Abstract
Aims
Person-centred care (PCC) emphasizes a partnership in care between patients and healthcare professionals and is advocated by WHO as a key component of quality health care. We evaluated outcomes of PCC in hospitalized patients with chronic heart failure (CHF) with respect to the length of hospital stay (LOS), activities of daily living (ADL), health-related quality of life (HRQL) and 6-month readmission rate.
Methods and results
During 2008–2010, 248 consecutive patients hospitalized for symptoms of worsening CHF were enrolled in a controlled before and after designed study. A Usual care group (n= 123) was recruited according to pre-defined criteria to map usual CHF care and assess outcomes at five designated hospital wards. Based on the mapping, a panel of in-house clinicians and researchers developed measures aimed at aligning usual care with basic PCC principles. These measures were incorporated into a study protocol to guide care procedures at the same five wards. Person-centred care was then implemented at these wards and evaluated in 125 patients. Both length of hospital stay and 6-month readmission were extracted from patient records. Activities of daily living were evaluated at baseline and discharge and HRQL was evaluated at baseline and after 3 months. In the analysis of all patients, the LOS was reduced by 1 day (P= 0.16) while retaining ADL (P= 0.07). When PCC was fully implemented (per protocol analysis), LOS was reduced by 2.5 days (P= 0.01) and the ADL-level better preserved (P= 0.04). Health-related quality of life and time-to-first readmission did not differ.
Conclusion
In this proof-of-concept study, our findings suggest that a fully implemented PCC approach shortens hospital stay and maintains functional performance in patients hospitalized for worsening CHF, without increasing risk for readmission or jeopardizing patients' HRQL.
Keywords: Patient-centred care, Chronic heart failure, Disease management programmes, Person-centered medicine, Person-centered care
Introduction
Chronic heart failure (CHF) is a disabling lifelong progressive condition.1 In general, patients with CHF are elderly and commonly require hospital care. Although pharmacological therapy has improved outcome markedly over the last 10–15 years, management programmes are needed to optimize care.2 Active patient involvement is a self-evident requisite for effective self-management.1 Nevertheless, chronic care management programmes are often characterized by a professional monologue and inclusion of the patient as a central partner in the team has not yet been evaluated in these programmes.3,4 The World Health Organization and Institute of Medicine at the US National Academy of Sciences have identified person-centred care (PCC) as a core ingredient of quality care for the chronically ill patient.5,6 A central component of PCC is that the professional and patient jointly develop a care and treatment plan using resources identified in each patient's illness history but also by defining potential barriers.7
Over the last decades, the length of hospital stay (LOS) for CHF has decreased drastically8 and today the average care time in Europe is 9–11 days.9 Hospital readmission for worsening CHF remains high,10,11 and it has been argued that shorter LOS may impact hospital readmission negatively.12,13 No clear association has been found between shorter hospital LOS and increased readmissions,12 and the benefits of shorter vs. longer LOS on quality of care are debated.14 Hospitalized patients with CHF experience activities in the hospital as incomprehensible and caregivers as an amorphous whole,15 while growing evidence shows that patients who are actively involved and receive regular follow-up in a coordinated system report better outcomes in their activities of daily living (ADL) and greater satisfaction with care.16–18 This suggests that partnerships between patients and healthcare professionals in CHF management could improve outcomes. We therefore designed the PCC in patients with heart failure (PCC-HF) study to evaluate whether PCC reduces LOS, improves ADL, and impacts on health-related quality of life (HRQL) and on hospital readmission (time-to-first-event and frequency).
Methods
Study design
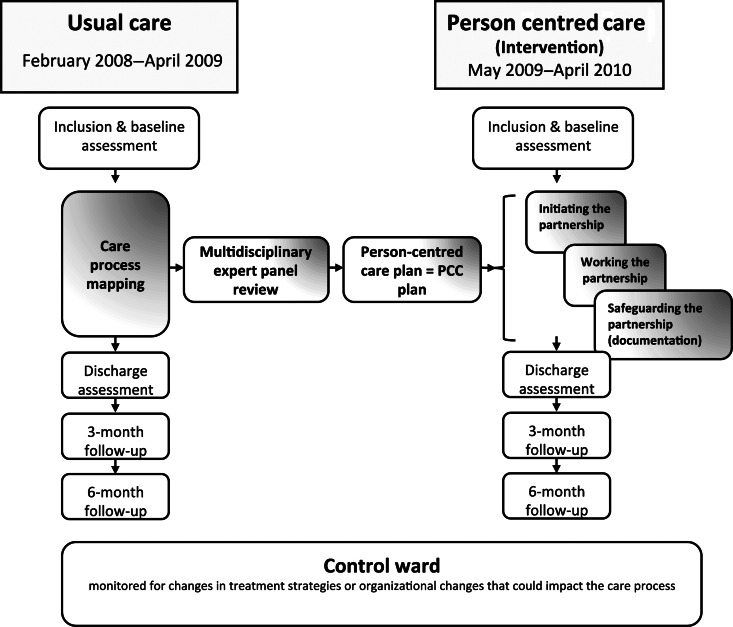
The study was designed as a proof-of-concept study and had a controlled before and after design. A Usual care group was recruited both to map usual care of patients with CHF and to assess outcomes of that care. The mapping served as a basis for designing the PCC intervention (see Figure 1). Subsequently, PCC outcomes were evaluated in an intervention group. During the study period, a control ward at the same hospital was monitored for changes in treatment strategies or organizational changes that could impact the care process. The LOS did not change in the control ward between 2008 and 2010.
Figure 1.
Study design, data-collection illustrated by white boxes. The Usual care group was used to map the care and formed the basis for the intervention with person-centred care as developed by the expert team (physicians, nurses, physiotherapists, and patients).
Patients
The study population comprised all patients with a prior diagnosis of CHF admitted for symptoms (mainly dyspnoea and/or fatigue) of worsening CHF from February 2008 to April 2010 (except during the study close-out periods) to five designated wards at the Department of Medicine at Sahlgrenska University Hospital/Östra in Gothenburg, Sweden. All patients admitted for heart failure were screened for worsening symptoms. Before study inclusion, a physician confirmed the tentative diagnosis of worsening CHF. Exclusion criteria were: acute myocardial infarction, chest pain and age <50 years, primary valvular disorder, severe concomitant illness (e.g. cancer), survival expectancy <3 months, planned surgical intervention, and cognitive impairment or reluctance to participate. All eligible patients who received a final diagnosis at discharge of CHF (i500–i509) or cardiomyopathy (i420) were included. The diagnosis was made according to the ESC guidelines for CHF1 and a separate committee was involved in the adjudication of the diagnosis. All patients received oral information about the study and gave signed informed consent. The Regional Ethical Review Board approved the study and the investigation conforms to the principles outlined in the Declaration of Helsinki.
Usual care group
Patients in the Usual care group were enrolled into the study during February 2008 until April 2009. After the signed, informed consent, eligible patients were informed that they would be treated according to the usual care routines for heart failure at the department. They were also asked to complete questionnaires at baseline and follow-up (3 months). The Usual care group served as a comparison group against which to evaluate intervention outcomes, and in addition this group was used to map usual care at the department. The mapping thus constituted the basis upon which the PCC intervention was designed.
PCC intervention group
Subsequent to the recruitment of the Usual care group, the intervention group was recruited using the same inclusion criteria from April 2009 until April of 2010. The intervention aimed to implement PCC systematically combined with evidence-based guidelines1 and clinical knowledge. The intervention was developed by a group of experienced staff nurses, physicians, physiotherapists, and occupational therapists, as well as representatives from the local patient association and the research team. The expert group met ∼10 times during a 2-month period to review usual care practices and to discuss and propose measures to align the care to PCC. When the PCC intervention was approved by the head of the department, all staff (∼300 persons) received a 3h introduction to the theory and application of PCC. During the intervention period, dedicated study nurses monitored and supported the staff in PCC.
The PCC strategy was specifically designed to identify each patient's resources for and barriers to recovery and to guide the planning and performance of care. The PCC plan consisted of three steps:
Initiating the partnership: at admission, a comprehensive narrative was obtained from the patient, including information regarding everyday life prior to and during the worsening of their condition, symptoms, and his/her motivation/goals. The patient narrative was summarized in an assessment protocol to provide easily accessible and comprehensive understanding of how the patient's situation and symptoms impact on daily life. The assessment protocol included an evaluation of the patients' social situation, need for additional support after discharge, ADL level and self-rated symptom severity. Based on this and other clinical information, a tentative PCC plan was then drawn up by the care provider, which included planned investigations, treatment goals, and length of stay. The PCC plan was discussed with the patient and finalized, when an agreement was reached, preferably within 24 h or up to 48 h.
Working the partnership: patients were encouraged to be as active as possible, e.g. getting out of bed and staying up, and urine catheters were avoided. Patients rated their symptoms of dyspnoea and fatigue on a daily basis using a five-step Likert scale.19 These ratings were used as a process indicator for the medical treatment. Additional or new information that could affect the PCC plan was checked after 72 h from admission and every 48 h thereafter in order to evaluate and adjust the PCC plan. This structured evaluation aimed at maintaining and reinforcing a partnership with the patient and at promoting shared decision-making with healthcare professionals. The PCC plan also served to support decision-making and procedures at discharge to ensure continuity in the patient's care.
Safeguarding the partnership (documentation): the PCC plan stipulated that decisions and assessments be documented throughout the care process in the assessment record form.
All three steps were considered equally crucial in implementing the main goal of PCC, namely an improved partnership between the patient and healthcare professionals.
Endpoints
The primary endpoint was LOS, computed as the number of whole inpatient days from admission to discharge. Secondary endpoints included ADL, HRQL, and hospital readmission. ADL was self-assessed at admission and assessed by a nurse at discharge with the Katz–ADL index.20 The Katz-ADL index assesses the functional performance in six activities, including bathing, dressing, toileting, transferring continence, and feeding. The HRQL was assessed at admission and 3 months after discharge using the Swedish version of the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated 25-item disease-specific HRQL instrument.21–23 KCCQ ratings were aggregated using standardized scoring procedures into an overall summary score (general health status) and a clinical summary scale (symptom impact).23 Hospital readmissions for CHF within 6 months were obtained from patient records.
Statistics
Sample size estimates were based on a 2-day reduction in length of stay from 8.5 days, with α = 0.05 and 1 – β = 0.80. Accordingly, at least 91 patients were needed in each of the two groups.
To compensate for withdrawals, 120 patients in each group were targeted. Intention-to-treat analysis included all patients who fulfilled all inclusion criteria mentioned above and no exclusion criteria. The per-protocol (PP) analysis excluded patients from the analysis if the re-evaluation of the PCC plan was not conducted by the healthcare professionals either within the first 72 h or within the subsequent evaluations of the PCC plan every 48 h until discharge. Descriptive statistics were used to characterize the study groups. Between-group differences were tested using Fisher's exact test for dichotomous variables, Mantel–Haenszel χ2-test for ordered categorical variables, χ2-test for non-ordered categorical variables, and Mann–Whitney U test for continuous variables. Adjusted P-values were obtained by logistic regression adjusting for important variables that differed significantly at baseline (NYHA class and Dyspnoea score). In addition, in the analysis of all patients, we adjusted the analysis for age. All statistical tests were two-sided with a significant level of P≤ 0.05. The data were analysed using SAS version v9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
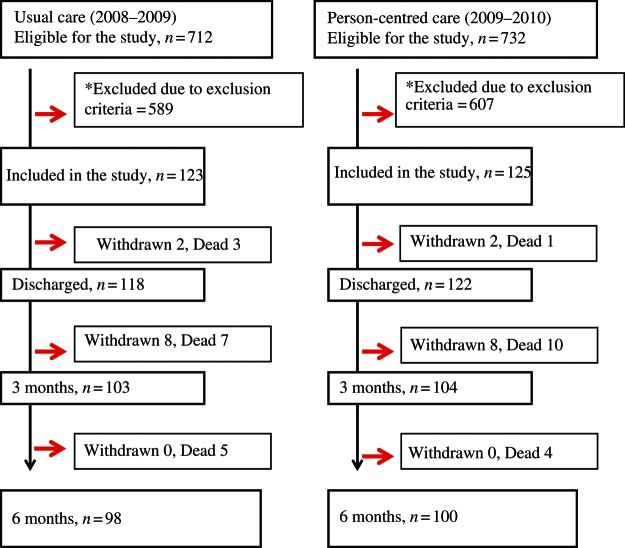
Figure 2 shows the study profile after the initial screening. In total, 248 patients were included in the trial, 125 patients in the PCC intervention and 123 in the Usual care group. Most of the excluded patients declined participation (PCC 60% vs. Usual care 61%). Fifteen patients in each group died during the study (n= 15) and 10 withdrew consent to continued follow-up and were censored from the date of withdrawal. Table 1 shows baseline characteristics for the two groups. The study groups were similar regarding age, sex, comorbidities, ADL function, vital signs, and biomarkers. Compared with the Usual care group, PCC patients were significantly more symptomatic (NYHA; P= 0.002) and reported worse dyspnoea (P= 0.03). Per-protocol analyses included 74 patients from the PCC group and all patients in the Usual care group. Their baseline characteristics were similar regarding age, sex, comorbidities, ADL function, vital signs, and biomarkers; PCC patients were also more symptomatic (NYHA class, P= 0.03).
Figure 2.
Study profile.
Table 1.
Baseline characteristics
| Characteristics | Usual care group (n= 123) | PCC group (n= 125) | P-value, Usual vs. PCC group | PCC group (per-protocol) (n= 74) |
|---|---|---|---|---|
| Female (%) | 41 | 42 | 1.0 | 49 |
| Age at inclusion | 80 ± 9 | 77 ± 11 | 0.08 | 78 ± 10 |
| NYHA class level | ||||
| Class I (%) | 4 | 0 | 0 | |
| Class II (%) | 41 | 31 | 30 | |
| Class III (%) | 52 | 58 | 0.002 | 64 |
| Class IV (%) | 3 | 11 | 6 | |
| Vital signs | ||||
| Heart rate (b.p.m.) | 80 ± 19 | 81 ± 19 | 0.7 | 81 ± 19 |
| Breathing rate (breaths/min) | 21 ± 6 | 21 ± 5 | 0.5 | 20 ± 5 |
| Systolic BP (mmHg) | 139 ± 26 | 133 ± 22 | 0.08 | 135 ± 23 |
| Diastolic BP (mmHg) | 78 ± 15 | 77 ± 16 | 0.4 | 77 ± 15 |
| Serum creatinine (µmol/L) | 114 ± 44 | 115 ± 70 | 0.4 | 115 ± 78 |
| Serum sodium (mmol/L) | 138 ± 4 | 139 ± 3 | 0.4 | 139 ± 3 |
| Serum potassium (mmol/L) | 4.2 ± 0.6 | 4.3 ± 0.5 | 0.03 | 4.3 ± 0.5 |
| Medical history | ||||
| Number of comorbidities (SD) | 4 ± 2 | 4 ± 2 | 0.7 | 4 ± 2 |
| Myocardial infarction (%) | 41 | 34 | 0.2 | 38 |
| Pacemaker (%) | 16 | 16 | 1.0 | 15 |
| Percutaneous coronary intervention (%) | 9 | 6 | 0.6 | 4 |
| Cardiac resynchronization therapy (%) | 0 | 1 | 1.0 | 0 |
| Coronary artery bypass surgery (%) | 20 | 17 | 0.6 | 16 |
| Implantable cardioverter-defibrillator (%) | 2 | 0 | 0.5 | 0 |
| Asthma (%) | 8 | 11 | 0.5 | 13 |
| Chronic obstructive pulmonary disease (%) | 19 | 10 | 0.09 | 13 |
| Atrial fibrillation (%) | 59 | 61 | 0.9 | 63 |
| Diabetes (%) | 24 | 30 | 0.4 | 32 |
| Smoking (%) | 7 | 11 | 0.3 | 12 |
| Hypertension (%) | 47 | 47 | 1.0 | 50 |
| Hypercholesterolaemia (%) | 25 | 33 | 0.2 | 33 |
| Medication at inclusion | ||||
| ACE-I/ARB (%) | 69 | 66 | 0.7 | 66 |
| Beta-blockers (%) | 72 | 82 | 0.07 | 86 |
| Dyspnoea (five-grade Likert scale) | ||||
| 1 (%) | 6 | 6 | 7 | |
| 2 (%) | 32 | 24 | 26 | |
| 3 (%) | 24 | 19 | 0.03 | 18 |
| 4 (%) | 31 | 35 | 37 | |
| 5 (%) | 6 | 16 | 11 | |
| Activity-of-daily living (ADL) | ||||
| A (%) | 71 | 79 | 78 | |
| B (%) | 17 | 12 | 13 | |
| C (%) | 6 | 2 | 0.7 | 1 |
| D (%) | 2 | 2 | 3 | |
| E (%) | 0 | 3 | 4 | |
| F (%) | 3 | 2 | 0 | |
Mann–Whitney U test was used for continuous variables, presented as mean ± standard deviation (SD). Categorical variables Categorical variables are given as percent (%) and were compared using χ2 tests.
Length of stay
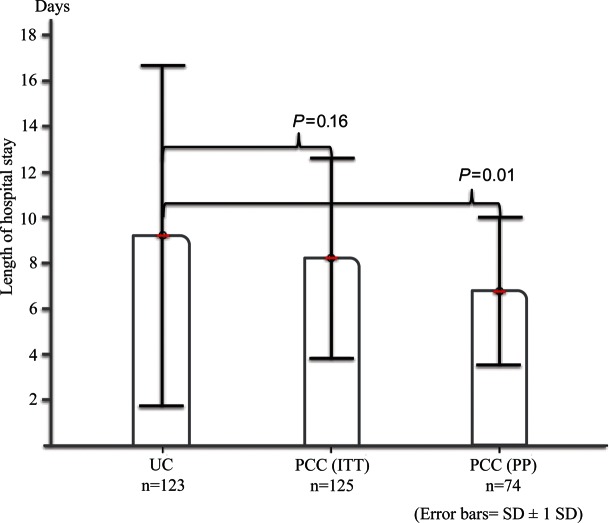
The mean LOS in the Usual care group was 9.22 days (SD 7.4, median 7, IQR 5, range 2–44 days) compared with 8.22 days (SD 4.4, median 8, IQR 5, range 2–31 days) in the PCC group (P= 0.16). In the PP analysis, LOS was significantly shorter (2.5 days) in the PCC group (6.77 days, SD 3.2, median 6.5, IQR 3, range 2–25; P= 0.01), Figure 3.
Figure 3.
Length of stay: the Usual care group vs. the PCC group for both analysis of all patients and per-protocol analysis. The analysis of all patients was adjusted for New York Heart Association (NYHA) class, dyspnoea (five-grade Likert scale), and age using logistic regression. The PP analysis was adjusted for NYHA class.
Activities of daily living
Physical functional performance as assessed with the Katz–ADL index was similar at baseline between the two groups in the analysis of all patients as well as in the PP analysis. At discharge, ADL levels were better in the PCC group (all patients, P= 0.07; the PP group, P= 0.04), Table 2. This difference owed to the fact that ADL was preserved or improved, primarily in the lowest ADL categories, in the PCC group and worsened across all ADL levels in the Usual care group.
Table 2.
ADL at discharge
| ADL-level | PCC group (%) | UC group (%) | P-value |
|---|---|---|---|
| PCC group (all patients) vs. Usual care (UC) group | n = 122 | n = 118 | |
| A | 79 | 68 | 0.07 |
| B | 14 | 20 | |
| C | 4 | 4 | |
| D | 0 | 1 | |
| E | 2 | 1 | |
| F | 1 | 5 | |
| PCC group (PP analysis) vs. Usual care (UC) group | n = 72 | n = 118 | |
| A | 79 | 68 | 0.04 |
| B | 15 | 20 | |
| C | 6 | 4 | |
| D | 0 | 1 | |
| E | 0 | 1 | |
| F | 0 | 5 | |
Health-related quality of life
There were no differences in the KCCQ Overall Summary Score or the Clinical Summary score after 3 months (Table 3).
Table 3.
Kansas City Cardiomyopathy Questionnaire clinical summary score and overall summary score
| KCCQ | PCC group | Usual care group | P-value |
|---|---|---|---|
| Analysis of all patients at baseline and change at 3 months | n = 125 | n = 123 | |
| Baseline clinical summary score | 48 ± 18 | 53 ± 22 | 0.5a |
| Change from baseline to 3 months | 10 ± 24 | 5 ± 23 | 0.3a |
| Baseline overall summary score | 46 ± 18 | 51 ± 22 | 0.8a |
| Change from baseline to 3 months | 10 ± 25 | 7 ± 23 | 0.6a |
| PP analysis of baseline and change | n = 74 | n = 123 | |
| Baseline clinical summary score | 51 ± 17 | 53 ± 22 | 0.9b |
| Score change from baseline to 3 months | 7 ± 21 | 5 ± 23 | 0.6b |
| Baseline overall summary score | 50 ± 17 | 51 ± 22 | 0.5b |
| Score change from baseline to 3 months | 8 ± 20 | 7 ± 23 | 0.7b |
Values are mean ± SD.
n= is presented.
aAdjusting for NYHA class level, dyspnoea (five-grade Likert scale), and age using logistic regression.
bAdjusting for NYHA class level using logistic regression.
Readmission
Readmission within 6 months occurred in 49% of the patients in the PCC group compared with 59% in the Usual care group (P= 0.16). Time-to-first readmission did not differ significantly between the groups (data not shown).
Discussion
To our knowledge, this is the first study to evaluate a structured PCC approach in patients hospitalized for a cardiac reason. In our proof-of-concept study, we found that when PCC was implemented in patients with CHF who had been admitted to hospital for worsening heart failure, a 1-day non-significant reduction in LOS was achieved in the PCC group (P= 0.16), while maintaining the patients' physical independence (ADL) compared with the Usual care group (P= 0.07). We found that it is also important to analyse the results in patients where the intervention was implemented according to protocol during the entire hospital stay. When PCC was implemented as planned, a significant 2.5-day reduction in LOS was achieved, as well as a significant improvement in functional performance compared with that of the Usual care group. As anticipated, the PCC programme also lead to less variability in the distribution of in-patient days as seen in the SD range (PP 3.2 vs. Usual care 7.4). Furthermore, there were no differences in 6-month readmission rates, time-to-first readmission within 6 months, or HRQL between the groups. These findings suggest that a fully implemented PCC approach shortens the hospital stay and preserves the functional performance, without increasing risk for readmission or jeopardizing patients' HRQL.
This was not a randomized controlled study. We chose a controlled before and after design because a randomized controlled trial was considered not feasible for several reasons. Hospital staff often work in several different wards and it is not possible for them to apply two entirely different approaches to care at the same time, as described by Olsson et al.24 An alternative, to randomize between different hospitals, would have implied several additional confounding factors and required a much larger trial. Although random assignment was not applied, the control and intervention groups were recruited by the same pre-defined criteria and they were comparable at baseline with respect to a large number of clinical and socio-demographic variables.
Randomized controlled trials are widely viewed to provide greatest evidentiary value for assessing the efficacy of treatment interventions and are, in the context of evidence-based medicine, hailed as the gold standard for evaluation; nevertheless, they may not always be feasible, or adequate for evaluating the effectiveness of a care intervention.25–27 ‘Weaker’ experimental or quasi-experimental designs may often be the only recourse to evaluate complex interventions and may provide invaluable data on the effectiveness of an intervention to support evidence-based recommendations for everyday clinical practice.28–30 However, since there are few circumstances under which non-RCT designs can yield reliable estimates of effect, conclusions from such studies, as ours, should be made with caution.
Although hospital care for patients with worsening CHF has been shortened during the last decades,8 there is conflicting evidence regarding its impact on readmission rates.12,13,31 Our findings may be seen to support proposals that shortening LOS has no effect on time-to-first readmission or 6-month readmission rates. This is especially important in CHF and other chronic conditions where relapses and rehospitalization are common. However, in line with Kossovsky et al.14 and Clarke,32 we believe that hospital stays may effectively be reduced only through an improved quality of care. We interpret our finding of improved functional performance in our PCC patients to indicate that PCC also improves the quality of care. On the other hand, we believe that the lack of a PCC effect on HRQL may be due to the fact that follow-up assessments were conducted 3 months after discharge and hence potential PCC impacts may have been mitigated in the interim.
The PCC intervention was planned jointly by patient representatives, staff physicians, registered nurses, and other care professions in collaboration with the research team. Our intention was to establish a working consensus to facilitate and safeguard the implementation of PCC in the designated hospital wards. Nevertheless, only 60% of the patients received the entire PCC intervention according to the protocol. The protocol non-adherence in this study reflects the challenges of instituting wide-ranging changes in daily hospital practice not only to care routines but also to approaches to care. It is likely that better compliance by the staff would have been realized by restricting the setting to a single ward and thereby focusing information and training, and concentrating support and monitoring to a limited number of staff members.
Our findings are in agreement with results from one of our previous studies evaluating PCC in elderly patients with hip fractures.18 In that study we achieved a 50% reduction in length of stay compared with the 30% reduction in the present study. There are important differences between patients hospitalized for CHF and elderly patients undergoing orthopaedic surgery that may explain our poorer results. Worsening CHF develops over a relatively long time and delay in seeking care at the emergency department can be up to 2 weeks.33 The condition itself is therefore more diffuse and rehabilitation plans and goals are not as clear as in patients' with an orthopaedic condition. Nevertheless, in both studies, the patients' narrative allowed early identification of each patient's physical and social resources before the actual deterioration of the condition, thereby setting the target level for the patients' discharge capacity. Previous studies suggest that a decreased physical functional performance (ADL) is a major predictor for institutionalization and frailty.34 In our present and previous studies,18 Person-centred care had significant advantages over conventional care with respect to ADL, while conventional care does not seem to impact positively on patients' ADL level at discharge.16,18
Limitations
Our findings have several limitations. The PCC group was more symptomatic and younger; however, these differences were adjusted for in the follow-up analyses. Secondly, the present study was a single-centre study designed to investigate proof-of-concept of PCC in chronic care management; hence, the generalization of the findings should be seen in the light of this study design. Thirdly, the number of patients who declined participation was high. Possible reasons for patient refusal may be their advanced symptoms and our need for an early inclusion into the study within 24–48 h from admission into the study. The requirements and signing the informed consent and the battery of questionnaires during the study could also have contributed to the declined participation.
Conclusions
Our findings suggest that a fully implemented PCC approach shortens hospital stay and maintains functional performance in hospitalized patients with worsening CHF without increasing the risk for readmission or jeopardizing patients' HRQL.
The importance of PCC in clinical practice needs to be assessed in studies including both hospital, primary and community care in order to further develop the structured PCC approach.
Funding
This work was supported by funding from the Swedish Research Council and the agreement between the government and the county councils concerning economic support for providing an infrastructure for research and education of doctors. Funding to pay the Open Access publication charges for this article was provided by the Swedish Research Council.
Conflict of interest: none declared.
Acknowledgement
We gratefully acknowledge Kerstin Frid, patient research partner from the Heart and Lung Association in Gothenburg and the Swedish Disability Foundation, for invaluable advice and assistance in this study and Nils-Gunnar Pehrsson (PhD) for the statistical analysis.
Footnotes
See page 1037 for the editorial comment on this article (doi:10.1093/eurheartj/ehr354)
References
- 1.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark AM, Thompson DR. What heart failure programme works best? Wrong question, wrong assumptions. Eur J Heart Fail. 2010;12:1271–1273. doi: 10.1093/eurjhf/hfq164. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan M. The new subjective medicine: taking the patient's point of view on health care and health. Soc Sci Med. 2003;56:1595–1604. doi: 10.1016/s0277-9536(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Quality of Health Care in America IoM. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: The National Academies Press; 2001. [Google Scholar]
- 6.Epping-Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of health care for chronic conditions. Qual Saf Health Care. 2004;13:299–305. doi: 10.1136/qshc.2004.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospitalization for heart failure in Scotland, 1990–1996. An epidemic that has reached its peak? Eur Heart J. 2001;22:209–217. doi: 10.1053/euhj.2000.2291. [DOI] [PubMed] [Google Scholar]
- 9.Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Hochadel M, Komajda M, Lopez-Sendon JL, Ponikowski P, Tavazzi L. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–248. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 10.Tavazzi L, Maggioni AP, Lucci D, Cacciatore G, Ansalone G, Oliva F, Porcu M. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J. 2006;27:1207–1215. doi: 10.1093/eurheartj/ehi845. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 12.Westert GP, Lagoe RJ, Keskimaki I, Leyland A, Murphy M. An international study of hospital readmissions and related utilization in Europe and the USA. Health Policy. 2002;61:269–278. doi: 10.1016/s0168-8510(01)00236-6. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56:362–368. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Kossovsky MP, Sarasin FP, Chopard P, Louis-Simonet M, Sigaud P, Perneger TV, Gaspoz JM. Relationship between hospital length of stay and quality of care in patients with congestive heart failure. Qual Saf Health Care. 2002;11:219–223. doi: 10.1136/qhc.11.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekman I, Lundman B, Norberg A. The meaning of hospital care, as narrated by elderly patients with chronic heart failure. Heart Lung. 1999;28:203–209. doi: 10.1016/s0147-9563(99)70060-9. [DOI] [PubMed] [Google Scholar]
- 16.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 17.Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 18.Olsson LE, Karlsson J, Ekman I. The integrated care pathway reduced the number of hospital days by half: a prospective comparative study of patients with acute hip fracture. J Orthop Surg Res. 2006;1:3. doi: 10.1186/1749-799X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11:288–292. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 22.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Patel H, Ekman I, Spertus JA, Wasserman SM, Persson LO. Psychometric properties of a Swedish version of the Kansas City Cardiomyopathy Questionnaire in a chronic heart failure population. Eur J Cardiovasc Nurs. 2008;7:214–221. doi: 10.1016/j.ejcnurse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Olsson L-E. Effects of nursing interventions within an integrated care pathway for. J Adv Nurs. 2007;58:116. doi: 10.1111/j.1365-2648.2007.04209.x. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong R, Waters E, Moore L, Riggs E, Cuervo LG, Lumbiganon P, Hawe P. Improving the reporting of public health intervention research: advancing TREND and CONSORT. J Public Health. 2008;30:103–109. doi: 10.1093/pubmed/fdm082. [DOI] [PubMed] [Google Scholar]
- 26.Glasziou P, Chalmers I, Rawlins M, McCulloch P. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007;334:349–351. doi: 10.1136/bmj.39070.527986.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victora CG, Habicht JP, Bryce J. Evidence-based public health: moving beyond randomized trials. Am J Public Health. 2004;94:400–405. doi: 10.2105/ajph.94.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, Guthrie B, Lester H, Wilson P, Kinmonth AL. Designing and evaluating complex interventions to improve health care. BMJ. 2007;334:455–459. doi: 10.1136/bmj.39108.379965.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creegan C HA. Towards a policy evaluation service: developing infrastructure to support the use of experimental and quasi-experimental methods. In: Justice Mo., editor. Ministry of Justice Research Series. London: 2007. ISBN:978 1 84099 088 1. [Google Scholar]
- 31.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. J Am Med Assoc. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke A. Length of in-hospital stay and its relationship to quality of care. Qual Saf Health Care. 2002;11:209–210. doi: 10.1136/qhc.11.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravely-Witte S, Jurgens CY, Tamim H, Grace SL. Length of delay in seeking medical care by patients with heart failure symptoms and the role of symptom-related factors: a narrative review. Eur J Heart Fail. 2010;12:1122–1129. doi: 10.1093/eurjhf/hfq122. [DOI] [PubMed] [Google Scholar]
- 34.Luppa M, Luck T, Weyerer S, Konig HH, Brahler E, Riedel-Heller SG. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39:31–38. doi: 10.1093/ageing/afp202. [DOI] [PubMed] [Google Scholar]