Abstract
Objective
This study investigated the effects of acculturation on cortisol, a biological correlate of maternal psychological distress, and perinatal infant outcomes, specifically gestational age at birth and birth weight.
Methods
Fifty-five pregnant women of Mexican descent were recruited from a community hospital and collected saliva samples at home over 3 days during pregnancy at 15–18 (early), 26–2 (mid), and 32+ (late) weeks gestation and once in the postpartum period (4–12 weeks). These values were used to determine the diurnal cortisol slope at each phase of pregnancy. Mothers also completed an acculturation survey and gave permission for a medical chart review to obtain neonate information.
Results
Multiple regression analyses determined that greater acculturation levels significantly predicted earlier infant gestational age at birth (R2=0.09, p=0.03). T-tests revealed that mothers of low birth weight infants weight (<2500g) had significantly higher acculturation scores than mothers of infants with birth weight >2500g (t=−2.95, p=0.005). A blunted maternal cortisol slope during pregnancy was also correlated with low birth weight (r=−0.29, p=0.05), but not gestational age (r=−0.08, p=0.59). In addition, more acculturated women had a flatter diurnal cortisol slope late in pregnancy (R2=0.21, p=0.01). Finally diurnal maternal cortisol rhythms were identified as a potential mediator between increased acculturation and birth weight.
Conclusions
This study associated increased acculturation with perinatal outcomes in the US Mexican population. This relationship may be mediated by prenatal maternal diurnal cortisol, which can program the health of the fetus leading to several adverse perinatal outcomes.
Keywords: perinatal, hypothalamic-pituitary-adrenal axis, mother, immigrant, gestational age, birth weight
Introduction
In the United States Mexican population, acculturation, defined as members of one cultural group adapting to the beliefs and norms of another cultural group, has been associated with various negative perinatal outcomes, including low infant birth weight and preterm birth (1). However, the physiological mechanism through which acculturation leads to these negative effects is unknown. Prenatal exposure to dysregulated maternal cortisol, a hormone product of the hypothalamic adrenal pituitary axis, has been associated with various negative perinatal outcomes, such as growth retardation, preterm birth, and attention and temperament problems in infancy (2). As acculturation also seems to affect these outcomes, cortisol may be a promising physiological mechanism involved in mediating some of these relationships. This study collectively evaluates the relationship between acculturation and cortisol in a US Mexican population as well as its association with infant birth weight and gestational age at birth.
Overall, less acculturated Mexican, US immigrants have lower rates of morbidity and mortality despite lower socioeconomic status, limited education and decreased access to healthcare (3). This is referred as the “Latino health paradox.” This hypothesis suggests that acculturation of Mexican immigrants into US mainstream culture can have deleterious effects on their health. Traditional cultural values among the Mexican population in the US have been associated with a reduction of negative health behaviors, including smoking, alcohol consumption and unhealthy eating habits (3–5). High cultural orientation (less acculturated) is also associated with robust social support (6–8), an important protective factor for a variety of health behaviors and disorders (9–11). These paradoxical morbidity and mortality rates are especially pertinent at a time of greater vulnerability such as pregnancy. For example, acculturated women of Mexican descent are more likely to engage in non-healthy prenatal behaviors, such as smoking and to have inadequate social support relative to those less acculturated [15]. In addition, more acculturated US-born Mexicans have a higher number of unintended pregnancies and greater negative attitudes regarding their pregnancies (12). Acculturation is also a critical risk factor for increased stressors and negative perinatal outcomes in this population, such as low-birth weight [6], declines in breastfeeding [17, 18] and an increased risk of postpartum depression in many [5, 19], but not all studies [20]. These studies demonstrate a clear link between acculturation and negative perinatal outcomes in the US Mexican population.
On the other hand, the mechanism by which acculturation may be leading to these negative outcomes is less clear. One possibility is that acculturation may be associated with increased psychological stress (13–15). Elevation in the stress-related hormone cortisol is associated with many of the same negative perinatal outcomes as acculturation, such as premature birth and decreased birth weight (2, 16). However, little is known about maternal cortisol regulation in the Mexican population during pregnancy. One study did not report any significant ethnic differences in cortisol levels across pregnancy, although cortisol levels early in pregnancy in Hispanic women (Mexican and Central American) did predict later high circulating corticotropin-releasing hormone (CRH), a regulatory component of the HPA-axis cascade (17). Differing CRH levels between Hispanic and non-Hispanic Caucasian women in mid-pregnancy have been reported in some (18), but not all studies (17). Together these data suggest that pregnant Mexican women may have alterations in their HPA-axis functioning, the end product of which is cortisol, which may have lasting consequences for perinatal outcomes. The present study focuses on this possible relationship.
Salivary cortisol during pregnancy has been assessed by single measurements (19), area under the curve (20), cortisol awakening response (21, 22) as well as diurnal decline (23–25). Evaluation of the diurnal rhythm of cortisol across the day is an informative measure of HPA-axis functioning (23, 26). Generally, diurnal cortisol levels peak within 30 min of waking and steeply decline across the day (20, 27). A recent meta-analysis found chronic stress was associated with a flatter diurnal rhythm, higher daily cortisol output, greater afternoon cortisol levels and lower morning cortisol (26). These blunted cortisol profiles have been shown in stressed populations, such women with breast cancer (28, 29) and children with histories of trauma, neglect and maltreatment (30, 31). Chronically depressed persons exhibit a flattened cortisol decline across the day as well (32, 33). Thus, diurnal profiles can provide a measure of HPA-axis regulation during pregnancy. Since a blunted slope is associated with stress and acculturation is considered a cultural stressor (20, 34, 35), the diurnal decline of cortisol may provide important information related to challenges associated with acculturation.
The present study assessed diurnal decline of salivary cortisol levels and maternal self-reports of acculturation along with infant birth weight and gestational age. We tested the hypothesis that high levels of acculturation, as measured by the Short Acculturation Scale for Hispanics (SASH), would be associated with a blunted cortisol decline along with a decrease in both infant birth weight and gestational age at birth. It was also hypothesized that a blunted cortisol decline would mediate (36) the relationship between acculturation and birth outcomes.
Methods
Sample
Seventy Mexican and Mexican-American women were recruited to complete this study. The sample consisted of pregnant women (ages 18–45), without a history of current or past illicit drug use, who were recruited by staff at a local county hospital. Visits occurred at normally scheduled obstetrics clinic appointments between February 2009 and July 2010. The hospital was located in an urban setting, in which 92% of patient families are living below the poverty line and 42% of patients are uninsured (37). One participant withdrew from the study citing a lack of available time, four participants switched their care to other clinics and one participant tested positive for an illicit substance and were removed from the study. Eight participants were not included in the analysis due to failure to provide saliva for cortisol analysis at more than one of the three pregnancy phases (early, mid, late). Only women with complete salivary cortisol for at least two of three pregnancy phases were included in the analyses. A final sample of 55 mothers was evaluated (Table 1). Prior to beginning the analysis, a power analysis determined adequate power such that a sample size of 55 has 80% power to detect an r2= 0.12 (a small effect size) attributed to an independent variable using an F-Test with α=0.05. The variables tested were adjusted for the additional independent variable age with an r2=0.12. There is limited power to examine mediation.
Table 1.
Maternal characteristics
| Characteristic | N | % |
|---|---|---|
| Age | ||
| <20 | 2 | 3.6 |
| 20–29 | 29 | 52.8 |
| 30–39 | 22 | 40.0 |
| ≥40 | 2 | 3.6 |
| Place of birth | ||
| Mexico | 31 | 56.4 |
| United States | 24 | 43.6 |
| Primary language | ||
| English | 32 | 58.2 |
| Spanish | 20 | 36.4 |
| Bilingual | 3 | 5.4 |
| Employment status | ||
| Unemployed | 18 | 32.7 |
| Homemaker | 22 | 40.0 |
| Part-time | 9 | 16.4 |
| Full-time | 6 | 10.9 |
| Years of education | ||
| <12 | 33 | 60.0 |
| 12 | 17 | 30.9 |
| >12 | 2 | 3.6 |
| Unknown | 3 | 5.5 |
| Marital status | ||
| Married | 19 | 34.5 |
| Living together, not married | 20 | 36.4 |
| Not living together, not married | 13 | 23.6 |
| Separated | 3 | 5.5 |
| Current Smoking | ||
| No | 53 | 94.6 |
| Yes | 3 | 5.4 |
| Family Income | ||
| <$10,000 | 18 | 32.7 |
| $10,000–$20,000 | 14 | 25.4 |
| $21,000–$25,000 | 11 | 20.0 |
| $26,000–$30,000 | 6 | 10.9 |
| >$31,000 | 3 | 5.5 |
| Unknown | 3 | 5.5 |
| Pre-pregnancy BMI | ||
| <18.5 | 3 | 5.5 |
| 18.5–<25 | 18 | 32.7 |
| 25–<30 | 13 | 23.6 |
| ≥30 | 18 | 32.7 |
| Unknown | 3 | 5.5 |
Procedure
At home cortisol samples were collected during early (15–18 weeks gestation), mid (26–29 weeks gestation) and late pregnancy (32–36 weeks gestation) and in the postpartum period (4–12 weeks). Mothers filled out the acculturation survey at the late pregnancy visit. All demographic variables are listed in Table 1. Self-report of smoking status was routinely evaluated early in pregnancy by clinic nurses and obtained via chart review. All research subjects provided informed consent and all procedures were approved by the Colorado Multiple Institutional Review Board.
Maternal acculturation status
Acculturative status was measured once during pregnancy at 32–36 weeks gestation using the Short Acculturation Scale for Hispanics (SASH) (38). This short (12-item) acculturation scale evaluates three factors: language use, media and ethnic social relations. Respondents are asked what language they use during various activities ranging from “Only Spanish” (1) to “Only English” (5) or who they associate with ranging from “All Latinos/Hispanics” (1) to “All Americans” (5). This scale has a reliability coefficient α=0.92 and is highly correlated with respondents’ generation, length of residence in the U.S., age at arrival and ethnic self-identification, though is not a direct measure of cultural self-identity, per se. Higher scores indicate higher levels of acculturation. Surveys were presented to participants in their preferred language. Originally, questions were developed in English or Spanish and translated using a double translation procedure (38). This scale has been shown to be reliable in both Mexican and Central American populations (38) and has previously been used when evaluating health outcomes in Mexican women (39) and acculturation in Latina mothers (40). Acculturation was only measured once during pregnancy. In adults, acculturation is a fairly stable construct tied to personal identity, another stable construct (41), and generally refers to generational differences. As pregnancy lasts 9 months or less, a relatively limited time period, changes in acculturation are expected to be minimal. Information of maternal birthplace (US or Mexico) was also collected to compare with the continuous measure of acculturation on the SASH.
Life Event Information
At each study visit, participants were presented with a list of 31 events that may have happened to them. Participants were asked to check each event that has occurred in their life over the past few months. If an event happened more than once, the number of times was indicated. Events included: death of a spouse, divorce, marital separation, jail term, death of family member, personal injury/illness, marriage, fired at work, marital reconciliation, change in health of family member, change in work responsibilities, child leaving home, trouble with in-laws, outstanding personal achievement, spouse starts/stops work, begin/end school, change in living conditions, revision of personal habits, trouble with boss, change in work hours/conditions, change in residence, change in sleeping habits, change in eating habits, vacation, minor law violation, change in health of family member, change in financial state, sex difficulties, death of close friend, change in line of work, or arguments with spouse. The total events were determined for early, mid and late pregnancy. As the number of life events did not vary over pregnancy (see Results), they were totaled over pregnancy for a final life event score.
Maternal salivary cortisol collection
Saliva was collected using filter paper contained in specially constructed booklets for saliva collection as previously described (23, 42–44) and validated (44). Saliva was collected on three days at each phase (17.3±1.8, 28.1±1.5, 34.3±1.4 weeks gestation and 7.4±2.8 weeks postpartum). In brief, collection filters (Whatman Grade 42) were assembled in a small booklet that contained three filters for collection labeled “30 minutes after waking”, “before lunch,” and “4PM”. Three booklets were provided, one for each day of collection. This sampling schedule reduces problems associated with food intake on salivary cortisol levels. Tooth brushing was not allowed prior to sampling and fluids were withheld for at least 20 min prior to sampling. Filters were separated from each other in the collection booklet by waxed paper to prevent cross contamination. Color tabs on the waxed paper dividers matched color codes on the cover of the booklet and ensured that the correct filter in the booklet was wetted. Subjects were asked to enter time of each collection on the cover of the collection booklet. Proper saliva collection was taught via personal instruction.
Filters were carefully cut and extracted in 0.25 ml of assay buffer as previously described in detail (44). In brief, the buffer containing the cut filter was shaken overnight in a microcentrifuge tube. The 25 µl of extraction buffer was added in duplicate to the appropriate wells of the assay plate. Extraction dilutes the saliva approximately 1:5. Salivary cortisol concentration in the extraction buffer was determined using a commercial expanded range high sensitivity enzyme immunosorbent assay kit (No. 1-3002/1-3012, Salimetrics, LLC) that detects cortisol levels in the range of 0.003 – 3.0 µg /dl (0.08 – 82.77 nmol/L). Standard curves were fit by a weighted regression analysis using commercial software (Gen 5) for the plate reader (BioTek). After taking dilution into consideration, the detection limit was 0.018 µg/dl (0.50 nmol/L). This kit shows minimal cross reactivity (≤4%) with other steroids present in the saliva. Controls run on every plate for determination of inter-assay coefficients variability were less than 7.5% for high and low control levels. Intra-assay coefficients of variation for duplicate determinations were less than 3%. This enzyme immunoassay has been cross validated with outside laboratories with excellent correspondence to both DELFIA and mass spectrographic procedures (r2 = 0.85). Extraction losses ranged from 10–15%. The slope of the diurnal decline in salivary cortisol for each participant between 30 min after waking until 1600hr was estimated from the means at each time for the three days at each phase during pregnancy using commercial software SPSS (45).
Infant outcome data collection
Chart review of infant birth records were used to collect infant birth weight and gestational age at delivery (in weeks). Gestational age was determined by last menstrual period. If this was not known or not consistent with ultrasound dating, the earliest ultrasound was used to determine gestational age. Early gestational age/preterm birth is considered infants born <37 weeks and low birth weight is infants weighing <2500g at birth.
Statistical Analysis
Correlations were used to determine associations of the continuous variables age, body mass index (BMI) and parity on both acculturation and diurnal cortisol slope. One-way analyses of variance (ANOVAs) were used to determine the effects of the categorical variables education, employment status, marital status, family income and smoking history (groups as represented in Table 1) on acculturation and diurnal cortisol slope. None of the demographic variables were associated with either acculturation or cortisol slope (data not shown), except for maternal age (see Results). Given evidence that maternal age is associated with adverse perinatal outcomes, including early gestational age and low infant birth weight (46, 47), it was included as a covariate in the following analyses. Since preterm birth is a strong predictor of low birth weight (48), analyses of birth weight were co-varied for gestational age at birth. Only three women reported smoking behavior early in pregnancy, albeit minimal. Women who admitted to smoking early in pregnancy did not exhibit adverse perinatal outcomes (early gestational age or low birth weight) and were included in analyses.
To capture acculturation quantitatively, this measure was evaluated as a continuous variable. Multiple linear regression analysis was completed with perinatal outcomes (infant birth weight and gestational age) as dependent variables and acculturation and maternal age as predictor variables to identify models explaining the perinatal outcomes. Multiple regression tests assessed the relationship between diurnal cortisol slopes (the dependent variable) and acculturation and maternal age as predictor variables. T-tests were used to compare maternal birthplace (US or Mexico) and acculturation scores as well as to compare dichotomized perinatal outcome variables (low birth weight <2500g and preterm birth <37 weeks gestation) and acculturation scores. In addition, a t-test was used to compare number of life events and acculturation. Correlations were used to determine the relationship between perinatal outcomes and maternal cortisol slope. These analyses were conducted using a commercial statistical package, SigmaStat 3.0 (SPSS Inc., Chicago). For those relationships that reached significance, mediation analyses were then conducted in SAS using the steps identified by Baron and Kenny (36).
Results
Sample characteristics
All women in this study self-identified as Hispanic/Latino and were of Mexican ancestral origin. The mean age (± standard deviation) of mothers in the study was 28± 6 years. Parity ranged from 0–7 with a mean parity of (± standard deviation) of 2.1±1.6. Only three women in the study were primiparous. All other maternal demographics are included in Table 1.
Maternal age was significantly negatively correlated with acculturation indicating that less acculturated women were more likely to be older (means±std; acculturated: 24.3±5.4, non-acculturated: 30.1±4.5; r=−0.48, p<0.001; Pearson’s correlation). Mothers with an acculturation score greater than 2.99 were considered acculturated as previously determined (38). No other demographic variables were associated with acculturation or diurnal cortisol slope.
Relationship between maternal acculturation and maternal birthplace
Maternal birthplace was significantly related to acculturation. Women born in the US had significantly higher acculturation scores (mean±SEM: 3.51±0.18) than women born in Mexico (mean±SEM: 1.40±0.06) (t=11.42, df= 54, p<0.001; t-test).
Relationship between maternal acculturation and perinatal outcomes
Greater acculturation marginally predicted lower infant birth weight when maternal age and gestational age of the infant were considered as covariates (Table 2; Figure 1A), with higher levels of acculturation tending to be associated with lower birth weights (p=0.07). However, when considered as a categorical variable, birth weight was significantly related to maternal acculturation scores. A t-test reveaed that mothers who gave birth to infants of low birth weight (<2500g, clinical definition of low birth weight; n=9) infants had significantly higher acculturation scores than mothers of infants with birth weight >2500g (n=46) (t=−2.95, df=54, p=0.005; Figure 1B).
Table 2.
Multiple linear regression analysis of acculturation predicting perinatal outcomes (n=55) Bold indicates significant predictor variables, p<0.05.
| Outcome variable | R2 | Variables included | Regression coefficient |
SE | t value | Sig (p) |
|---|---|---|---|---|---|---|
| Birth weight | 0.556 | Acculturation | −83.48 | 46.25 | −1.81 | 0.07 |
| Maternal age | 13.71 | 23.68 | 6.41 | 0.20 | ||
| Gestational age | 13.71 | 10.65 | 1.29 | <0.001 | ||
| Gestational age | 0.091 | Acculturation | −0.06 | 0.26 | −2.28 | 0.03 |
| Maternal age | −0.07 | 0.06 | −1.15 | 0.26 |
Figure 1.
Acculturation predicts perinatal outcomes. A, Greater maternal acculturation marginally predicts low infant birth weight (R2=0.56, p = .07, multiple regression). B, Mothers of low-birth-weight infants (<2500g; n = 9) had significantly higher acculturation scores than mothers of infants with birth weight greater than 2500 g (n = 45) (t = −2.21, df = 53, p = 0.005). Analyses are covaried for age, but age is not included in the figures. SASH = Short Acculturation Scale for Hispanics.
Acculturation significantly predicted gestational age as a dependent variable, even when maternal age was considered as a covariate (Table 2; Figure 2A). Infants of more acculturated mothers were more likely to be born earlier. Additional analyses investigating the effects of acculturation on gestational age as a categorical dichotomy examined differences in acculturation scores between those women who gave birth to preterm (<37 weeks; n=11) and term (>37 weeks; n=44) infants. A t-test revealed that mothers of preterm infants had higher acculturation than mothers with term infants (t=−2.21, df=54, p=0.03; Figure 2B).
Figure 2.
Acculturation predicts gestational age. A, Greater maternal acculturation predicts early gestational age at birth (R2 = 0.09, p = .03, multiple regression). B, Mothers of preterm infants (<37 weeks; n = 11) had marginally higher acculturation than mothers with term infants (n = 43; t = −2.95, df = 53, p = 0.03). Analyses are covaried for age, but age is not included in the figures. SASH = Short Acculturation Scale for Hispanics.
Relationship between acculturation and maternal diurnal cortisol slope
Greater acculturation predicted a flatter diurnal cortisol slope late in pregnancy, even when maternal age was considered as a covariate (Linear Regression; Table 3; Figure 3). There were no significant relationships based on multiple linear regression analyses observed in early pregnancy, mid pregnancy or during the postpartum period (Table 3).
Table 3.
Multiple regression models explaining maternal cortisol slopes (n=55) Bold indicates significant predictor variable, p<0.05.
| Outcome Phase | R2 | Variables included | Regression coefficient |
SE | t value | Sig (p) |
|---|---|---|---|---|---|---|
| Early Pregnancy | 0.02 | Acculturation | <0.001 | 0.01 | 0.03 | 0.98 |
| Maternal age | <−0.001 | 0.002 | −0.71 | 0.48 | ||
| Mid Pregnancy | 0.07 | Acculturation | −0.009 | 0.008 | −1.16 | 0.25 |
| Maternal age | −0.003 | 0.001 | −1.85 | 0.08 | ||
| Late Pregnancy | 0.17 | Acculturation | 0.014 | 0.007 | 1.84 | 0.01 |
| Maternal age | −0.001 | 0.002 | −0.35 | 0.35 | ||
| Postpartum | 0.17 | Acculturation | 0.01 | 0.007 | 1.55 | 0.13 |
| Maternal age | −0.002 | 0.002 | −1.46 | 0.15 |
Figure 3.
Maternal acculturation score predicts maternal cortisol slope during late (34.3 [1.4] weeks of gestation) pregnancy. A, A high acculturation score on the SASH predicts a flatter maternal diurnal cortisol slope late in pregnancy (34.3[1.4] weeks of gestation; p = .01, multiple regression). B, The diurnal curve for acculturated women is flat in comparison to nonacculturated women in late pregnancy. Acculturation cutoffs were previously predetermined: less than 2.99 for nonacculturated women and greater than 2.99 for acculturated women (38). SASH = Short Acculturation Scale for Hispanics.
Relationship between maternal diurnal cortisol and perinatal outcomes
Infant birth weight was positively correlated with diurnal cortisol slope in late pregnancy. Women who gave birth to lower birth weight infants exhibited lower diurnal cortisol slopes (r=−0.29, p=0.05). However, no significant correlations were observed between infant birth weight and maternal cortisol slope early in pregnancy (r=−.07, p=0.63), at mid-pregnancy (r=−0.15, p=0.28), or during the postpartum period (r=−0.07, p=0.63). There was no significant correlation between gestational age at birth and maternal diurnal slope cortisol throughout pregnancy, including early pregnancy (r=−0.05, p=0.70), mid-pregnancy (r=−0.003, p=0.97), late pregnancy (r=−0.08, p=0.59) or postpartum (r=0.03, p=0.82).
Mediation analysis between acculturation, diurnal cortisol slope and infant birth weight
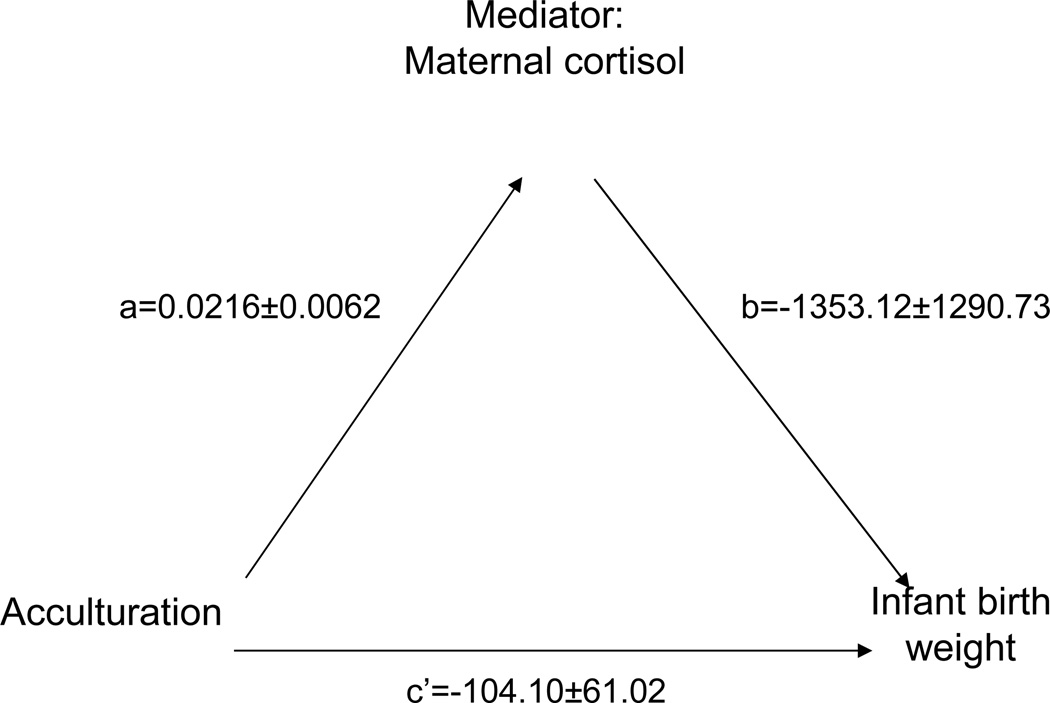
Acculturation predicted infant birth weight (c=−133.70±54.32, p=0.04). The c statistic is defined as the direct path between acculturation and infant birth weight. Based on the mediation model of Baron and Kenny (36), a multiple linear regression was performed with maternal cortisol and acculturation as predictors of infant birth weight. See Figure 4. Maternal acculturation no longer significantly predicted birth weight (c’= −140.10±61.02, p=0.30) when cortisol was included in the model. This suggests a shared variance of acculturation and cortisol that predicts birth weight. In addition, significance in Sobel’s test (ab= −27.0, p<0.01) points to cortisol as a potential mediator in the relationship between greater acculturation and lower infant birth weight.
Figure 4.
Maternal cortisol late in pregnancy may potentially mediate the relationship between acculturation and low infant birth weight. Pathways a = 0.02 [0.006] (between acculturation and mediator variable maternal cortisol) and b = −1353.12 [1290.73] (between mediator cortisol and outcome variable infant birth weight) were both significant (p = .046 and p = .30, respectively). Pathway c’= −104.10 [61.02] (acculturation predicting birth weight in presence of cortisol) approached 0 (p = .31) and demonstrated that, once cortisol was includedin the model, there was an absence of an association between acculturation and birth weight (p = .31). Thus, this is consistent with the possibility that cortisol mediates the relationship between the initial variable (acculturation) and outcome variable (birth weight). Values are partial regression coefficients [standard errors].
Relationship between maternal acculturation and total life events
Maternal self-report of life event(s) was significantly related to acculturation. As number of life events report did not differ across pregnancy (F (2,53)= 0.94, p=0.39; one way repeated measures ANOVA), a total sum number of life events across pregnancy was determined for each pregnant women. Following a natural log transform to normalize the data, non-acculturated women (mean±SEM: 8.9±1.3) reported a higher number of life events than acculturated women (mean±SEM: 5.4±1.5) (t=2.05, df=53, p=0.046; t-test).
Relationship between maternal cortisol and total life events
Total life events during pregnancy was not significantly correlated with diurnal cortisol slope early in pregnancy (r=−.02, p=0.87), at mid-pregnancy (r=−0.10, p=0.50), late pregnancy (r= −0.254, p=0.08) or the postpartum period (r=−0.20, p=0.18).
Relationship between total life events and perinatal outcomes
Total life events during pregnancy were not significantly related to gestational age ( r=0.07, p=0.59) or birth weight ( r=0.02 and p=0.88) as determined by Pearson’s correlation.
Discussion
The Mexican and Mexican-American population is the most rapidly growing population in the United States (49), thus, the healthcare needs of women of Mexican descent must be addressed in a culturally competent manner. This study is one of the first to examine a possible mechanism, maternal cortisol during pregnancy, by which acculturation may negatively affect perinatal outcomes in the US Mexican population. This study replicated previous findings that higher levels of acculturation are associated with both earlier gestational age and lower birth weight. Low birth weight, but not gestational age, was associated with a blunted maternal cortisol slope late in pregnancy. In addition, higher levels of acculturation alone predicted blunted maternal cortisol levels; implying acculturation may have physiological consequences in the perinatal period that affect offspring outcomes.
The Mexican population living in the U.S. experiences the social phenomenon of acculturation. Those who have spent more time in the US often experience the negative effects of acculturation, such that as the Mexican population adapts to mainstream US culture, the worse their health outcomes (3). This can be especially detrimental during the perinatal period, when proper development of a fetus and subsequent child development is crucial. In the current study, greater acculturation levels were associated with decreased infant birth weight and earlier gestational, both adverse perinatal outcome associated with a host of negative childhood developmental outcomes (50–52). High acculturation has previously predicted low infant birth weight (53) and early gestational age (54, 55), replicated by this study. Acculturated women gave birth on average a day before 37 weeks, which is clinically considered preterm birth (56) (means ± standard error: 38.9±0.2 and 36..9±0.7 weeks gestation, respectively). Birth outcomes can be significantly effected by small changes in gestational age (57) and/or birth weight (58) reinforcing the significance of the present observations. In addition, of the infants in the study, more preterm infants were born to acculturated mothers (44.4%) than non-acculturated mothers (8.3%) and more low birth weight infants were born to acculturated mothers (44.4%) relative to non-acculturated mothers (2.7%). Given our data along with previous work, increased acculturation status may be a risk factor for decreased birth weight and earlier gestational age at birth in the US Mexican population.
A novel association between increased acculturation and a blunted maternal cortisol decline across the day during late pregnancy was also noted. Flat diurnal declines have been seen in stressed and depressed populations (26, 28, 31, 59, 60), suggesting the effects of acculturation may share a final common pathway with similar adverse physiological effects. This is especially likely since acculturation is considered a cultural stressor (34, 35). The current results replicate previous work associating increased trauma (61) and depression (62) with a blunted cortisol response in the non-pregnant US Mexican population. In addition, ethnicity has been related to cortisol levels as both older Hispanic women (63) and Hispanic adolescents (both male and female) exhibit flatter diurnal salivary cortisol patterns (64) in comparison to their white non-Hispanic counterparts. In addition, higher allostatic load, as determined by a variety of factors including various psychosocial, immune and plasma cortisol measures (57), have been reported in US-born Mexican Americans relative to foreign-born Mexicans (65). Increased allostatic load is associated with negative health outcomes (66–68) and in the aforementioned study, acculturation negatively affected metabolic measures (65). Other studies report higher perceived stress and depression during pregnancy with an increase in acculturative status (69–71). The current study only reported on number of life events, not perceived stress associated with these events, and found acculturated women reported fewer life events than non-acculturated women. However, despite this, acculturated women exhibited the flatter cortisol diurnal decline. Thus, it is unlikely that exposure to stressors alone can explain why the acculturated women are showing this altered physiological response, but less acculturation may play a role in the buffering of negative perception of events, stressful or otherwise. The current study extends previous work by monitoring cortisol diurnal slope during a critical developmental period for the fetus, pregnancy, and related it to perinatal outcomes; birth weight and gestational age.
There is not a consensus in the literature on the timing of effects of stress or cortisol on adverse perinatal outcomes (2). However, as found in other perinatal studies (72, 73), our data identifies the third trimester as an important time during pregnancy when maternal cortisol may have unfavorable effects on perinatal outcomes via acculturation. In the current study, acculturation was only measured once during pregnancy. Acculturation is a fairly stable trait (41) tied to generation and should remain stable during pregnancy. Thus, changes in acculturation during pregnancy are unlikely. Low birth weight was also associated with a blunted maternal cortisol slope late in pregnancy. While high acculturation has previously predicted low infant birth weight (53) and increased cortisol is associated with low infant birth weight in non-Latino Caucasian populations (2), this is one of the first studies to identify that acculturation affects a physiological mechanism that may be leading to adverse perinatal outcomes. There was no effect of maternal cortisol on gestational age at birth. Separate relationships have previously been found between acculturation and preterm birth (54, 55) and cortisol and preterm birth (2, 74), but no studies link these two relationships in the US Mexican population. Thus, these data (i.e. more acculturated, altered biomarker) may help us to identify which women may be at risk for delivering a low birth weight infant.
The slope of the maternal diurnal cortisol decline was found to statistically mediate the relationship between acculturation and low birth weight, suggesting diurnal cortisol regulation may be an important factor that contributes to the detrimental effects of acculturation on perinatal outcomes. This is the first study to date to identify a potential mediator between acculturation and its negative health outcomes. However, it is unlikely that cortisol is acting alone. Aberrant cortisol rhythms are also associated with other physiological mediators such as immune-related processes and alterations in additional endocrine processes (74–76). These changes have also have been associated with low birth weight (77, 78). Thus, further research on the interactions between cortisol and other biomarkers such as inflammatory markers will help elucidate the role of cortisol in low infant birth weight in at-risk populations.
There is considerable variability in acculturation measures (69, 79). Many measurements of acculturation do not take into account the individuals perception of their own acculturation. The SASH is a measure of language use and media (radio, TV) and ethnic social relations. While the SASH has been associated with measures of time in US, age at arrival, generational status, and cultural identity (38), it does not directly measure group and/or self-identity which would have provided further information about the role of acculturation in poor birth outcomes. More research needs to investigate the role of acculturation in risk for adverse perinatal outcomes using a more comprehensive assessment that includes measures of ethnic affiliation, social interactions and both group and self-identity in a larger sample.
This research adds to an understanding of factors that make more acculturated women of Mexican descent more susceptible to negative perinatal outcomes. This study identified maternal salivary cortisol as a potential risk factor in relationship with acculturation and pregnancy outcome in the Mexican population. Together cortisol and acculturation information may provide a means for assessing risk for perinatal outcomes in this vulnerable population. By identifying interactions between psychosocial factors and biological risks in the perinatal period, healthcare providers can identify risk for negative perinatal outcomes in the Mexican community and determine effective forms of intervention.
Acknowledgments
The authors would like to acknowledge Maribel Perea, Jazmin Garcia, Marianne Kreither, Rachel Grzywa and Crystal Natvig for their contributions to this research. We would also to thank the families for their participation. This work was funded by research grants from the University of Colorado Dean’s Academic Enrichment Fund (MCH), Developmental Psychobiology Endowment Fund (University of Colorado Denver, Department of Psychiatry) and NIH AA013973 (MLL), CA126971(MLL), MH086383 (RGR)]. In addition, Dr. Kimberly D’Anna was supported by an institutional NIH NRSA postdoctoral research training program, MH015442 and a NSF Minority Postdoctoral Research Fellowship.
The work was supported by National Institutes of Health
Acronyms
- ANOVA
analysis of variance
- BMI
body mass index
- HPA
hypothalamic pituitary axis
- SASH
Short Acculturation Scale for Hispanics
- STD
standard deviation
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: none
References
- 1.Callister LC, Birkhead A. Acculturation and perinatal outcomes in mexican immigrant childbearing women: An integrative review. J Perinat Neonatal Nurs. 2002;16:22–38. doi: 10.1097/00005237-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Field T, Diego M. Cortisol: The culprit prenatal stress variable. Int J Neurosci. 2008;118:1181–1205. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- 3.Morales L, Marielena L, Kington R, Valdez R, Escarce J. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J Health Care Poor Underserved. 2002;13:477–503. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger JB, Reynolds K, Shakib S, Spruijt-Metz D, Sun P, Johnson CA. Acculturation, physical activity, and fast-food consumption among Asian-American and Hispanic adolescents. J Community Health. 2004;29:467–481. doi: 10.1007/s10900-004-3395-3. [DOI] [PubMed] [Google Scholar]
- 5.Abraído-Lanza AF, Chao MT, Flórez KR. Do healthy behaviors decline with greater acculturation?: Implications for the Latino mortality paradox. Soc Sci Med. 2005;61:1243–1255. doi: 10.1016/j.socscimed.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos B, Schetter CD, Abdou C, Hobel C, Glynn LM, Sandman CA. Familialism, social support, and stress: Positive implications for pregnant Latinas. Cultural Diversity and Ethnic Minority Psychology. 2008;14:155–162. doi: 10.1037/1099-9809.14.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabogal F, MarÃn G, Otero-Sabogal R, MarÃn BV, Perez-Stable EJ. Hispanic Familism and Acculturation: What Changes and What Doesn't? Hispanic Journal of Behavioral Sciences. 1987;9:397–412. [Google Scholar]
- 8.Vaux A. Variations in social support associated with gender, ethnicity, and age. J Soc Iss. 1985;41:89–110. [Google Scholar]
- 9.Berton HK, Cassel JC, Gore S. Social Support and Health. Med Care. 1977;15:47–58. doi: 10.1097/00005650-197705001-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cámara RJA, Lukas PS, Begré S, Pittet V, von Känel R. Swiss Inflammatory Bowel Disease Cohort Study G. Effects of social support on the clinical course of Crohn's disease. Inflamm Bowel Dis. 2011;17:1277–1286. doi: 10.1002/ibd.21481. [DOI] [PubMed] [Google Scholar]
- 11.Hurdle D. Social support: A critical factor in women's health and health promotion. Health Soc Work. 2001;26:72–9. doi: 10.1093/hsw/26.2.72. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EK. Acculturation and changes in the likelihood of pregnancy and feelings about pregnancy among women of mexican origin. Women Health. 2008;47:45–64. doi: 10.1300/J013v47n01_03. [DOI] [PubMed] [Google Scholar]
- 13.Berry JW. Immigration, acculturation, and adaptation. Appl Psychol. 1997;46:5–34. [Google Scholar]
- 14.Vega W, Amarto H. Latino Outlook: Good health, uncertain prognosis. Annu Rev Public Health. 1994;15:39–67. doi: 10.1146/annurev.pu.15.050194.000351. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan MS, Marks G. Adverse effects of acculturation: Psychological distress among Mexican American young adults. Soc Sci Med. 1990;31:1313–1319. doi: 10.1016/0277-9536(90)90070-9. [DOI] [PubMed] [Google Scholar]
- 16.Diego M, Jones N, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Garcia A. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosom Med. 2006;68:747–753. doi: 10.1097/01.psy.0000238212.21598.7b. [DOI] [PubMed] [Google Scholar]
- 17.Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28:1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Siler-Khodr TM, Forthman G, Khodr C, Matyszczyk S, Khodr Z, Khodr G. Maternal Serum Corticotropin-Releasing Hormone at Midgestation in Hispanic and White Women. Obstet Gynecol. 2003;101:557–564. doi: 10.1016/s0029-7844(02)03072-7. [DOI] [PubMed] [Google Scholar]
- 19.O'Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: A pilot study. J Affect Disord. 130:300–305. doi: 10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 20.D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.02.041. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 13:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kivlighan K, Dipietro JA, Costigan KA, Laudenslager ML. Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, Schulte HM. Diurnal salivary cortisol patterns during pregnancy and after delivery: Relationship to plasma corticotrophin-releasing-hormone. Clin Endocrinol (Oxf) 1990;33:279–289. doi: 10.1111/j.1365-2265.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Scott EM, McGarrigle HHG, Lachelin GCL. The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. J Clin Endocrinol Metab. 1990;71:639–644. doi: 10.1210/jcem-71-3-639. [DOI] [PubMed] [Google Scholar]
- 26.Miller G, Chen E, Zhou E. It it goes up, must it come down? Chronic stress and hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci U S A. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Giese-Davis J, Sephton S, Abercrombie H, Duran R, Spiegel D. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:654–660. doi: 10.1037/0278-6133.23.6.645. [DOI] [PubMed] [Google Scholar]
- 30.Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev Med. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- 31.Gunnar M, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 32.Deuschle M, Schweiger U, Weber B, Gotthardt U, Körner A, Schmider J, Standhardt H, Lammers C-H, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 33.den Hartog HM, Nicolson NA, Derix MMA, van Bemmel AL, Kremer B, Jolles J. Salivary cortisol patterns and cognitive speed in major depression: a comparison with allergic rhinitis and healthy control subjects. Biol Psychol. 2003;63:1–14. doi: 10.1016/s0301-0511(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 34.Berry JW, Kim U, Minde T, Mok D. Comparative studies of acculturative stress. Int Migr Rev. 1987;21:491–511. [Google Scholar]
- 35.Wong PTP, Wong LCJ, Berry JW. Acculturative Stress. Springer US: Handbook of Multicultural Perspectives on Stress and Coping; 2006. pp. 287–298. [Google Scholar]
- 36.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, stratetic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 37.Denver Health Annual Report. Denver; 2009. Available from: www.denverhealth.org. [Google Scholar]
- 38.Marin G, Sabogal F, Marin B, Otero-Sabogal R, Perez-Stable E. Development of a short acculturation scale for Hispanics. Hispanic Journal of Behavioral Sciences. 1987;9:183–205. [Google Scholar]
- 39.Vella C, Ontiveros D, Zubia R, Bader J. Acculturation and metabolic syndrome risk factors in young Mexican and Mexican–American women. Journal of Immigrant and Minority Health. 2009:1–8. doi: 10.1007/s10903-009-9299-7. [DOI] [PubMed] [Google Scholar]
- 40.Guilamo-Ramos V, Bouris A, Jaccard J, Lesesne C, Gonzalez B, Kalogerogiannis K. Family mediators of acculturation and adolescent sexual behavior among Latino youth. The Journal of Primary Prevention. 2009;30:395–419. doi: 10.1007/s10935-009-0180-1. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz S, Montgomery M, Briones E. The role of identity in acculturation among immigrant people: Theoretical propositions, empirical questions, and applied recommendations. Hum Dev. 2006;49:1–30. [Google Scholar]
- 42.DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Dev Psychobiol. 2009;51:505–512. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laudenslager ML, Noonan C, Jacobsen C, Goldberg J, Buchwald D, Bremner JD, Vaccarino V, Manson SM. Salivary cortisol among American Indians with and without posttraumatic stress disorder (PTSD): Gender and alcohol influences. Brain Behav Immun. 2009;23:658–662. doi: 10.1016/j.bbi.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neu M, Goldstein M, Gao D, Laudenslager ML. Salivary cortisol in preterm infants: Validation of a simple method for collecting saliva for cortisol determination. Early Hum Dev. 2007;83:47–54. doi: 10.1016/j.earlhumdev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 45.SPSS I. PASW Statistics 18.0. Chicago2009. [Google Scholar]
- 46.Fraser AM, Brockert JE, Ward RH. Association of Young Maternal Age with Adverse Reproductive Outcomes. New Engl J Med. 1995;332:1113–1118. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsson B, Ladfors L, Milsom I. Advanced Maternal Age and Adverse Perinatal Outcome. Obstet Gynecol. 2004;104:727–733. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 48.Paneth NS. The Problem of Low Birth Weight. The Future of Children. 1995;5:19–34. [PubMed] [Google Scholar]
- 49.US Census Bureau PD. US Census Demographic Profiles. Washington, D.C: 2010. [Google Scholar]
- 50.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-Age Outcomes in Children with Birth Weights under 750 g. New Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 51.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. New Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 52.Saigal S, Doyle L. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371 doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 53.Beck C. Acculturation: implications for perinatal research. MCN Am J Matern Child Nurs. 2006;31:114–120. doi: 10.1097/00005721-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz R, Saade G, Brown C, Nelson-Becker C, Tan A, Bishop S, Bukowski R. The effect of acculturation on progesterone/estriol ratios and preterm birth in Hispanics. Obstet Gynecol. 2008;111:309–316. doi: 10.1097/01.AOG.0000297896.00491.2c. [DOI] [PubMed] [Google Scholar]
- 55.Crump C, Lipsky S, Mueller B. Adverse birth outcomes among Mexican-Americans: Are US-born women at greater risk than Mexico-born women? Ethn Health. 1999;4:29–34. doi: 10.1080/13557859998164. [DOI] [PubMed] [Google Scholar]
- 56.Beck S, Wojdyla D, Say L, Betran A, Merialdi M, Requejo J, Rubens C, Menon R, Van Look P. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull WHO. 2010;88 doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Kramer MS. Variations in Mortality and Morbidity by Gestational Age among Infants Born at Term. The Journal of Pediatrics. 2009;154:358–362. doi: 10.1016/j.jpeds.2008.09.013. e1. [DOI] [PubMed] [Google Scholar]
- 58.Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Hennekens CH, Willet WC. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McEwen B. Stress, adaptation and disease: Allostasis and allostatic load. Ann N Y Acad Sci. 1998;84:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 60.Kivlighan KT, DiPietro JA, Costigan KA, Laudenslager ML. Diurnal rhythm of cortisol during late pregnancy: Associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology. 2008;33:1225–1235. doi: 10.1016/j.psyneuen.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm Behav. 2010;58:637–646. doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burke HM, Fernald LC, Gertler PJ, Adler NE. Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosom Med. 2005;67:211–216. doi: 10.1097/01.psy.0000156939.89050.28. [DOI] [PubMed] [Google Scholar]
- 63.Gallagher-Thompson D, Shurgot G, Rider K, Gray H, McKibbin C, Kraemer H, Sephton S, Thompson L. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and non-caregivers. Am J Geriatr Psychiatry. 2006;14:334–342. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- 64.DeSantis A, Adam E, Doane L, Mineka S, Zinbarg R, Craske M. Racial/Ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Peek MK, Cutchin MP, Salinas JJ, Sheffield KM, Eschbach K, Stowe RP, Goodwin JS. Allostatic load among non-Hispanic whites, non-Hispanic blacks, and people of Mexican origin: Effects of ethnicity, nativity, and acculturation. Am J Public Health. 2011;100:940–946. doi: 10.2105/AJPH.2007.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Logan JG, Barksdale DJ. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. Journal of Clinical Nursing. 2008;17:201–208. doi: 10.1111/j.1365-2702.2008.02347.x. [DOI] [PubMed] [Google Scholar]
- 67.McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 68.McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 69.Campos B, Schetter CD, Walsh JA, Schenker M. Sharpening the focus on acculturative change. Hispanic Journal of Behavioral Sciences. 2007;29:209–224. [Google Scholar]
- 70.Heilemann M, Frutos L, Lee K, Kury F. Protective strength factors, resources, and risks in relation to depressive symptoms among childbearing women of mexican descent. Health Care Women Int. 2004;25:88–106. doi: 10.1080/07399330490253265. [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Schallmoser L, Telleen S. The effect of social support and acculturation on postpartum depression in mexican american women. J Transcult Nurs. 2003;14:329–338. doi: 10.1177/1043659603257162. [DOI] [PubMed] [Google Scholar]
- 72.Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational Effects of Posttraumatic Stress Disorder in Babies of Mothers Exposed to the World Trade Center Attacks during Pregnancy. J Clin Endocrinol Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 73.Poggi E, Glynn LM, Dunkel-Schetter C, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 74.Kamel R. The onset of human parturition. Arch Gynecol Obstet. 2010;281:975–982. doi: 10.1007/s00404-010-1365-9. [DOI] [PubMed] [Google Scholar]
- 75.Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav Immun. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 76.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41:224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]
- 77.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, Lee-Parritz A, Wood RA, Kattan M, Bloomberg GR, Burger M, Togias A, Witter FR, Sperling RS, Sadovsky Y, Gern JE. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. Association of maternal serum CRP, IL-6, TNF-alpha, homocysteine, folic acid and vitamin B12 levels with the severity of preeclampsia and fetal birth weight. Hypertens Pregnancy. 2009;28:190–200. doi: 10.1080/10641950802601179. [DOI] [PubMed] [Google Scholar]
- 79.Cabassa L. Measuring acculturation: Where we are where we need to go. Hispanic Journal of Behavioral Sciences. 2003;25 [Google Scholar]