Abstract
Monoclonal antibody neutralization of interleukin-10 (IL-10) increased Johnin purified protein derivative-induced whole-blood gamma interferon (IFN-γ) secretion 23-fold and also increased IFN-γ secretion ninefold following in vitro Mycobacterium avium subsp. paratuberculosis infection of peripheral blood mononuclear cells. These results demonstrate the suppressive effect of IL-10 on immune responses to M. avium subsp. paratuberculosis infection in cattle.
Immune responses to Mycobacterium avium subsp. paratuberculosis infection in cattle are characterized by a progressive shift from the protective cell-mediated (Th1) response in the subclinical stages of the disease towards the nonprotective antibody-mediated (Th2) response during the clinical stages of the disease (3, 4, 6, 9, 10, 12). It has been suggested that cytokines with an immunosuppressive effect on Th1 responses, in particular interleukin-10 (IL-10), may mediate this change (12, 13). The cytokine IL-10 is produced by Th2 CD4+ T cells, B cells, and macrophages and has many functions including inhibition of cytokine production by Th1 cells while promoting B-cell proliferation and differentiation (5, 8). However, the effect of IL-10 on immune responses to M. avium subsp. paratuberculosis in cattle has never been demonstrated.
Animals used in this study were 40-month-old castrated male Holstein cattle comprising the infected group in the subclinical stage of experimental M. avium subsp. paratuberculosis infection and an uninfected group, each containing five animals. Details of the experimental infection were described previously (2).
The study first investigated whether IL-10 has an influence on the purified protein derivative (PPD)-induced whole-blood gamma interferon (IFN-γ) response (1). Blood was stimulated as previously described (8) with few modifications. Stimulants and monoclonal antibodies (MAbs) were added into wells of 24-well round-bottomed tissue culture plates (Sumilon; Sumitomo Bakelite, Tokyo, Japan) to a final volume of 1 ml comprising 1/10-diluted blood, 5 μg of M. avium subsp. paratuberculosis PPD/ml, and different fourfold dilutions of anti-IL-10 MAb CC320 or an immunoglobulin G1 isotype control MAb (Sigma, St. Louis, Mo.). The diluent was RPMI 1640 (Sigma) containing 1% penicillin-streptomycin and l-glutamine. The blood was incubated at 37°C and 5% CO2 in air. Positive controls included blood stimulated with concanavalin A (ConA; type IV; Sigma) at a final concentration of 10 μg/ml, and unstimulated cells served as negative control. Supernatants were measured for IFN-γ concentration with the bovine IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (Bio-X SPRL, Marche-en-Famenne, Belgium) after 24 h of incubation. For measurement of cell proliferation, triplicate 100-μl aliquots of PPD-stimulated blood were pulsed with 0.5 μCi of [3H]thymidine at 48 h and plates were incubated for an additional 24 h at 37°C in 5% CO2 in air. The cells were then harvested, and radioactivity was quantified in a liquid scintillation counter (Tri-Carb 1600TR; Packard, Meriden, Conn.).
To investigate whether PPD induces a differential IL-10 or transforming growth factor β (TGF-β) mRNA expression in the M. avium subsp. paratuberculosis-infected and uninfected animals, 1 ml of undiluted blood was stimulated with 5 μg of M. avium subsp. paratuberculosis PPD/ml and at 0, 1, 3, 6, 9, 12, and 24 h after stimulation, the blood cell fraction was processed for RNA isolation with the QIAamp RNA blood kit (Qiagen GmbH, Hilden, Germany). The RNA was reverse transcribed and subsequently amplified according to the instructions of the manufacturer (Applied Biosystems). The primers and TaqMan probes used are described in Table 1. The PCR conditions were 2 min at 50°C, 10 min at 95°C, and 40 cycles of 95°C for 15 s and 60°C for 1 min.
TABLE 1.
Primers and probes for real-time PCR
| Target and type | Designationb | Sequence | Reference no. |
|---|---|---|---|
| IL-10 | U00799 | ||
| Forward | F280 | GTGATGCCACAGGCTGAGAA | |
| Reverse | R414 | CGCCTTGCTCTTGTTTTCG | |
| Probe | T364 | CGGCTGCGGCGCTGTCATC | |
| TGF-β | U62110 | ||
| Forward | F748 | GGCCCTGCCCTTACATCTG | |
| Reverse | R822 | CCGGGTTGTGCTGGTTGT | |
| Probe | T770 | CCTGGATACACAGTACAGCAAG GTCCTGGC | |
| GAPDHa | U85042 | ||
| Forward | F364 | GGCGTGAACCACGAGAAGTATAA | |
| Reverse | R481 | CCTCCACGATGCCAAAGTG | |
| Probe | T393 | CCTCAAGATTGTCAGCAATGCCT CCTG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Numbers indicate the sequence positions.
Finally the study investigated whether IL-10 has an effect on IFN-γ responses to infection with live M. avium subsp. paratuberculosis. Peripheral blood mononuclear cells were isolated from jugular blood samples with the use of Ficoll-Paque PLUS (Amersham Bioscience, Uppsala, Sweden) density gradient centrifugation per the manufacturer's instructions. The cells were resuspended in RPMI 1640 medium (Sigma) containing 10% fetal calf serum and dispensed into wells of 24-well tissue culture plates (Costar, Cambridge, Mass.) at 3 × 106 cells per well. To each well was added 3 × 106 CFU of M. avium subsp. paratuberculosis (14) in the presence of 1/1,000-diluted anti-IL-10 MAb CC320 or 5 μg of isotype control MAb/ml, and after 24, 48, and 96 h of infection, the supernatant was collected for IFN-γ quantification. Data in this study were analyzed by analysis of variance, and a P value of <0.05 was considered significant.
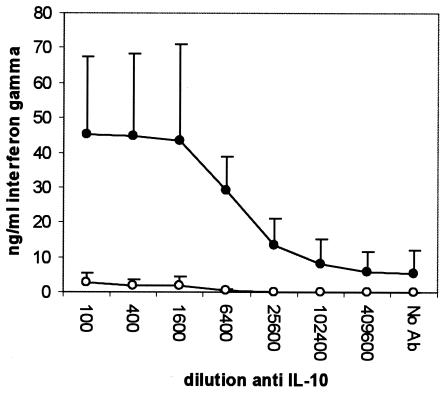
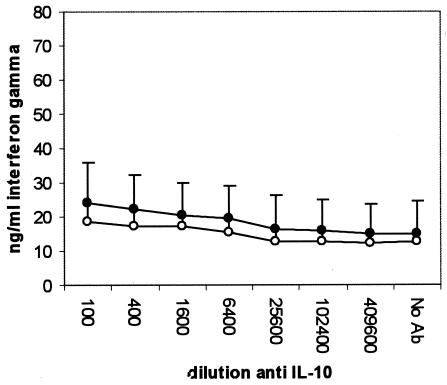
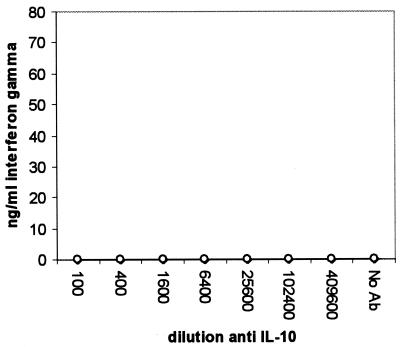
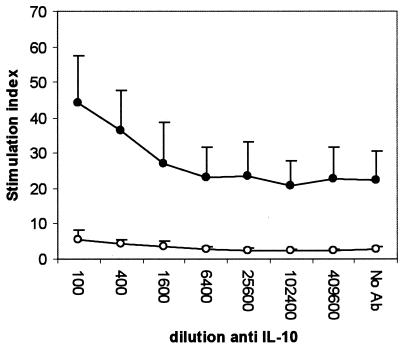
Compared to samples stimulated in the absence of anti-IL-10 MAb, the PPD-induced IFN-γ level was significantly increased in the infected group at a MAb dilution of 1/1,600 and below (P < 0.05), representing a 23-fold increase (Fig. 1). The anti-IL-10 MAb, however, did not change the PPD-induced IFN-γ level in the uninfected group, and levels in the infected group were higher at a MAb dilution of 1/6,400 or lower (P < 0.01). The neutralization of IL-10 had no effect on IFN-γ secretion by ConA-stimulated (Fig. 2) or nonstimulated (Fig. 3) cells. Unlike the anti-IL-10 MAb, an isotype control MAb had no effect on IFN-γ secretion in response to PPD- or ConA-stimulated or unstimulated cells (data not shown). The PPD-induced proliferation in samples from the infected group was significantly increased at antibody dilutions of 1/400 or lower, representing up to a twofold increase over that in samples stimulated in the absence of IL-10 neutralization (Fig. 4). Parallel to findings for IFN-γ production, the anti-IL-10 MAb had no effect on ConA-stimulated or nonstimulated samples, and similarly, the isotype control MAb had no effect on cell proliferation (data not shown).
FIG. 1.
Changes in IFN-γ secretion by whole blood from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after 24 h of stimulation with Johnin PPD in the presence of an IL-10-neutralizing MAb.
FIG. 2.
Changes in IFN-γ secretion by whole blood from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after 24 h of stimulation with ConA in the presence of an IL-10-neutralizing MAb.
FIG. 3.
Changes in IFN-γ secretion by whole blood from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after 24 h of incubation without stimulation in the presence of an IL-10-neutralizing MAb.
FIG. 4.
Changes in Johnin PPD-induced proliferation by whole-blood cells from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle in the presence of an IL-10-neutralizing MAb. Whole blood diluted 1/10 was stimulated with 5 μg of Johnin PPD/ml, and after 48 h of stimulation, the cells were pulsed with 0.5 μCi of [3H]thymidine and incubated for an additional 24 h. Radioactivity was measured with a liquid scintillation counter.
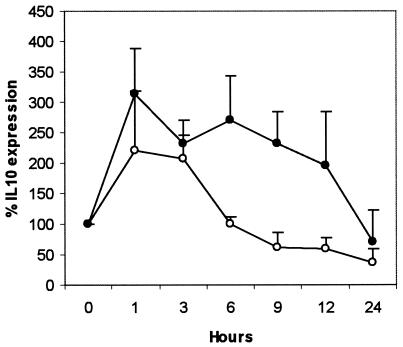
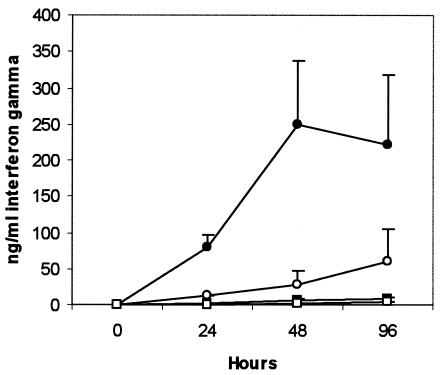
The PPD-induced IL-10 mRNA expression rose sharply and was significantly increased at 1 h in the infected and uninfected groups (P < 0.05 and P < 0.01, respectively) (Fig. 5). After 3 h, however, while expression in the infected group was sustained, the expression in the uninfected group declined sharply and was significantly lower at 6 (P < 0.001), 9 (P < 0.01), and 12 (P < 0.01) h. The TGF-β expression was not different between the infected and the uninfected groups (data not shown). Live M. avium subsp. paratuberculosis infection of peripheral blood mononuclear cells under conditions of IL-10 neutralization significantly increased IFN-γ secretion at 48 and 96 h (P < 0.001) in the infected and uninfected groups, representing as much as a ninefold and threefold increase, respectively (Fig. 6). However, peak IFN-γ levels were significantly higher (P < 0.001) in cells from the infected group to a magnitude of 28-fold.
FIG. 5.
Changes in IL-10 mRNA expression by whole blood from M. avium subsp. paratuberculosis-infected (•) and uninfected (○) cattle after stimulation with Johnin PPD. RNA was purified from whole blood by use of the QIAamp kit and reverse transcribed using the TaqMan reverse transcription reagents. The resulting cDNA was amplified for IL-10 and glyceraldehyde-3-phosphate dehydrogenase by real-time PCR with the TaqMan Universal PCR Mastermix (Applied Biosystems). Results are expressed as IL-10 mRNA expression of PPD-stimulated blood as a percentage of that in unstimulated whole blood.
FIG. 6.
Changes in IFN-γ secretion by peripheral blood mononuclear cells after in vitro infection with live M. avium subsp. paratuberculosis in the presence of anti-IL-10 neutralizing MAb or an immunoglobulin G1 isotype control MAb. The different series represent infected cattle cells in the presence of IL-10-neutralizing MAb (•) or isotype control MAb (○) and uninfected cattle cells in the presence of IL-10-neutralizing MAb (▪) or isotype control MAb (□). The anti-IL-10 MAb was used at a 1/1,000 dilution while the isotype control MAb was used at 5 μg/ml.
Results from this study demonstrate for the first time the suppressive effect of IL-10 on Th1 immune responses to M. avium subsp. paratuberculosis and its antigens in infected cattle and provide an approach for improvement of diagnosis and further studies of the disease's pathogenesis.
The whole-blood IFN-γ ELISA is a useful test for diagnosis of M. avium subsp. paratuberculosis infection in cattle but becomes less effective late in the disease due to unresponsiveness of the infected animals (6). The amplification of the whole-blood PPD-induced IFN-γ response by the IL-10-neutralizing MAb may help improve diagnosis of M. avium subsp. paratuberculosis infection, particularly late in the subclinical stage, for animals whose IFN-γ response is below the limit of detection by the IFN-γ ELISA but who have not developed sufficient antibodies for detection by serological tests.
Results from this study showed that M. avium subsp. paratuberculosis antigens induced a more sustained IL-10 response in infected animals than in uninfected animals, extending previous observations from uninfected animals (13). The suppressive effect of IL-10 on M. avium subsp. paratuberculosis-specific T-cell IFN-γ and cell proliferation responses may contribute greatly to the observed immunopathology associated with the disease. The suppression of proliferative responses, if maintained during the infection period, will reduce the numbers of M. avium subsp. paratuberculosis-specific T cells, leading to the progressive reduction in IFN-γ responses and the decrease in peripheral and intestinal cell-mediated immune responses normally observed (7, 9, 11). Furthermore, since IFN-γ plays a crucial role in antimycobacterial responses (14), its suppression will likely result in suboptimal killing of M. avium subsp. paratuberculosis, leading to the chronic infections associated with the disease. However, studies with cells from the infected sites are still required for comparison with observations for peripheral blood.
Acknowledgments
This work was supported by the Japan Bio-oriented Technology Research Advancement Institute (BRAIN).
We thank C. J. Howard of the Institute of Animal Health, Berkshire, United Kingdom, for donating the IL-10-neutralizing MAb. We also thank T. Kamio of the Parasitic Disease Section, National Institute of Animal Health, Tsukuba, Japan, for granting access to some animals used in this study and S. Enoki of the Paratuberculosis and IBD research team for assistance in the preparation of various experiments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Billman-Jacobe, H., M. Carrigan, and F. Cochram. 1992. A comparison of the interferon gamma assay with absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 2.Buza, J. J., Y. Mori, A. M. Bari, H. Hikono, Aodon-geril, S. Hirayama, Y. Shu, and E. Momotani. 2003. Mycobacterium avium subsp. paratuberculosis infection causes suppression of RANTES, monocyte chemoattractant protein 1, and tumor necrosis factor alpha expression in peripheral blood of experimentally infected cattle. Infect. Immun. 71:7223-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiodini, R. J., H. J. Van Kruningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 4.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino, D. F., A. Zlotnik, P. Vieira, T. R. Mosmann, M. Howard, K. W. Moore, and A. O'Garra. 1991. IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146:3444-3451. [PubMed] [Google Scholar]
- 6.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Müller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong, L. S., J. C. Hope, M. L. Thom, P. Sopp, S. Duggan, G. P. Bembridge, and C. J. Howard. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85:213-223. [DOI] [PubMed] [Google Scholar]
- 9.Perez, V., J. Tellechea, J. J. Badiola, M. Gutierrez, and J. F. Garcia Marin. 1997. Relation between serologic response and pathologic findings in sheep with naturally acquired paratuberculosis. Am. J. Vet. Res. 58:799-803. [PubMed] [Google Scholar]
- 10.Stabel, J. R. 1996. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of preclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 11.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney, R. W., D. E. Jones, P. Habecker, and P. Scott. 1998. Interferon-γ and interleukin-4 expression in cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 59:842-847. [PubMed] [Google Scholar]
- 13.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao, B., M. T. Collins, and C. J. Czuprynski. 1997. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect. Immun. 65:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]