Abstract
Using a metacyclic promastigote ear infection model of cutaneous leishmaniasis, we examined the phenotype, parasite load, and cytokine production of dendritic cells in the skin and draining lymph nodes of resistant C57BL/6J and susceptible BALB/c mice. Five dendritic cell populations were isolated from the skin and lymph nodes, and the main difference between the groups of mice was an increased number of plasmacytoid dendritic cells in the lymph nodes of the susceptible mice. Although similar cell types were present in the skin emigrants of both strains, there was a 10-fold larger number of cells in BALB/c mouse skin early in infection than in C57BL/6J mouse skin. None of the dendritic cells in the lymph nodes harbored parasites until 3 weeks after infection, with the Langerhans cells having the largest load and the plasmacytoid dendritic cells having the smallest load but the longest lasting infection. Although parasites could be detected in the lymph nodes a few hours after infection, none of the skin emigrants harbored parasites, indicating that they are not the vehicle that ferries the parasites from the skin to the lymph nodes. The presence of larger numbers of plasmacytoid cells in infected BALB/c mice, the more protracted infection of these cells, and their production of alpha interferon point to a complex and important role for the plasmacytoid cells in leishmaniasis.
Leishmania species are obligatory intracellular parasites of the mononuclear phagocyte system. For these organisms, macrophages perform dual functions, as they are both the permissive host for the parasite and the effector arm of a successful T-cell-mediated immune response (1).
The clinical manifestations of human cutaneous leishmaniasis display a spectrum of disease severity, which can be reproduced in the laboratory by the infection of different inbred strains of mice with Leishmania major. In particular, two strains, the highly susceptible BALB/c strain and the resistant C57BL/6J strain, have been widely used to study the biology of the host response to infection. With this system, the marked polarization of the T-helper cell immune responses observed upon infection has been shown to determine the severity of disease. BALB/c mice produce Th2-type cytokines, in particular interleukin-4 (IL-4) and IL-10, which have been shown to be associated with disease progression and susceptibility (reviewed in reference 30). In contrast, recovery from infection of resistant C57BL/6J mice depends upon the induction of a Th1-type response, resulting in the activation of macrophages and the killing of the intracellular organisms.
However, the effective eradication of microbial pathogens requires interplay between the adaptive and innate immune systems (3, 33). Dendritic cells (DCs) are a unique population of circulating leukocytes that specialize in the uptake, transport, and presentation of antigen to naïve T cells (3, 16, 34). DCs interact with microbial pathogens, including L. major, through pattern recognition receptors such as the Toll-like receptors (10, 19, 24). It has been suggested that DC subsets, through their interactions with different pathogens and their products, may be modified to direct the differentiation of T cells into Th1 or Th2 cells (6, 34).
The available data support an important role for DCs in leishmaniasis because they can be infected in vitro, they were shown to transport amastigotes from lesions to the draining lymph nodes and to harbor long-term persistent parasites in otherwise immune animals, and they protected BALB/c mice from infection (when pulsed with antigen) (12, 22, 23, 39). However, much of this information was obtained before it became clear that DCs are phenotypically and functionally heterogeneous. More recently, an analysis of distinct populations of DCs revealed that not all DC subtypes are equally susceptible to infection with L. major amastigotes, at least in vitro (12). Moreover, the least Leishmania-permissive CD8+ DCs were the only producers of IL-12, the cytokine associated with the induction of Th1 immune responses (12). These data prompted us to undertake an in-depth investigation of the in vivo interaction of L. major parasites with the different subpopulations of DCs derived from susceptible BALB/c and resistant C57BL/6J mice.
We used an ear infection model (4, 5) to investigate whether (and which type of) DCs are infected in vivo in the skin and draining lymph nodes from the very early stages of infection and throughout the course of disease. We also examined the role of DC subpopulations in parasite dissemination from the skin to the draining lymph nodes.
MATERIALS AND METHODS
Mice.
BALB/c and C57BL/6J female mice of 6 to 7 weeks of age were obtained from the specific-pathogen-free animal breeding facility at The Walter and Eliza Hall Institute of Medical Research and were subsequently maintained in a conventional animal facility. The mice were 7 to 8 weeks of age at the commencement of experiments.
Parasites.
The parasites used in these studies were of the cloned line V121 derived from the L. major human isolate LRC-L137 (MHOM/IL/67/Jericho II) and were obtained from the WHO Reference Center for Leishmaniasis, Jerusalem, Israel. Promastigotes were maintained in vitro at 26°C in M199 medium supplemented with 10% fetal bovine serum (HyClone, South Logan, Utah). For infection of mice, the parasites were grown in biphasic blood agar medium (25). In order to keep virulent stocks of L. major for infection purposes, we maintained the parasites in CBA/H-nu/nu mice and cultured them in vitro for a maximum of 4 weeks.
Infection and monitoring of disease pattern.
For infections, L. major V121 metacyclic promastigotes were prepared as previously described (8). Briefly, a primoculture initiated with 5 × 105 logarithmic-phase promastigotes per ml was grown for 6 days and then washed with phosphate-buffered saline, pH 7.3, before incubation with peanut agglutinin (Vector Labs) at a final concentration of 100 μg/ml. After 30 min at room temperature, the agglutinated nonmetacyclic parasites were removed by centrifugation, and the metacyclic promastigotes remaining in suspension were washed and resuspended at 103/10 μl for injection into mice (31, 32).
For each experiment, infected and naïve groups of 24 to 50 mice were used. After they were anesthetized with an inhalant anesthetic, methoxyflurane (Medical Development Australia Pty. Ltd.), the mice were injected in the pinna of the ear with 103 V121 metacyclic promastigotes in 10 μl of saline. Control mice received a similar volume of phosphate-buffered saline.
Ear lesion development was assessed weekly by measurement of the diameter of the lesion, essentially as previously described (20). Lesions were assigned a score from one to four, with one representing a small localized swelling and four representing a diameter of 10 mm (20). Disease progression was expressed in mean lesion scores plus standard deviations.
At weeks 1, 3, 6, 9, and 12 postinfection, DC subpopulations were purified from the auricular lymph nodes that drained the injection site and were analyzed for cytokine production (with the exception of week 1, when only plasmacytoid predendritic cells [PDCs] and mixed conventional DCs [CDCs] were examined) and parasite load as described below.
For examination of the time required for transmigration of the parasites from the skin to the draining auricular lymph nodes, in vitro ear explant cultures and parasite load assays were performed with DCs and macrophages collected at days 3, 5, 7, and 21 postinfection, as described below.
DC isolation.
At each time point, PDCs and CDCs were isolated from the draining auricular lymph nodes of Leishmania-infected and naïve mice as described previously (13, 40). Briefly, the tissue was cut into small fragments and digested at room temperature with 1 mg of collagenase type II (Worthington Biochemical, Freehold, N.J.)/ml and 0.1 M DNase I (Boehringer Mannheim, Mannheim, Germany) in 6 ml of RPMI medium containing 2% fetal bovine serum. Frequent mixing at room temperature for 25 min was sufficient to disperse the cells, after which 600 μl of 0.1 M EDTA, pH 7.2, was added and stirred continuously to break up the DC-T-cell conjugates. Undigested fibrous material was removed by filtration through a 70-μm-pore-size cell strainer (Falcon) before isolation of the low-density cells by use of 1.082 g of Nycodenz AG (Nycomed Pharma AS, Oslo, Norway)/cm3. The depletion of non-DC-lineage cells was then completed by using a cocktail of monoclonal antibodies and anti-rat immunoglobulin-coupled magnetic beads (Dynabeads; Dynal, Oslo, Norway). The monoclonal antibody cocktail (10 μl/106 cells) used contained the following: anti-CD3, KT3-1.1; anti-Thy-1, T24/31.7; anti-Gr-1, RB68C5; anti-B220, RA36B2; and anti-erythrocyte, TER119 (all used at optimal levels for maximum staining). Notably, for purification of B220+ PDCs, the anti-B220 antibody RA36B2 was replaced with the anti-CD19 antibody ID3. In the presence of propidium iodide, the remaining lymph node DC preparation was then subjected to presorting to remove autofluorescent cells (mostly macrophages, as determined by microscopic examination) before immunofluorescent labeling and analysis by flow cytometry (MoFlo; Cytomation Inc., Fort Collins, Colo.). For purification and segregation of the CDC subpopulations, anti-CD8α (YTS169.4)-Cy5, anti-CD205 (Dec-205; clone NLDC-145)-fluorescein isothiocyanate, and anti-CD11c (N418)-phycoerythrin antibodies were used. For definition of the discrete population of CD11cint CD45RAhi PDCs, anti-CD11c (N418)-fluorescein isothiocyanate and anti-CD45RA (14.8)-phycoerythrin antibodies were used. Reanalyses of the CDCs and PDCs were routinely performed with sorted populations to ensure that the purity was more than 95 and 98%, respectively, before further differentiation and activation experiments were conducted.
Limiting dilution analysis of parasite load.
The parasite burden of the total lymph node and of each sorted DC subpopulation was determined by limiting dilution analysis (36). The total number of cells was determined, and 1 × 105 to 3 × 106 cells/ml from each cell suspension was prepared in Schneider's Drosophila medium (Life Technologies, Rockville, Md.) containing 10% fetal bovine serum and was titrated in a 96-well plate, using threefold dilutions. After 5 to 10 days in culture at 26°C, the highest dilution containing parasites was determined and the parasite burden per 106 cells was calculated.
In vitro cell migration from mouse ear explants.
At days 3, 5, 7, and 21 postinfection, ears were removed from BALB/c and C57BL/6J mice, cleared of hair, and washed in 70% ethanol before being placed ventral side down upon a folded cloth to dry. Once dry, each dorsal skin layer was separated from the cartilage and placed split side down on 1 ml of Dulbecco's modified Eagle's medium containing 10% fetal bovine serum for 4 h to remove residual erythrocytes. After 4 h, each ear was moved to a fresh well containing a 13-mm-wide glass coverslip (Menzel-glaser) to which adherent macrophages could bind. The tissue samples were incubated for 24 h at 37°C in a humidified 10% CO2 in-air incubator in order to eliminate the many non-DCs that migrated into the culture medium. After 24 h, each ear explant was transferred to a fresh well containing a coverslip (Menzel-glaser) and incubated for a further 48 h, enabling migratory DCs to move into the medium. The adherent cells, primarily macrophages, that had attached to the coverslips were fixed, stained with 10% Giemsa stain (Merck Pty. Ltd., Victoria, Australia), and then examined for the presence of intracellular parasites. The nonadherent DCs were either harvested from the supernatant and examined as unsorted DCs (at days 3 and 5 postinfection) or sorted (at days 7 and 21 postinfection) by the monoclonal antibody selection process and flow cytometry and then cultured for limiting dilution analysis as described above. Unsorted DCs were incubated for 2 h on 13-mm-wide coverslips that were precoated with 10 μg of anti-major histocompatibility complex (MHC) class II (N22) monoclonal antibody/ml; bound cells were then gently fixed, stained, and examined as described above.
In vitro infection of sorted CDCs and PDCs with V121 amastigotes.
After sorting, DC populations were suspended in RPMI 1640 medium at a concentration of 106 cells/ml, and 100 μl of the suspension was added to each well of a 96-well plate. V121 amastigotes were added to the wells at a ratio of 10 parasites to 1 cell before incubation overnight at 37°C with 10% CO2. Viability rates were examined by ethidium bromide-acridine orange staining. Cells were bound to the MHC class II monoclonal antibody as described above before fixation and staining with Wright-Giemsa stain. The infection and attachment rates were monitored by counting of at least 200 cells in duplicate samples.
In vitro differentiation and activation of CDCs and PDCs by use of cytokines.
At each time point, sorted subpopulations of CDCs and PDCs were incubated with various cytokine and bacterial combinations in order to determine their abilities to stimulate IL-12, alpha interferon (IFN-α), and IFN-γ production as described previously (14), using the following reagents: for IL-12, 200 U of murine recombinant granulocyte-macrophage colony-stimulating factor (gift from Immunex Corp., Seattle, Wash.)/ml, 20 ng of murine recombinant IFN-γ (rIFN-γ) (Pepro Tech Inc., Rocky Hill, N.J.)/ml, 100 U of murine rIL-4 (gift from Immunex Corp.)/ml, and 0.5 μM fully phosphorothioated CpG motif according to a published sequence, Cp1668 (GeneWorks Pty. Ltd, Adelaide, Australia); and for IFN-γ, 10 ng of murine rIL-12p70 (R&D Systems, Minneapolis, Minn.)/ml and 10 ng of murine rIL-18 (Pepro Tech Inc.)/ml.
Sorted DCs (5 × 105 cells/ml) were cultured in 96-well round-bottomed tissue culture plates in a final volume of 200 μl of modified RPMI 1640 medium containing 10% fetal bovine serum, 5 × 10−5 M 2-beta-mercaptoethanol (Univar), and cytokine stimuli. Depending upon the cytokine being investigated, cells were incubated for 24 to 72 h at 37°C with 10% CO2 before the collection of supernatants and the analysis.
Quantitation of cytokine production.
The analysis of IL-12 and IFN-γ production in culture supernatants was carried out by two-site enzyme-linked immunosorbent assay (ELISA) as previously described (14). IFN-α was quantitated in the supernatants taken from DCs stimulated with CpG. Briefly, 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories) were coated with the R2-9A5 (anti-mouse IL-12 p70; American Type Culture Collection), C15.6 (anti-mouse IL-12 p40; BD PharMingen), RMMA-1 (anti-mouse IFN-α; PBL Biomedical Labs), or R4-6A2 (anti-mouse IFN-γ; BD PharMingen) capture antibody. Serial twofold dilutions of all supernatants were used initially to determine the four optimal dilutions, which were then selected for each ELISA. The IL-12, IFN-α, and IFN-γ standards used were recombinant mouse IL-12 (Pepro Tech Inc.), IFN-α (PBL Biomedical Laboratories), and IFN-γ (Pepro Tech Inc.), respectively. Cytokine binding was detected by use of biotinylated C17.8 (anti-mouse IL-12 p70 and p40; hybridoma provided by L. Schofield, The Walter and Eliza Hall Institute) and XMG1.2 (anti-mouse IFN-γ; BD PharMingen) monoclonal antibodies. For IFN-α, a rabbit polyclonal antiserum against IFN-α (PBL Biomedical Laboratories) was used. For IL-12 and IFN-γ, detection was achieved with a streptavidin-horseradish peroxidase conjugate (Amersham Pharmacia Biotech) followed by 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS; Sigma-Aldrich) and 0.001% hydrogen peroxide (Ajax Chemicals) in 0.1 M citric acid, pH 4.2. Plates were scanned at an optical density of 405 to 490 nm. For the detection of IFN-α, a peroxidase-conjugated goat anti-rabbit antibody was followed by 3,3′,5,5′-tetramethylbenzidine and 0.02% peroxidase in a citric acid buffer (TMB peroxidase substrate system; KPL). Readings were recorded at 450 nm.
Nitric oxide production by DC populations.
Cultured supernatants collected from in vitro differentiation assays and from the cells stimulated with the cytokines described above were also used to measure nitrite contents. Fifty microliters of supernatant was incubated for 5 min at room temperature with an equal volume of the colorimetric Griess reagent [0.5% sulfanilamide and 0.05% N-(1-napthyl)ethylenediamine dihydrochloride in phosphoric acid] in round-bottomed 96-well plates. The absorbance at 540 nm was measured, and the nitrite content was quantitated by comparison with a standard curve generated with NaNO2 in the range of 0 to 220 μM.
RESULTS
Disease pattern in BALB/c and C57BL/6J mice infected with V121 in the ear pinna.
In order to examine the functions of DCs during the course of cutaneous leishmaniasis in a low-dose model of ear infections, we infected susceptible BALB/c and resistant C57BL/6J mice intradermally with 103 L. major V121 metacyclic promastigotes. For this series of experiments, mice were infected for 1, 3, 6, 9, or 12 weeks, at which point they were killed and their DCs were purified and characterized.
The recorded weekly lesion measurements showed the expected pattern of lesion development (Fig. 1). The BALB/c mice developed small lesions at 1 to 2 weeks postinfection which grew to nonhealing, large, open ulcers as the course of infection progressed. In contrast, the genetically resistant C57BL/6J mice developed moderate swelling 4 weeks after infection, with resolution occurring by week 10 to 12. These disease profiles were concordant with the disease pattern described originally by Belkaid et al. (4, 5), who used the Friedlin strain of L. major, and confirmed in our laboratory with both the Friedlin strain and the cloned line V121 (2).
FIG. 1.
Disease pattern in BALB/c and C57BL/6J mice infected intradermally in the ear with 103 L. major V121 metacyclic promastigotes. The mean lesion scores and standard deviations are shown as a function of time. Gray circles, BALB/c mice; black triangles, C57BL/6J mice. The data represent at least 100 mice for the 1- to 6-week time points and at least 50 mice for the 7- to 12-week time points.
Purification and characterization of DC populations from the auricular lymph nodes of L. major-infected mice.
At 1, 3, 6, 9, and 12 weeks postinfection, PDCs and DCs were isolated from the draining auricular lymph nodes of infected and naïve BALB/c and C57BL/6J mice. A set of cell surface markers, including Langerin and MHC II fluorescence, was initially used as the selection criterion for the various DC populations, based on previous studies from our laboratory (12, 13). For the purpose of DC collection in these experiments, a more conservative gating system was applied to the populations to ensure purity.
For purification of the PDCs, the selection criteria involved the simultaneous sorting of cells carrying intermediate levels of CD11c and high levels of CD45RA (Fig. 2A). From the same flow cytometry experiment, a mixed population of CDCs was also obtained (Fig. 2A). For the CDCs, the expression of CD8 and CD205 was used to isolate the subpopulations expressing these markers from the CD11c-positive population. By this criterion, the cells could be divided into the following four DC populations (Fig. 2B): CD8int Dec-205hi (Langerhans cells [LCs]), CD8hi Dec-205int (CD8H+), CD8int Dec-205int (dermal DCs [DDCs]), and CD8− Dec-205− double-negative cells (−/−). Notably, the dot plots depicting the sorted DC populations from naïve BALB/c and C57BL/6J mice were typically well defined, with most cells falling within the conservative gate boundaries (Fig. 2A), while the infected groups tended to have a larger number of cells outside the gates (Fig. 2B). These outliers may be indicative of DC activation by infection, rather than distinct subpopulations, as determined by their surface marker phenotypes (S. Henri, unpublished data).
FIG. 2.
Representative dot plots of CD11c+ DC populations purified from naïve and infected BALB/c and C57BL/6J mice. (A) Sorted naïve BALB/c PDCs and unsorted CDCs. (B) Sorted infected C57BL/6 mature CDCs. (−/−), double-negative (CD8− Dec-205−) cells; CD8H+, CD8hi Dec-205int cells; DDC, CD8int Dec-205int cells; LC, CD8int Dec-205hi cells.
Three of the DC populations purified from the draining lymph nodes were similar to those isolated from the spleen (26, 40, 41), while the LC and DDC populations were consistent with those observed for the skin.
In contrast to the case in naïve mice, the total cellularity of the lymph nodes in infected BALB/c and C57BL/6J mice increased during the progression of disease. This increase correlated with the increased number of DCs isolated. Although the BALB/c and C57BL/6J mice had similar mature DC profiles at each time point, there were distinct differences observed for the PDCs. For the purified PDC populations, the naïve and infected groups had similar values within each mouse strain, but there were differences between the strains; in C57BL/6J mice, the ratio of DCs to PDCs was about 1:1, whereas in BALB/c mice the ratio was 1:3.
Parasite load in DC populations isolated from draining lymph nodes.
A controversial issue in the cutaneous leishmaniasis literature is the nature of the cells in the skin that harbor and transport parasites to the draining lymph nodes over the entire period of disease (21, 29). We set out to examine systematically the parasite load in the different DC subpopulations isolated from the draining lymph nodes of susceptible BALB/c and resistant C57BL/6J mice at weeks 1, 3, 9, and 12 postinfection with 103 L. major V121 metacyclic promastigotes. In vitro cultures of the isolated cells were used in order to check if the various DC populations harbored parasites and to quantitate the parasite burdens (Fig. 3).
FIG. 3.
Parasite loads per 106 lymph node cells and in individual DC populations isolated from the draining lymph nodes 1, 3, 9, and 12 weeks after infection with 103 L. major V121 metacyclic promastigotes in the ear pinna of 24 to 50 BALB/c (A) and C57BL/6J (B) mice. (−/−), double-negative (CD8− Dec-205−) cells; CD8H+, CD8hi Dec-205int cells; DDC, CD8int Dec-205int cells; LC, CD8int Dec-205hi cells; TLN, total lymph node cell population.
In cultures of CDCs derived from BALB/c mice, no parasites were detected at week 1 postinfection, but from week 3 onward all DC subpopulations contained parasites (Fig. 3A). In contrast, large numbers of parasites were detected in the lymph nodes throughout the infection, including at the 1-week time point, indicating that at the early time they were present in macrophages rather than DCs (Fig. 3A). The continuous presence of parasites is indicative of either a continuous migration of infected DCs from the lesion or an invasion of DCs in the lymph nodes by parasites carried from the skin by infected macrophages. Once inside the DCs, the persistence of the parasites may be facilitated by the absence of NO production by DCs (see below). Of the five populations, the skin-derived dermal (CD8int Dec-205int) and epidermal (CD8int Dec-205hi) LCs consistently contained large parasite loads, while the CD8hi Dec-205int loads were consistently half a log smaller than those in the CD8int Dec-205int DCs. Interestingly, the PDCs had the smallest parasite loads at week 3, yet carried similar parasite loads as the skin DCs at weeks 9 and 12 postinfection. Likewise, the CD8− Dec-205− DCs also appeared to harbor smaller numbers of parasites at week 3, which may correspond with the delayed arrival of these particular cells from their place of origin. This population was seen to peak at week 9 before decreasing somewhat at week 12.
Figure 3B shows the parasite load in C57BL/6J mice. Like those from the BALB/c mice, the DC populations sorted from the C57BL/6J mice had no detectable parasites at day 8, but all contained parasites at week 3 and all had equivalent parasite loads. As was the case in BALB/c mice, parasites were detected in the lymph nodes at week 1 postinfection, most likely in macrophages (Fig. 3B). However, unlike in BALB/c mice, the mature DC populations from C57BL/6J mice had no parasites by week 9, and the immature PDCs had no detectable parasites by week 12. It was interesting that in the C57BL/6J mice, the PDCs not only had the longest parasite persistence time, but at the early time point of 3 weeks, they harbored a 10-fold larger parasite load than the BALB/c mice.
The absence of parasites in the C57BL/6J mice at the late time points most likely reflects the healing of the lesions and hence the lack of parasites translocating from the ear to the lymph node. In addition, parasite absence may also be due to local killing, either in the DCs themselves or in activated macrophages. This is supported by the fact that there was a time-dependent sharp decline in the total parasite burden in the lymph nodes in the C57BL/6J mice, in contrast to the case in the BALB/c mice (Fig. 3).
Purification and characterization of DC populations from the ear pinna of L. major-infected mice.
In order to validate that the DCs were not the shuttle by which the parasites migrated to the lymph nodes, we collected week 1 and 3 ear explants from BALB/c and C57BL/6J mice and cultured them for 72 h to enable the DCs in the skin to migrate out. The cells were characterized in terms of DC subpopulation. The cells that were collected and sorted from the ear explant cultures contained mainly LCs (CD8int Dec-205hi) and DDCs (CD8int Dec-205int) (13). A percentage value was obtained for each subpopulation by calculating the number of cells present within each population compared to the total number of cells sorted (Fig. 4A). As the data obtained for the BALB/c and C57BL/6J mice were very similar, Fig. 4A contains the values for BALB/c mice only. The data indicate that the majority of the DCs migrating from the skin at weeks 1 and 3 were LCs in both mouse strains. The percentage of LCs in BALB/c naïve mice was 21.6%, while those for the infected mice were 16% at week 1 and 25.2% at week 3. For the C57BL/6J mice, these values were 20, 15, and 19%, respectively. The DDCs were also present but represented only about 1 to 2% of the population in naïve and infected BALB/c and C57BL/6J mice at week 1. At week 3, the DDC population in the BALB/c mice doubled while that in the C57BL/6J infected mice rose by a factor of 8. As anticipated, the non-skin-derived CD8− Dec-205− and the CD8H+ (CD8hi Dec-205int) DC populations present in the naïve and infected mice of both strains at weeks 1 and 3 were negligible (12).
FIG. 4.
Distribution of DCs and infection rates. (A) Representative experiment showing the distribution of CDC populations emigrating from ear explants of 13 to 20 BALB/c mice at 1 and 3 weeks postinfection with 103 L. major V121 metacyclic promastigotes. (B) Infection rates of BALB/c and C57BL/6J macrophages isolated on days 3, 5, and 7 postinfection from ear explants cultured for 24 h. The data represent the means and standard deviations from two independent experiments performed with 16 mice per group.
Parasite load in DC populations and macrophages isolated from the ear pinna.
Macrophages have long been recognized as the preferred host of the obligatory intracellular Leishmania parasites and as such have generally been considered to be the primary means by which transportation to the lymph node is achieved. Using ear skin explants, we examined the importance of the macrophage as a shuttle vehicle and further examined whether the DCs have a role in this process during the early and late stages of infection, at days 3, 5, 7, and 21 postinfection. At all time points examined, C57BL/6J mice appeared to have 1 log fewer cells migrating from the skin than did BALB/c mice.
For BALB/c mice, the infection rate in macrophages cultured from ear explants over a 24-h period was similar over the entire period of days 3 (8.7% ± 1.8%), 5 (6.4% ± 1.6%), and 7 (6.6% ± 1.8%) postinfection (Fig. 4B). For C57BL/6J macrophages, the infection rate was also maintained during the infection, but it was somewhat lower than that for BALB/c mice throughout the period of observation (4.5% ± 0.5%, 2.9% ± 0.9%, and 4.8% ± 1.1%, respectively). Parasites were also present in the day 21 macrophage samples, but the infection rate was not quantitated.
For the quantitation of parasites in the DCs collected from ear explant cultures at 3, 5, 7, and 21 days postinfection, we used microscopic examination as described in Materials and Methods. By microscopy, no parasites were detected in any of the DC populations at any time, suggesting that DCs play a minor role, if any, in the translocation of parasites from the skin to the lymph node. These results point to the macrophages as the main mode of transportation between the skin and lymph nodes.
In vitro infection of CDCs and PDCs with L. major.
Infections of BALB/c and C57BL/6J PDCs with L. major V121 amastigotes were compared to those of CDCs by use of a ratio of 10 parasites to each cell. All groups of infected and control uninfected cells were incubated overnight at 37°C before viability (Fig. 5A) and infection rates (Fig. 5B) were determined.
FIG. 5.
Viabilities and infection and attachment rates of BALB/c and C57BL/6J PDCs and CDCs infected in vitro with L. major V121 amastigotes at a ratio of 10 parasites to each cell. (A) Percentages of viable cells present after 18 h. (B) Infection and attachment rates. The data represent two experiments conducted with all samples in duplicate.
Since the life span of DCs is limited in culture, it was important to determine the viability of the infected and uninfected cells. Figure 5A shows that about 70% of control CDCs maintained their viability in culture, whereas only about 45 to 50% of the PDCs were alive after 18 h. Moreover, while the infection had no effect on the viability of CDCs purified from either BALB/c or C57BL/6J mice, it reduced the viability of PDCs by half. It is possible that the difference in viability between the CDCs and the PDCs may be related to their state of activation. A microscopic examination of the cultures showed that the CDCs were aggregated in clusters, suggesting activation, while the PDCs were spread evenly in the culture dish, indicating a quiescent state.
For the viable BALB/c and C57BL/6J PDCs, the rates of infection by amastigotes at 18 h were similar, at 22% ± 0.5% and 18%, respectively. Interestingly, a very large number of parasites remained attached to the surface rather than being phagocytosed (Fig. 5B). For the CDC groups, the rates of infection were somewhat higher, at 31.6% ± 3.9% for BALB/c mice and 28.9% ± 2.9% for C57BL/6J mice, and the percentages of parasites that were phagocytosed were also higher (Fig. 5B). Whether this reflects the more active nature of the CDCs remains to be determined.
Cytokine production by DC populations.
IFN-γ, IL-12 p70, IL-12 p40, and IFN-α production were measured by capture ELISA with culture supernatants from DC populations derived from naïve and infected BALB/c and C57BL/6J mice. Without in vitro stimulation, negligible levels of cytokine production were detected for any of the sorted DC populations, whether they were infected or not. This situation may indicate that only a few cells within a sorted population contained the necessary stimulatory parasite material, that the cytokine production had occurred earlier, or that the production level fell below the detection level of the assays.
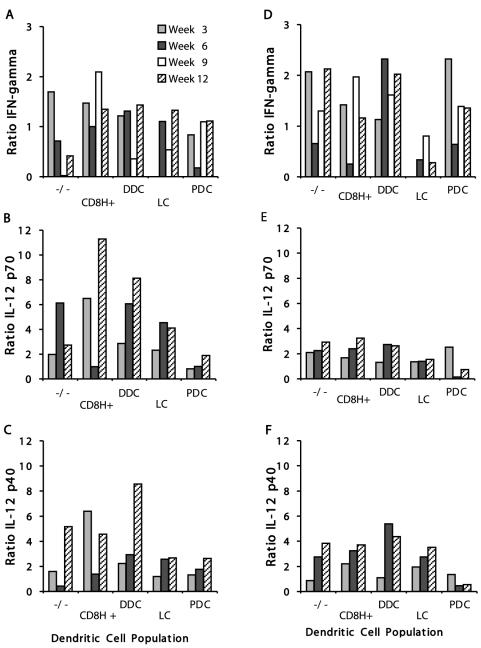
In these experiments, cytokine production was measured upon exogenous stimulation of the cells. For these measurements, the ratios of IFN-γ production in infected mice to those in control mice are shown in Fig. 6A and D for the BALB/c and C57BL/6J mouse strains. In the susceptible BALB/c mice, there appeared to be a low to moderate level of IFN-γ produced by some but not all of the DC populations examined. Early during the infection, the double-negative and CD8H+ DCs were the only ones to produce up to twofold more IFN-γ than the naïve controls (Fig. 6A). Later in the infection, only the CD8H+ cells produced cytokine at a level above the background at week 9. In contrast, several of the C57BL/6J DC populations showed moderate but sustained IFN-γ production (Fig. 6D). It was interesting that early in the infection, only the CD8− Dec-205− DCs and the PDCs showed an increase in the level of IFN-γ compared to controls. Later in the infection, most DC types produced IFN-γ, except the PDCs, which produced IFN-γ only at the 3-week time point. The most striking observation was that the LCs produced no IFN-γ at any time in susceptible or resistant mice. Unlike the pattern of IFN-γ response, it was surprising that overall, the IL-12 p70 production level observed for the DC populations of the BALB/c mice seemed to be higher than that in the C57BL/6J lymph nodes (Fig. 6B and E). Early in the infection, for BALB/c mice, the CD8H+ DCs showed the largest increase in cytokine production compared to controls. Later on, the CD8H+ DCs and DDCs showed significant increases in IL-12 p70 production. In BALB/c mice, the cells producing the least IL-12 p70 were the PDCs, consistent with their low IFN-γ levels. The PDCs were also the cells that produced the lowest levels of IL-12 p70 in C57BL/6J mice (Fig. 6E). However, for the C57BL/6J mice, at week 3 of infection there was a twofold increase in cytokine level compared to controls, in line with the increase in production of IFN-γ detected at this time (Fig. 6D and E).
FIG. 6.
Ratios of IFN-γ, IL-12 p70, and IL-12 p40 levels observed for DC populations isolated from infected versus naïve BALB/c and C57BL/6J lymph nodes at 3, 6, 9 (IFN-γ only), and 12 weeks postinfection with 103 L. major V121 metacyclic promastigotes. Twenty-four to 50 mice were used per time point. For the evaluation of IFN-γ, cells were stimulated in vitro with murine rIL-12 and rIL-18 for 72 h. For IL-12 cytokine production, cells were incubated for 24 h with murine recombinant granulocyte-macrophage colony-stimulating factor, rIL-4, rIFN-γ, and CpG, as described in Materials and Methods. All stimulations were done in duplicate or triplicate. For some time points, two independent experiments were performed, with all results being similar. Data are shown for IFN-γ production in BALB/c (A) and C57BL/6J (D) mice; IL-12 p70 production in BALB/c (B) and C57BL/6J (E) mice; and IL-12 p40 production in BALB/c (C) and C57BL/6J (F) mice. (−/−), double-negative (CD8− Dec-205−) cells; CD8H+, CD8hi Dec-205int cells; DDC, CD8int Dec-205int cells; LC, CD8int Dec-205hi cells.
In contrast to the case for p70 production, there were no major differences in the levels of p40 between the mouse strains (Fig. 6C and F), with the exception of the PDCs, which produced very little p40 throughout the course of infection in C57BL/6J mice but produced low to moderate levels in BALB/c mice. The DDCs stood out, with the largest increase in p40 production at week 12 of infection of BALB/c mice, when the lesion pathology was most severe. The CD8H+ DCs from C57BL/6J mice initially showed relatively low IL-12 p40 production, which increased somewhat during the course of infection to reach an almost fourfold increase compared to controls on week 12 of infection, when the skin pathology had subsided. In contrast, the CD8H+ population sorted from BALB/c mice showed variable amounts of IL-12 p40, with high and moderate levels at weeks 3 and 12, respectively, while the production at week 6 was low. There appeared to be a direct correlation between the IL-12 p70 and IL-12 p40 production levels measured for the mature CDC populations.
Although IFN-α production was examined for all purified DC populations, cytokine production was detected only in the PDCs (Fig. 7). In both naïve BALB/c and C57BL/6J mice, a low level of 100-U/ml IFN-α production was detected in the PDCs after stimulation with CpG. In the case of the PDCs from C57BL/6J mice, IFN-α was detected above the level induced by the activation of control cells only at week 3 postinfection. In contrast, the BALB/c infected mice produced continually increasing amounts of IFN-α, peaking at 290 U/ml at week 9 (Fig. 7).
FIG. 7.
IFN-α production in PDCs purified from BALB/c and C57BL/6J lymph nodes at 1, 3, 6, and 9 weeks postinfection with 103 L. major V121 metacyclic promastigotes and stimulated in vitro with CpG oligonucleotides for 18 h at 37°C with 10% CO2. Twenty-four to 50 mice were used per time point.
NO production by DC populations.
NO production by macrophages has been shown to correlate with Leishmania killing in vivo and in vitro (17, 18). For the first time, we examined the ability of DC subpopulations to produce NO in response to an infection with L. major. An evaluation of purified DC populations from naïve and infected BALB/c and C57BL/6J mice indicated that there was no significant NO production. As there was no difference between the responses recorded for stimulation with IFN-γ alone or in combination with L. major parasites, we suggest that DCs are not involved in the direct killing of Leishmania by NO production.
DISCUSSION
DCs and macrophages are important accessory cells that regulate T-cell responses to Leishmania and thus the host's ability to control disease. Macrophages are the main host cell for the parasite and are required for parasite clearance, mainly through nitric oxide production (15, 18). DCs are the main antigen-presenting cells for naïve T cells, and in this capacity they kick-start the antiparasite immune response (21). Moreover, different subpopulations of DCs which differ in phenotype, function, and localization (34) may direct the T-cell immune response towards a Th1 or Th2 response and may affect the severity of disease (11). DCs are also known to affect the expansion and differentiation of regulatory T lymphocytes, NK cells, and NKT cells, but little is known about the mechanisms or the subpopulation of DCs involved (35). Genetically determined differences in the DC populations and functions may therefore be associated with resistance or susceptibility to infection with a given pathogen (21). These genetically determined differences may control the quality and quantity of the immune response through the production of particular cytokines (37) or growth and suppressive factors, such as thiols and indoleamine 2,3-dioxygenase (35). On the other hand, pathogens have evolved mechanisms to subvert DC functions by interfering with their ability to present antigens to T cells (38).
For this study, we examined the populations of DCs emigrating from infected skin and those present in the lymph nodes draining the lesion site in BALB/c and C57BL/6J mice, from very early times after infection and over a 12-week period.
We identified five DC populations (Fig. 2) in the draining lymph nodes of infected mice based on their unique surface marker profiles (26, 41). These were similar to the subtypes described in a previous study of the normal lymph node (13) plus the additional PDC group, which was identified in mice since that time (7, 26). We compared susceptible BALB/c and resistant C57BL/6J mice and found that for both there was an increased cellularity of the lymph node during the course of infection. This increase correlated directly with the increased number of mature DCs isolated. The only striking exception was the larger number of PDCs observed in the susceptible BALB/c mice than in the C57BL/6J mice. The significance of this observation is not clear, in particular since plasmacytoid cells have been implicated in the induction of Th1-type immune responses (6, 7). However, they perform a variety of other functions. For example, through their production of IFN-α they induce the maturation of other subpopulations of DCs and potentiate humoral immunity (35). It is possible that the PDCs affect the quality of the immune response by controlling the balance between Th1- and Th2-type responses. Thus, in the genetically susceptible BALB/c mice, they may skew this balance through an early effect on the maturation of particular subpopulations of DCs which may then determine the type of cytokines produced.
An examination of the parasite loads (Fig. 3) of the five DC populations indicated that at the 3-week time point the PDCs harbored the smallest parasite burdens. Although the parasite burdens over the ensuing period seemed to be similar for all the populations, the PDCs continued to harbor parasites for the longest period. They were the only DC population that contained parasites in the C57BL/6J mice at week 9 of infection, at a time when the lesions had begun to heal. Although the 2-week life span of the plasmacytoid cells is longer than those of the other DCs, it is unlikely that they harbor parasites for extended periods (26) given that no parasites were seen in the resistant C57BL/6J mice at week 12 postinfection.
Our study has shown that the LCs were the dominant population migrating from the skin, followed by the DDCs (Fig. 4A). It also showed that both populations were present in naïve and infected BALB/c and C57BL/6J mice at weeks 1 and 3 postinfection. However, the C57BL/6J mice consistently had 10-fold fewer DCs than did the BALB/c mice. In contrast, the DC populations detected in the draining lymph nodes were similar for all mice, suggesting that there may be a difference in the migratory behaviors of these cells due to differences in chemokines attracting them to the lymph nodes, retaining them in the skin (9, 27), or enabling rapid migration to the lymph node. Despite the fact that emigrating skin DCs could be efficiently infected in vitro, no parasites were detected in the DCs emigrating from infected skin early (3 to 7 days) or 3 weeks after infection, suggesting that DCs do not play a role in the transport of parasites from the skin to the lymph nodes. In contrast to the DCs, macrophages emigrating from the skin explant harbored significant numbers of parasites at all time points examined (Fig. 4B), suggesting that the macrophages are the vehicle for parasite dissemination. It is likely that DCs take up parasites released from infected macrophages in the lymph nodes and not in the skin. This idea is supported by the fact that although parasites are detected in the lymph node a few hours after infection (data not shown), they are not detected in DCs in the draining lymph nodes until 3 weeks after infection for both resistant and susceptible mice. It is still possible that infected DCs are present in the skin but that their migration is impaired and therefore they do not move out of the skin (9, 28). These data do not support the conclusions of early studies of Moll and colleagues (23). These studies showed that in vitro-infected skin DCs reinjected into the skin migrated to the draining lymph nodes, and they concluded that the skin DCs are the cells that transport the parasites from the skin to the lymph node (23). They also showed that the skin-derived LCs were the host cells harboring persistent parasites in the draining lymph nodes of cured mice (22). In our study, no parasites were detected in DCs from cured mice.
Since this is the first time that PDCs have been isolated from lymph nodes of infected BALB/c and C57BL/6J mice and since BALB/c mice were found to harbor parasites throughout infection, we were interested to see whether they could be infected in vitro and whether they are susceptible to infection by promastigotes as well as amastigotes. As observed in earlier studies using conventional DCs (12), the amastigote stage of L. major is more infectious than the promastigote form (data not shown). Both PDCs and CDCs could be infected in vitro, but the CDCs were more susceptible to infection than the PDCs (Fig. 5B).
It was interesting that many more parasites were attached to cells than were internalized by both CDCs and PDCs. However, this may not be functionally important, because attachment has been shown to be as effective as internalization for triggering the innate immune responses to gram-negative bacteria (21). Another surprising observation was that despite the lower infection rate of the PDCs compared to the CDCs, the PDCs died in infected cultures (Fig. 5A). One of the striking differences between the CDC and PDC cultures was the lack of cell clusters and signs of activation in the PDC cultures. It is not clear if there is any correlation between a lack of activation and cell death.
We also examined the production of several cytokines which have been described to be different in various DC populations in an attempt to determine some functional characteristics of these cells in infected BALB/c and C57BL/6J mice. In the absence of exogenous stimulation, no cytokine production was detected, suggesting that the number of sorted cells containing antigen may be too low to detect in the assays we used. With this study, we examined the ability of the various DC populations to produce several cytokines in the presence of stimulation, and the pattern observed was quite complex. It was surprising that some DC populations from susceptible BALB/c mice, in particular the double-negative and CD8H+ DCs, produced moderate levels of IFN-γ (Fig. 6A) as well as significant levels of IL-12 p70 (Fig. 6B). In addition to these populations, the DDCs also showed a significant increase in IL-12 p70 compared to controls. The functional significance of these observations is not clear, but it may suggest that other aspects of DC function lead to the Th2-type responses and associated pathologies observed with these mice. In the case of the resistant C57BL/6J mice, as expected, most DC populations produced IFN-γ (Fig. 6D) throughout the infection, including the time when the healing process was complete. A striking exception was the LCs, which did not seem to produce any IFN-γ and produced very little IL-12 p70 (Fig. 6D and E). The C57BL/6J DCs also produced low to moderate levels of IL-12 p70 and p40. For several experiments, the data obtained by ELISA were confirmed by Western blot analysis (data not shown).
It was interesting that the PDCs from susceptible BALB/c mice produced no IFN-γ and negligible amounts of IL-12 p70, whereas in resistant mice, there was a modest but clear twofold increase in IL-12 p70 early in infection (Fig. 6C and F), consistent with the idea that the PDCs are uniquely able among the DC subpopulations to produce both IL-12 and IFN-α and to induce Th1-type immune responses (7). In this context, we examined and compared the production of IFN-α by the BALB/c and C57BL/6J PDCs during infection (Fig. 7). IFN-α was detected in the resistant C57BL/6J mice early in infection, but it disappeared well before the healing of the lesions. However, a significant amount of IFN-α was also detected in the susceptible BALB/c mice until late in infection. This observation, together with the presence of larger numbers of PDCs in infected BALB/c mice than in C57BL/6J mice and the more protracted infection of these cells compared to other DCs, points to an important role for PDCs in leishmaniasis.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alexander, J., A. R. Satoskar, and D. G. Russell. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, T., C. Elso, J. Curtis, L. Buckingham, and E. Handman. 2003. The site of Leishmania major infection determines the disease severity and immune responses. Infect. Immun. 71:6830-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Nogen-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 6.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y. J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373-379. [DOI] [PubMed] [Google Scholar]
- 8.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 9.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 10.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822-2831. [DOI] [PubMed] [Google Scholar]
- 11.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henri, S., J. Curtis, H. Hochrein, D. Vremec, K. Shortman, and E. Handman. 2002. Hierarchy of susceptibility of dendritic cell subsets to infection by Leishmania major: inverse relationship to interleukin-12 production. Infect. Immun. 70:3874-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henri, S., D. Vremec, A. Kamath, J. Waithman, S. Williams, C. Benoist, K. Burnham, S. Saeland, E. Handman, and K. Shortman. 2001. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167:741-748. [DOI] [PubMed] [Google Scholar]
- 14.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 166:5448-5455. [DOI] [PubMed] [Google Scholar]
- 15.Iniesta, V., L. C. Gomez-Nieto, and I. Corraliza. 2001. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J. Exp. Med. 193:777-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 17.Liew, F. Y., Y. Li, D. Moss, C. Parkinson, M. V. Rogers, and S. Moncada. 1991. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur. J. Immunol. 21:3009-3014. [DOI] [PubMed] [Google Scholar]
- 18.Liew, F. Y., X. Q. Wei, and L. Proudfoot. 1997. Cytokines and nitric oxide as effector molecules against parasitic infections. Phil. Trans. R. Soc. Lond. B 352:1311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, G. F. 1983. Murine cutaneous leishmaniasis: resistance in reconstituted nude mice and several F1 hybrids infected with Leishmania tropica major. J. Immunogenet. 10:395-412. [DOI] [PubMed] [Google Scholar]
- 21.Moll, H. 2003. Dendritic cells and host resistance to infection. Cell. Microbiol. 5:493-500. [DOI] [PubMed] [Google Scholar]
- 22.Moll, H., S. Flohe, and M. Rollinghoff. 1995. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur. J. Immunol. 25:693-699. [DOI] [PubMed] [Google Scholar]
- 23.Moll, H., H. Fuchs, C. Blank, and M. Rollinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595-1601. [DOI] [PubMed] [Google Scholar]
- 24.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 170:4237-4241. [DOI] [PubMed] [Google Scholar]
- 25.Nicolle, C. H. 1908. Cultures des corps de Leishman isoles de la rate dans trois cas d'anemic sptenique infantile. Bull. Soc. Pathol. Exot. 1:121. [Google Scholar]
- 26.O'Keeffe, M., H. Hochrein, D. Vremec, I. Caminschi, J. L. Miller, E. M. Anders, L. Wu, M. H. Lahoud, S. Henri, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2002. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J. Exp. Med. 196:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritter, U., and H. Korner. 2002. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunol. 24:295-301. [DOI] [PubMed] [Google Scholar]
- 28.Ritter, U., H. Moll, T. Laskay, E.-B. Bröcker, O. Velazco, I. Bercker, and R. Gillitzer. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699-709. [DOI] [PubMed] [Google Scholar]
- 29.Rittig, M. H., and C. Bogdan. 2000. Leishmania-host-cell interaction: complexities and alternative views. Parasitology 16:292-297. [DOI] [PubMed] [Google Scholar]
- 30.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 31.Sacks, D. L., and R. P. da Silva. 1987. The generation of infective stage Leishmania major promastigotes is associated with the cell-surface expression and release of a developmentally regulated glycolipid. J. Immunol. 139:3099-3106. [PubMed] [Google Scholar]
- 32.Sacks, D. L., and P. V. Perkins. 1984. Identification of an infective stage of Leishmania promastigotes. Science 223:1417-1419. [DOI] [PubMed] [Google Scholar]
- 33.Sher, A., E. Pearce, and P. M. Kaye. 2003. Shaping the immune response to parasites: role of dendritic cells. Curr. Opin. Immunol. 15:421-429. [DOI] [PubMed] [Google Scholar]
- 34.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 35.Steinman, R. M. 2003. Some interfaces of dendritic cell biology. APMIS 111:675-697. [DOI] [PubMed] [Google Scholar]
- 36.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 37.Trinchieri, G., S. Pfkanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 38.Urban, B. C., and D. J. Robers. 2002. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr. Opin. Immunol. 14:458-465. [DOI] [PubMed] [Google Scholar]
- 39.von Stebut, E., Y. Belkaid, B. V. Nguyen, M. Cushing, D. L. Sacks, and M. C. Udey. 2000. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 30:3498-3506. [DOI] [PubMed] [Google Scholar]
- 40.Vremec, D., J. Pooley, H. Hochrein, L. Wi, and K. Shortman. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978-2986. [DOI] [PubMed] [Google Scholar]
- 41.Vremec, D., and K. Shortman. 1997. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes on incubation and differences among thymus, spleen and lymph nodes. J. Immunol. 159:565-573. [PubMed] [Google Scholar]