Abstract
Aims
A randomized, double-blind, placebo-controlled study was conducted to investigate the safety and efficacy of mipomersen, an apolipoprotein B-100 (apoB) synthesis inhibitor, in patients who are statin intolerant and at high risk for cardiovascular disease (CVD).
Methods and results
Thirty-three subjects, not receiving statin therapy because of statin intolerance, received a weekly subcutaneous dose of 200 mg mipomersen or placebo (2:1 randomization) for 26 weeks. The primary endpoint was per cent change in LDL cholesterol (LDL-c) from the baseline to Week 28. The other efficacy endpoints were per cent change in apoB and lipoprotein a [Lp(a)]. Safety was determined using the incidence of treatment-emergent adverse events (AEs) and clinical laboratory evaluations. After 26 weeks of mipomersen administration, LDL-c was reduced by 47 ± 18% (P < 0.001 vs. placebo). apoB and Lp(a) were also significantly reduced by 46 and 27%, respectively (P < 0.001 vs. placebo). Four mipomersen (19%) and two placebo subjects (17%) discontinued dosing prematurely due to AEs. Persistent liver transaminase increases ≥3× the upper limit of normal were observed in seven (33%) subjects assigned to mipomersen. In selected subjects, liver fat content was assessed, during and after treatment, using magnetic resonance spectroscopy. Liver fat content in these patients ranged from 0.8 to 47.3%. Liver needle biopsy was performed in two of these subjects, confirming hepatic steatosis with minimal inflammation or fibrosis.
Conclusion
The present data suggest that mipomersen is a potential therapeutic option in statin-intolerant patients at high risk for CVD. The long-term follow-up of liver safety is required.
Clinical Trial Registration: ClinicalTrials.gov identifier: NCT00707746
Keywords: Lipoproteins, Hypercholesterolaemia, Hepatic steatosis, Antisense oligonucleotides, Statin intolerance
Introduction
Whereas statins, the first-line treatment in patients at increased risk for cardiovascular disease (CVD),1 are well tolerated, adverse events (AEs) such as liver transaminase increases and myalgia occur.2,3 In a minority, side effects may even lead to discontinuation of therapy. The incidence of statin ‘intolerance’ is rising, most likely reflecting the use of higher statin doses required to achieve more stringent LDL cholesterol (LDL-c) targets.1,4 Available alternatives to lower LDL-c levels in statin-intolerant patients include switching to other statins, non-daily or low-dosing regimens,4 and the use of non-statin LDL-lowering drugs such as ezetimibe and bile acid-binding resins.5 The efficacy of these therapeutic strategies is, however, limited.
Mipomersen is a second-generation antisense oligonucleotide which inhibits the synthesis of apolipoprotein B-100 (apoB).6 apoB is the main structural component of all atherogenic lipid particles and is required for the secretion of very low-density lipoprotein (VLDL) from the liver. In previous clinical trials, mipomersen has been shown to induce dose-dependent reductions in LDL-c and all other apoB-containing lipoproteins, in patients with various extents of hypercholesterolaemia including patients with familial hypercholesterolaemia (FH).7–9 Injection site reactions and flu-like symptoms are the most common AEs with mipomersen. In addition, liver transaminase increases have been observed. Since previous attempts to inhibit VLDL production with microsomal transport protein inhibitors were complicated by profound increases in intrahepatic triglyceride (IHTG) content,10 safety concerns regarding mipomersen have focused on the liver.11
In the present report, we describe the results of a randomized, double-blind, placebo-controlled Phase 2 study designed to evaluate the safety and efficacy of mipomersen in statin-intolerant subjects at high risk for CVD.
Methods
Study participants
Forty-two hypercholesterolaemic subjects, who were statin intolerant and at high risk for CVD events, were screened for participation; 34 were randomized. High risk was defined as meeting National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria.1 Heterozygous FH subjects (≥30 years for men and ≥45 years for women) were also classified as high risk.12–14 Familial hypercholesterolaemia was diagnosed by genotyping or by fulfilling the criteria as outlined by the World Health Organization (FH: report of the WHO, 1998).
In the present study, patients were considered to be ‘statin intolerant’ if they were unable to tolerate at least two different statins due to side effects of any kind. Participants did not use other lipid-lowering drugs unless the dose had been stable for >8 weeks prior to screening. At screening, fasting LDL-c was ≥3.4 mmol/L and plasma triglyceride levels <2.3 mmol/L. Haemoglobin A1c (HbA1c) was ≤8.0, alanine aminotransferase (ALT) ≤1.5× the upper limit of normal (ULN), and serum creatine phosphokinase <3× ULN. Alcohol consumption had to be ≤3 U (30 g) per day and ≤12 U (120 g) per week for male subjects, and ≤2 U (20 g) per day and ≤8 U (80 g) per week for female subjects. All study participants were enrolled at one site in the Netherlands. The study protocol was approved by the local institutional review board. All subjects gave written informed consent. The study was performed in compliance with the standards of Good Clinical Practice (CPMP/ICH/135/95) and the declaration of Helsinki (Washington 2002). During the study, the protocol was amended to allow the inclusion of subjects with FH as well as subjects with controlled type II diabetes mellitus.
Study design
Participants were randomized at a 2:1 ratio, active to placebo. Participants, investigators, and study staff were blinded to the treatment assignment with the exception of the personnel who prepared the study drug. The study drug was administered subcutaneously at a dose of 200 mg/week from Week 1 until Week 26.11 Pre-specified efficacy endpoints included per cent change in LDL-c from the baseline to 2 weeks after the last dose. The other endpoints included per cent change in total cholesterol, apoB, HDL-c, triglycerides, non-HDL-c, VLDL, LDL/HDL ratio, ApoA1 and lipoprotein a [Lp(a)] concentrations as well as change in the particle size and number from the baseline to 2 weeks after the last dose. Safety was determined using the incidence of treatment-emergent AEs, clinical laboratory evaluations, vital signs, electrocardiograms, and physical examination findings. Due to the long half-life of mipomersen, the treatment period was followed by a 6-month evaluation period with visits at Weeks 28, 32, 40, and 50.
Lipid and lipoprotein analysis
Fasting blood and urine samples were taken after at least 10 h of fasting at visit during Weeks 1, 3, 5, 7, 9, 11, 13, 17, 21, 25, 28, 32, 40, and 50. Fasting blood samples were analysed for lipids and lipoproteins by MedPace (Cincinnati, OH, USA). apoB, apoA1, and Lp(a) concentrations were determined by rate nephelometry; and total cholesterol and triglycerides were measured by standard enzyme-based colorimetric assays. High-density lipoprotein cholesterol (HDL-c) was determined by an enzyme-based colorimetric assay after dextran sulfate precipitation. Low-density lipoprotein cholesterol and non-HDL-c were calculated. Lipoprotein particles were analysed by nuclear magnetic resonance spectroscopy (MRS) as described previously.15
Safety monitoring
The safety and tolerability of mipomersen was assessed by determining the incidence, severity, and possible relationship to the study drug of AEs and laboratory parameters, including blood chemistry, routine haematology, coagulation, and urinalysis. Vital signs were recorded at Weeks 1, 2, 3, 5, 7, 9, 11, 13, 15, 17, 21, 25, 28, 32, 40, and 50. Full physical examination was performed at screening and at Weeks 13, 28, and 50. A 12-lead electrocardiogram was recorded at screening and at Week 28.
Liver assessment
Three-Tesla proton MRS was used to quantify IHTG concentration.16,17 The IHTG concentration of >5.6% was defined as reflecting hepatic steatosis.18 Intrahepatic triglyceride values were quantified by one assessor who was masked to treatment assignment. Initially, MRS was recommended for subjects with persistent transaminase levels ≥3× ULN or for medical reasons. Following the observation of moderate hepatic steatosis in one patient, MRS was performed if ALT levels ≥2× ULN at any time during treatment. If IHTG content was ≥10%, MRS measurements were repeated around Weeks 28 and 50. In case hepatic steatosis persisted, MRS was repeated until IHTG was <10% or stabilized. Subjects with persistent transaminase increases ≥2× ULN and IHTG ≥ 20% were referred to a hepatologist. In patients requiring liver biopsy, the hepatic macrovesicular steatosis and steatohepatitis score was determined according to Kleiner et al.19
Statistical analysis
A sample size of 30 patients (20 mipomersen and 10 placebo) was planned for this study assuming a standard deviation of per cent change in LDL-c ≤ 20%. A two-sided t-test with an α level of 0.05 was expected to provide ≥90% power to detect a 30% difference in LDL-c per cent reduction between the two groups.
The study database was housed by an electronic data collection vendor (Almac, Souderton, PA, USA). Investigators had full access to the data. Data analysis as defined in the protocol was performed by a clinical research organization MedPace. Post hoc analysis was performed by the investigators. The sponsor had no influence on the interpretation of the results. Baseline characteristics were summarized using descriptive statistics. For the efficacy parameters, baseline was defined as the mean of the value at screening and the last value prior to the first dose. For the safety parameters, baseline was defined as the last value prior to the first dose. The primary efficacy time point was defined as the visit closest to 2 weeks after the last dose of study treatment.
Percentage change from the baseline for lipid parameters was compared between treatment groups using the t-test or the Wilcoxon rank-sum test for data with a skewed distribution. The difference between the highest and lowest IHTG content during follow-up was used to estimate the increase in IHTG content attributable to mipomersen. In a post hoc analysis, a comparison of each patient's highest and lowest IHTG content was tested using the Wilcoxon signed-rank test. Spearman's rank correlation coefficients were calculated to assess the relationship between ALT increases, IHTG content, and apoB levels. Software utilized for the analyses was SAS version 9.2 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided with a significance level of 0.05. Data were expressed as mean ± standard deviation, unless specified otherwise.
Results
Study subjects
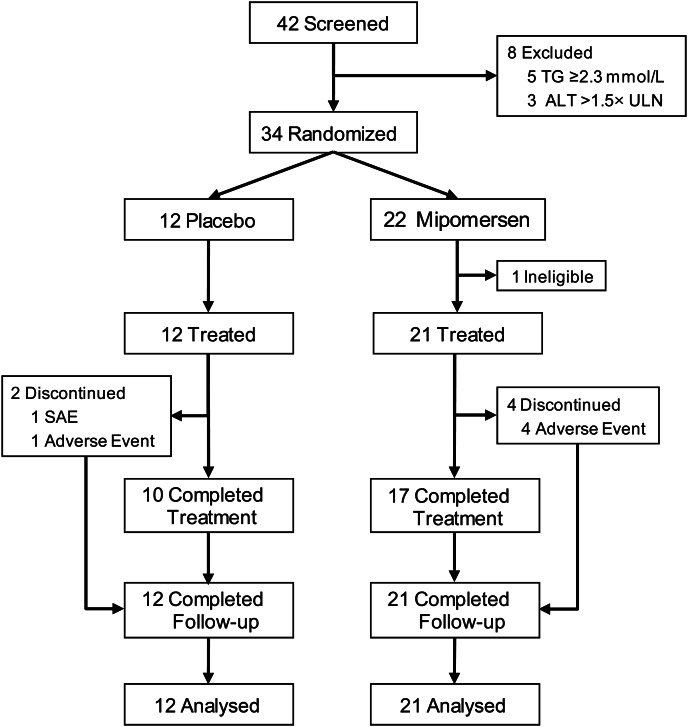
Thirty-four subjects with high CVD risk were enrolled from 42 candidates screened (Figure 1). Subjects were randomly assigned to either mipomersen (n= 22) or placebo (n= 12). One subject assigned to mipomersen was excluded from participation before the start of treatment because of corticosteroid use. All 33 subjects who were treated met the protocol-specified definition of statin intolerance. The reasons given for intolerance included myalgia (n= 30, 91%), liver enzyme elevations (n= 1, 3%), neurological symptoms (n= 3, 9%), and other reasons (n= 10, 30%). Twenty-seven of 33 subjects (82%) treated completed the study protocol. Four (18%) mipomersen-treated subjects discontinued treatment after 5–23 doses, whereas two (17%) placebo-treated subjects discontinued treatment after single and eight doses, respectively. Baseline characteristics are summarized in Table 1.
Figure 1.
Flow chart of study participants.
Table 1.
Patient demographics and baseline characteristics
| Placebo | Mipomersen | |
|---|---|---|
| Demographics | n = 12 | n = 21 |
| Gender (M:F), n (%) | 4 (33):8 (67) | 11 (52):10 (48) |
| Agea (years) | 52 (39–68) | 55 (46–69) |
| BMIa (kg/m2) | 26 (22–29) | 27 (21–32) |
| Metabolic syndrome, n (%) | 8 (67) | 9 (43) |
| FH, n (%) | 8 (67) | 11 (52) |
| DMII, n (%) | 1 (8) | 1 (5) |
| CVD, n (%) | 5 (42) | 7 (33) |
| Lipid-lowering therapy, n (%) | ||
| Any lipid-lowering medication | 6 (50) | 12 (57) |
| Ezetimibe | 3 (25) | 7 (33) |
| Colesevelam | 0 (0) | 2 (10) |
| Ciprofibrate | 1 (8) | 0 (0) |
| Nicotinic acid | 2 (17) | 1 (5) |
| Fish oil or omega-3 triglycerides | 2 (17) | 4 (19) |
| Serum aminotransferase activity (U/L) | ||
| ALTb | 25.0 ± 6.7 | 26.5 ± 11.8 |
| ASTb | 23.8 ± 4.0 | 25.5 ± 11.6 |
M, male; F, female; FH, familial hypercholesterolaemia; DMII, type 2 diabetes; CVD, cardiovascular disease.
aData are expressed as median (min–max).
bData are expressed as mean ± standard deviation.
Efficacy
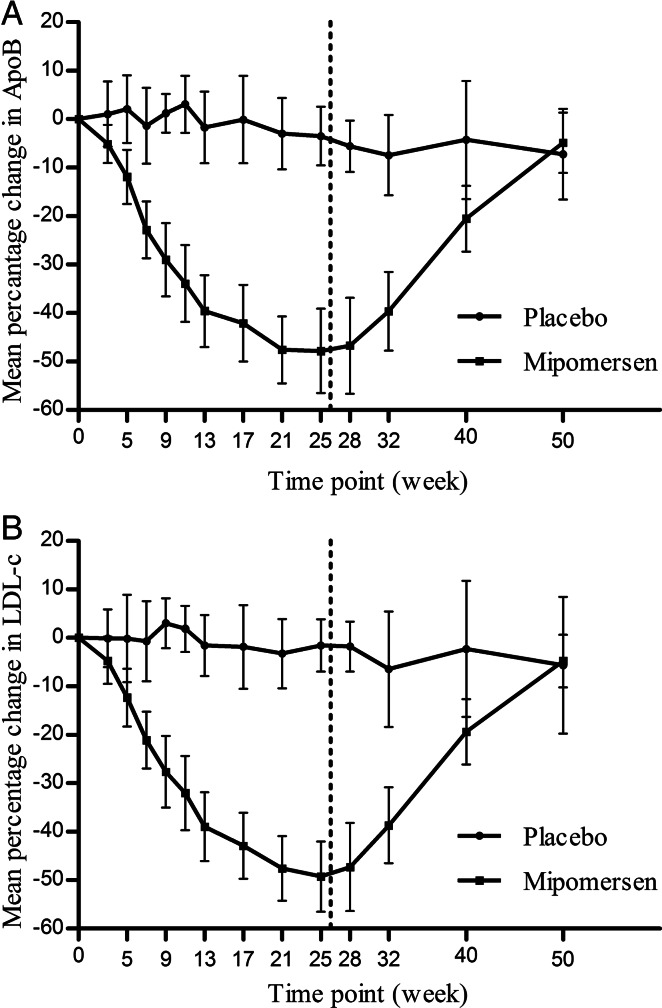
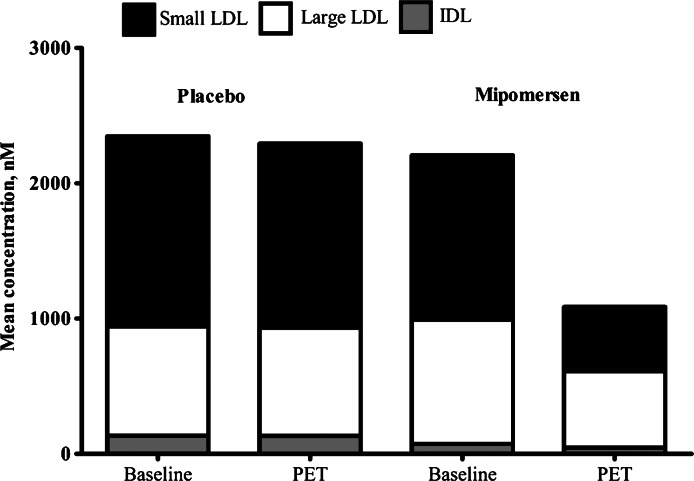
Efficacy results are summarized in Table 2 and Figure 2. Treatment with mipomersen 200 mg/week resulted in significant reductions in LDL-c of 47% (±18) (P < 0.001 vs. placebo) with a range of −19 to −77%. The observed reductions in LDL-c corresponded to mean apoB reductions of 46% (±20) (P < 0.001 vs. placebo) with a mean apoB of 0.98 (±0.51) g/L at the endpoint. Mipomersen treatment also significantly lowered total cholesterol, triglycerides, and Lp(a) but did not affect HDL-c and apoA1. Mipomersen differentially lowered LDL particle numbers with largest reductions in the small LDL particles [−729 ± 647 (−56%±47); P= 0.001 vs. placebo], when compared with the large LDL particles [−335 ± 345 (−4% ± 116); P < 0.017 vs. placebo] (Table 3 and Figure 3). The small LDL particles include medium small LDL (19.8–21.2 nm) and very small LDL (18–19.8 nm).
Table 2.
Lipid concentrations at the baseline and primary efficacy time point
| Lipid parameter (mmol/L) | Placebo (n = 12) |
Mipomersen 200 mg (n = 21) |
||||
|---|---|---|---|---|---|---|
| Baseline | PET | % change | Baseline | PET | % change | |
| LDL cholesterol | 6.3 ± 1.7 | 6.1 ± 1.4 | −2.0 ± 8.4 | 6.3 ± 2.4 | 3.3 ± 1.9 | −47.3 ± 18.5† |
| ApoB (g/L) | 1.8 ± 0.4 | 1.7 ± 0.4 | −4.3 ± 7.5 | 1.8 ± 0.5 | 1.0 ± 0.5 | −46.2 ± 19.5† |
| Total cholesterol | 8.4 ± 1.7 | 8.2 ± 1.4 | −1.8 ± 6.5 | 8.3 ± 2.4 | 5.2 ± 2.0 | −36.9 ± 14.7† |
| Non-HDL cholesterol | 7.1 ± 1.7 | 6.9 ± 1.4 | −1.9 ± 7.1 | 7.0 ± 2.4 | 3.8 ± 2.1 | −45.6 ± 18.2† |
| Triglyceridea | 1.5 (1.2, 1.9) | 1.6 (1.2, 1.9) | 5.8 (−9.5, 21.6) | 1.5 (1.1, 2.2) | 1.0 (0.8, 1.3) | −28.0 (−50.0, −9.6)‡ |
| Lp(a) | 0.4 ± 0.8 | 0.4 ± 0.9 | 0.0 ± 8.6 | 0.5 ± 0.5 | 0.4 ± 0.5 | −27.1 ± 31.2‡ |
| VLDL cholesterol | 0.8 ± 0.3 | 0.8 ± 0.3 | 4.5 ± 26.7 | 0.7 ± 0.3 | 0.5 ± 0.2 | −27.0 ± 30.8‡ |
| LDL/HDLa | 4.8 (3.8, 6.2) | 5.0 (3.9, 6.7) | 4.1 (−9.5, 11.2) | 5.0 (3.8, 6.0) | 2.5 (1.4, 2.9) | −47.7 (−68.5, −37.0)† |
| ApoA1 | 1.5 ± 0.2 | 1.5 ± 0.3 | −1.2 ± 11.1 | 1.5 ± 0.3 | 1.5 ± 0.2 | −0.0 ± 12.4 |
| HDL cholesterol | 1.3 ± 0.3 | 1.3 ± 0.4 | −2.2 ± 12.8 | 1.3 ± 0.3 | 1.4 ± 0.3 | 8.1 ± 17.2 |
PET, primary efficacy time point. Data are expressed as mean ± standard deviation. P-values are for the difference in the percentage change from the baseline between the mipomersen and placebo treatment groups.
aData are expressed as median (inter-quartile range).
†P < 0.001.
‡P < 0.01.
Figure 2.
Effect of mipomersen on apolipoprotein B-100 (A) and low-density lipoprotein cholesterol (B) over time. Low-density lipoprotein cholesterol is presented as the mean per cent change from baseline ± 95% confidence interval. Dotted line represents the end of the treatment period.
Table 3.
Low-density lipoprotein particle numbers and size at the baseline and primary efficacy time point
| LDL Particle | Placebo |
Mipomersen 200 mg |
||||
|---|---|---|---|---|---|---|
| Baseline (n= 12) | PET (n= 12) | % change (n= 12) | Baseline (n= 20) | PET (n= 20) | % change (n= 20) | |
| Total number, nmol/L | 2347 ± 742 | 2293 ± 594 | −0.7 ± 11 | 2207 ± 962 | 1086 ± 875 | −49 ± 22* |
| IDL | 135 ± 64 | 134 ± 91 | 14 ± 71 | 75 ± 78 | 47 ± 44 | 32 ± 212 |
| Large LDL | 807 ± 459 | 800 ± 417 | 15 ± 64 | 917 ± 439 | 563 ± 210 | −4 ± 116† |
| Small LDL | 1406 ± 854 | 1359 ± 665 | 13 ± 48 | 1215 ± 1033 | 476 ± 807 | −56 ± 47* |
| Particle size (nm) | 21.0 ± 1.0 | 20.9 ± 0.8 | −0.1 ± 2.5 | 21.2 ± 0.9 | 21.9 ± 0.8 | 3.3 ± 3.9† |
PET, primary efficacy endpoint. Data are expressed as mean ± standard deviation. The total LDL particle number includes IDL (23–27 nm), large LDL (21.2–23 nm), and small LDL (18–21.2 nm) subclasses. P-values are for the difference in the percentage change from the baseline between the mipomersen and placebo treatment groups.
*P < 0.001.
†P < 0.01.
Figure 3.
Effect of mipomersen on low-density lipoprotein particle subclass distribution.
Safety
Two serious AEs were reported: an on-treatment serious AE of acute myocardial infarction in the placebo treatment group and a coronary artery re-stenosis during the follow-up period in the mipomersen treatment group. In the active treatment group, four subjects discontinued treatment due to one or more AEs (flu-like symptoms, malaise, myalgia, and transaminase increase). One of these subjects met a stopping rule for liver transaminases with an ALT increased ≥10× ULN in Week 8. Further evaluation with MRS showed an IHTG content of only 0.8% in Week 9. Liver transaminase levels returned to normal within 4 weeks. One subject from the placebo treatment group discontinued treatment due to diarrhoea.
The most common AEs were injection site reactions following subcutaneous administration. Twenty (95%) subjects treated with mipomersen compared (83%) subjects on placebo treatment experienced at least one injection site reaction. In the mipomersen treatment group, these events were most frequently characterized by erythema of mild severity. No subject discontinued treatment due to an injection site reaction. Other AEs with a more than 10% incidence in the mipomersen treatment group are listed in Supplementary material online, Table S1.
Increases in ALT above the ULN were more common in the mipomersen treatment group [n= 17 (81%)] compared with the placebo treatment group [n= 3 (25%)] (see Supplementary material online, Table S3). Persistent increases in ALT (≥3× ULN on two consecutive occasions at least 7 days apart) were observed in seven subjects (33%) from the active treatment group. In a post hoc analysis in the mipomersen treatment group, ALT activities at the endpoint were found to correlate to apoB concentrations at the endpoint (r = − 0.644, P= 0.002). No increases in total bilirubin, alkaline phosphatase, or prothrombin time were observed. After discontinuation of treatment, transaminases returned to normal (<1.5× ULN) in all subjects. All persistent increases in ALT > 3× ULN were considered AEs probably related to the study drug by the investigators.
There was no evidence of treatment-related effects on renal function based on serum chemistry. Vital signs, electrocardiography, urinalysis, and other safety laboratory assessments, including fasting serum glucose and HbA1c fraction, did not show any clinically significant changes.
Hepatic magnetic resonance spectroscopy
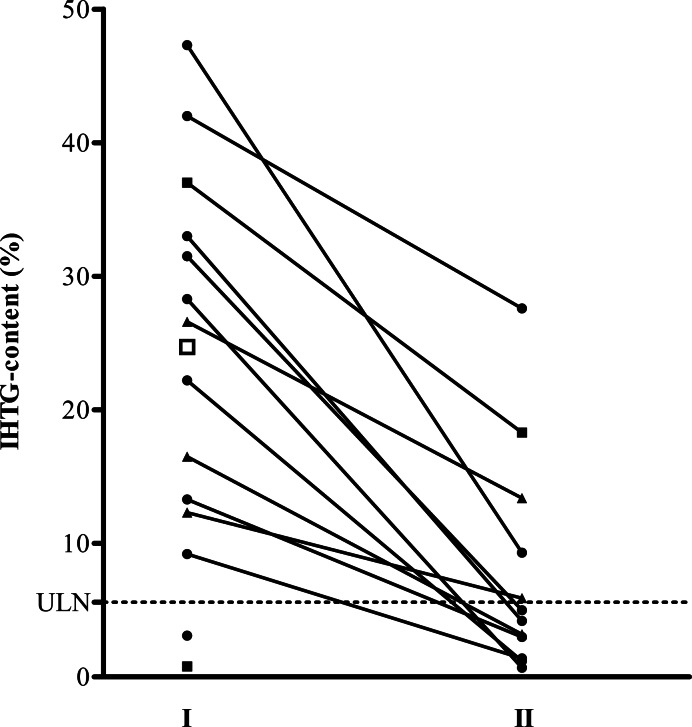
Hepatic MRS was performed at least twice in 14 of 21 subjects from the active treatment group and in 1 of 12 subjects from the placebo treatment group, because of an increase in ALT of at least ≥2× ULN. A summary of the results for on-treatment and post-treatment MRS is shown in Figure 4. The median of the highest IHTG content measured in all 14 mipomersen-treated subjects was 24.4% ranging from 0.8 to 47.3%. Hepatic steatosis (IHTG content >5.6%) was detected in 12 of the 14 subjects treated with mipomersen in whom MRS was performed and in 1 of the 1 placebo-treated subjects (P= 0.022 mipomersen vs. placebo). Highest IHTG content was correlated to apoB levels at the endpoint (r = − 0.699, P= 0.011, n= 12). During follow-up, subjects showed either stabilization or reduction in IHTG content (see Supplementary material online, Figure S1).
Figure 4.
Change in intrahepatic triglyceride (IHTG) content. Intrahepatic triglyceride content for all subjects with increases in alanine aminotransferase >2× the upper limit of normal (ULN) (n = 15). I, the highest measurement performed; filled circle, measurements performed between Weeks 24 and 31; filled triangle, measurement performed after Week 35; filled square, measurements performed at early termination in Weeks 7 and 15; open square, measurement in a patient from the placebo group who refused follow-up because of claustrophobia; II, the lowest value measured during follow-up between Weeks 50 and 90. Horizontal dotted line represents the upper limit of normal of 5.6% for intrahepatic triglyceride content. The median absolute change from highest intrahepatic triglyceride content to lowest intrahepatic triglyceride content at follow-up was −17.7% (−6.4 to −38.0; n= 12, P= 0.0005).
Liver biopsy
Four subjects with IHTG > 20% and persistent ALT ≥ 2× ULN were referred to a hepatologist. A liver biopsy was performed in two of these subjects. The first biopsy (Week 22) was performed in a subject with IHTG content increasing from 17.8% in Week 4 to 34.7% in Week 18. It showed severe macrovesicular steatosis in >66% of the hepatocytes, with minimal lobular inflammation, few ballooning cells, and minimal fibrosis (score: 5/8, fibrosis grade 1) (see Supplementary material online, Figure S2). A second biopsy (Week 21) was performed in a subject with IHTG content increasing from 23.7% in Week 10 to 47.3% in Week 30. It showed severe macrovesicular hepatic steatosis with minimal lobular inflammation and few ballooning cells, but no significant fibrosis (score 5/8, fibrosis grade 0) (see Supplementary material online, Figure S2). Low-density lipoprotein-c levels in these subjects reached values as low as 2.2 and 1.3 mmol/L.
Discussion
In subjects intolerant to statins and at high risk for CVD, mipomersen achieved robust reductions in LDL-c, apoB, and Lp(a) and was overall well tolerated. A significantly elevated IHTG content was observed predominantly in subjects with concomitant ALT increases ≥2× ULN, which was reversible after discontinuation of treatment. Liver biopsies performed in two cases confirmed hepatic steatosis. Pending long-term safety data, these findings suggest that mipomersen may be a potential therapeutic strategy for patients who are statin intolerant and at high risk for CVD.
Efficacy
Low-density lipoprotein cholesterol reductions were comparable to those achieved by high doses of potent statins such as atorvastatin and rosuvastatin20,21 and exceeded those achieved in previous reports on clinical trials with mipomersen 200 mg once weekly on top of lipid-lowering therapy.7,9,22,23 Equipotent reductions were observed following 13 weeks of mipomersen treatment in subjects with mild-to-moderate hyperlipidaemia not using statins.24 Results from the 26-week Phase 3 studies on mipomersen suggest that LDL-c reductions in patients with FH may be less profound (−25 to −28%) compared with patients with moderate hypercholesterolaemia. Although these observations may imply a role for the LDL-receptor in contributing to the LDL-lowering effect of mipomersen, studies in LDL receptor knock-out mice did not show an attenuation of mipomersens' efficacy.25 The concomitant reduction in Lp(a) is in line with previous clinical trials.7,9,22,23 The relation between Lp(a) change and reduction in apoB in a quantitative and temporal manner suggests that apoB production is a limiting factor in the generation of Lp(a) particles.26
Safety
Injection site reactions and flu-like symptoms were the most common AEs. These events did not interfere with continued dosing. Overall adherence to mipomersen exceeded 80% at the end of the study. These data compare favourably with adherence rates in patients not tolerating statin therapy, using alternative dosing regiments of different statins, whereas LDL-c reductions in these trials were much less profound.27–29
Persistent increases in liver transaminases ≥3× ULN were more common (33%) compared with earlier studies (6–15%).7–9,22,23,30 Alanine aminotransferase increases following mipomersen treatment may result from a direct pharmacological effect or may be related to hepatic fat accumulation. In support of the latter, hepatic steatosis was observed in a substantial proportion of subjects from the mipomersen treatment group who had ALT increases ≥2× ULN (12 of 14). Results from earlier trials did not provide consistent evidence for the induction of hepatic steatosis. In pre-clinical animal model studies, no increases in hepatic transaminases were observed due to compensatory mechanisms including down-regulation of hepatic fatty acid synthase and increased fatty acid oxidation.25,31 In subsequent Phase 2 clinical trials of 13 weeks or less, hepatic steatosis was reported only incidentally.9,22 In a dedicated 12-week study in patients with FH, mipomersen resulted in a trend towards increased IHTG content.30 Preliminary data from a Phase 3 trial in heterozygous FH patients reported an absolute median increase in per cent liver fat of 4.9% (inter-quartile range: 1.3–13.3%) following 26 weeks of mipomersen administration.23
Several factors may have contributed to the high incidence of hepatic steatosis in the present study. First, a recent post hoc analysis of the GREACE study, a randomized, controlled trial comparing atorvastatin vs. usual care, reported that statins reduce transaminase increases in patients with suspected non-alcoholic fatty liver disease (NAFLD).32 Similarly, others have reported that statins reverse ultrasonographic evidence of NAFLD.33,34 The absence of statin therapy in our patients may have contributed to increased hepatic fat accumulation compared with participants on statin therapy.7–9,22 Secondly, apoB reductions in the present study were higher compared with previous trials with mipomersen, at similar dose and with similar treatment duration (−26 to −37% compared with −47% in the present study).8,9,23 Liver fat concentrations inversely correlated with the level of apoB at the endpoint, compatible with the concept that impaired excretion of VLDL may enhance accumulation of triglycerides in the liver. Thus, the higher reductions in apoB in the present study may also have contributed to a higher incidence of hepatic steatosis.
Biopsies
Evidence is accumulating to show that steatosis following apoB synthesis inhibition may differ from NAFLD, the most common cause of hepatic steatosis which carries the risk of progressive liver disease.35,36 Notably, the majority of patients with familial hypobetalipoproteinaemia (FHBL), the ‘genetic homologue’ of apoB synthesis inhibition, are characterized by severe hepatic steatosis.30,37,38 In contrast to NAFLD, reports on fibrosis and cirrhosis in these patients are scarce.38,39 In addition to this observation, our recent finding that hepatic steatosis in FHBL was not associated with insulin resistance lends further support to the concept that liver fat accumulation associated with apoB inhibition is without metabolic sequelae and therefore differs from NAFLD.40 This concept needs to be validated in long-term safety studies following prolonged mipomersen administration.
Limitations
Several aspects of our study deserve closer attention. First, determination of IHTG content was performed only in subjects with transaminase increases. As a consequence, baseline and highest values as well as values for IHTG content in subjects without liver transaminase increases were missing, leaving the effect of mipomersen on IHTG content incomplete. Secondly, conclusions based on the biopsy findings are hampered by its small sample size, the short treatment duration, and the lack of pre-treatment biopsy. Therefore, in future clinical trials with mipomersen, the repetitive measurement of IHTG content should be considered in all participants, whereas liver biopsies may be required following prolonged treatment to fully explore the effects of apoB synthesis inhibition on liver tissue.
Conclusions
Pending long-term safety data, apoB synthesis inhibition may offer a potential therapeutic strategy for patients at high risk for CVD with statin intolerance for whom currently limited alternative options are available to effectively lower LDL-c.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by ISIS Pharmaceuticals, Carlsbad, CA, USA. Funding to pay the Open Access publication charges for this article was provided by Genzyme Corporate, Boston, USA.
Conflict of interest: J.J.P.K. and E.S.G.S. have received advisory fees for consultation on Mipomersen. G.W. and J.M.D. are employees of Genzyme. R.S.G. and B.F.B. are employees of ISIS Pharmaceuticals.
Supplementary Material
Acknowledgements
We thank all patients for participating in the trial.
Footnotes
See page 1040 for the editorial comment on this article (doi:10.1093/eurheartj/ehs062)
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 3.Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol. 2007;18:401–408. doi: 10.1097/MOL.0b013e32825a6773. [DOI] [PubMed] [Google Scholar]
- 4.Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Crooke RM, Baker BF, Wedel M. Cardiovascular Therapeutic Applications in Antisense Drug Technology; Principles, Strategies and Applications. 2nd ed. Boca Raton, FL: CRC Press; 2007. pp. 601–639. [Google Scholar]
- 7.Akdim F, Stroes ES, Sijbrands EJ, Tribble DL, Trip MD, Jukema JW, Flaim JD, Su J, Yu R, Baker BF, Wedel MK, Kastelein JJ. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55:1611–1618. doi: 10.1016/j.jacc.2009.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 9.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 10.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 11.Visser ME, Kastelein JJ, Stroes ES. Apolipoprotein B synthesis inhibition: results from clinical trials. Curr Opin Lipidol. 2010;21:319–323. doi: 10.1097/MOL.0b013e32833af4c1. [DOI] [PubMed] [Google Scholar]
- 12.Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. Br Med J. 1991;303:893–896. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 1969;2:1380–1382. doi: 10.1016/s0140-6736(69)90930-1. [DOI] [PubMed] [Google Scholar]
- 14.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ, Sijbrands EJ. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. Br Med J. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Werven J, Hoogduin JM, Nederveen AJ, van Vliet AA, Wajs E, Vandenberk P, Stroes ES, Stoker J. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444–448. doi: 10.1002/jmri.21837. [DOI] [PubMed] [Google Scholar]
- 18.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. J Am Med Assoc. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 21.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. J Am Med Assoc. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 22.Akdim F, Visser M, Tribble DL, Baker B, Stroes E, Yu R, Flaim J, Su J, Stein E, Kastelein J. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density-lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105:1413–1419. doi: 10.1016/j.amjcard.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Stein E, Dufour R, Gagne C, Gaudet D, East C, Tribble D, Donovan J, Chin W, Mcgowan M. A randomized, double-blind, placebo-controlled study to assess efficacy and safety of mipomersen as add-on therapy in heterozygous familial hypercholesterolemia patients with coronary artery disease. Eur Heart J. 2010;31:898. doi: 10.1161/CIRCULATIONAHA.112.104125. (abstract supplement) [DOI] [PubMed] [Google Scholar]
- 24.Akdim F, Tribble DL, Flaim JD, Yu R, Su J, Geary RS, Baker BF, Fuhr R, Wedel MK, Kastelein JJ. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011;32:2650–2659. doi: 10.1093/eurheartj/ehr148. [DOI] [PubMed] [Google Scholar]
- 25.Mullick AE, Fu W, Graham MJ, Lee RG, Witchell D, Bell TA, Whipple CP, Crooke RM. Antisense oligonucleotide reduction of apoB-ameliorated atherosclerosis in LDL receptor-deficient mice. J Lipid Res. 2011;52:885–896. doi: 10.1194/jlr.M011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merki E, Graham MJ, Mullick AE, Miller ER, Crooke RM, Pitas RE, Witztum JL, Tsimikas S. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118:743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 27.Degreef LE, Opdam FL, Teepe-Twiss IM, Jukema JW, Guchelaar HJ, Tamsma JT. The tolerability and efficacy of low-dose simvastatin in statin-intolerant patients. Eur J Intern Med. 2010;21:293–296. doi: 10.1016/j.ejim.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol. 2008;101:1747–1748. doi: 10.1016/j.amjcard.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 29.Ruisinger JF, Backes JM, Gibson CA, Moriarty PM. Once-a-week rosuvastatin (2.5–20 mg) in patients with a previous statin intolerance. Am J Cardiol. 2009;103:393–394. doi: 10.1016/j.amjcard.2008.09.095. [DOI] [PubMed] [Google Scholar]
- 30.Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJ, Trip MD, Stroes ES. Effect of apolipoprotein-B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res. 2010;51:1057–1062. doi: 10.1194/jlr.M002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooke RM, Graham MJ, Lemonidis KM, Whipple CP, Koo S, Perera RJ. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J Lipid Res. 2005;46:872–884. doi: 10.1194/jlr.M400492-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22:873–883. doi: 10.1185/030079906X104696. [DOI] [PubMed] [Google Scholar]
- 33.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 34.Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, Karagiannopoulou G, Tzioufa V, Dimitrios K. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23:131–134. [PubMed] [Google Scholar]
- 35.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl. 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 36.Musso G, Gambino R, Cassader M. Non-alcoholic fatty liver disease from pathogenesis to management: an update. Obes Rev. 2010;11:430–445. doi: 10.1111/j.1467-789X.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Tarugi P, Averna M, Di LE, Cefalu AB, Noto D, Magnolo L, Cattin L, Bertolini S, Calandra S. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007;195:e19–e27. doi: 10.1016/j.atherosclerosis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, Adinolfi LE, Ruggiero G, Carulli N, Loria P. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190–196. doi: 10.1016/j.jhep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Visser ME, Lammers NM, Nederveen AJ, van der Graaf M, Heerschap A, Ackermans MT, Sauerwein HP, Stroes ES, Serlie MJ. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54:2113–2121. doi: 10.1007/s00125-011-2157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.