Abstract
We compared the relative reservoir competence of European wood mice for two genospecies of Lyme disease spirochetes by analyzing susceptibility, intrinsic incubation period, and degree and duration of infectivity. Borrelia afzelii, specializing in particular reservoir hosts, is better adapted to those hosts than is the more generalist genospecies B. burgdorferi sensu stricto.
Diverse Lyme disease spirochetes infect European vector ticks. The local distribution of genospecies varies. In seven sites in Germany and France that we have examined, Borrelia afzelii consistently infects ticks more frequently than does B. burgdorferi sensu stricto (14, 17, 18). For each tick infected by B. burgdorferi sensu stricto, more than six ticks are infected by B. afzelii.
The composition of reservoir hosts in a site appears to affect the local genospecies distribution. B. afzelii is said to perpetuate mainly in rodents, whereas B. garinii is said to perpetuate in birds (7). No such host association, however, has been suggested for B. burgdorferi sensu stricto. Indeed, both murine and avian hosts are reservoir competent for B. burgdorferi sensu stricto in the northeastern United States (1, 19), where it is the sole genospecies that is pathogenic for humans. White-footed mice, Peromyscus leucopus, and American robins, Turdus migratorius, readily acquire B. burgdorferi sensu stricto infection from infected nymphal ticks, maintain the infection, and infect most ticks feeding on them. If both murine and avian hosts were similarly reservoir competent for B. burgdorferi sensu stricto in Central Europe, it would seem paradoxical that this variant is less prevalent in questing ticks than is B. afzelii, which parasitizes rodents but not birds. The differential reservoir competence of rodents for B. burgdorferi sensu stricto and B. afzelii remains unknown.
It may be that European rodents are more competent reservoir hosts for B. afzelii than for B. burgdorferi sensu stricto. Accordingly, we analyzed the susceptibility, the intrinsic incubation period, and the degree and duration of infectivity of these spirochetes in rodents. In particular, we compared the competence of a natural European reservoir rodent, the wood mouse Apodemus sylvaticus, to that of an experimental host, the Mongolian jird Meriones unguiculatus.
(Portions of this research were conducted in partial fulfillment of the requirements for a doctoral degree [to B.K.] from the Freie Universität Berlin, Berlin, Germany.)
Each of the B. afzelii and B. burgdorferi sensu stricto isolates originated from an individual naturally infected nymphal tick collected from vegetation in suburban Berlin, Germany. The genospecies of each was identified by amplification and sequence analysis of their 16S rRNA genes (18).
To infect rodents and to determine their infectivity for ticks, we permitted nymphal ticks infected by B. afzelii or by B. burgdorferi sensu stricto to feed on each host. Hosts were exposed to ticks as described previously (12). Noninfected larvae were permitted to attach simultaneously to each animal and again every 2 or 3 days throughout the first 3 weeks. This xenodiagnostic procedure was repeated every 6 weeks for 7 months thereafter. Spirochetal infection in xenodiagnostic ticks was diagnosed after molting by examining their gut contents by dark-field microscopy.
First, we determined whether B. afzelii infects rodents more readily than does B. burgdorferi sensu stricto. Twelve nymphal ticks infected by one or another spirochete genospecies were permitted to attach to wood mice, and 12 B. burgdorferi sensu stricto-infected ticks or 8 B. afzelii-infected ticks were allowed to attach to jirds. About half as many engorged ticks were recovered from the wood mice as from jirds (Table 1). At least one infected tick engorged on each of the rodents, as verified by dark-field microscopy. All hosts exposed to B. afzelii-infected ticks became infectious to at least one xenodiagnostic tick. Somewhat fewer of the rodents that had been exposed to tick-borne B. burgdorferi sensu stricto infected xenodiagnostic ticks.
TABLE 1.
Susceptibility of wood mice, A. sylvaticus, and jirds, M. unguiculatus, to infection by tick-borne B. afzelii and B. burgdorferi sensu stricto
| Host
|
Data for infecting nymphs
|
No. of hosts infecting ≥1 xenodiagnostic tick | ||||
|---|---|---|---|---|---|---|
| Species | No. | Containing genospecies | % Infected | No. | % Recovered | |
| A. sylvaticus | 6 | B. afzelii | 100 | 74 | 31.1 | 6 |
| 7 | B. burgdorferi | 70 | 84 | 20.2 | 5 | |
| M. unguiculatus | 6 | B. afzelii | 100 | 48 | 60.4 | 6 |
| 6 | B. burgdorferi | 70 | 72 | 38.9 | 5 | |
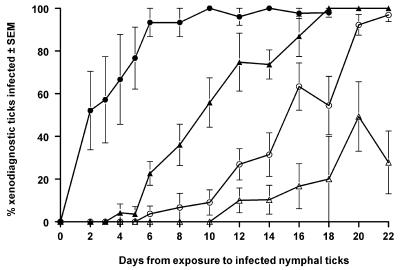
We then compared the intrinsic incubation periods of B. afzelii and B. burgdorferi sensu stricto in rodents, as measured from the time of initial exposure of a rodent to infected nymphs until the time that xenodiagnostic larvae first acquired infection from these rodents. About half of the larvae acquired B. afzelii from infected jirds as early as 2 days after infected nymphs had been permitted to attach (Fig. 1). Wood mice, in contrast, infected appreciable numbers of xenodiagnostic larvae with this spirochete 6 days after initial exposure to infected ticks. B. burgdorferi sensu stricto-infected rodents infected numerous xenodiagnostic larvae about 12 days after initial infection, and wood mice became infectious more slowly than did jirds. We conclude that the incubation period of B. afzelii in rodents is shorter than that of B. burgdorferi sensu stricto (P = 0.0024, Mann-Whitney test) and that jirds become infectious to ticks more rapidly than do wood mice (P = 0.036, Mann-Whitney test).
FIG. 1.
Infectivity to larval vector ticks of wood mice (A. sylvaticus [triangles]) and jirds (M. unguiculatus [circles]) throughout the first 3 weeks following exposure to nymphal I. ricinus ticks infected with B. afzelii (solid symbols) or B. burgdorferi sensu stricto (open symbols). SEM, standard error of the mean.
We determined how infectious rodents may become after exposure to spirochete-infected ticks. Virtually all larvae became infected when feeding on B. afzelii-infected rodents (Fig. 1). Jirds reached this degree of infectivity during the first week after exposure to infected nymphs, whereas wood mice lagged about 2 weeks behind. B. burgdorferi sensu stricto-infected rodents never became so infectious to larval ticks, and infected jirds were more infectious than wood mice during the acute first 3 weeks of infection. B. afzelii-infected wood mice appear to be more infectious to larval ticks than are B. burgdorferi sensu stricto-infected rodents.
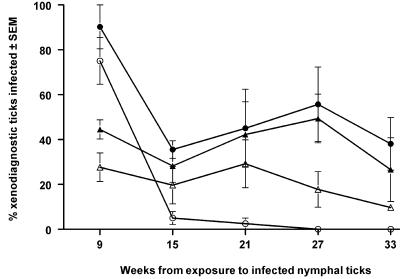
We observed the infectivity of rodents chronically infected by B. afzelii or B. burgdorferi sensu stricto. Infectivity generally has waned by about 15 weeks (Fig. 2). Although infectivity of B. burgdorferi sensu stricto in jirds waned to nil, that of the other combinations of spirochetes and hosts appeared to plateau. Particular rodents retain infectivity of B. afzelii and of B. burgdorferi sensu stricto long enough to perpetuate the infection between transmission seasons.
FIG. 2.
Duration of infectivity to larval vector ticks of wood mice (A. sylvaticus [triangles]) and jirds (M. unguiculatus [circles]) exposed to nymphal I. ricinus ticks infected with B. afzelii (solid symbols) or B. burgdorferi sensu stricto (open symbols). SEM, standard error of the mean.
Finally, we compared the cumulative potential of B. afzelii- or B. burgdorferi sensu stricto-infected rodents to infect the ticks that might parasitize them throughout an 8-month transmission season. B. afzelii-infected rodents infected at least twice as many of the larval ticks as did B. burgdorferi sensu stricto-infected rodents, regardless of the rodent species (P < 0.0001, Fisher's exact test) (Table 2). At least in the case of B. afzelii, more ticks became infected after feeding on infected jirds than on wood mice (P < 0.0001, Fisher's exact test). B. afzelii-infected rodents would infect more ticks in the course of a transmission season than would B. burgdorferi sensu stricto-infected rodents.
TABLE 2.
Efficiency of the natural reservoir host, wood mouse (A. sylvaticus) and the laboratory model, Mongolian jird (M. unguiculatus) to infect larval I. ricinus ticks with B. afzelii or B. burgdorferi sensu stricto over a period of 8 months
| Host
|
Genospecies | Data for xenodiagnostic ticks
|
||
|---|---|---|---|---|
| Species | No. | No. examined | % Infected | |
| A. sylvaticus | 6 | B. afzelii | 604 | 48.0 |
| 5 | B. burgdorferi | 339 | 21.2 | |
| M. unguiculatus | 6 | B. afzelii | 493 | 68.8 |
| 4 | B. burgdorferi | 415 | 27.5 | |
Infected wood mice remain infectious to ticks long enough to span the transmission season. Considering that such reservoir rodents encounter an infected nymphal tick only infrequently (13), such persistent infectivity would be required for efficient perpetuation of the pathogen. Indeed, naturally infected wood mice remain infectious to ticks in the laboratory for several years, which is longer than such mice normally live (2). Natural rodent hosts, therefore, may contribute infected ticks throughout the entire season of tick activity. Such an extended period of infectivity may also facilitate overwinter survival of the pathogen within its reservoir host.
Our demonstration that B. afzelii is better adapted to mice than is B. burgdorferi sensu stricto corresponds to field observations. The majority of spirochete-infected, field-derived mice harbor B. afzelii infections (4, 6). Although B. burgdorferi sensu stricto is much less prevalent in Central Europe than is B. afzelii, these spirochetes have been isolated from a broad array of hosts, including various rodents and birds as well as their attached ticks (4, 15, 17). Other genospecies, such as B. garinii and B. valaisiana, seem better adapted to birds. Indeed, isolates taken from European birds most frequently prove to be one of these spirochetes (3, 5, 8, 15). Numerous ticks acquire spirochetes from pheasants experimentally infected by B. garinii, but only few ticks do so from B. burgdorferi sensu stricto-infected pheasants (9). Whereas B. afzelii appears to be well adapted to rodents and B. garinii seems to be adapted to birds, B. burgdorferi sensu stricto is less likely to perpetuate in either of these hosts. Our observations constitute the first experimental demonstration of relative competence of particular genospecies in rodents. We conclude that the more specialized genospecies, B. afzelii, appears better adapted to rodents than is the more generalized genospecies, B. burgdorferi sensu stricto.
The B. burgdorferi sensu stricto genospecies appears to behave differently in North America than in Europe. The American B. burgdorferi sensu stricto generally infects about one-third of nymphal vector ticks. It perpetuates in birds and mice (10, 11, 16). In the rodent population, prevalence of infection becomes virtually universal. In Europe, B. burgdorferi sensu stricto infects only about 3% of questing nymphal ticks (18, 20, 21) and is far less prevalent in rodents (4, 17) and birds (15). Although they are considered the same species, their host adaptations appear to differ between continents. American B. burgdorferi sensu stricto passes readily in avian as well as rodent experimental hosts (1, 19). Although the European B. burgdorferi sensu stricto form is more closely related to its American equivalent than to B. afzelii or B. garinii, it is transmitted less efficiently. The force of transmission of B. burgdorferi sensu stricto in Europe is weaker than it is in North America.
The prevalence of B. afzelii in Central Europe exceeds that of B. burgdorferi sensu stricto but is similar to that of B. garinii (18). B. valaisiana and B. burgdorferi sensu stricto infect far fewer ticks, about 1 in 11 and 1 in 14 infected ticks, respectively. These relationships most likely reflect differences in the competence of their mutually shared reservoir hosts. Indeed, we found that one of the important European reservoir hosts, the wood mouse, is far more competent for B. afzelii than for B. burgdorferi sensu stricto. Because prevalence of infection reflects the force of transmission, which in turn reflects adaptation, B. afzelii would be better adapted than B. burgdorferi sensu stricto in much of Europe. The more specialized genospecies, B. afzelii, appears better adapted to rodents than is the more generalized genospecies, B. burgdorferi sensu stricto.
Acknowledgments
This study was supported by grant Ma 942/10-1 from the Deutsche Forschungsgemeinschaft.
Editor: D. L. Burns
REFERENCES
- 1.Donahue, J. G., J. Piesman, and A. Spielman. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 36:92-96. [DOI] [PubMed] [Google Scholar]
- 2.Gern, L., M. Siegenthaler, C. M. Hu, S. Leuba-Garcia, P. F. Humair, and J. Moret. 1994. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): duration and enhancement of infectivity for Ixodes ricinus ticks. Eur. J. Epidemiol. 10:75-80. [DOI] [PubMed] [Google Scholar]
- 3.Hanicova, K., V. Taragelova, J. Koci, S. M. Schäfer, R. Hails, A. J. Ullmann, J. Piesman, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69:2825-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu, C. M., P.-F. Humair, R. Wallich, and L. Gern. 1997. Apodemus sp. rodents, reservoir hosts for Borrelia afzelii in an endemic area in Switzerland. Zentbl. Bakteriol. 285:558-564. [DOI] [PubMed] [Google Scholar]
- 5.Humair, P.-F., D. Postic, R. Wallich, and L. Gern. 1998. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentbl. Bakteriol. 287:521-538. [PubMed] [Google Scholar]
- 6.Humair, P. F., O. Rais, and L. Gern. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33-42. [DOI] [PubMed] [Google Scholar]
- 7.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schäfer, H.-S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 8.Kurtenbach, K., M. Peacy, S. G. T. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtenbach, K., S. M. Schäfer, H.-S. Sewell, M. Peacey, A. Hoodless, P. A. Nuttall, and S. E. Randolph. 2002. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immun. 70:5893-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine, J. F., M. L. Wilson, and A. Spielman. 1985. Mice as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 34:355-360. [DOI] [PubMed] [Google Scholar]
- 11.Mather, T. N., M. L. Wilson, S. I. Moore, J. M. Ribeiro, and A. Spielman. 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130:143-150. [DOI] [PubMed] [Google Scholar]
- 12.Matuschka, F.-R., S. Endepols, D. Richter, and A. Spielman. 1997. Competence of urban rats as reservoir hosts for Lyme disease spirochetes. J. Med. Entomol. 34:489-493. [DOI] [PubMed] [Google Scholar]
- 13.Matuschka, F.-R., P. Fischer, M. Heiler, D. Richter, and A. Spielman. 1992. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J. Infect. Dis. 165:479-483. [DOI] [PubMed] [Google Scholar]
- 14.Ohlenbusch, A., F.-R. Matuschka, D. Richter, H.-J. Christen, R. Thomssen, A. Spielman, and H. Eiffert. 1996. Etiology of the acrodermatitis chronica atrophicans lesion in Lyme disease. J. Infect. Dis. 174:421-423. [DOI] [PubMed] [Google Scholar]
- 15.Olsén, B., T. G. Jaenson, and S. Bergström. 1995. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand, P. W., E. H. Lacombe, R. P. Smith, and J. Ficker. 1998. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. J. Med. Entomol. 35:270-276. [DOI] [PubMed] [Google Scholar]
- 17.Richter, D., S. Endepols, A. Ohlenbusch, H. Eiffert, A. Spielman, and F.-R. Matuschka. 1999. Genospecies diversity of Lyme disease spirochetes in rodent reservoirs. Emerg. Infect. Dis. 5:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter, D., D. B. Schlee, and F.-R. Matuschka. 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter, D., A. Spielman, N. Komar, and F.-R. Matuschka. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijpkema, S. G. T., D. Golubic, M. Molkenboer, N. Verbeek-de Kruif, and J. F. P. Schellekens. 1996. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 20:23-30. [DOI] [PubMed] [Google Scholar]
- 21.Rijpkema, S. G. T., M. J. C. H. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. P. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]