Abstract
Anti-GM1 antibodies of the immunoglobulin M (IgM) isotype are normal components of the antibody repertoire of adult human serum. Using a sensitive high-performance thin-layer chromatography (HPTLC) immunostaining assay, we found that these antibodies were absent in the umbilical vein and children <1 month of age but could be detected after 1 month of age. Although most of the children older than 6 months of age were positive, there were still a few negative children. The appearance of anti-GM1 IgM antibodies showed a perfect concordance with two well-characterized antibacterial antibodies, anti-Forssman and anti-blood group A, which indicates a similar origin. We also studied IgM reactivity with lipopolysaccharides (LPSs) from gram-negative bacteria isolated from stool samples from healthy babies and from Escherichia coli HB101 in serum from individuals of different ages. We found a positive reaction with both LPSs in all the children more than 1 month of age analyzed, even in those that were negative for anti-GM1 antibodies. Anti-GM1 IgM antibodies were purified from adult serum by affinity chromatography and tested for the ability to bind LPSs from different bacteria. This highly specific preparation showed reactivity only with LPS from a strain of Campylobacter jejuni isolated from a patient with diarrhea. We conclude that normally occurring IgM antibodies are generated after birth, probably during the immune defense against specific bacterial strains.
Antibodies reacting with GM1 are clearly associated with motor neuropathies (11, 14, 18). Although the pathogenic role of the antibodies in the disease is still uncertain, cumulative evidence suggests that they are primarily involved (1, 17, 19, 21, 22, 24). In contrast, very little information is available about how these autoantibodies are originated. Anti-GM1 immunoglobulin M (IgM) antibodies are part of the antibody repertoire of normal humans (9) and similar antibodies but with higher affinity have been found in patients with neuropathy (6, 10). Studies of anti-GM1 IgM antibodies (13) cloned from neuropathy patients have shown that they are encoded by somatically mutated diverse V genes. In contrast, although an antibody response can be obtained by immunization of animals with GM1, only low-affinity antibodies can be generated (5, 7). Consistent with these findings, all the mouse monoclonal antibodies characterized so far are encoded by genes close to the germ line configuration, with few mutations (25).
Based on comparative studies of experimentally induced versus disease-associated antibodies, high affinity has been postulated as a disease determinant factor in anti-GM1 antibodies (7), but the origin of the high-affinity antibodies is still hypothetical. Two different hypotheses have been proposed for explaining the appearance of anti-GM1 antibodies in disease, the antigen mimicry hypothesis (2, 3a, 15, 27) postulates a cross-reactive immune response originally directed to Campylobacter jejuni lipopolysaccharides (LPSs), and the binding site drift hypothesis (6, 8) proposes that the origin of disease-associated (high-affinity) antibodies is spontaneous mutations in the binding site of normally occurring antibodies. An unresolved point of the second hypothesis is the origin of normally occurring antibodies. Since the pioneering work of Springer (20), it had been widely accepted that naturally occurring antibodies recognizing defined glycans such as the Forssman and blood group antigens are produced in response to intestinal or respiratory tract bacteria. In the present paper, we present evidence indicating a similar origin for anti-GM1 IgM antibodies in healthy humans.
MATERIALS AND METHODS
Human serum.
Umbilical vein blood samples were obtained shortly after delivery. Adult blood was obtained from healthy volunteers with negative serology for common infectious diseases. After clot separation (usually less than 3 h after extraction), the serum was frozen at −70°C until use. For infants, we used serum samples extracted for neonatal screening (babies less than 1 week old) or presurgery control (children more than 1 month of age).
Glycolipids.
GM1, GD1a, and GD1b were obtained from human brains. Asialo-GM1 (GA1) was prepared by acid hydrolysis of cow brain gangliosides (3). Forssman and blood group A glycolipids were obtained from sheep erythrocytes and human A meconium, respectively. Glycolipids were purified by DEAE chromatography (26) and high-pressure liquid chromatography (HPLC) on an Iatrobeads silica gel column (23).
Bacterial growth conditions and extraction of LPS.
Campylobacter jejuni isolated from a patient with diarrhea was grown on blood agar (Columbia agar base supplemented with 5% human blood and fetal bovine serum) at 42°C for 48 h in a microaerobic atmosphere. Aerobic conditions, 37°C, 48 h, and EMB growth medium were used for Escherichia coli HB101, Salmonella enteritidis, Shigella flexneri, and cultures obtained from stool samples from the babies. Bacterial biomass was harvested and LPSs were extracted with the miniphenol-water method (16). LPS from Escherichia coli O127-B8 and Pseudomonas aeruginosa serotype 10 were from Sigma (St. Louis, Mo.).
HPTLC immunostaining.
Glycolipids (0.3 nmol each) and LPS (50 μg or amounts equivalent to 3 mg of bacteria) were separated on high-performance thin-layer chromatography (HPTLC) plates in running solvent containing chloroform-methanol-aqueous 0.2% CaCl2 (45:45:10) and 2-propanol-7% NH4(OH) (6:4), respectively, with a tank designed to obtain highly reproducible chromatograms (12). After air drying, the plates were coated by being dipped for 2 min in a 0.5% solution of polyisobutylmethacrylate (Plexigum P28; Röhm and Haas, Darmstadt, Germany) in n-hexane-chloroform (9:1). The plates were blocked with 1% bovine serum albumin in phosphate-buffered saline containing 0.05% Tween 20 (BSA-PBSt) for 1 h, incubated overnight with BSA-PBSt-diluted serum (1:10), and washed thoroughly with PBSt. Binding was detected after 2 h of incubation with BSA-PBSt-diluted (1:1,000) peroxidase-conjugated anti-human IgM (μ chain) goat antibodies (Sigma). All the incubation steps were performed at 4°C. After washing, color development was achieved in a substrate solution containing 2.8 mM 4-chloro-1-naphthol and 0.01% H2O2 in methanol-20 mM Tris-HCl buffer, pH 7.4 (1:29). The reaction was stopped after 20 min by washing the plates with PBSt.
Preparation of GM1 affinity column.
The method used, modified from that of Hirabayashi et al. (4), was previously found to be suitable for separation of anti-GM1 antibody populations (6). Briefly, 1.5 ml of methanol-aqueous 0.2 M KCl (1:1) containing 0.25 μmol of GM1 was added to 1 ml of octyl-Sepharose (Sigma). The mixture was rotated end over end for 1 h at room temperature. After removal of the solution, the gel was washed three times with 5 volumes of PBS. The remaining steps were performed at 4°C. Small portions of the gel were loaded in a calibrated column and washed with PBS. Serum (1 ml per 0.1 ml of gel) was passed through the column five times. The column was washed with PBS, and retained antibodies were eluted with 1 M KSCN in PBS (10 bed volumes) and desalted in Sephadex G-25 columns. Fractions were supplemented with BSA (1% final concentration), aliquoted, and frozen at −70°C until use.
RESULTS
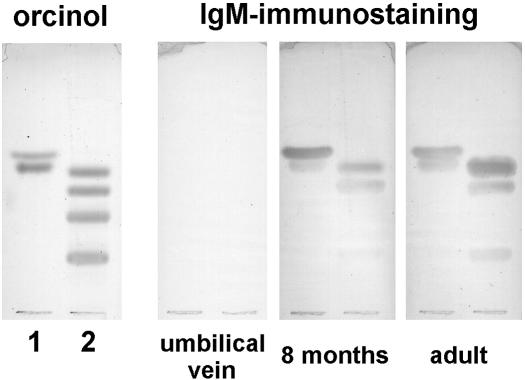
Using HPTLC immunostaining, we screened samples of normal human serum, searching for antibodies that reacted with GM1 and related glycolipids (Fig. 1). As we described previously (9), anti-GM1 IgM antibodies cross-reacting with GA1 and GD1b were detected in all the adults tested. In contrast, we failed to detect them in umbilical vein serum samples, even at very low serum dilutions (1:2). Antibody binding was observed when an appropriate amount of affinity-purified anti-GM1 antibodies from adult serum was mixed with umbilical vein serum (results not shown), indicating that if they were present, these antibodies should have been detected by the method used. Anti-GM1 antibody binding reactivity was also absent in very young babies (5 to 10 days old), but it began to be detectable after approximately 1 month of life. The incidence of these antibodies in serum samples from umbilical veins (n = 6), children 5 to 10 days old (n = 7), children 1 to 5 months old, 6 to 12 months old, and 13 to 24 months old (n = 42), and adults (n = 12) was 0, 0, 30, 88, 100, and 100%, respectively. Although most of the children older than 6 months had these antibodies, it is important to highlight that there were still some negative individuals.
FIG. 1.
Antiglycan IgM antibodies in normal human serum. Forssman and blood group A glycolipids (lane 1, upper and lower bands, respectively), and gangliosides GA1, GM1, GD1a, and GD1b (lane 2, top to bottom) were separated by HPTLC as described in Materials and Methods. Plates were incubated with the stated human serum, and after being washed, IgM antibody binding was detected with peroxidase-labeled anti-human IgM antibodies. One plate was stained with orcinol reagent for chemical detection of glycolipids.
In addition to anti-GM1 antibodies, we also searched for IgM antibodies reacting with two other glycans, the Forssman and blood group A glycolipids (Fig. 1). Comparing anti-GM1 with anti-Forssman antibodies, a perfect concordance was observed: when one reactivity was absent or present in a serum, the other was also absent or present, respectively. Similar results were observed with anti-A antibodies when only non-A individuals were considered. It have been clearly demonstrated that anti-Forssman and anti-A antibodies are produced in response to bacteria (20). Consequently, these results are strong evidence of a similar origin for normal anti-GM1 antibodies.
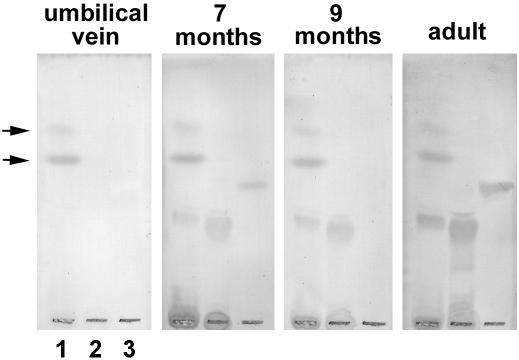
LPSs are the main glycan-carrying molecules in gram-negative bacteria. LPS from gram-negative bacteria isolated from stool samples from healthy babies and LPS from Escherichia coli HB101 were prepared and assayed for antibody binding. As shown in Fig. 2, adult serum contained IgM antibodies to both LPSs, but they were not detected in umbilical vein serum samples. Babies less than 1 week old were also devoid of anti-LPS antibodies (results not shown), and as expected, they started to appear later on. Interestingly, antibodies to LPS were detected in serum samples from children that were negative for anti-GM1 antibodies (Fig. 2). These results suggest that anti-GM1 antibodies are not related to the immune response to symbiotic bacteria.
FIG. 2.
Normal human serum IgM antibody binding to bacterial LPSs. LPSs from gram-negative bacteria isolated from stool samples from healthy babies (lane 1) and from Escherichia coli HB101 (lane 2) were prepared by the miniphenol-water method (16). Both preparations and GM1 (lane 3) were separated by HPTLC and assayed for IgM antibody binding as described in Materials and Methods. Serum samples from an umbilical vein, an adult, and two children were used. Notice the absence of anti-GM1 IgM antibody binding in the serum sample from the 9-month-old child. Arrows indicate a nonspecific spot originating from the culture medium.
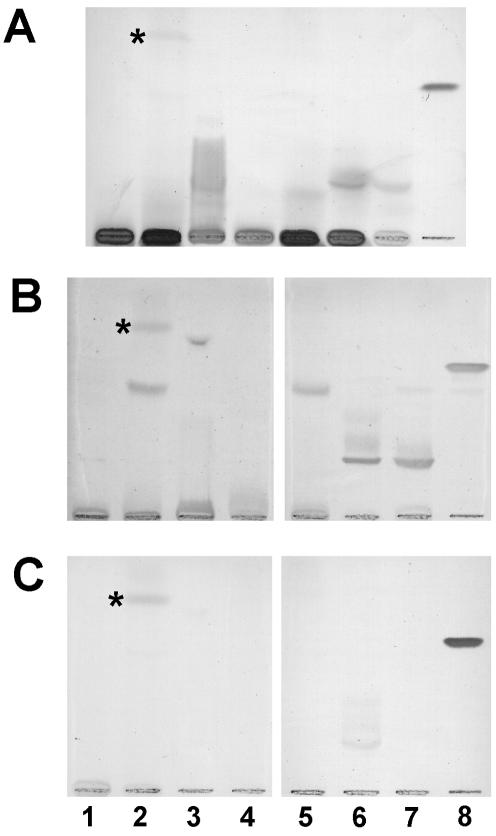
Adult anti-GM1 antibodies were purified with a GM1 column (6). The antibody preparation obtained was highly specific and did not contain anti-Forssman or anti-A antibodies (results not shown). When this preparation was assayed for binding to LPSs obtained from different bacteria, we found that most of the anti-LPS activity present in total serum disappeared (Fig. 3). However, anti-GM1 antibodies reacted with LPS from a local isolate of Campylobacter jejuni. When these LPSs were assayed with serum samples from children, we found results similar to those obtained with LPSs from normal flora bacteria; anti-LPS antibodies were not detected in samples from umbilical veins and babies less than 1 week old but were found in older children, even in those with negative reactivity to GM1 (results not shown).
FIG. 3.
Cross-reactivity of anti-GM1 antibodies with LPS from E. O127-B8 (lane 1), gram-negative bacteria isolated from stool samples from healthy babies (lane 2), Pseudomonas aeruginosa serotype 10 (lane 3), Shigella flexneri (lane 4), Salmonella enteritidis (lane 5), Campylobacter jejuni isolate 1 and C. jejuni isolate 2 (lane 6), were chromatographed together with GM1 (lane 8) and assayed for IgM antibody binding as described in Materials and Methods. HPTLC plates were incubated either with total adult serum samples (lane 7) or (B) their anti-GM1 IgM antibodies purified by affinity chromatography (C). One plate was stained with orcinol reagent (A). The asterisk indicates a nonspecific spot originating from the culture medium.
DISCUSSION
Normal adult human serum contains anti-GM1 antibodies of the IgM isotype (9). They are relatively low-affinity antibodies, cross-reacting with GA1 and GD1b and probably devoid of GM1-mediated biological activity (10). Since early experiments showed that they were not present in umbilical vein serum, we studied infant serum to search for the time of their appearance. As expected, they were also absent in babies less than 1 week old but could be detected after a few months of life. The appearance of anti-GM1 antibodies seems to be related to the immune response to bacteria, because they showed a perfect concordance (100%) with the presence of two anti-glycan IgM antibodies identified as typical antibacterial antibodies, anti-Forssman and anti-blood group A. Both antibodies are considered to originate in response to bacteria that colonize the intestinal or respiratory tract (20).
Triggering of the production of anti-GM1 antibody by bacteria can be achieved by at least two mechanisms, by polyclonal lymphocyte activation through a bystander effect of infection, if these antibodies are part of the collection of natural antibodies, and by an antigen-specific immune response. Considering that bacterial colonization of the body starts early after birth, the absence of antibodies in some children more than 1 month of age indicates that symbiotic bacteria are not involved in their generation. This idea is additionally supported by the facts that serum lacking anti-GM1 antibodies reacted with LPS obtained from gram-negative bacteria isolated from stool samples from babies and with E. coli HB101 and that affinity-purified anti-GM1 antibodies did not react with these LPSs. Although antibody concordance is a clear evidence of the antibacterial origin of the anti-GM1 antibodies, the rest of the results indicate that bacteria, at least those belonging to normal flora, are not mainly responsible for their generation.
These apparently contradictory results can be explained if we think of an infection with a particular bacterium. In order to search for a bacterial candidate that could act as the immunogen for anti-GM1 antibodies, LPSs from different bacteria were assayed for reactivity against anti-GM1 IgM antibodies affinity purified from an adult human. This highly specific antibody preparation only reacted with LPS from a C. jejuni strain insolated from the stool sample of a patient with diarrhea but not with other bacterial LPSs, including LPS from a second C. jejuni strain insolated from a different patient. Interestingly, children more than 1 month of age, even those negative for anti-GM1 antibodies, showed immunoreactivity with LPSs from both strains, highlighting the high strain specificity of anti-GM1 antibodies.
Our results indicate that normally occurring IgM antibodies are generated after birth during the immune defense against specific bacterial strains. In our study, a strain of C. jejuni was identified as a candidate for immunogenicity, but other bacteria not related to diarrhea can also be the immunogen responsible. If this bacterium is the immunogen for the antibody production, it appears that an episode of diarrhea is not necessary: in the general population, most children are positive for anti-GM1 after 1 year of life, but few of them have had the disease associated with C. jejuni infection. Subclinical or asymptomatic infection, commonly observed in developing countries (3a), could be sufficient to generate this immune response.
Finally, Guillain-Barré syndrome with anti-GM1 antibodies is clearly associated with a preceding infection with GM1 glycan-carrying strains of C. jejuni (2, 15, 27). The relationship between this fact and our results is still an open question, and the answer can shed light on the origin of anti-GM1 antibodies in disease.
Acknowledgments
This work was supported by grants (to G.A.N.) from the Ministerio de Salud (Beca Carrillo-Oñativia), SeCyT (UNC), ANPCYT, and CONICET, Argentina. M.E.A. and R.D.L. had fellowship assistance from CONICET.
We thank H. Fernandez (Universidad Austral de Chile), F. Irazoqui, J. Barra, and M. Maccioni (Universidad Nacional de Córdoba) for critical reading of the manuscript.
Editor: F. C. Fang
REFERENCES
- 1.Arasaki, K., S. Kusoniki, N. Kudo, and I. Kanasawa. 1993. Acute conduction block in vitro following exposure to anti-ganglioside sera. Muscle Nerve 16:587-593. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall, G. O., S. Fujimoto, A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect. Immun. 62:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumar, F. A., H. S. Barra, H. J. Maccioni, and R. Caputto. 1968. Sulfation of glycosphingolipids and related carbohydrates by brain preparation from young rats. J. Biol. Chem. 246:5075-5084. [PubMed] [Google Scholar]
- 3a.Glass, R. I., B. J. Stoll, M. I. Huq, M. J. Struelens, M. Blaser, and A. K. Kibriya. 1983. Epidemiologic and clinical features of endemic Campylobacter jejuni infection in Bangladesh. J. Infect. Dis. 148:292-296. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi, Y., T. Susuki, Y. Susuki, T. Taki, M. Matsumoto, H. Higashi, and S. Kato. 1983. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J. Biochem. 94:327-330. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima, I., O. Nakamura, and T. Tai. 1992. Antibody responses to ganglio-series gangliosides in different strains of imbred mice. Mol. Immunol. 29:625-632. [DOI] [PubMed] [Google Scholar]
- 6.Lopez, P. H. H., R. D. Lardone, F. J. Irazoqui, A. Villa, M. Di Egidio, G. Zaisar, R. E. P. Sica, and G. A. Nores. 2001. Variable patterns of anti-GM1 IgM antibody populations defined by affinity and fine specificity in patients with motor syndromes: evidence for their random origin. J. Neuroimmunol. 119:131-136. [DOI] [PubMed] [Google Scholar]
- 7.Lopez, P. H. H., A. M. Villa, R. E. P. Sica, and G. A. Nores. 2002. High affinity as a disease determinant factor in anti-GM1 antibodies: comparative characterization of experimentally-induced vs. disease-associated antibodies. J. Neuroimmunol. 128:69-76. [DOI] [PubMed] [Google Scholar]
- 8.Lopez, P. H. H., R. D. Lardone, F. J. Irazoqui, M. Maccioni, and G. A. Nores. 2002. The origin of anti-GM1 antibodies in neuropathy: the “binding site drift” hypothesis. Neurochem. Res. 27:687-695. [DOI] [PubMed] [Google Scholar]
- 9.Mizutamari, R. K., H. Wiegandt, and G. A. Nores. 1994. Characterization of anti-ganglioside antibodies present in normal human plasma. J. Neuroimmunol. 50:215-220. [DOI] [PubMed] [Google Scholar]
- 10.Mizutamari, R. K., L. J. Kremer, E. A. Basile, and G. A. Nores. 1998. Anti-GM1 ganglioside IgM-antibodies present in human plasma: affinity and biological activity changes in a patient with neuropathy. J. Neurosci. Res. 51:237-242. [DOI] [PubMed] [Google Scholar]
- 11.Nobile-Orazio, E., M. Carpo, G. Legname, N. Meucci, S. Sonnino, and G. Scarlatto. 1990. Anti-GM1 IgM antibodies in motor neuron disease and neuropathies. Neurology 40:1747-1750. [DOI] [PubMed] [Google Scholar]
- 12.Nores, G. A., R. K. Mizutamari, and D. M. Kremer. 1994. Chromatographic tank designed to obtain high reproducible high-performance thin-layer chromatograms of gangliosides and neutral glycosphingolipids. J. Chromatogr. A 686:155-157. [Google Scholar]
- 13.Paterson, G., G. Wilson, P. G. E. Kennedy, and H. J. Willinson. 1995. Analysis of anti-GM1 ganglioside IgM antibodies cloned from motor neuropathy patients demonstrates diverse V region gene usage with extensive somatic mutations. J. Immunol. 155:3049-3059. [PubMed] [Google Scholar]
- 14.Pestronk, A. 1991. Motor neuropathies, motor neuron disorders and antiglycolipid antibodies. Muscle Nerve 14:927-936. [DOI] [PubMed] [Google Scholar]
- 15.Prendergast, M. M., A. J. Lastovica, and A. P. Moran. 1998. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect. Immun. 66:3649-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prendergast, M. M., T. U. Kosunen, and A. P. Moran. 2001. Development of an immunoassay for rapid detection of ganglioside GM1 mimicry in Campylobacter jejuni strains. J. Clin. Microbiol. 39:1494-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, M., H. J. Willinson, A. Vincent, and J. Newson-Davis. 1995. Multifocal motor neuropathy human sera block distal motor nerve conduction in mice. Ann. Neurol. 38:569-576. [DOI] [PubMed] [Google Scholar]
- 18.Sadiq, S. A., F. P. Thomas, K. Kilidireas, S. Protopsaltis, A. P. Hays, K. W. Lee, S. N. Romas, N. Kumar, L. van den Berg, M. Santoro, D. J. Lange, D. S. Younger, R. E. Lovelace, W. Trojaborg, W. H. Sherman, J. R. Miller, J. Minuk, M. A. Fehr, R. I. Roelofs, D. Hollander, F. T. Nichols, H. Mitsumoto, J. J. Keller, T. R. Swift, T. L. Munsat, and N. Latov. 1990. The spectrum of neurologic disease associated with anti-GM1 antibodies. Neurology 40:1067-1072. [DOI] [PubMed] [Google Scholar]
- 19.Santoro, M. A., A. Uncini, M. Corbo, S. M. Staugaitis, F. P. Thomas, A. P. Hays, and N. Latov. 1992. Experimental conduction block induced by serum from a patient with anti-GM1 antibodies. Ann. Neurol. 31:385-390. [DOI] [PubMed] [Google Scholar]
- 20.Springer, G. F. 1971. Blood-group and Forssman antigenic determinants shared between microbes and mammalian cells. Prog. Allergy 15:9-77. [PubMed] [Google Scholar]
- 21.Takigawa, T., H. Yasuda, R. Kikkawa, Y. Shigeta, T. Saida, and H. Kitasato. 1995. Antibodies against GM1 ganglioside affect K+ and Na+ currents in isolated rat myelinated nerve fibers. Ann. Neurol. 37:436-442. [DOI] [PubMed] [Google Scholar]
- 22.van Sorge, N. M., L. H. van den Berg, K. Geleijns, J. A. van Strijp, B. C. Jacobs, P. A. van Doorn, J. H. J. Wokke, J. A. J. van de Winkel, J. H. W. Leusen, and W. L. van der Pol. 2003. Anti-GM1 IgG antibodies induce leukocyte effector functions via Fcγ receptors. Ann. Neurol. 53:570-579. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe, K., and Y. Arao. 1981. A new solvent system for the separation of neutral glycosphingolipids. J. Lipid Res. 22:1020-1026. [PubMed] [Google Scholar]
- 24.Weber, F., R. Rüdel, P. Aulkemeyer, and H. Brinkmeier. 2000. Anti-GM1 antibodies can block neuronal voltage-gated sodium channels. Muscle Nerve 23:1414-1420. [DOI] [PubMed] [Google Scholar]
- 25.Weng, N., E. Ritter, E. Yucel, D. Zhang, G. Ritter, and D. M. Marcus. 1994. Specificity and structure of murine monoclonal antibodies against GM1 ganglioside. J. Neuroimmunol. 55:61-68. [DOI] [PubMed] [Google Scholar]
- 26.Yu, R. K., and R. W. Ledeen. 1972. Gangliosides of human, bovine and rabbit plasma. J. Lipid Res. 13:680-687. [PubMed] [Google Scholar]
- 27.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and M. Miyatake. 1993. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]