Abstract
Visceral (VAT) and abdominal subcutaneous (SAT) adipose tissues contribute to obesity but may have different metabolic and atherosclerosis risk profiles. Among obese participants in the Dallas Heart Study, we examined the cross-sectional associations of abdominal VAT and SAT mass, assessed by magnetic resonance imaging (MRI) and indexed to body surface area (BSA), with circulating biomarkers of insulin resistance, dyslipidemia, and inflammation (n=942); and with aortic plaque and liver fat by MRI and coronary calcium by computed tomography (n=1200). Associations of VAT/BSA and SAT/BSA were examined after adjustment for age, sex, race, menopause, and body mass index. In multivariable models, VAT significantly associated with the homeostasis model assessment of insulin resistance (HOMA-IR), lower adiponectin, smaller LDL and HDL particle size, larger VLDL size, and increased LDL and VLDL particle number (p<0.001 for each). VAT also associated with prevalent diabetes, metabolic syndrome, hepatic steatosis, and aortic plaque (p<0.001 for each). VAT independently associated with C-reactive protein but not with any other inflammatory biomarkers tested. In contrast, SAT associated with leptin and inflammatory biomarkers, but not with dyslipidemia or atherosclerosis. Associations between SAT and HOMA-IR were significant in univariable analyses but attenuated after multivariable adjustment. In conclusion, VAT associated with an adverse metabolic, dyslipidemic, and atherogenic obesity phenotype. In contrast, SAT demonstrated a more benign phenotype, characterized by modest associations with inflammatory biomarkers and leptin, but no independent association with dyslipidemia, insulin resistance, or atherosclerosis in obese individuals. These findings suggest that abdominal fat distribution defines distinct obesity sub-phenotypes with heterogeneous metabolic and atherosclerosis risk.
Keywords: Obesity, Lipoproteins, Inflammation, Atherosclerosis, Diabetes
Introduction
Abdominal obesity is a major risk factor for diabetes and cardiovascular disease, but its manifestations are heterogeneous across individuals (1). Excess visceral (VAT) and subcutaneous (SAT) adipose tissues are key contributors to abdominal obesity but differ in their structural composition, metabolic activity, and functional significance (2). Current evidence suggests that positive caloric balance in individuals with impaired adipogenesis may result in abdominal SAT adipocyte hypertrophy leading to impaired energy storage and SAT dysfunction (2). An inadequate SAT reservoir for fat storage, in turn, promotes redistribution of free fatty acids to ectopic tissues such as VAT, liver, and skeletal muscle, predisposing to increased metabolic risk (3).
Prospective studies have shown VAT and SAT to be associated with multiple cardiovascular risk factors (4–6), markers of inflammation and oxidative stress (7), hepatic steatosis (8), and atherosclerosis (9) in the general population. However, data regarding the differential contributions of VAT and abdominal SAT to cardiac and metabolic risk in obese persons are conflicting (10–12) and studies to date vary in design, sample population, and methods applied and have not comprehensively compared the associations of VAT and SAT with metabolic, lipid, inflammatory, and atherosclerosis biomarkers specifically in the obese population. Additionally, because abdominal fat distribution varies significantly between racial and ethnic groups (13), and because obesity is more prevalent among racial and ethnic minorities (14), it is important to define the associations of VAT and SAT with metabolic and atherosclerosis risk in a representative population with a large number of ethnic minorities.
Therefore, we sought to determine the associations of abdominal VAT and SAT mass assessed by magnetic resonance imaging (MRI) with biomarkers of insulin resistance, dyslipidemia, inflammation, and imaging-based measures of liver fat and subclinical atherosclerosis in a multi-ethnic, population-based sample of obese adults with extensive cardiac and metabolic phenotyping. We hypothesized that VAT would associate with an adverse metabolic, inflammatory, and atherogenic phenotype, whereas SAT would associate with a generally more benign phenotype.
Methods and Procedures
Study population
The Dallas Heart Study (DHS) is a multi-ethnic, population-based, probability sample of Dallas County residents, with deliberate over-sampling of African-Americans. Details of the study design have been described previously (15). A subject selection diagram for the current study is provided in Supplementary Appendix Figure 1. Briefly, among the 2971 subjects who completed all three DHS visits, including a detailed in-home survey, laboratory testing, and imaging studies, we excluded 248 participants who had incomplete MRI imaging or biomarker data, a co-existing malignancy, connective tissue disease, or HIV infection, or who died within 1 year of the last visit. Of the remaining 2723 subjects, the current study further excluded all 1523 non-obese subjects (BMI< 30 kg/m2), resulting in a final sample size of 1200 obese individuals with characterization of abdominal adipose tissue distribution by MRI and full survey and biomarker data.
All participants were included for analyses of traditional risk factors, liver fat, and atherosclerosis endpoints. For analyses of biomarkers and lipoproteins, we excluded an additional 258 participants receiving aspirin, lipid-lowering, or glucose-lowering medications since these medications may influence levels of biomarkers. As expected, participants excluded due to medication use were older, with higher rates of hypertension, diabetes, and the metabolic syndrome, and had more VAT and SAT compared with those included. However, these participants did not substantially differ from those included with regard to sex, race, BMI, or percent total body fat (Supplementary Appendix Table 1). All participants provided informed consent, and the protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
Variable definitions
Race/ethnicity, history of cardiovascular diseases, and smoking status were self-reported. Hypercholesterolemia was defined as a calculated low-density lipoprotein (LDL) cholesterol ≥160 mg/dL on a fasting sample, direct LDL ≥160 mg/dL on a non-fasting sample, total cholesterol ≥240 mg/dL, or use of statin medication. Low high-density lipoprotein (HDL) cholesterol was defined as HDL <40 mg/dL in men and <50 mg/dL in women. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg from an average of five sequential blood pressure measurements done at the first visit, or use of antihypertensive medication. Diabetes was defined by a fasting glucose level ≥126 mg/dL or use of hypoglycemic medication. Presence of the metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report (16). Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease equation (17). The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated by fasting insulin (μIU/ml)×fasting glucose (mmol/liter)/22.5 (18).
Body composition measurements
Waist and hip circumference were measured in centimeters and waist to hip ratio was calculated as waist circumference (cm)/hip circumference (cm). BMI was calculated as weight (kilograms)/height (meters)2 and body surface area (BSA, cm2) was calculated as 128.1×weight (kg)0.44×height (cm)0.60 for men and 147.4×weight (kg)0.47×height (cm)0.55 for women by the method of Tikuisis et. al (19).
MRI measurements of abdominal fat mass were performed as previously described using a validated method of fat mass prediction from a single MRI slice at the L2-L3 inter-vertebral level (Figure 1) (20). Single slice measurement of subcutaneous and visceral fat mass at this inter-vertebral level has been previously validated in multiple studies to provide highly concordant results (intra-class correlation coefficient, ICC=92–98%), compared with total fat mass measured at all inter-vertebral levels (20–22). Since this method had been previously validated and is less time consuming than measurement of total abdominal fat, it was prospectively used for measurement of abdominal fat in the DHS. Subjects were imaged by a 1.5 Tesla MRI scanner (Intera, Philips Medical Systems, Best, The Netherlands) and abdominal adipose tissue was separated into visceral and subcutaneous compartments by manually circumscribing contours using anatomical landmarks. Fat volumes were converted to mass using 0.9196 kg/L as the density of adipose tissue. MRI fat mass variables used in the current study include visceral fat mass and subcutaneous fat mass indexed to BSA (subsequently referred to as VAT and SAT, respectively) to account for variations in lean body mass and overall body size. BSA was selected as the indexing variable since it was available in all patients and correlated strongly with lean mass in our cohort (R=0.93 between VAT/lean body mass and VAT/BSA). Hepatic triglyceride content (HTGC) was measured using 1.5T 1H magnetic resonance spectroscopy, and the presence of hepatic steatosis was defined as a HTGC>5.5% as determined for a low-risk sub-population from this study cohort, as previously described (23).
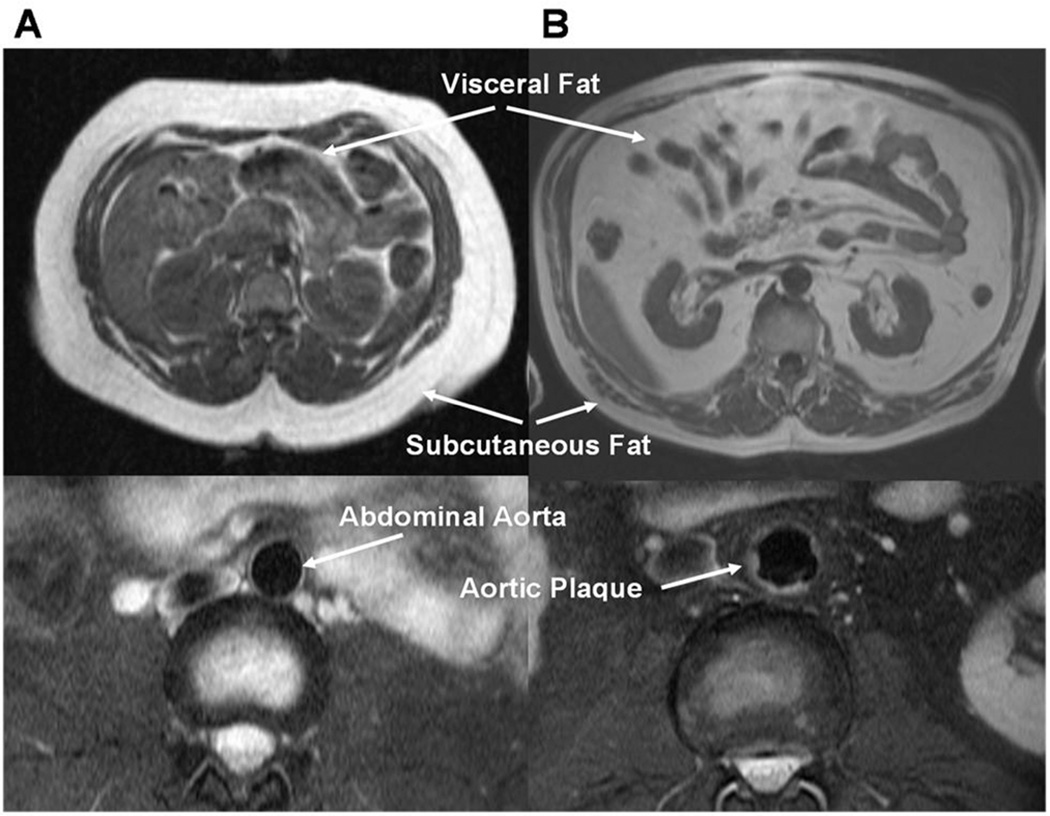
Figure 1. Representative Examples of Abdominal Fat and Aortic Plaque by MRI in Two Subjects with Divergent Cardiovascular and Metabolic Phenotypes.
Panel A: Transverse abdominal MRI images of VAT and SAT (upper panel) and aortic plaque (lower panel) in a 21 year old black female with BMI of 36 kg/m2 and total body fat of 4.2 kg (41%) demonstrate very low VAT (0.22 kg/m2) and high SAT (4.45 kg/m2), and no aortic plaque (0%). Panel B: In contrast, images of VAT and SAT (upper panel) and aortic plaque (lower panel) in a 59 year old white male with a BMI of 31.4 kg/m2 and total body fat of 4.0 kg (34%) demonstrate very high VAT (1.80 kg/m2) and low SAT (1.46 kg/m2), and high aortic plaque (18%).
BMI, body mass index; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue
Dual-energy x-ray absorptiometry (DEXA, Delphi W scanner, Hologic Inc., Bedford, MA, USA and Discovery software [version 12.2]) was used to measure total fat mass and fat-free mass of the following body compartments: trunk, upper and lower extremities, and head (24) The trunk was defined as the region delineated by a horizontal line below the chin, vertical lines within the left and right glenoid fossae and bordering laterally to the ribs, and by oblique lines crossing the femoral necks and converging below the pubic symphysis. Lower extremity fat was defined as all fat mass below these oblique lines.
Biomarker measurements
Venous blood was collected in EDTA tubes and maintained at 4 C for ≤ 4 hours before centrifugation at 1430×g for 15min. Plasma was then removed and frozen at −80 C until assays were performed by individuals blinded to all clinical data. All biomarkers reported here have been previously measured and the analytical methods described, including fasting blood glucose, insulin, and plasma lipids (15), leptin (25), adiponectin (26), and high-sensitivity C-reactive protein (hs-CRP) (27). Particle sizes and concentrations of LDL, HDL, and very low-density lipoprotein (VLDL) sub-classes were measured by LipoScience, Inc. (Raleigh, NC, USA) using NMR spectroscopy (28). Other biomarkers measured in the DHS and reported in this analysis are listed in the Supplementary Appendix Methods.
Atherosclerosis measurements
Electron beam computed tomography measurements of coronary artery calcium (CAC) were performed in duplicate 1–2 minutes apart using an Imatron 150 XP scanner (Imatron Inc., San Bruno, CA, USA). Scores were expressed in Agatston units and the mean of two consecutive scans was used as the final score (29).
Using the same 1.5T MRI system described above, six transverse slices of the infrarenal abdominal aorta were obtained using a free-breathing, ECG-gated, T2-weighted turbo spin-echo (black-blood) sequence. Images were analyzed by trained observers blinded to all subject data using the Magnetic Resonance Analytic Software Systems cardiac analysis software package (Version 4.2 beta, Medis Medical Imaging Systems Inc). Atherosclerotic plaque was identified as hyperintense signal volume that protruded ≥1 mm from the endoluminal surface of the aortic wall (Figure 1). Aortic plaque burden (APB) was calculated as the sum of the total plaque area divided by the total vessel area for all slices, multiplied by 100. Aortic wall thickness (AWT) was calculated by dividing the total vessel wall area by the aortic circumference in each slice and averaged over all slices (30).
Statistical analysis
Dichotomous variables were compared using chi-square tests and continuous variables were compared using Student’s T-test for normally distributed variables or the Wilcoxon ranksum test for non-normally distributed variables. Correlation analysis was performed using Spearman’s rank coefficients. Linear regression modeling was used to assess associations of VAT and SAT with continuous biomarker variables, with the log-transformed biomarker value as the dependent variable and VAT/BSA and SAT/BSA as the independent variables. Standardized β coefficients were used for both dependent and independent variables to facilitate comparisons between VAT, SAT, and biomarkers. The β coefficient for the fat parameter represents the unit change in 1 standard deviation of the log-transformed biomarker for a 1 standard deviation change in the fat parameter. Logistic regression was used to determine associations of VAT/BSA and SAT/BSA with categorical outcome variables. The odds ratio represents the odds of the outcome for each 1 standard deviation increase in the fat parameter. To determine independent associations of VAT/BSA and SAT/BSA with cardiac and metabolic phenotypes, all models were adjusted for age, sex, race, menopausal status (if female), VAT/BSA, and SAT/BSA; models were further adjusted for BMI to evaluate for attenuation of these associations by overall adiposity. Associations with lipoproteins and inflammatory biomarkers were additionally adjusted for HOMA-IR to determine independence from insulin resistance, and models of atherosclerosis were additionally adjusted for traditional coronary disease risk factors and glucose lowering, lipid-lowering, or aspirin medication use to evaluate for attenuation by risk factors or medications that improve the risk factor profile. Adjusted aortic plaque prevalence across sex-specific tertiles of VAT and SAT was calculated using the average probability for prevalent aortic plaque from the fully adjusted multivariable logistic regression model and compared using the Jonckheere-Terpstra trend test. Due to potential collinearity by inclusion of VAT or SAT with BMI in multivariable models, we tested for correlations (correlation coefficients of BMI with VAT/BSA and SAT/BSA were −0.02 and 0.68, respectively) and by examining variance inflation factors after inclusion of BMI in multivariable models, which were consistently less than 3. Because the continuous measure of APB included many zero values and a non-normal distribution of the non-zero measurements, Tobit linear regression (using PROC LIFEREG) was performed for log (APB+1) (31). All analyses were adjusted for multiple testing and Bonferroni-corrected p-values <0.0016 were considered statistically significant. All statistical analyses were performed using SAS version 9.2 software (SAS Corporation, Cary, NC, USA).
Results
Among the 1200 obese participants, the median age (25th, 75th percentile) was 44 (36, 52) years and median BMI was 35 (32, 39) kg/m2. The prevalence of metabolic syndrome was 56%; other demographic characteristics of the study cohort are reported in Table 1. Women had more subcutaneous fat and less visceral fat compared with men (median [25th, 75th percentile] 7.6 [6.1, 9.7] vs. 4.9 [4.0, 6.5] kg and 1.4 [1.1, 1.8] vs. 2.0 [1.6, 2.4] kg, respectively, p<0.0001 for both). Blacks had less VAT and more SAT than either Whites or Hispanics (Supplementary Appendix Figure 2).
Table 1.
Demographic Characteristics of Obese Participants in the Dallas Heart Study (n=1200)
| Variable | Median (25th, 75th percentile) or Proportion (%) | |
|---|---|---|
| Age (years) | 44 (36, 52) | |
| Female | 746 (62%) | |
| Race | ||
| White | 318 (26%) | |
| Black | 660 (55%) | |
| Hispanic | 222 (19%) | |
| Weight (kg) | 98.0 (87.5, 110.2) | |
| Height (cm) | 165.1 (158.8, 172.7) | |
| BMI (kg/m2) | 35 (32, 39) | |
| Waist Circumference (cm) | 110 (102.0, 118.0) | |
| Hip Circumference (cm) | 118.0 (111.0, 127.8) | |
| Waist/Hip ratio | 0.92 (0.87, 0.98) | |
| DEXA Fat Measures | ||
| Fat Mass (kg) | 35.0 (28.8, 42.6) | |
| Lean Mass (kg) | 57.9 (50.7, 68.4) | |
| Total Body Fat (%) | 39.7 (30.5, 44.1) | |
| Lower Body Fat (kg) | 12.1 (9.2, 15.5) | |
| Truncal Fat (kg) | 17.6 (14.8, 21.3) | |
| MRI Fat Measures | ||
| SAT (kg) | 6.6 (5.0, 8.9) | |
| VAT (kg) | 1.6 (1.2, 2.0) | |
| Hypertension | 510 (43%) | |
| Diabetes | 209 (17%) | |
| Hypercholesterolemia | 181 (15%) | |
| Low HDL | 617 (51%) | |
| Metabolic Syndrome | 675 (56%) | |
| Current Smoking | 268 (22%) | |
| Prior CVD | 96 (8%) | |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; DEXA, dual energy x-ray absorptiometry; HDL, high-density lipoprotein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue
Univariable correlations
VAT and SAT were not significantly correlated with each other in this sample (Spearman’s rho=0.02, p=0.44). Unadjusted Spearman’s rank correlation coefficients for VAT and SAT with all variables tested are listed in Supplementary Appendix Table 2. In univariable analyses, VAT and SAT positively correlated with HOMA-IR, but showed differential correlations with fructosamine, leptin, and adiponectin levels. VAT correlated with a dyslipidemic phenotype, whereas SAT was inversely correlated with dyslipidemic markers (Supplementary Appendix Table 2). Both VAT and SAT were modestly correlated with selected biomarkers of adipogenesis, inflammation, and endothelial and renal function, but VAT more strongly correlated with measures of subclinical atherosclerosis compared with SAT (Supplementary Appendix Table 2).
Multivariable-Adjusted Regressions
Biomarkers (n=942)
In multivariable analyses including both VAT and SAT as independent variables, VAT remained associated with markers of hyperglycemia and insulin resistance whereas associations with SAT were no longer significant after adjustment for BMI (Table 2). VAT, but not SAT, was inversely associated with adiponectin levels. Conversely, SAT was more strongly associated with leptin than VAT. VAT was associated with a dyslipidemic lipoprotein profile including increased LDL and VLDL particle number, smaller LDL and HDL size and larger VLDL size, increased triglycerides, and lower HDL cholesterol (Table 2). After further adjustment for HOMA-IR, VAT remained associated with these lipoprotein parameters. In contrast, SAT showed no significant independent associations with any lipid markers in fully-adjusted multivariable models (Table 2).
Table 2.
Multivariable-Adjusted Linear Regression Models of Relation of VAT or SAT to Biomarkers: Standardized β coefficient of VAT/BSA or SAT/BSA†
| VAT/BSA | SAT/BSA | ||||||
|---|---|---|---|---|---|---|---|
| Biomarker | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Insulin Resistance | |||||||
| Glucose (mg/dL) | 0.19* | 0.18* | -- | 0.07 | 0.01 | -- | |
| Insulin (µU/mL) | 0.32* | 0.30* | -- | 0.26* | 0.13 | -- | |
| HOMA-IR | 0.34* | 0.31* | -- | 0.26* | 0.12 | -- | |
| Fructosamine (µmol/L) | 0.02 | 0.03 | -- | −0.11* | −0.06 | -- | |
| Adipokines | |||||||
| Leptin (µg/L) | 0.07 | 0.05 | 0.04 | 0.43* | 0.36* | 0.35* | |
| Adiponectin (ng/mL) | −0.17* | −0.16* | −0.12* | −0.02 | 0.06 | 0.07 | |
| Lipids | |||||||
| LDL-C (mg/dL) | 0.07 | 0.09 | 0.08 | 0.02 | 0.13 | 0.13 | |
| LDL particle no. (nmol/L) | 0.21* | 0.22* | 0.18* | −0.03 | 0.05 | 0.04 | |
| LDL size (nm) | −0.23* | −0.23* | −0.20* | 0.08 | 0.08 | 0.10 | |
| Triglycerides (mg/dL) | 0.22* | 0.22* | 0.16* | −0.11* | −0.12 | −0.14 | |
| VLDL particle no. (nmol/L) | 0.17* | 0.18* | 0.15* | −0.08 | −0.03 | −0.04 | |
| VLDL size (nm) | 0.16* | 0.15* | 0.11 | 0.03 | 0.03 | 0.01 | |
| HDL-C (mg/dL) | −0.18* | −0.17* | −0.15* | −0.001 | 0.06 | 0.08 | |
| HDL particle no. (µmol/L) | 0.03 | 0.04 | 0.04 | 0.01 | 0.07 | 0.06 | |
| HDL size (nm) | −0.22* | −0.21* | −0.18* | −0.03 | 0.01 | 0.03 | |
| Inflammation | |||||||
| hs-CRP (mg/L) | 0.20* | 0.17* | 0.16* | 0.37* | 0.24* | 0.23* | |
| LTβR (ng/mL) | 0.04 | 0.04 | 0.05 | 0.22* | 0.20* | 0.20* | |
| sESAM (ng/mL) | 0.10 | 0.11 | 0.10 | 0.17* | 0.20* | 0.19* | |
p<0.0016
Model 1 adjusted for age + sex + race + menopausal status (women only) + VAT/BSA + SAT/BSA
Model 2 adjusted for Model 1 + BMI
Model 3 adjusted for Model 2 + HOMA-IR
Data presented are β coefficients that represent the estimated unit change in 1 standard deviation of the log-transformed biomarker for a 1 standard deviation increase in the fat parameter.
Only biomarkers with significant associations in multivariable analyses are shown in this table. See Supplementary Appendix Table 3 for list of multivariable associations with all other biomarkers tested.
Abbreviations: BMI, body mass index; BSA, body surface area; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity Creactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; LTβR, lymphotoxin β receptor; SAT, subcutaneous tissue; sESAM, soluble endothelial cell-selective molecule; VAT, visceral adipose tissue
Both VAT and SAT were modestly associated with hs-CRP, but only SAT was significantly associated with lymphotoxin β receptor (LTβR) and soluble endothelial cell-selective adhesion molecule (sESAM) (Table 2). However, the large majority of inflammatory biomarkers tested showed no association with either VAT or SAT in adjusted models (Supplementary Appendix Table 3).
Traditional Risk Factors, Liver Fat, and Atherosclerosis (n=1200)
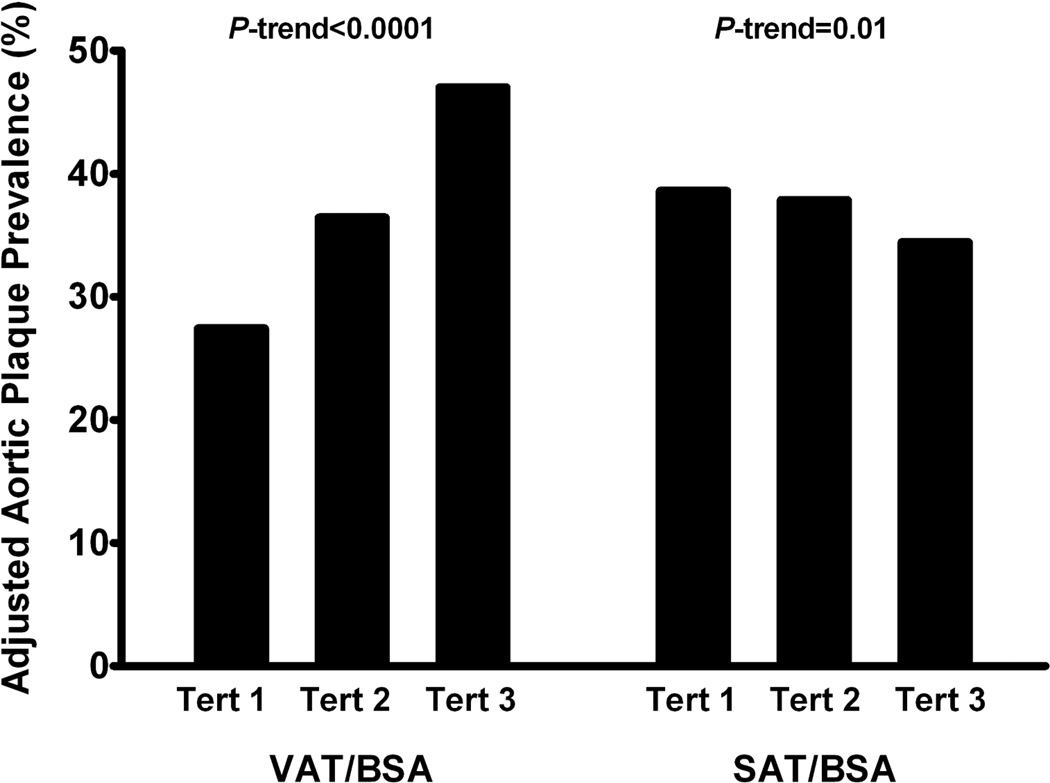
In fully adjusted models, VAT was significantly associated with prevalent diabetes and the metabolic syndrome (p<0.0001 for each). VAT also strongly associated with percent liver fat, aortic plaque burden, and aortic wall thickness (Table 3). Associations remained significant after further adjustment for traditional atherosclerotic risk factors and medication use. In contrast, no associations between SAT and traditional risk factors, liver fat, or atherosclerosis phenotypes were observed in fully adjusted models (Table 3). Whereas a significant increase in adjusted aortic plaque prevalence was observed across tertiles of VAT in multivariable analyses (p-trend<0.0001), a significant inverse relationship with aortic plaque was seen across tertiles of SAT (p-trend=0.01, Figure 2). Neither VAT nor SAT was independently associated with CAC in adjusted models; results were unchanged in analyses of CAC as a continuous or dichotomous variable with varying cut-points.
Table 3.
Multivariable-Adjusted Logistic and Linear Regression Models of Relation of VAT or SAT to Traditional Risk Factors, Liver Fat, and Atherosclerosis: Odds Ratio (95% CI) or Standardized β coefficient of VAT/BSA or SAT/BSA
| VAT/BSA | SAT/BSA | |||||
|---|---|---|---|---|---|---|
| Biomarker | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Hypertension | 1.12 (0.95, 1.32) | 1.10 (0.93, 1.30) | 1.10 (0.92, 1.31) | 1.23 (1.07, 1.48) | 1.14 (0.90, 1.44) | 1.08 (0.84, 1.38) |
| Diabetes mellitus | 1.54 (1.27, 1.85)* | 1.49 (1.23, 1.80)* | 1.41 (1.06, 1.87)* | 1.39 (1.15, 1.66) | 1.17 (0.90, 1.53) | 1.02 (0.67, 1.55) |
| Metabolic syndrome | 1.69 (1.44, 1.98)* | 1.64 (1.39, 1.92)* | 1.54 (1.23, 1.92)* | 1.14 (0.98, 1.31) | 0.95 (0.77, 1.18) | 0.85 (0.62, 1.15) |
| Liver Fat (%) | 0.33* | 0.31* | 0.28* | 0.15* | 0.05 | 0.03 |
| Coronary artery calcium (Agatston units) | 0.06 | 0.04 | 0.05 | 0.15* | 0.08 | 0.07 |
| Aortic plaque burden (%) | 0.29* | 0.38* | 0.35* | −0.08 | 0.13 | 0.08 |
| Aortic wall thickness (mm) | 0.20* | 0.19* | 0.19* | 0.05 | 0.02 | 0.01 |
p <0.0016
Model 1 adjusted for age + sex + race + menopausal status (women only) + VAT/BSA + SAT/BSA
Model 2 adjusted for Model 1 + BMI
Model 3 adjusted for Model 2 + hypertension + diabetes + smoking + hypercholesterolemia + low HDL + glucose-lowering medication + lipid-lowering medication + aspirin
Data presented are β coefficients that represent the estimated unit change in 1 standard deviation of the log-transformed biomarker for a 1 standard deviation increase in the fat parameter or an odds ratio (95% CI) that represents the odds of the outcome for each 1 standard deviation increase in the fat parameter and associated 95% confidence interval.
Figure 2. Adjusted Prevalence of Aortic Plaque by Tertile of VAT/BSA or SAT/BSA in Obese Adults.
The adjusted prevalence of aortic plaque increases significantly across sex-specific tertiles of VAT, but decreases across tertiles of SAT, in obese adults. Adjusted for age, sex, race, menopausal status (women only), hypertension, diabetes, smoking, hypercholesterolemia, low HDL cholesterol, glucose-lowering medication, lipid-lowering medication, aspirin, VAT/BSA, and SAT/BSA. p-value for trend across tertiles
BSA, body surface area; SAT, subcutaneous adipose tissue; Tert, tertile; VAT, visceral adipose tissue
Findings were generally consistent and insensitive to stratification by sex or race, and to indexing VAT and SAT by total body fat. The only significant differences by sex and race observed were associations of VAT with leptin in men but not in women (standardized β=0.18, p<0.0016 vs. β=−0.05, p=0.40) and differential associations of VAT with VLDL particle number in black vs. white participants (standardized β=0.19, p<0.001 vs. β=0.09, p=0.23).
Discussion
In this study designed to examine the cross-sectional associations of abdominal fat distribution with cardiac and metabolic risk markers in a large, multi-ethnic, population-based sample of obese adults, we demonstrate that visceral and subcutaneous abdominal adipose tissue mass are not significantly correlated with each other in the obese population, and differentially associate with markers of dyslipidemia, insulin resistance, hepatic steatosis, and subclinical atherosclerosis. VAT associates with an atherogenic and dysmetabolic obesity phenotype whereas SAT demonstrates no association with atherosclerosis and inconsistent, and generally weaker, associations with metabolic phenotypes despite advanced obesity. Novel findings include 1) strong associations between VAT and aortic atherosclerosis not previously described in a large, population-based study, 2) no independent associations observed between SAT and dyslipidemia or atherosclerosis, with only modest associations seen with insulin resistance, liver fat, and traditional risk factors that were no longer significant after further adjustment for BMI, and 3) the absence of a robust inflammatory phenotype specifically associated with either fat depot, an observation that challenges conventional paradigms about obesity and circulating inflammatory biomarkers. Findings were generally insensitive to stratification by sex or race suggesting that, although fat distribution differs by sex and race, the associations of abdominal fat distribution with metabolic and cardiac phenotypes are similar in men and women and across race/ethnicity. These observations suggest that clinically relevant sub-phenotypes of obesity can be defined by abdominal fat distribution, supporting the notion of obesity as a heterogeneous disorder with varying cardiac and metabolic manifestations.
Previous studies in community-based cohorts have generally concluded that visceral, and to a lesser extent subcutaneous, adiposity correlates with an adverse metabolic profile (4–7, 9). However, the average BMI in these populations ranged from normal to overweight, with obese subjects comprising a minority of the overall sample. Because SAT function may be modified by increasing weight gain and amount of VAT (32), examining relations of SAT with biomarkers may be particularly important among obese individuals. Additionally, it is unclear if an obesity threshold exists beyond which VAT no longer contributes to the dysmetabolic state. Our findings, in a cohort with an average BMI meeting criteria for class II obesity, show clear associations between progressively larger amounts of VAT and a detrimental dyslipidemic and atherosclerotic phenotype, independent of BMI. In contrast, we do not find compelling evidence that SAT independently associates with this phenotype in advanced obesity.
While our study provides strong evidence of independent associations between VAT and markers of insulin resistance among obese individuals, the findings with regard to SAT were less consistent, and sensitive to whether adjustment was performed for BMI. Previous physiologic studies of glucose metabolism reported that the rate of glucose disposal (a marker of insulin sensitivity) was more strongly correlated with abdominal SAT than with VAT, and that the majority of postprandial free fatty acids (FFA) originate from abdominal SAT, rather than VAT (33–34). However, a cross-sectional study of abdominal fat distribution in 3000 community-dwelling individuals found that abdominal SAT was associated with the metabolic syndrome in normal-weight, but not obese, men (35). Similarly, the Framingham Heart Study demonstrated that SAT was associated with adverse risk factors in those with low VAT but was relatively neutral in those with high VAT (11). Because our study focused only on obese participants, it does not address the role of SAT on insulin resistance among individuals with lower BMI and less abdominal fat. It is possible that the development of insulin resistance in earlier stages of adiposity may be related to both abdominal VAT and SAT accumulation but that once obesity is present, VAT is the more important fat depot.
Few studies have examined the association between abdominal fat distribution and subclinical atherosclerosis. In one study, carotid intima-media thickness was positively associated with VAT and negatively associated with SAT, but this analysis was restricted to middle-age women and was not adjusted for BMI (9). In our study, VAT also associated with measures of aortic atherosclerosis not previously described, including aortic plaque burden and wall thickness, independent of BMI. In contrast, although CAC associated with VAT in univariable analyses, the association was attenuated after adjustment. This finding is consistent with a recent study that did not demonstrate an independent association between VAT and coronary atherosclerosis after BMI adjustment (36). Others, however, have described independent associations between anthropometric measures of obesity and CAC (37–38). Explanations for the absence of association with CAC in our study may include the relatively low prevalence of CAC, a 2:1 ratio of women to men, and the relatively young age of our sample population. Further investigation into relationships between VAT and measures of atherosclerosis in higher risk individuals is warranted.
Previous studies have reported a possible “protective” effect of lower body fat, which is composed primarily of SAT, with regard to metabolic risk factors (24) and biomarkers such as adiponectin (26). In our study, SAT associated with leptin and inflammatory markers to a greater degree than with dyslipidemia or atherosclerosis. Some associations with SAT suggest potential protective effects against atherosclerosis and metabolic disease, including inverse associations with fructosamine, an intermediate-term marker of glucose control, and with triglycerides and VLDL particle number. However, no protective effects were seen with increasing SAT for insulin resistance, liver fat, or atherosclerosis in multivariable models. A possible explanation for these discrepant findings may relate to the distribution of SAT. Peripheral SAT may be associated with a metabolically more favorable phenotype, but abdominal SAT may have a more complex relationship with metabolic risk. Abdominal SAT has both superficial and deep components that may have opposing effects (32) leading to an overall neutral association with metabolic and cardiovascular markers in the obese population. Thus, the preponderance of evidence does not suggest a protective effect of increasing abdominal SAT in obese individuals.
Potential mechanisms
What is the reason for the apparent stronger association between VAT and metabolic risk factors compared with SAT? One explanation may be that in persons with greater VAT, the subcutaneous adipose depot (the preferred site of fat storage) does not provide an adequate reservoir for body fat. Excess fatty acids may then be redistributed to ectopic tissues such as visceral fat, liver, and skeletal muscle, predisposing to metabolic risk. This may be especially apparent in obese persons in whom SAT is overwhelmed by excess energy input. In these individuals, VAT size may be a strong indicator of deficient body fat storage capacity in subcutaneous adipose tissue.
The mechanistic link between visceral obesity and inflammation remains unclear. Previous studies have generally investigated adipose tissue from biopsy samples and measured locally expressed or secreted tissue markers. The absence of a robust inflammatory phenotype specific to VAT in our study could reflect the failure of measurements of circulating biomarkers to accurately characterize actions of inflammatory cytokines in the local tissue microenvironment. Alternatively, our findings of generally similar associations of inflammatory biomarkers with VAT and SAT may suggest that inflammation associated with obesity is more related to overall fat mass than to fat distribution. In contrast to our findings with insulin resistance and dyslipidemia, inflammation may be related to expansion of all fat compartments and not represent a distinct visceral adipose phenotype.
Limitations
Several limitations of our study must be acknowledged. Our study is cross-sectional and causal relationships between VAT, SAT, and phenotypes cannot be determined. We also acknowledge that our study may yield some false-positive findings due to multiple testing given that we examine associations with multiple biomarkers and imaging variables. Additionally, because participants receiving aspirin, lipid-lowering, or glucose-lowering medications were excluded a priori from lipoprotein and biomarker analyses, our findings cannot be generalized to individuals taking these medications. Finally, our cohort is relatively young and therefore extrapolations to older populations in whom fat mass physiology may be different cannot be made.
Clinical Implications
Considerable heterogeneity exists in the cardiovascular manifestations of obesity. BMI is a simple measure for clinical use, but it does not differentiate between fat and lean mass, and obscures differences between various body mass compartments. Other measures, such as waist circumference or waist-to-hip ratio, offer more information regarding fat distribution, but add only modest clinical information to the prediction of incident CVD, with divergent results across studies (39–40). Because the consequences of obesity on the cardiovascular system are not predictable based on simple anthropometric measurements, new tools are needed to identify appropriate candidates for intensive CVD risk reduction, and possibly also to select candidates for weight reduction surgery or pharmacological therapy. The observation that VAT and SAT associate with divergent phenotypes suggests that MRI and other imaging tests merit further exploration as tools to guide clinical decision-making among obese individuals. However, while MRI is a simple and safe tool to differentiate VAT and SAT components, clinical application may be limited by cost, availability, and expertise required for image analysis. Moreover, it should be acknowledged that while the associations of VAT with atherosclerosis phenotypes were statistically robust, and likely of pathophysiological significance, they are quantitatively modest and thus assessment of VAT may not substantially improve risk prediction. VAT may represent only one key feature of dysfunctional adiposity; in the future, it is possible that measurement of circulating biomarkers (such as lipoprotein sub-classes) may provide useful surrogate information for the risk associated with VAT, and may be more feasible when MRI is not available.
Conclusions
In a large, multi-ethnic, population-based sample of obese adults, we found that VAT and SAT are associated with different obesity sub-phenotypes: an adverse metabolic, dyslipidemic, and atherogenic phenotype with VAT, and a moderately inflammatory but generally more benign phenotype with SAT. These findings suggest that abdominal obesity is a heterogeneous disorder comprised of clinically distinguishable sub-phenotypes defined by abdominal fat distribution. Further research is needed to determine if measurement of VAT and SAT improves cardiac and metabolic risk assessment among obese individuals.
Supplementary Material
Acknowledgments
Sources of Funding and Acknowledgments
This work was supported by Award Number T32HL007360 from the National Heart, Lung, and Blood Institute, the Donald W. Reynolds Foundation (Las Vegas, NV), and by United States Public Health Service General Clinical Research Center (USPHS GCRC) grant #M01-RR00633 from National Institutes of Health/National Center for Research Resources-Clinical Research (NCRRCR). Biomarker measurements were supported by investigator-initiated grants from Alere Inc (San Diego CA) and Roche Diagnostics (Indianapolis IN). We would like to acknowledge Ron Peshock, MD for his assistance with the selection of representative abdominal fat and aortic plaque magnetic resonance images for inclusion in the manuscript.
Abbreviations
- VAT
Visceral Adipose Tissue
- SAT
Subcutaneous Adipose Tissue
- BSA
Body Surface Area
- BMI
Body Mass Index
- MRI
Magnetic Resonance Imaging
- DEXA
Dual Energy X-Ray Absorptiometry
Footnotes
Supplementary Material is available at www.nature.com/obesity
Disclosures
Dr. de Lemos has received grant support from Roche Diagnostics; consulting income from Tethys Bioscience, AstraZeneca, and Daiichi Sankyo; and lecture honoraria from BMS/Sanofi-Aventis. Dr. McGuire has received consulting income from F. Hoffmann LaRoche, Genentech, Sanofi-Aventis, Daiichi Sankyo, Novo Nordisk, and Tethys Bioscience.
References
- 1.Cornier MA, Despres JP, Davis N, et al. Assessing Adiposity: A Scientific Statement From the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 2.Bays HE, Gonzalez-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 3.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008;36:935–940. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Oka R, Miura K, Sakurai M, et al. Impacts of visceral adipose tissue and subcutaneous adipose tissue on metabolic risk factors in middle-aged Japanese. Obesity (Silver Spring) 2010;18:153–160. doi: 10.1038/oby.2009.180. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Fox CS, Hickson DA, et al . Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 8.Ducluzeau PH, Manchec-Poilblanc P, Roullier V, et al. Distribution of abdominal adipose tissue as a predictor of hepatic steatosis assessed by MRI. Clin Radiol. 2010;65:695–700. doi: 10.1016/j.crad.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Wildman RP, Janssen I, Khan UI, et al. Subcutaneous adipose tissue in relation to subclinical atherosclerosis and cardiometabolic risk factors in midlife women. Am J Clin Nutr. 2011;93:719–726. doi: 10.3945/ajcn.110.007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bays HE, Fox KM, Grandy S. Anthropometric measurements and diabetes mellitus: clues to the "pathogenic" and "protective" potential of adipose tissue. Metab Syndr Relat Disord. 2010;8:307–315. doi: 10.1089/met.2009.0089. [DOI] [PubMed] [Google Scholar]
- 11.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depotspecific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:740–744. [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1996;27:652–663. doi: 10.1016/s0272-6386(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Tikuisis P, Meunier P, Jubenville CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. 2001;85:264–271. doi: 10.1007/s004210100484. [DOI] [PubMed] [Google Scholar]
- 20.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 21.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–795. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 22.Armellini F, Zamboni M, Perdichizzi G, et al. Computed tomography visceral adipose tissue volume measurements of Italians. Predictive equations. Eur J Clin Nutr. 1996;50:290–294. [PubMed] [Google Scholar]
- 23.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 24.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 25.Abdullah SM, Khera A, Leonard D, et al. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195:404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Turer AT, Khera A, Ayers CR, et al. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Jain T, Peshock R, McGuire DK, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–107. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 30.Rohatgi A, Ayers CR, Khera A, et al. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: Observations from the Dallas Heart Study. Atherosclerosis. 2009;203:569–575. doi: 10.1016/j.atherosclerosis.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Long J. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, California: Sage; 1997. pp. 187–203. [Google Scholar]
- 32.Goedecke JH, Levitt NS, Lambert EV, et al. Differential effects of abdominal adipose tissue distribution on insulin sensitivity in black and white South African women. Obesity (Silver Spring) 2009;17:1506–1512. doi: 10.1038/oby.2009.73. [DOI] [PubMed] [Google Scholar]
- 33.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity (Silver Spring) 2006;14(Suppl 1):20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 36.Khashper A, Gaspar T, Azencot M, et al. Visceral abdominal adipose tissue and coronary atherosclerosis in asymptomatic diabetics. Int J Cardiol. 2011 Jun 2; doi: 10.1016/j.ijcard.2011.05.059. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Blaha MJ, Rivera JJ, Budoff MJ, et al. Association between obesity, high-sensitivity Creactive protein >/=2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1430–1438. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 39.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 40.Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52:605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.