21,22].
Figure 5.
PL excitation and emission spectra of Fe3O4@Y2O3:Tb3+composite particles.
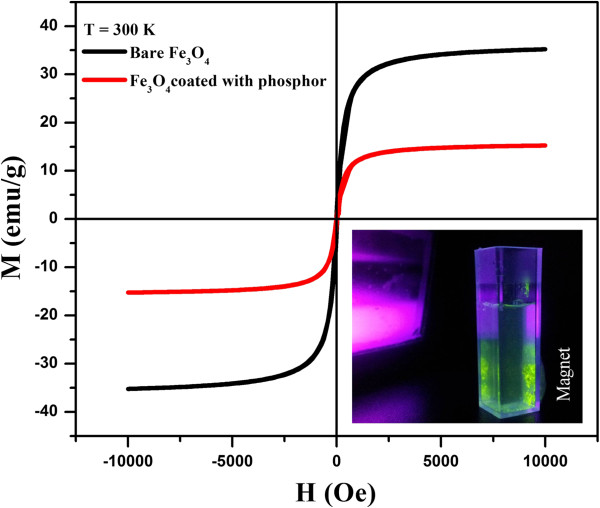
To examine the magnetic properties of the bare Fe3O4 and core-shell Fe3O4@Y2O3:Tb3+ particles, the magnetization curves were measured by QD-VSM with a magnetic field cycle between −10 and +10 kOe at 300 K, as shown in Figure 6. The saturation magnetization value of the Fe3O4@Y2O3:Tb3+ particles was 15.12 emu/g. This value is much lower than that (34.97 emu/g) of the bare Fe3O4 due to diamagnetic Y2O3:Tb3+ thin shell coating. The coercivity at 300 K was negligible, indicating typical superparamagnetic behavior. Although thin shell coating reduces the magnetization of the bare Fe3O4 significantly, the Fe3O4@Y2O3:Tb3+ composites still showed strong magnetization, which suggests their suitability for magnetic targeting and separation. The inset in Figure 6 shows that bifunctional Fe3O4@Y2O3:Tb3+ composites can be attracted easily by an external magnet and show strong eye-visible green luminescence upon the excitation of a commercially available 254-nm UV lamp. Therefore, bifunctional Fe3O4@Y2O3:Tb3+ composites exhibit good magnetic and optical properties and have potential applications in targeting and bioseparation.
Figure 6.
Room temperature magnetization curves of bare Fe3O4and Fe3O4@Y2O3:Tb3+composite particles.
Conclusions
Bifunctional Fe3O4@Y2O3:Tb3+ composites were prepared using a facile urea-based homogeneous precipitation method. These composite particles offer two distinct functionalities: an inner Fe3O4 core, which gives the composites strong magnetic properties, making them easy to manipulate magnetically, and an outer Y2O3:Tb3+ shell with strong luminescent properties. A similar approach can be used to develop certain bifunctional composites with different core-shell structures. In addition, the simple design concept for bifunctional composites might open up new opportunities in bioanalytical and biomedical applications.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All specimens used in this study and the initial manuscript were prepared by TSA. HKK and YHH added a valuable discussion and coordinated the present study as principal investigators. All authors read and approved the final manuscript.
Contributor Information
Timur Sh Atabaev, Email: atabaev@snu.ac.kr.
Hyung-Kook Kim, Email: hkkim@pusan.ac.kr.
Yoon-Hwae Hwang, Email: yhwang@pusan.ac.kr.
Acknowledgements
This work was supported by the National Research Foundation of Korea (grant no. 2012R1A1B3001357).
References
- Doyle PS, Bibette J, Bancaud A, Viovy JL. Self-assembled magnetic matrices for DNA separation in lab on a chip. Science. 2002;8:227. doi: 10.1126/science.295.5553.227a. [DOI] [PubMed] [Google Scholar]
- Pankhurst QA, Thanh NKT, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2009;8:224001. doi: 10.1088/0022-3727/42/22/224001. [DOI] [Google Scholar]
- Gao JH, Gu HW, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res. 2009;8:1097. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- Guardia P, Labarta A, Batlle X. Tuning the size, the shape, and the magnetic properties of iron oxide nanoparticles. J Phys Chem C. 2011;8:390. doi: 10.1021/jp1084982. [DOI] [Google Scholar]
- Schladt TD, Schneider K, Schild H, Tremel W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Trans. 2011;8:6315. doi: 10.1039/c0dt00689k. [DOI] [PubMed] [Google Scholar]
- Wang D, He J, Rosenzweig N, Rosenzweig Z. Superparamagnetic Fe2O3beads–CdSe/ZnS quantum dots core–shell nanocomposite particles for cell separation. Nano Lett. 2004;8:409. doi: 10.1021/nl035010n. [DOI] [Google Scholar]
- Leng Y, Sato K, Shi Y, Li JG, Ishigaki T, Yoshida T, Kamiya H. Oxidation-resistant silica coating on gas-phase-reduced iron nanoparticles and influence on magnetic properties. J Phys Chem C. 2009;8:16681. doi: 10.1021/jp902655r. [DOI] [Google Scholar]
- Gee SH, Hong YK, Erickson DW, Park MH, Sur JC. Synthesis and aging effect of spherical magnetite (Fe3O4) nanoparticles for biosensor applications. J Appl Phys. 2003;8:7560. doi: 10.1063/1.1540177. [DOI] [Google Scholar]
- Lin YS, Wu SH, Hung Y, Chou YH, Chang C, Lin ML, Tsai CP, Mou CU. Multifunctional composite nanoparticles: magnetic, luminescent, and mesoporous. Chem Mater. 2006;8:5170–5172. doi: 10.1021/cm061976z. [DOI] [Google Scholar]
- Atabaev TS, Lee JH, Lee JJ, Han DW, Hwang YH, Kim HK, Nguyen HH. Mesoporous silica with fibrous morphology: a multifunctional core–shell platform for biomedical applications. Nanotechnology. 2013;8:345603. doi: 10.1088/0957-4484/24/34/345603. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee JE, Lee J, Yu JH, Kim BC, An K, Hwang Y, Shin CH, Park JG, Kim J, Hyeon T. Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J Am Chem Soc. 2006;8:688–689. doi: 10.1021/ja0565875. [DOI] [PubMed] [Google Scholar]
- Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J Am Chem Soc. 2005;8:4990–4991. doi: 10.1021/ja0428863. [DOI] [PubMed] [Google Scholar]
- Cheng L, Yang K, Li Y, Zeng X, Shao M, Lee SH, Liu Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 2012;8:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069. [DOI] [PubMed] [Google Scholar]
- Yang P, Quan Z, Hou Z, Li C, Kang X, Cheng Z, Lin J. A magnetic, luminescent and mesoporous core–shell structured composite material as drug carrier. Biomaterials. 2009;8:4786–4795. doi: 10.1016/j.biomaterials.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Gai S, Yang P, Li C, Wang W, Dai Y, Niu N, Lin J. Synthesis of magnetic, up-conversion luminescent, and mesoporous core–shell-structured nanocomposites as drug carriers. Adv Funct Mater. 2010;8:1166–1172. doi: 10.1002/adfm.200902274. [DOI] [Google Scholar]
- Guo S, Li D, Zhang L, Li J, Wang E. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials. 2009;8:1881–1889. doi: 10.1016/j.biomaterials.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Atabaev TS, Jin OS, Lee JH, Han DW, Vu HHT, Hwang YH, Kim HK. Facile synthesis of bifunctional silica-coated core-shell Y2O3:Eu3+, Co2+ composite particles for biomedical applications. RSC Adv. 2012;8:9495–9501. doi: 10.1039/c2ra21332j. [DOI] [Google Scholar]
- Ajmal M, Atabaev TS. Facile fabrication and luminescent properties enhancement of bimodal Y2O3:Eu3+particles by simultaneous Gd3+codoping. Opt Mater. 2013;8:1288–1292. doi: 10.1016/j.optmat.2013.01.036. [DOI] [Google Scholar]
- Atabaev TS, Hwang YH, Kim HK. Color-tunable properties of Eu3+and Dy3+ codoped Y2O3 phosphor particles. Nanoscale Res Lett. 2012;8:556. doi: 10.1186/1556-276X-7-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JG, Li X, Sun X, Ishigaki T. Monodispersed colloidal spheres for uniform Y2O3:Eu3+ red-phosphor particles and greatly enhanced luminescence by simultaneous Gd3+ doping. J Phys Chem C. 2008;8:11707–11716. doi: 10.1021/jp802383a. [DOI] [Google Scholar]
- Sung JM, Lin SE, Wei WCJ. Synthesis and reaction kinetics for monodispersive Y2O3:Tb3+ spherical phosphor particles. J Eur Ceram Soc. 2007;8:2605–2611. doi: 10.1016/j.jeurceramsoc.2006.11.065. [DOI] [Google Scholar]
- Flores-Gonzales MA, Ledoux G, Roux S, Lebbou K, Perriat P, Tillement O. Preparing nanometer scaled Tb-doped Y2O3 luminescent powders by the polyol method. J Solid State Chem. 2005;8:989–997. doi: 10.1016/j.jssc.2004.10.029. [DOI] [Google Scholar]