Abstract
Oral squamous cell carcinoma is common in cats and humans and invades oral bone. We hypothesized that the cyclooxygenase-2 inhibitor, meloxicam, with the bisphosphonate, zoledronic acid (ZOL), would inhibit tumor growth, osteolysis and invasion in feline oral squamous cell carcinoma (OSCC) xenografts in mice. Human and feline OSCC cell lines expressed cyclooxygenase (COX)-1 and 2 and the SCCF2 cells had increased COX-2 mRNA expression with bone conditioned medium. Luciferase-expressing feline SCCF2Luc cells were injected beneath the perimaxillary gingiva and mice were treated with 0.1 mg/kg ZOL twice weekly, 0.3 mg/kg meloxicam daily, combined ZOL and meloxicam, or vehicle. ZOL inhibited osteoclastic bone resorption at the tumor-bone interface. Meloxicam was more effective than ZOL at reducing xenograft growth but did not affect osteoclastic bone resorption. Although a synergistic effect of combined ZOL and meloxicam was not observed, combination therapy was well tolerated and may be useful in the clinical management of bone-invasive feline OSCC.
Keywords: zoledronic acid, meloxicam, feline, oral squamous cell carcinoma, bone invasion, bone resorption
Introduction
Oral squamous cell carcinoma (OSCC) is an extremely aggressive cancer in cats, and is the most commonly diagnosed oral malignancy in this species.1 There is currently no effective treatment for feline OSCC, and cats typically live an average of only 2 months following diagnosis.2 In most cases of gingival OSCC there is invasion and lysis of adjacent bone which is characterized by osteoclastic bone resorption.3 Since most cats present with deep invasion of bone, treatment options are limited because feline OSCC is resistant to both chemotherapy and radiation.2
Human OSCC has been shown to produces cytokines that can serve as agonists of osteoclastic bone resorption including prostaglandin E2 (PGE2),4,5 parathyroid hormone related-protein (PTHrP),6,7 tumor necrosis factor-alpha (TNF-α), interleukin-6 and interleukin-11.8,9 In human OSCC, high levels of PGE2 are due to up-regulation of COX-2.10 As cancer-induced bone lysis occurs, TGF-β can be released from the bone matrix and induces further secretion of tumor-derived factors that promote bone resorption including PTHrP11–13 and PGE214 and can increase the expression of PTHrP receptors.15 The authors have shown that feline OSCC produces PTHrP in vitro and in vivo,3,6,16 and others have shown that COX-2 is also over-expressed in feline OSCC.17 This combination of bone resorption and stimulation of OSCC cells to produce osteolytic factors may fuel a ‘vicious cycle’ of cancer growth and bone loss.11
Up-regulation of COX-2 and increased synthesis of prostaglandins are known to promote cell proliferation,18 angiogenesis19 and tumor invasiveness20 while inhibiting immune surveillance21 and apoptosis.20 Additionally, PGE2 has been shown to stimulate osteoclastogenesis through the up-regulation of RANKL and IL-1 expression in osteoblasts, bone marrowstromal cells, and periodontal ligament cells.22–24
Nitrogen-containing bisphosphonates such as zoledronic acid (ZOL) exert anti-resorptive effects by interference with the mevalonate pathway within osteoclasts.25–28 The authors have previously shown that ZOL therapy reduced OSCC xenograft tumor growth and tumor-associated bone resorption in a nude mouse model of feline OSCC.29 Additionally, YM529 (a nitrogen-containing bisphosphonate) prevented bone invasion and inhibited tumor growth of human OSCC xenografts in a nude mouse model.30 The ability of COX-2 inhibitors to reduce tumor growth has been documented in rodent models of human OSCC31–33 as well as in lung adenocarcinoma34, bladder transitional cell carcinoma35 and mammary carcinoma.36 Piroxicam, in particular, prevented tumors in a rat model of 4-nitroquinoline 1-oxide-(4-NQO)-induced tongue carcinogenesis.37
There are no published reports of a bisphosphonate being used in combination therapy for bone invasive OSCC, in humans or domestic animals. The hypothesis of this study was that the combination of ZOL and a selective COX-2 inhibitor (meloxicam) will reduce bone resorption and tumor growth in a unique, orthotopic model of bone invasive feline OSCC in nude mice.
We used real-time reverse transcriptase polymerase chain reaction to measure expression of COX-1 and COX-2 in feline and human OSCC cell lines, and determined that culture of SCCF2 cells (feline bone-invasive OSCC) in bone conditioned medium stimulated expression of COX-2. The effect of ZOL and meloxicam therapy on OSCC growth and tumor-associated osteolysis was investigated using bioluminescent imaging, Faxitron radiography, micro-computed tomography and maxillary histomorphometry. We found that combination therapy of ZOL and meloxicam reduced tumor growth (attributed to meloxicam) and bone loss (attributed to ZOL), was well tolerated, and may be an effective treatment for bone-invasive OSCC.
Materials and Methods
Cells and reagents
Feline OSCC cell lines (SCCF1, laryngeal SCC; SCCF2, gingival SCC and SCCF3, lingual SCC) and feline OSCC tumor-associated fibroblasts (TAF) were derived as previously described.29 Human cell lines (A253, salivary squamous cell carcinoma; SCC25, lingual squamous cell carcinoma) and murine preosteoblast cells (MC3T3-E1). The human laryngeal squamous cell carcinoma cell line, UMSCC12, was provided by Dr. Thomas Carey at the University of Michigan. Maintenance of cell lines was previously described.16 Zoledronic acid (Zometa; Novartis, East Hanover, NJ) was purchased from the James Cancer Center at The Ohio State University. Meloxicam (Metacam; Boehringer Ingelheim, Ridgefield, CT) was purchased from the Veterinary Medical Center at The Ohio State University. SCCF2 cells were previously transfected with a luciferase-yellow fluorescent protein (YFP) fusion construct (pCDNA3.1(+) Luc-YFP), kindly provided by Christopher Contag (Stanford University, Stanford, CA), as previously described.29
Real-time RT-PCR
OSCC cells (UMSCC12, A253, SCCC25, SCCF1, SCCF2 and SCCF3) were evaluated using real-time reverse transcriptase polymerase chain reaction (RT-PCR) for expression of COX-1 and COX-2 mRNA relative to β-2 microglobulin mRNA. RNA extraction, reverse transcription, PCR, and real-time RT-PCR were performed as previously described.38 The feline primers for real-time RT-PCR included β2-microglobulin (B2M) (forward: CTACTTCTGGCGCTGCTCTG and reverse: CCTGAACCTTTGGAGAATGC), COX-1 (forward: CTGGGGTGATGAGCAACTCT and reverse: GGAAGTAACCGCTCAACTGC) and COX-2 (forward: AGGACTGGGCCATGGGGTGG and reverse: CTGGCCCACAGCAAACCGCA). The human primers included COX-1 (forward: GAGCAGCTTTTCCAGACGAC and reverse: GCAGGAAATAGCCACTCAGC) and COX-2 (forward: GCAGTTGTTCCAGACAAGCA and reverse: GCCACTCAAGTGTTGCACAT). Primer sequences for human B2M were previously described.38
OSCC cells were seeded at 1x105 cells per well in six-well plates and grown for 48 hours in growth medium followed by overnight serum starvation and 6-hour stimulation with serum-containing growth medium. Gene expression was expressed as relative expression compared to the cell line with lowest expression.
To determine if bone-conditioned medium contained factors that stimulate COX-2 expression, SCCF2 cells were cultured in serum-free, MC3T3-conditioned medium (murine preosteoblasts), or murine bone-conditioned medium. Bone-conditioned medium was prepared by culturing 1 calvarium per 2 ml of serum-free medium (high glucose DMEM). Medium was harvested every 96 hours for 8 days. MC3T3-conditioned medium was prepared from confluent cultures grown in serum free high-glucose DMEM every 96 hours for 8 days. Conditioned medium was filter sterilized and stored at −30°C until use. Unconditioned medium was prepared as conditioned medium except there were no cells or bone in the flasks. SCCF2 cells were seeded in 6-well plates at a density of 1x105 cells per well in growth medium for 48 hours followed by serum-free medium for 24 hours. Medium was replaced with 50% unconditioned serum-free medium and 50% conditioned serum-free medium for 3 hours. RNA extraction, reverse transcriptase PCR and real-time PCR was performed as described above.
Animals and treatments
All animals were 6-week-old male nu/nu mice (NCI, Frederick, MD). Mice were housed in micro-isolator cages and provided with food pellets and water ad libitum. Animal care procedures were approved by the Institutional Lab Animal Care and Use Committee using criteria based on both the Animal Welfare Act and the Public Health Services “Guide for the Care and Use of Laboratory Animals”. Mice were systematically randomized to 1 of 6 groups; nontumor-bearing vehicle-treated mice, nontumor-bearing ZOL-treated mice, tumor-bearing vehicle-treated mice, tumor-bearing meloxicam-treated mice, tumor-bearing ZOL-treated mice, and tumor-bearing combination-treated mice.
One mouse died of undetermined cause within 24 hours of injection, 2 mice were removed because of significant leakage of tumor suspension, 3 mice were removed because of low initial signal (ROI bioluminescence < 1x107 photons), and one mouse was removed because it was a high BLI outlier at day 0 (Grubbs’ test). In order to evaluate the effect of treatment on OSCC-associated bone resorption, all mice were microscopically evaluated at the end of the study to confirm contact between the xenograft and the maxilla. Mice with xenografts demonstrating minimal-to-no histologic contact with the maxillary bone were removed from the study and not included in the analyses. This included four vehicle-treated mice, four meloxicam-treated mice, four zoledronic-acid-treated mice, and two combination-treated mice. There was no apparent treatment-related effect on bone contact based on the relatively equal numbers of mice removed from each group.
The final number of mice included in each group was 5 nontumor-bearing, untreated mice; 5 nontumor-bearing, ZOL-treated mice; 11 vehicle-treated, tumor-bearing mice; 11 meloxicam-treated, tumor-bearing mice; 12 ZOL-treated, tumor-bearing mice; and 10 combination-treated, tumor-bearing mice. The xenograft of 1 mouse completely regressed (meloxicam monotherapy group), and was not removed from analysis because there was evidence of prior contact with bone (resolving bone resorption and surface remodeling) and statistical analysis (Grubbs’ test) did not identify the mouse as a bioluminescent outlier. One meloxicam-treated mouse met early removal criteria for weight loss at day 21, but had tumor growth and bone invasion similar to other mice in the group and was kept in the analysis.
Mice were anesthetized with isoflurane and injected with 1x106 SCCF2Luc cells suspended in 0.1 ml of sterile PBS below the maxillary gingiva adjacent to the maxillary incisors as previously described.16 Nontumor-bearing mice were similarly anesthetized and injected with cell-free PBS. Treatment was initiated 3 days following injection of SCCF2Luc cells, and consisted of twice weekly subcutaneous injections of ZOL at 0.1 mg/kg diluted in 0.9% sodium chloride (saline). Meloxicam was diluted in saline and administered by intraperitoneal (ip) injection at a dose of 0.3 mg/kg once daily. Vehicle-treated mice received daily ip injections of saline. Mice were weighed weekly. Bioluminescent images were collected at the onset of treatment and following 14 and 21 days of treatment. Mice were euthanized by carbon dioxide inhalation and cervical dislocation following 25 days of treatment (28 days of xenograft growth). Tissues were fixed in 4°C 10% buffered formalin for 48 hours, followed by storage in 4°C 70% ethanol until routine histologic processing and evaluation.
Bioluminescent imaging
Mice were injected ip with 4.3 mg D-Luciferin (Caliper Life Sciences, Hopkinton, MA) dissolved in sterile PBS, and imaged while under isoflurane anesthesia. In vivo bioluminescent imaging was performed using an IVIS 100 system (Caliper Life Sciences) and analyzed using LivingImageR software, version 2.2 (Caliper Life Sciences) as previously described.16,39 Region of interest (ROI) bioluminescence photon values were normalized by dividing values at day 28 by the values at the onset of treatment for each mouse and are expressed as the fold-change.
Faxitron radiography and micro-computed tomography
Five random mice from each tumor-bearing group, and all 5 mice in each of the nontumor-bearing groups, were selected for Faxitron radiography and microcomputed tomography (using a random number generator from www.random.org). The mandible was removed from each skull and the degree of maxillary and premaxillary bone loss was evaluated qualitatively using a Faxitron cabinet X-ray system (Hewlett-Packard, McMinnville, OR) as previously described.16 Bone loss was measured using microcomputed tomography (microCT) (Siemens Inveon Preclinical CT scanner and Inveon Research Workplace 3-Dimensional Image Software, Siemens AG, Munich, Germany).
Images were acquired in 400 exposures over 360 degrees, at 80 KVp, 500MA, 175 millisecond exposure, Bin 4 and a pixel width of 38.8 μm. Image data were reconstructed using Cobra software (Exxim, Pleasanton CA) and analyzed using 3D analysis software (Inveon Research Workplace 3-Dimensional Image Software, Siemens). A 2 mm thick ROI that extended caudally from the rostral commissure of the palatine fossae and included the region of xenograft growth was selected. Intensity thresholds for extracting bone and teeth from surrounding soft tissue were kept constant for all mice. ROI bone volume was compared between treatment groups. Higher resolution acquisitions were taken for figures.
Histopathology, TRAP histochemistry and histomorphometry
Skulls were decalcified and processed for microscopic examination as previously described.29 The degree of invasiveness was determined by visually identifying tumor cells at the level of the periodontal ligament of the maxillary incisor, and within the nasal cavity (tumor cells observed immediately beneath nasal respiratory epithelium). HE-stained slides were scanned using the Aperio ScanScope slide scanner (Aperio, Vista CA). The degree of maxillary bone loss was measured by expressing bone area on the tumor-bearing side as a percentage of bone area on the nontumor-bearing side. Maxillary bone was classified as either pre-existing (mature) bone or new bone (immature) based on collagen pattern (woven or lamellar), osteocyte density, and anatomic location.
Enzymatic histochemistry for tartrate-resistant acid phosphatase (TRAP, Sigma-Aldrich kit 387A, St. Louis, MO) was completed as previously described.27 Bone histomorphometry was performed with Imagescope software (Aperio). The average percentage of eroded bone, number of activated osteoclasts, osteoclast area and number of nuclei per osteoclast were determined for the lateral aspect of the maxillary bone at the invasive tumor and compared between treatment groups.
Statistical analysis
Results are displayed as means ± standard error. Normalized gene expression data (ΔCT) was analyzed for statistical significance using one-way ANOVA and Bonferroni’s post hoc test, and graphically represented by showing relative expression compared to the cell line with the lowest expression. Data from the in vivo experiment was analyzed by comparing each treatment group to the control group using Student’s t-test. In the event that data was not normally distributed, a Wilcoxon rank sum (Mann-Whitney) test was performed. The in vivo data was evaluated by comparing each treatment group to the vehicle group using three separate tests; therefore, a standard P value of 0.05 divided by 3 was considered significant (adjusted for multiple comparisons, P value of 0.017). Categorical data (presence of invasion) was analyzed using Fisher’s exact test. All comparisons were performed with STATA intercooled 10 (Cary, NC). Outliers were detected using Grubbs’ test (GraphPad QuickCalcs; www.graphpad.com).
Results
OSCC expression of COX-1 and COX-2
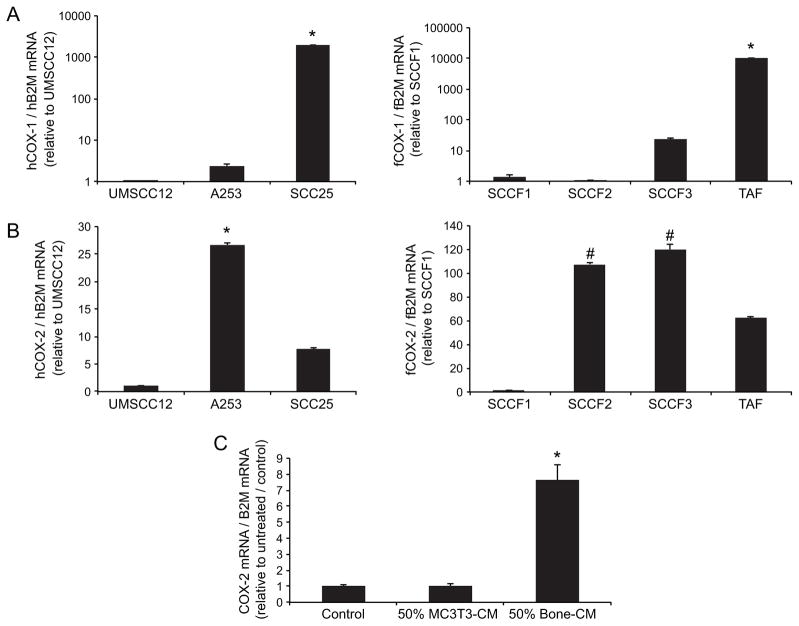
In order to determine if feline OSCC cell lines expressed COX-1 and COX-2, semi-quantitative real-time RT-PCR was performed on a panel of feline and human OSCC cell lines. We previously reported that SCCF2 cells and UMSCC12 cells induced the greatest degree of osteoclastic bone resorption16 compared to SCCF1, SCCF3 and A253 cells. COX-1 expression was detected at the mRNA level in all OSCC cell lines (figure 1A), but was not associated with the osteolytic phenotype (SCCF2 and UMSCC12 expressed the lowest levels of COX-1). Interestingly, TAF cells (feline OSCC tumor-associated fibroblasts) expressed the highest amount of COX-1. COX-2 mRNA was detectable in all OSCC cell lines (figure 1B). Similar to COX-1, COX-2 was not associated with osteolytic activity (A253 and SCCF3 expressed the most COX-2, but did not stimulate the most bone resorption).
Figure 1. OSCC cells express COX-1 and COX-2.
SCCF2 and UMSCC12 cells have been previously shown to stimulate osteoclastic bone resorption. COX expression was measured using real-time RT-PCR and mRNA levels were expressed relative to the lowest-expressing cell line. A. COX-1 mRNA was detected in all OSCC cell lines, and was lowest in the osteolytic SCCF2 and UMSCC12 cells. TAF cells (feline OSCC tumor-associated fibroblasts) expressed the greatest amount of COX-1. B. COX-2 mRNA was detectable in all OSCC cell lines, but was highest in A253, SCCF2 and SCCF3 cells (A253 and SCCF3 cells had the lowest levels of osteoclast activity). (*highest expression compared to all other cell lines, P<0.05, ANOVA and Bonferroni post hoc test; # SCCF2 and SCCF3 had higher expression than the other cell lines, P<0.05 ANOVA and Bonferroni post hoc test). C. SCCF2 cells were cultured for 3 hours in unconditioned medium (control), MC3T3 conditioned medium or murine bone conditioned medium. Bone-conditioned medium stimulated COX-2 expression in SCCF2 cells (*P<0.05 ANOVA and Bonferroni post hoc test), but MC3T3-conditioned medium did not (NS = not significant compared to control).
In order to determine if COX-2 could be stimulated by factors in bone-conditioned medium, SCCF2 cells were cultured for 24 hours in various types of conditioned medium; unconditioned (control medium), MC3T3 (murine preosteoblasts) or murine bone-conditioned medium. Bone-conditioned medium stimulated COX-2 expression in SCCF2 cells but MC3T3-conditioned medium did not (figure 1C).
Effect of therapy on tumor growth and invasion
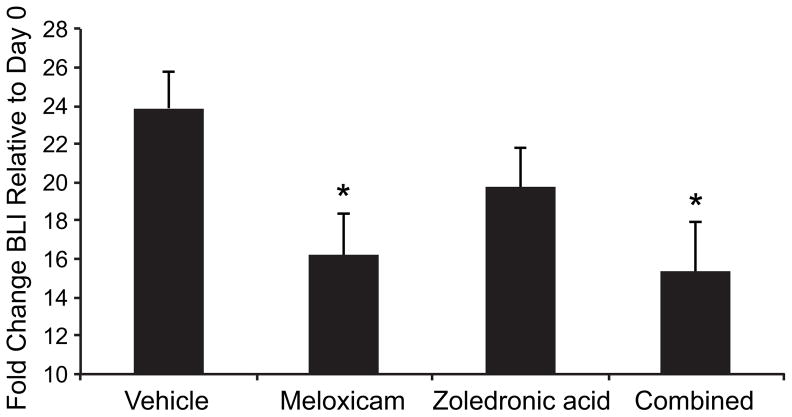
At the 21st day of treatment, meloxicam reduced tumor bioluminescence by 32% compared to vehicle-treated mice (P=0.0143, 2-tailed t-test), and combination therapy reduced bioluminescence by 36% (P=0.0134, 2-tailed t-test, figure 2). ZOL monotherapy resulted in a non-statistically significant 17% reduction in bioluminescence (P=0.1509, 2-tailed t-test, figure 2). This degree of tumor growth suppression is comparable to what we observed in our previous study of ZOL monotherapy following 3 weeks of treatment.29
Figure 2. Meloxicam reduced tumor growth.
On day 21 of treatment, meloxicam reduced tumor bioluminescence by 32% compared to the vehicle-treated mice, and combination therapy reduced tumor bioluminescence by 36%. *P<0.0167. ZOL monotherapy resulted in a non-statistically significant 17% reduction in tumor bioluminescence.
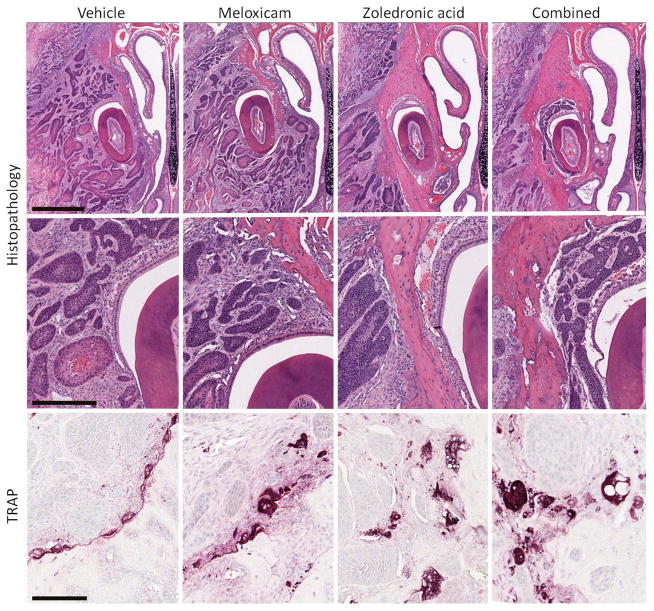
SCCF2Luc xenografts were composed of islands and cords of malignant epithelial cells demonstrating variable degrees of squamous differentiation and keratinization with regions of central necrosis. Maxillary bone loss was most evident in vehicle- or meloxicam-treated mice compared to ZOL- and combination-treated mice (figures 3A and 3B). There was no difference in the incidence of tumor invasion into the sulcus of the maxillary incisor between treatment groups (figure 3B), which was observed in 90% of vehicle-treated mice, 91% of meloxicam-treated mice, 92% of ZOL-treated mice, and 80% of combination-treated mice. ZOL, alone or combined with meloxicam, caused a non-statistically significant reduction in the incidence of tumor invasion into the nasal cavity; which was observed in 64% of vehicle-treated mice, 73% of meloxicam-treated mice, 33% of ZOL-treated mice and 40% of combination-treated mice (P=0.192, Pearson chi square).
Figure 3.
Zoledronic acid reduced bone loss and was associated with osteoclast vacuolar degeneration. A, upper panel: Maxillary bone loss was most common in vehicle- and meloxicam-treated mice. Bar=1000 μm. B, middle panel: Tumor invasion around the maxillary incisor occurred with similar frequency between treatment groups. Bar=300 μm. C, lower panel: Bone invasion was associated with TRAP-positive osteoclasts in resorption pits at the tumor-bone interface, and vacuolar degeneration of osteoclasts was observed in ZOL-treated mice (alone and in combination with meloxicam). Bar=100 μm. Images are representative of the mice in each of the groups.
Xenograft invasion into bone was associated with numerous TRAP-positive osteoclasts in resorption pits at the tumor-bone interface (figure 3C). Cytoplasmic vacuolation of some osteoclasts was observed in ZOL-treated mice (alone and in combination with meloxicam).
Tumor-bearing mice occasionally demonstrated mild to moderate bone necrosis at the invasive front of the tumor. Bone necrosis was observed in 5 of 10 ZOL + meloxicam treated-mice, 5 of 12 ZOL-only treated mice, 1 of 10 meloxicam-only treated-mice, and 1 of 11 vehicle-treated mice. Bone necrosis was only observed in xenografts with significant bone resorption. Bone and tooth necrosis was not observed on the non-tumor-bearing side of the maxilla, and was not observed in ZOL-treated, non-tumor-bearing mice. Two (2) combination treated mice and 1 ZOL-only treated mouse had evidence of tooth necrosis in addition to bone necrosis, and only occurred in mice with pronounced tumor invasion.
Zoledronic acid decreased loss of bone volume
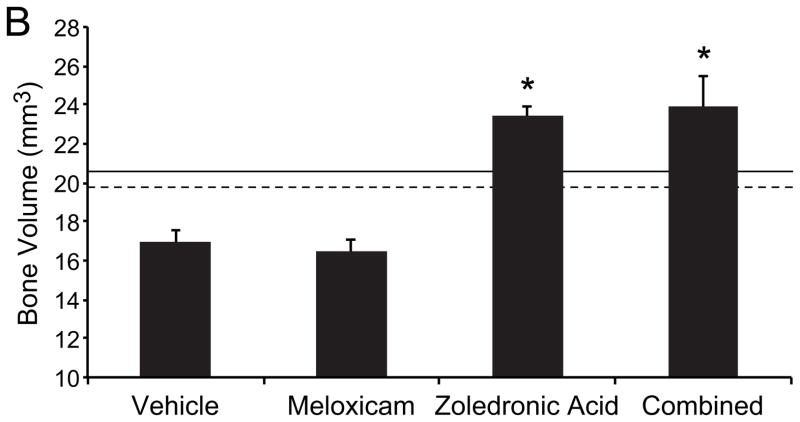
In order to demonstrate the effect of treatment on maxillary bone loss, Faxitron radiography and microcomputed tomography (microCT) were performed. There was a qualitative reduction in bone loss and increased periosteal new bone formation in the region of xenograft growth in the ZOL-treated mice (monotherapy or combined with meloxicam) compared to vehicle-treated mice (figures 4A and B). MicroCT of 5 randomly selected mice from each treatment group was performed to quantify changes in bone volume in a 2mm thick region of interest adjacent to the xenograft. Meloxicam monotherapy had no effect on bone volume compared to vehicle-treated mice; however, ZOL therapy (alone and combined with meloxicam), was associated with significantly increased maxillary bone volume compared to vehicle-treated mice (figure 4B; P<0.0167, Students t-test). Combination treatment did not result in an additional increase in bone volume compared to ZOL-monotherapy. Additionally, ZOL-treatment resulted in increased bone volume compared to vehicle-treated, non-tumor-bearing mice (dashed line) and ZOL-treated, nontumor-bearing mice (solid line). The increased bone volume was attributed to new bone formation.
Figure 4. Zoledronic acid reduced loss of bone volume.
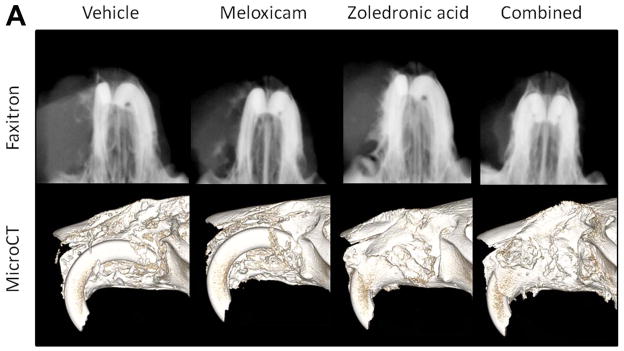
A, upper panel: Faxitron radiography of a representative mouse from each group. There was reduced maxillary bone loss and increased new bone formation in the ZOL-treated mice (monotherapy or combined with meloxicam) compared to vehicle-treated mice and meloxicam-treated mice. A, lower panel: Reconstructed microCT images of a representative mouse from each group showing reduced maxillary bone loss and increased new bone formation in the ZOL-treated mice (monotherapy or combined with meloxicam) compared to vehicle-treated mice or meloxicam-treated mice. B, graph: Quantitative microCT analysis of bone volume was performed on five randomly selected mice from each treatment group. Meloxicam monotherapy had no effect on bone volume compared to vehicle-treated mice; however, ZOL therapy (alone and combined with meloxicam), was associated with significantly increased maxillary bone volume compared to vehicle-treated mice (*P<0.0167). ZOL-treatment resulted in increased bone volume compared to vehicle-treated, non-tumor-bearing mice (dashed line) and ZOL-treated, non-tumor-bearing mice (solid line). Increased bone volume was attributed to new bone formation.
Effect of zoledronic acid and meloxicam on bone area
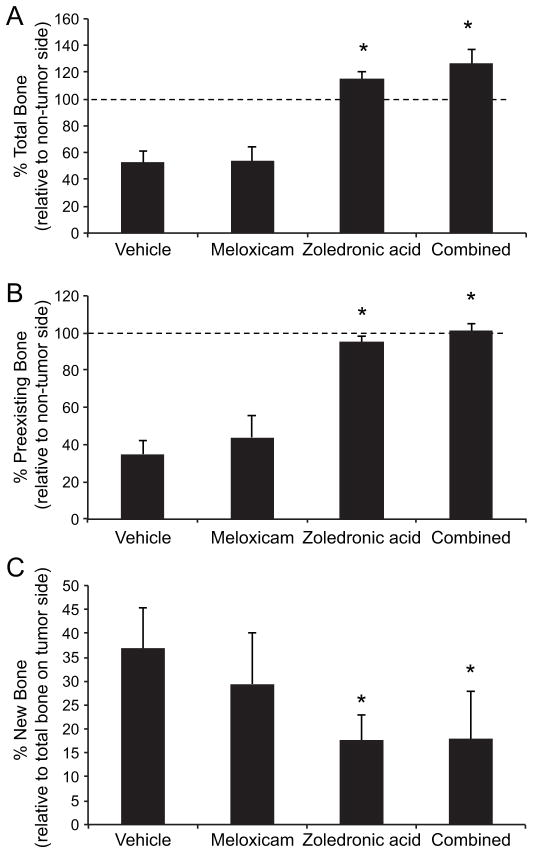
Maxillary bone area on the nontumor-bearing side and tumor-bearing side was measured using Imagescope software. Total bone (pre-existing bone and new bone combined) on the tumor-bearing side was expressed as a percentage of bone area on the nontumor-bearing side (figure 5A). Xenograft growth in vehicle-treated mice was associated with a 47% reduction in total bone area compared to the non-tumor-bearing side (dashed line). There was no effect of meloxicam treatment on bone area (loss of 46%) compared to vehicle-treated mice. ZOL monotherapy and ZOL-meloxicam combination therapy reduced loss of total bone compared to vehicle-treated mice (P<0.00001 for both comparisons, 2-tailed t-test). Mice treated with ZOL or combination therapy had increased total bone compared to the nontumor-bearing side (15% and 27% more total bone, respectively).
Figure 5. Zoledronic acid reduced loss of bone area.
Maxillary bone area on the non-tumor-bearing side and tumor-bearing side was measured using Imagescope software. A: Xenograft growth in vehicle-treated mice was associated with a 47% reduction in total bone area compared to the non-tumor-bearing side (dashed line). There was no effect of meloxicam treatment on bone area (loss of 46%). ZOL monotherapy and ZOL-meloxicam combination therapy eliminated loss of total bone compared to vehicle-treated mice (*P<0.0167). Mice treated with ZOL or combination therapy had increased total bone compared to the nontumor-bearing side (15% and 27% more total bone, respectively). B: Xenograft growth was associated with a 65% reduction in pre-existing bone compared to the nontumor-bearing side (dashed line). Meloxicam treatment resulted in a non-statistically significant reduction in pre-existing bone loss. Mice treated with ZOL monotherapy retained greater pre-existing bone compared to vehicle-treated mice (*P<0.0167). ZOL-meloxicam also retained more pre-existing bone compared to vehicle-treated mice (*P<0.0167) and completely inhibited loss of pre-existing bone area. C: New bone area was determined and expressed as a percentage of the total bone on the tumor-bearing side. New bone represented the lowest percentage of total bone in the ZOL and ZOL-meloxicam combination treated mice (*P<0.0167), which was attributed the high retention of pre-existing bone.
Xenograft growth was associated with a 65% reduction in pre-existing bone compared to the nontumor-bearing side (dashed line, figure 4.5B). Meloxicam treatment resulted in a small reduction in pre-existing bone loss (56% compared to 65% in vehicle-treated mice) that did not reach statistical significance. Mice treated with ZOL monotherapy retained greater pre-existing bone compared to vehicle-treated mice (P=0.0003, Wilcoxon rank-sum, corresponding to a loss of 4.7%). Loss of pre-existing bone was inhibited by ZOL + meloxicam (P=0.0002, Wilcoxon rank-sum). To determine how much of the bone on the tumor-bearing side was composed of immature, reactive periosteal bone, the area of new bone was measured and expressed as a percentage of the total bone on the tumor-bearing side (figure 5C). New bone represented the lowest percentage of total bone in the ZOL and ZOL + meloxicam-treated mice (P=0.0013 and P=0.0054, 2-tailed t-tests) which was attributed the high retention of pre-existing bone. P values < 0.0167 were considered statistically significant (adjusted for multiple comparisons). There was no difference in body weights between the treatment groups.
Zoledronic acid reduced the number of osteoclasts and increased osteoclast size and number of nuclei
Tissue sections were evaluated for the presence of activated osteoclasts at the xenograft-bone interface using TRAP enzyme histochemistry. Osteoclasts appeared as multinucleated TRAP-positive cells in resorption pits on the bone surface. ZOL monotherapy and ZOL-meloxicam combination therapy significantly reduced osteoclast number (P<0.0001 for both comparisons, 2-tailed t-test, figure 4.6A). There was no effect of meloxicam monotherapy on the number of osteoclasts per millimeter of bone surface. Addition of meloxicam to ZOL did not reduce osteoclast number compared to ZOL monotherapy.
Figure 6. Zoledronic acid reduced osteoclast number and increased osteoclast size and number of nuclei.
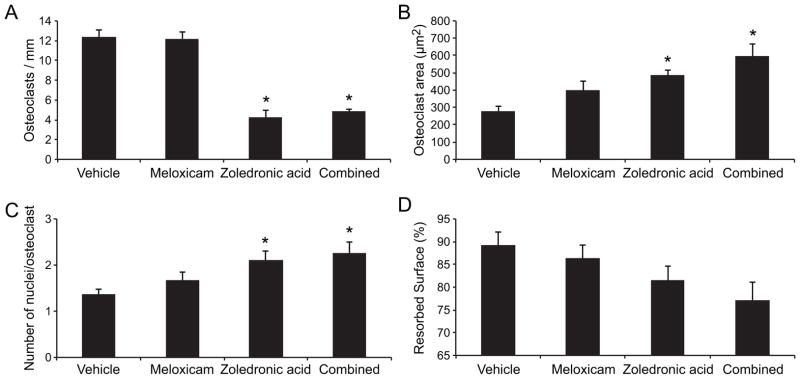
A. ZOL monotherapy and ZOL-meloxicam combination therapy significantly reduced osteoclast number (*P<0.0167) compared to vehicle-treated mice, but there was no effect of meloxicam monotherapy on the number of osteoclasts. B. The largest osteoclasts were observed in combination-treated mice (*P<0.0167), followed by ZOL-treated mice (*P<0.0167). There was a trend for meloxicam monotherapy to result in larger osteoclasts compared to vehicle-treated mice, but there was no statistical difference (P>0.0167). C. ZOL-monotherapy and ZOL-meloxicam-combination therapy resulted in the greatest number of osteoclast nuclei per cell (*P<0.0167). D. There was a trend for ZOL + meloxicam-treated mice to have the lowest percentage of eroded bone surface (P>0.0167).
The largest osteoclasts (figure 6B) were observed in combination-treated mice (P=0.0018, 2-tailed t-test, unequal variances), followed by ZOL-monotherapy-treated mice (P=0.0001, 2-tailed t-test, equal variances). There was a trend for meloxicam monotherapy to result in larger osteoclasts compared to vehicle-treated mice, but there was no statistical difference (P=0.0594, 2-tailed t-test). The effect of treatment on the number of nuclei per osteoclast (figure 6C) was similar to the effect on osteoclast size. ZOL-monotherapy and ZOL-meloxicam-combination therapy resulted in the highest numbers of osteoclast nuclei (P=0.004 and P=0.007 respectively, 2-tailed t-tests).
There was no statistically significant difference in the ratio of eroded to total bone surface between each of the treatment groups and the vehicle-treated group (figure 6D), despite an apparent trend for reduced eroded surfaces in ZOL-treated mice and ZOL + meloxicam-treated mice (P=0.0218 for combination-treated mice, 2-tailed t-test, greater than the adjusted P value of 0.0167).
Discussion
The purpose of this study was to determine if the inhibitory effect of ZOL on OSCC tumor growth and bone invasion could be enhanced by the addition of a preferential COX-2 inhibitor, Meloxicam. We previously reported that ZOL monotherapy reduced tumor growth and osteoclastic bone resorption in an orthotopic mouse model of maxillary invasive OSCC,16 and COX inhibitors have been shown to reduce tumor growth in rodent models of OSCC.31–33
COX-1 and COX-2 mRNA were detectable in all human and feline OSCC cell lines; however, there was no apparent association with osteolytic activity. For example, COX-1 and COX-2 expression was greatest in SCCF3 cells, but SCCF3 cells were not associated with a strong osteoclastic response.29 The OSCC cell lines that were associated with robust in vitro bone resorption and osteoclast formation (UMSCC12 and SCCF2) had relatively low COX-1 and COX-2 expression. These findings suggest that COX expression in OSCC cell lines is not important in the pathogenesis of bone invasion; however, Cox-1 and Cox-2 proteins and enzyme activities were not measured in this study and the SCCF2 cells did have increased COX-2 mRNA expression when stimulated with bone-conditioned medium. It is possible that OSCC cells have greater COX-2 enzymatic activity and produce more PGE2, when in close proximity with resorbing bone. Interestingly, feline tumor-associated fibroblasts expressed much more COX-1 mRNA compared to feline OSCC cells, suggesting that the tumor stroma or periodontal fibroblasts may be a significant source of PGE2, which could be available to stimulate bone resorption regardless of PGE2 production from the tumor cells themselves.
SCCF2 cells expressed more COX-2 when exposed to bone-conditioned medium. In gastrointestinal tumors, increased COX-2 expression has been attributed to transcriptional and post-translational regulation rather than copy number gain of the COX-2 gene.40 Transcriptional factors that have been associated with increased COX-2 expression include nuclear factor kappaB (NFκB), cAMP response element-binding protein (CREB), nuclear factor of activated T-cells (NFAT), activator protein-1 (AP-1), peroxisome proliferator-activated receptor (PPAR) and hypoxia-inducible factor 1, alpha subunit (HIF1A).40
The mechanism by which meloxicam inhibited tumor growth in this model is unknown. PGE2 has been shown to be increased in a variety of tumor tissues, and has been shown to stimulate tumor cell proliferation while inhibiting apoptosis, in addition to promoting angiogenesis, tumor invasiveness, escape from immune surveillance, and resistance to chemotherapeutic drugs.40 The antiproliferative and pro-apoptotic effects of cyclooxygenase inhibitors have been associated with both COX-dependent and COX-independent mechanisms.41
COX-2 inhibitors have demonstrated antiproliferative effects on OSCC in vitro, albeit at concentrations higher than needed to inhibit PGE2 production.41 While there are no published reports of the effects of meloxicam on clinical or preclinical in vivo OSCC studies, Meloxicam has been shown to induce tumor cell apoptosis in human patients with esophageal cancer42 and reduce tumor growth in animal models of other forms of cancer including osteosarcoma,43 hepatocellular carcinoma44 and ovarian carcinoma.45
Tumor cell expression of COX-2 and subsequent production of PGE2 is not the only target of a systemically administered COX-2 inhibitor. Meloxicam administration may have reduced PGE2 production in the tumor stroma or in the resorbing bone (COX expression was demonstrated in feline OSCC-associated fibroblasts). Regardless of the source, PGE2 activation of prostaglandin E (EP) receptors is capable of transactivating epidermal growth factor receptor (EGFR), potentially leading to increased angiogenesis, tumor invasion, and reduced apoptosis.40 EGFR is known to be overexpressed in human OSCC,46,47 and EGFR expression has been demonstrated in spontaneous feline OSCC tumors48 and in the three feline OSCC cell lines used in this study (unpublished data and Bergkvist et al.49).
As expected, ZOL therapy reduced bone loss in vivo that was characterized by increased bone volume and reduced numbers of osteoclasts. ZOL is known to interfere with the mevalonate (MVA) pathway by inhibiting farnesyl pyrophosphate synthase25,50,51 leading to reduced prenylation of small guanosine-triphosphate (GTP)-binding proteins required for osteoclast function and survival.52,53 ZOL-treatment increased osteoclast size and number of nuclei in this study, consistent with previously reported data.16,54 Interestingly, the presence of giant, hypernucleated osteoclasts has been observed in women on long term bisphosphonate therapy for the treatment of osteoporosis, but increased numbers of osteoclasts were also observed.55 The mechanism of hypernucleation is not known; however, Weinstein et al. speculated that inhibition of bone resorption would have caused reduced local calcium concentration and reduced apoptotic signals in the osteoclast, leading to increased duration of survival and more time for fusion of osteoclasts with mononuclear progenitors.55 Since we observed reduced osteoclast number in the ZOL-treated mice with OSCC xenografts, it is unlikely that reduced osteoclast apoptosis and increased osteoclast lifespan was the reason for increased osteoclast size and number of nuclei observed in this study.
We previously reported that ZOL reduced, but did not eliminate, loss of pre-existing bone in a bone invasive model of OSCC.16 In contrast, this study showed that ZOL almost completely inhibited loss of pre-existing bone. The improved effectiveness of ZOL in this study may be due to the fact that treatment was initiated earlier in the course of xenograft growth (3 days following tumor cell injection compared to 7 days in the previous study). Mice were treated earlier in this study to model smaller primary tumors compared to advanced cancers because zoledronic acid prevents rather than reverses cancer-induced bone loss. Additionally, the total duration of xenograft growth in this study was 7 days shorter compared to the previous study. The combination of earlier treatment and shorter duration of tumor growth may be the reason for the increased effectiveness of ZOL against tumor-associated bone loss and indicates that early diagnosis and treatment in cats is desirable.
Osteonecrosis of the jaw (ONJ) occurs in a low percentage of human cancer patients treated with bisphosphonates (estimated incidence of 5% to 10%).56,57 Small amounts of tumor-associated necrotic bone within the xenografts of ZOL-treated mice was attributed to the retention of bone undergoing necrosis as a result of tumor infiltration, since the antiresorptive effects of ZOL would inhibit removal of necrotic bone. Bone necrosis was rarely observed in non ZOL-treated animals (1 vehicle-treated mouse and 1 meloxicam-treated mouse). Tooth necrosis appeared as an extension of bone necrosis and was observed in few mice (3 of 22 mice). Bone necrosis was associated with invasive behavior, and suggests that OSCC patients with existing bone disease may be at increased risk for the development of ONJ. ONJ has not been reported in cats treated with bisphosphonates, but this should be considered a potential risk of bisphosphonate therapy in cats with oral cancer.
The data suggested that meloxicam mildly increased retention of pre-existing bone, reduced eroded surfaces and increased osteoclast size; however, the differences did not reach statistical significance. This trend was observed when meloxicam monotherapy was compared to untreated mice and when ZOL + meloxicam combination therapy was compared to ZOL monotherapy, suggesting that COX-2 inhibition may have had a mild inhibitory effect on osteoclastic bone resorption in this model of feline OSCC.
Meloxicam was more effective than ZOL at reducing xenograft growth but did not have a significant effect on bone resorption. The combination of meloxicam and ZOL was well tolerated but did not stimulate additional tumor suppression or inhibition of bone loss compared to meloxicam- or ZOL-monotherapy. Although a synergistic effect on tumor progression was not observed, the results indicate that meloxicam and zoledronic acid could be of benefit in the management of oral squamous cell carcinoma and support the need for future clinical trials in cats.
Acknowledgments
This work was supported by the Morris Animal Foundation (D08FE-023 and D09FE-404) and the National Institutes of Health, National Cancer Center (F32 CA130458).
References
- 1.Dorn CR, Priester WA. Epidemiologic analysis of oral and pharyngeal cancer in dogs, cats, horses, and cattle. J Am Vet Med Assoc. 1976;169(11):1202–6. [PubMed] [Google Scholar]
- 2.Morris J, Dobson J. Head and Neck. In: Morris J, Dobson J, editors. Small Animal Oncology. Alder Press Ltd; Oxford: Blackwell Science Ltd; 2001. pp. 94–124. [Google Scholar]
- 3.Martin CK, Tannehill-Gregg SH, Wolfe TD, Rosol TJ. Bone-invasive oral squamous cell carcinoma in cats: pathology and expression of parathyroid hormone-related protein. Vet Pathol. 2011;48(1):302–12. doi: 10.1177/0300985810384414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmali RA, Wustrow T, Thaler HT, Strong EW. Prostaglandins in carcinomas of the head and neck. Cancer Lett. 1984;22(3):333–6. doi: 10.1016/0304-3835(84)90171-x. [DOI] [PubMed] [Google Scholar]
- 5.Jung TT, Berlinger NT, Juhn SK. Prostaglandins in squamous cell carcinoma of the head and neck: a preliminary study. Laryngoscope. 1985;95(3):307–12. doi: 10.1288/00005537-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Tannehill-Gregg S, Kergosien E, Rosol TJ. Feline head and neck squamous cell carcinoma cell line: characterization, production of parathyroid hormone-related protein, and regulation by transforming growth factor-beta. In Vitro Cell Dev Biol Anim. 2001;37(10):676–83. doi: 10.1290/1071-2690(2001)037<0676:FHANSC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Kukreja SC, Rosol TJ, Wimbiscus SA, Shevrin DH, Grill V, Barengolts EI, Martin TJ. Tumor resection and antibodies to parathyroid hormone-related protein cause similar changes on bone histomorphometry in hypercalcemia of cancer. Endocrinology. 1990;127(1):305–10. doi: 10.1210/endo-127-1-305. [DOI] [PubMed] [Google Scholar]
- 8.Shibahara T, Nomura T, Cui NH, Noma H. A study of osteoclast-related cytokines in mandibular invasion by squamous cell carcinoma. Int J Oral Maxillofac Surg. 2005;34(7):789–93. doi: 10.1016/j.ijom.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto M, Hiura K, Ohe G, Ohba Y, Terai K, Oshikawa T, Furuichi S, Nishikawa H, Moriyama K, Yoshida H, Sato M. Mechanism for bone invasion of oral cancer cells mediated by interleukin-6 in vitro and in vivo. Cancer. 2000;89(9):1966–75. doi: 10.1002/1097-0142(20001101)89:9<1966::aid-cncr13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59(5):991–4. [PubMed] [Google Scholar]
- 11.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10(2):159–78. [PubMed] [Google Scholar]
- 12.Werkmeister JR, Blomme EA, Weckmann MT, Grone A, McCauley LK, Wade AB, O’Rourke J, Capen CC, Rosol TJ. Effect of transforming growth factor-beta1 on parathyroid hormone-related protein secretion and mRNA expression by normal human keratinocytes in vitro. Endocrine. 1998;8(3):291–9. doi: 10.1385/ENDO:8:3:291. [DOI] [PubMed] [Google Scholar]
- 13.Rosol TJ. Pathogenesis of bone metastases: role of tumor-related proteins. J Bone Miner Res. 2000;15(5):844–50. doi: 10.1359/jbmr.2000.15.5.844. [DOI] [PubMed] [Google Scholar]
- 14.Hiraga T, Myoui A, Choi ME, Yoshikawa H, Yoneda T. Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor-beta enhances bone metastases in breast cancer. Cancer Res. 2006;66(4):2067–73. doi: 10.1158/0008-5472.CAN-05-2012. [DOI] [PubMed] [Google Scholar]
- 15.McCauley LK, Beecher CA, Melton ME, Werkmeister JR, Juppner H, Abou-Samra AB, Segre GV, Rosol TJ. Transforming growth factor-beta1 regulates steady-state PTH/PTHrP receptor mRNA levels and PTHrP binding in ROS 17/2.8 osteosarcoma cells. Mol Cell Endocrinol. 1994;101(1–2):331–6. doi: 10.1016/0303-7207(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 16.Martin CK, Dirksen WP, Shu ST, Werbeck JL, Thudi NK, Yamaguchi M, Wolfe TD, Heller KN, Rosol TJ. Characterization of bone resorption in novel in vitro and in vivo models of oral squamous cell carcinoma. Oral Oncol. 2012;48(6):491–9. doi: 10.1016/j.oraloncology.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes A, Scase T, Miller J, Murphy S, Sparkes A, Adams V. COX-1 and COX-2 expression in feline oral squamous cell carcinoma. J Comp Pathol. 2006;135(2–3):93–9. doi: 10.1016/j.jcpa.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–6. [PubMed] [Google Scholar]
- 19.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 20.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94(7):3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58(6):1208–16. [PubMed] [Google Scholar]
- 22.Akatsu T, Takahashi N, Debari K, Morita I, Murota S, Nagata N, Takatani O, Suda T. Prostaglandins promote osteoclastlike cell formation by a mechanism involving cyclic adenosine 3′,5′-monophosphate in mouse bone marrow cell cultures. J Bone Miner Res. 1989;4(1):29–35. doi: 10.1002/jbmr.5650040106. [DOI] [PubMed] [Google Scholar]
- 23.Saito J, Kohn AD, Roth RA, Noguchi Y, Tatsumo I, Hirai A, Suzuki K, Kohn LD, Saji M, Ringel MD. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid. 2001;11(4):339–51. doi: 10.1089/10507250152039073. [DOI] [PubMed] [Google Scholar]
- 24.Saito S, Rosol TJ, Saito M, Ngan PW, Shanfeld J, Davidovitch Z. Bone-resorbing activity and prostaglandin E produced by human periodontal ligament cells in vitro. J Bone Miner Res. 1990;5(10):1013–8. doi: 10.1002/jbmr.5650051004. [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RG, Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006;103(20):7829–34. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannehill-Gregg SH, Levine AL, Nadella MV, Iguchi H, Rosol TJ. The effect of zoledronic acid and osteoprotegerin on growth of human lung cancer in the tibias of nude mice. Clin Exp Metastasis. 2006;23(1):19–31. doi: 10.1007/s10585-006-9008-z. [DOI] [PubMed] [Google Scholar]
- 27.Thudi NK, Martin CK, Nadella MV, Fernandez SA, Werbeck JL, Pinzone JJ, Rosol TJ. Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate. 2008;68(10):1116–25. doi: 10.1002/pros.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe TD, Pillai SP, Hildreth BE, 3rd, Lanigan LG, Martin CK, Werbeck JL, Rosol TJ. Effect of zoledronic acid and amputation on bone invasion and lung metastasis of canine osteosarcoma in nude mice. Clin Exp Metastasis. 2011;28(4):377–89. doi: 10.1007/s10585-011-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin CK, Werbeck JL, Thudi NK, Lanigan LG, Wolfe TD, Toribio RE, Rosol TJ. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res. 2010;70(21):8607–16. doi: 10.1158/0008-5472.CAN-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui N, Nomura T, Noma H, Yokoo K, Takagi R, Hashimoto S, Okamoto M, Sato M, Yu G, Guo C, Shibahala T. Effect of YM529 on a model of mandibular invasion by oral squamous cell carcinoma in mice. Clin Cancer Res. 2005;11(7):2713–9. doi: 10.1158/1078-0432.CCR-04-1767. [DOI] [PubMed] [Google Scholar]
- 31.Roh JL, Sung MW, Kim KH. Suppression of accelerated tumor growth in surgical wounds by celecoxib and indomethacin. Head Neck. 2005;27(4):326–32. doi: 10.1002/hed.20167. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Fuentes CF, Shapshay SM. Antiangiogenic and chemopreventive activities of celecoxib in oral carcinoma cell. Laryngoscope. 2002;112(5):839–43. doi: 10.1097/00005537-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, Yang CS, Chen X. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11(5):2089–96. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 34.Sievers EM, Bart RD, Backhus LM, Lin Y, Starnes M, Castanos R, Starnes VA, Bremner RM. Evaluation of cyclooxygenase-2 inhibition in an orthotopic murine model of lung cancer for dose-dependent effect. J Thorac Cardiovasc Surg. 2005;129(6):1242–9. doi: 10.1016/j.jtcvs.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 35.Smakman N, Schaap N, Snijckers CM, Borel Rinkes IH, Kranenburg O. NS-398, a selective cyclooxygenase-2 inhibitor, reduces experimental bladder carcinoma outgrowth by inhibiting tumor cell proliferation. Urology. 2005;66(2):434–40. doi: 10.1016/j.urology.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Yoshinaka R, Shibata MA, Morimoto J, Tanigawa N, Otsuki Y. COX-2 inhibitor celecoxib suppresses tumor growth and lung metastasis of a murine mammary cancer. Anticancer Res. 2006;26(6B):4245–54. [PubMed] [Google Scholar]
- 37.Tanaka T, Nishikawa A, Mori Y, Morishita Y, Mori H. Inhibitory effects of non-steroidal anti-inflammatory drugs, piroxicam and indomethacin on 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male ACI/N rats. Cancer Lett. 1989;48(3):177–82. doi: 10.1016/0304-3835(89)90115-8. [DOI] [PubMed] [Google Scholar]
- 38.Shu ST, Martin CK, Thudi NK, Dirksen WP, Rosol TJ. Osteolytic bone resorption in adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2010;51(4):702–14. doi: 10.3109/10428191003646697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannehill-Gregg SH, Levine AL, Rosol TJ. Feline head and neck squamous cell carcinoma: a natural model for the human disease and development of a mouse model. Vet Comp Oncol. 2006;4(2):84–97. doi: 10.1111/j.1476-5810.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295(1):7–16. doi: 10.1016/j.canlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Ko SH, Choi GJ, Lee JH, Han YA, Lim SJ, Kim SH. Differential effects of selective cyclooxygenase-2 inhibitors in inhibiting proliferation and induction of apoptosis in oral squamous cell carcinoma. Oncol Rep. 2008;19(2):425–33. [PubMed] [Google Scholar]
- 42.Liu JF, Zhang SW, Jamieson GG, Zhu GJ, Wu TC, Zhu TN, Shan BE, Drew PA. The effects of a COX-2 inhibitor meloxicam on squamous cell carcinoma of the esophagus in vivo. Int J Cancer. 2008;122(7):1639–44. doi: 10.1002/ijc.23288. [DOI] [PubMed] [Google Scholar]
- 43.Naruse T, Nishida Y, Hosono K, Ishiguro N. Meloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routes. Carcinogenesis. 2006;27(3):584–92. doi: 10.1093/carcin/bgi240. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X, Li H, Qiao H, Jiang H, Xu R, Sun X. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci. 2009;100(11):2226–33. doi: 10.1111/j.1349-7006.2009.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin B, Yokoyama Y, Shigeto T, Mizunuma H. Anti-tumor effect of non-steroidal anti-inflammatory drugs on human ovarian cancers. Pathol Oncol Res. 2007;13(4):365–9. doi: 10.1007/BF02940318. [DOI] [PubMed] [Google Scholar]
- 46.Laimer K, Spizzo G, Gastl G, Obrist P, Brunhuber T, Fong D, Barbieri V, Jank S, Doppler W, Rasse M, Norer B. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: a TMA-based immunohistochemical analysis. Oral Oncol. 2007;43(2):193–8. doi: 10.1016/j.oraloncology.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Ezquerra G, Salgado R, Toll A, Gilaberte M, Baro T, Alameda Quitllet F, Yebenes M, Sole F, Garcia-Muret M, Espinet B, Pujol RM. Multiple genetic copy number alterations in oral squamous cell carcinoma: study of MYC, TP53, CCDN1, EGFR and ERBB2 status in primary and metastatic tumours. Br J Dermatol. 2010;163(5):1028–35. doi: 10.1111/j.1365-2133.2010.09947.x. [DOI] [PubMed] [Google Scholar]
- 48.Looper JS, Malarkey DE, Ruslander D, Proulx D, Thrall DE. Epidermal growth factor receptor expression in feline oral squamous cell carcinomas. Vet Comp Oncol. 2006;4(1):33–40. doi: 10.1111/j.1476-5810.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 49.Bergkvist GT, Argyle DJ, Morrison L, MacIntyre N, Hayes A, Yool DA. Expression of epidermal growth factor receptor (EGFR) and Ki67 in feline oral squamous cell carcinomas (FOSCC) Vet Comp Oncol. 2011;9(2):106–17. doi: 10.1111/j.1476-5829.2010.00239.x. [DOI] [PubMed] [Google Scholar]
- 50.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296(2):235–42. [PubMed] [Google Scholar]
- 51.Green JR, Clezardin P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am J Clin Oncol. 2002;25(6 Suppl 1):S3–9. doi: 10.1097/00000421-200212001-00002. [DOI] [PubMed] [Google Scholar]
- 52.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 53.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 54.Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, Shi S, Zhang L. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010;177(1):280–90. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards BJ, Gounder M, McKoy JM, Boyd I, Farrugia M, Migliorati C, Marx R, Ruggiero S, Dimopoulos M, Raisch DW, Singhal S, Carson K, Obadina E, Trifilio S, West D, Mehta J, Bennett CL. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9(12):1166–72. doi: 10.1016/S1470-2045(08)70305-X. [DOI] [PubMed] [Google Scholar]
- 57.Yamashita J, McCauley LK. Antiresorptives and osteonecrosis of the jaw. J Evid Based Dent Pract. 2012;12(3 Suppl):233–47. doi: 10.1016/S1532-3382(12)70046-5. [DOI] [PubMed] [Google Scholar]