Abstract
Objective
Aging and obesity contribute to the initiation and progression of osteoarthritis with little information on their relation to gene expression in joint tissues, particularly the meniscus. Here, we test the hypothesis that patient age and body mass index (BMI) correlate with the expression of osteoarthritis- and obesity-related gene signatures in the meniscus.
Design
Meniscus was obtained from patients (N=68) undergoing arthroscopic partial meniscectomy. The mRNA expression of twenty-four osteoarthritis-related and four obesity-related genes in meniscus was assessed by qRT-PCR. The relationship between gene expression and patient age and BMI was analyzed using Spearman’s rank-order correlation. Hierarchical cluster dendrogram and heat maps were generated to study inter-gene associations.
Results
Age was negatively correlated (P<0.05) with the expression of MMP-1 (r=−0.447), NFκB2 (r=−0.361), NFκBIA (r=−0.312), IκBA (r=−0.308), IL-8 (r=−0.305), ADAMTS-4 (r=−0.294), APLN (r=−0.250) and IL-6 (r=−0.244). Likewise, BMI was negatively correlated with the expression of APLN (r=−0.328), ACAN (r=−0.268) and MMP-1 (r=−0.261). After adjusting for the correlation between age and BMI (r=0.310; P=0.008), the only independent effect of BMI on gene expression was for APLN (r=−0.272). However, age had an independent effect on expression on ADAMTS-4 (r=−0.253), MMP-1 (r=−0.399), IL-8 (r=−0.327), COL1A1 (r=−0.287), NFκBIA (r=−0.278), NFκB2 (r=−0.312) and IκBA (r=−0.299). The gene-correlation analysis identified four clusters of potentially relevant genes: transcription factors, matrix degrading enzymes, cytokines and chemokines, and obesity genes.
Conclusion
Age and BMI were negatively correlated with several osteoarthritis- and obesity-related genes. While the bulk of these changes appeared to be driven by age, expression of APLN was related to BMI. Inter-gene correlations implicated a common regulatory role of strongly correlated genes. Although age-related variations in gene expression are potentially more pertinent than obesity-related differences for the role of the meniscus in osteoarthritis development, further investigation into the role of APLN in meniscus and joint health is warranted.
Keywords: Meniscus tear, gene expression, osteoarthritis, age, body mass index, obesity
INTRODUCTION
Osteoarthritis is a degenerative joint disorder with severe consequences in terms of both disability and the financial burden in society1, 2. Today, osteoarthritis is not considered to be merely a disease of cartilage but a disease of the whole joint and its related tissues3. The pathologic changes in the arthritic knee are not confined only to articular cartilage (degradation, calcification) but also affect subchondral bone (thickening, osteophyte formation), synovium (synovitis), joint capsule (hypertrophy), ligaments (degeneration) and meniscus (extrusion, degeneration, calcification)4, 5.
The meniscus (pl. menisci) is a semi-lunar biconcave fibrocartilaginous disk that resides within the medial and lateral tibiofemoral articulations (i.e. interposed between the femoral condyles and tibial plateaux) and contributes to tibiofemoral congruence, joint lubrication, stability and load distribution6–10. In each knee joint, there are two menisci: a medial and a lateral and both are attached to bone via insertional ligaments and provide protection to underlying articular cartilage. The menisci increase the contact area in the femorotibial joint and thus decrease the stress on the articular cartilage6, 11, 12. Dispersal of synovial fluid over and through the menisci decreases friction across articular surfaces and contributes to joint lubrication10. Normal joint movement causes compression of the menisci and the resulting extrusion of synovial fluid bathes the articular cartilage to provide nutrition13.
A meniscus tear may occur as a consequence of trauma, twisting of the knee, deceleration and landing from a jump or getting up from a squatting or crouching position14, 15. Younger patients are more likely to have acute, traumatic meniscal tears whereas older patients are more likely to have degenerative tears16. It is believed that meniscal tears are associated with osteoarthritis development8, 17–21, but it is unclear how degenerative changes in the menisci affect cartilage homeostasis22–25.
A tear is usually treated by arthroscopic repair or partial meniscectomy. While meniscal repair is preferred when possible26, partial meniscectomy is the most commonly performed procedure 16 and is currently preferred over total meniscectomy27, 28, particularly since total meniscectomy has been associated with more cartilage degeneration and osteoarthritis than partial meniscectomy29, 30.
Several factors contribute to the initiation and progression of primary knee osteoarthritis including aging and obesity31–33. Aging undoubtedly contributes to osteoarthritis development through cartilage degeneration 34 but the explicit mechanisms for this contribution are not fully understood. Aging may contribute to the development of osteoarthritis through age-related changes at cellular and tissue levels making the joint more susceptible to damage with a decreased ability to maintain homeostasis5. Similarly, obesity, although a modifiable risk factor, represents another predictor of osteoarthritis35. Although the association between obesity and osteoarthritis is complex, multiple biochemical mediators (cytokines, chemokines and adipokines) that crosstalk with obesity and osteoarthritis are reported to contribute to this relationship36–38.
Our previous findings demonstrated that the expression of osteoarthritis-related genes in a torn meniscus decreases with age without sex-based differences39. In the literature, several studies have identified significant sexual dimorphism in the development and progression of knee osteoarthritis40, 41. However, there is limited information available on sex-based differences in gene expression in meniscus other than our recent finding of no substantial differences between women and men in the expression of osteoarthritis-related genes.
Little is known how gene expression signatures of a torn meniscus relate to osteoarthritis in the context of aging and obesity. Therefore, in this study we aim to gain insight into the interaction of osteoarthritis- and obesity-related gene signatures in the meniscus. Here, we test the hypothesis that gene expression patterns in menisci correlate with the age and body mass index (BMI) of patients. These findings have the potential to shed further light on how meniscal injury, age and obesity relate to the initiation and progression of osteoarthritis in the knee.
MATERIALS AND METHODS
Ethics statements
All procedures were approved by the Washington University School of Medicine human subjects institutional review board. Informed consent was obtained about the use of tissues for research purpose as approved by Human Research Protection Office.
Patients and clinical samples
A total of 68 meniscal samples were collected at the Department of Orthopaedic Surgery (Barnes-Jewish Hospital, Chesterfield, MO), between June 2010 and January 2012, at the time of arthroscopic partial meniscectomy for a symptomatic tear in their meniscus. Demographic data for age, sex, height and body weight was recorded and BMI was calculated (Table-1). None of the patients had advanced osteoarthritis or any ligament injury at the time of the meniscal surgery. Eligible patients indicated for partial meniscectomy voluntarily consented to participate in this study. Appropriate resection of non-repairable meniscus tears was performed and samples of the torn tissue were removed from the knee and transported to the laboratory in normal saline. We recorded whether the sample came from the inner half of the meniscus, the outer half of the meniscus or both.
Table-1.
Demographic information of patient cohort
| Female | Male | Overall | |
|---|---|---|---|
| N | 20 | 48 | 68 |
| Mean Age (years) | 41.85 | 46.70 | 45.28 |
| Range (years) | 14–67 | 15–72 | 14–72 |
| Mean Height±S.D. (in) | 64.2±1.64 | 70.7±3.41 | 68.8±4.23 |
| Mean Weight±S.D. (lb) | 160.85±31.96 | 198.88±42.99 | 187.69±43.48 |
| Mean BMI (kg/m2)±S.D. | 27.4±5.36 | 27.8±5.11 | 27.7±5.17 |
N = sample size; in = inches, lb = pounds; S.D = standard deviation
Tissue processing
The tissues, upon arrival in the lab, were washed with phosphate-buffered saline (PBS; HyClone; Thermo Fisher Scientific, Rockford, IL) and weighed. TRIzol reagent (Invitrogen, Carlsbad, CA) was added to each specimen (1mL/50–100 mg), which were then stored at −80°C prior to total RNA extraction.
Total RNA isolation
The thawed tissues were homogenized with use of a Polytron homogenizer (Brinkmann Instruments, Westbury, NY). Total RNA was isolated using a combination of phenol chloroform method and RNeasy mini spin columns (Qiagen Inc., Valencia, CA). Briefly, aliquots of the homogenized suspension were transferred to microfuge tubes and incubated at room-temperature for 5 min. Chloroform (200 µl) was added to each tube, vortexed vigorously, incubated for 5 min and centrifuged at 12000g for 15 min at 4°C. The upper aqueous phase, that contained RNA, was transferred to a clean tube and an equal volume of 70% RNase-free ethanol was added and the contents were mixed. The aqueous phase/ethanol mixture was applied to spin columns and spun for 15 sec at 8000 rpm at room-temperature. After decanting the flow-through, the column was washed twice with RW1 and RPE buffers. Finally, RNA was eluted in 30 µl of RNase-free water, quantified by a NanoDrop (Thermo Fisher Scientific, Pittsburg, PA) and stored at −80°C until reverse transcribed for complementary DNA (cDNA).
First strand cDNA synthesis
Total RNA was first subjected to DNase I (Life Technologies, Grand Island, NY) digestion to avoid contamination from genomic DNA. In 0.2-mL tubes, 150–300 ng of RNA, 2 units of amplification grade DNase I (1 U/µl), and 2 µl of 10X DNase I reaction buffer were added to a total 20-µl volume in RNase-free water and incubated at room-temperature for 15 min. DNase I was inactivated with 2 µl of 25 mM EDTA at 65°C for 10 min.
First strand cDNA synthesis was carried out by use of random hexamers and the SuperScript II First-Strand Synthesis System (Invitrogen, Carlsbad CA). In a 40-µl reaction volume, 2 µl of the random primer (50 ng/µl) and 2 µl of dNTP mix (10 mM) were added to DNase-I-treated RNA and incubated at 65°C for 5 min. The reaction mixture was chilled on ice and reverse-transcription was continued by adding 4 µl of 0.1M DTT (dithiothreitol), 400 units of SuperScript II (200 units/µl) and 5X first-strand buffer in the reaction mixture. Finally, the tube was incubated at 42°C for 50 min followed by deactivation at 70°C for 15 min.
Selection of candidate genes and oligonucleotide information
Several genes representative if pathways involved in osteoarthritis pathophysiology were selected including proinflammatory cytokines (IL-1α, IL-1β, IL-6, TNFα), chemokines (IL-8, CCL3, CCL3L1, CCL20, CXCL1, CXCL3, CXCL6), matrix degrading enzymes [aggrecanases (ADAMTS-4, ADAMTS-5), matrix metalloproteinases (MMP-1, MMP-3, MMP-9, MMP-13)], matrix genes (COL1A1, COL2A1, ACAN, BMP-2) and transcription factors (NFκBIA, NFκB2, IκBA). The sequence information for these primers has been previously published39. We also studied selected markers for obesity including adiponectin (ADIPOQ), apelin (APLN), leptin (LEP) 42 and resistin (RETN)43 (see respective references for primer sequences). All primers were obtained from Invitrogen, Carlsbad, CA.
Quantification of gene expression levels
Quantitative real-time PCR (qPCR) was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) on cDNA samples to assess expression levels of the genes listed above. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) acted as an endogenous reference gene for normalization of fluorescence threshold (Ct) values of target genes. In a 20-µl reaction volume, 10 µl of SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA), 1.5 µl of cDNA, and 200 nM of primers were added. Samples were amplified with an initial activation step at 95°C for 10 min, followed by forty cycles of denaturation at 95°C for 15 sec and annealing at 60°C for 60 sec. The Ct values for house-keeping gene and the genes of interest were measured and normalized to GAPDH for each sample (ΔCt). Rather than employing the traditional ΔΔCt method to identify increased or decreased expression of samples relative to a single reference sample and then comparing candidate genes to the multiple control genes, a regression strategy was used to correct all samples for interfering factors at once. This strategy eliminates amplification of error that would be created by introducing ratios when comparing candidate gene expression to control gene expression. Ratios of raw Ct values for the candidate gene relative to the Ct value for GAPDH removes the effect of total cellular material from inter-sample comparisons only if the slope of the regression between the scores is 1.0; otherwise, the ratio remains correlated with its denominator.
Inter-relations of gene expression levels
In order to determine the associations among gene expression levels, the values were subjected to a hierarchical cluster analysis using Ward Minimum Variance Method to produce a dendrogram. A manual heat map was then generated to display the pairwise correlations of candidate genes across all gene categories. To achieve this, a color code was assigned to the correlation strengths among various genes and finally the heat map was aligned with the hierarchical cluster dendrogram.
Statistical Analysis
All statistical analyses were performed using Systat-12 software (Systat, Chicago, IL.). Since the distribution of the gene expression values deviated from a normal distribution and contained several extreme values that would dominate any ordinary least squares linear regression analysis, Spearman's rank-order correlation was used for examining the associations between BMI and age and gene expression values on an ordinal scale.
The correlation between BMI and age is significantly positive, so we used rank-order partial correlation analysis to test for correlations between gene expression and BMI independent of age and between gene expression and age independent of BMI. The first-order partial correlation coefficient is given by
where X refers to the gene expression trait, Y to the factor of interest (either BMI or age), and Z to the factor controlled for in the analysis (either BMI or age).
Probability values for the Spearman’s partial correlations were computed by treating
as coming from a t-distribution with (n−k−2) degrees of freedom, where r is the partial correlation and k is the number of variables being partialled out.
RESULTS
Characteristics of the study population
The demographic information of the study population, from which meniscal samples were obtained, is presented in Table-1. The tissues were collected from 68 patients (20 females and 48 males) at the time of arthroscopic surgery. The mean age of patients was 45.3 years with a range of 14–72 years with BMI 27.70±5.17 kg/m2 (mean ± S.D.).
Gene expression is not correlated with sex
A one-way analysis of variance was first used to determine sex-based differences between 20 female and 48 male patients. The analysis showed no sexual dimorphism in gene expression (data not shown), therefore our results for age and BMI are based on combined data set (N=68) from both female and male subjects. The samples came predominantly from the inner rim (N=46) while only one sample came from the outer meniscus in isolation and the rest included a combination of the inner and outer zones of the meniscus. This distribution precluded any analysis based on zonal differences in gene expression.
Gene expression is negatively correlated with age
The present study confirms our previous 39 that ostearthritis- and obesity-related gene expression is significantly negatively correlated with age. The results from each gene category detailed below are depicted in Fig. 2 and Supplementary Table-1.
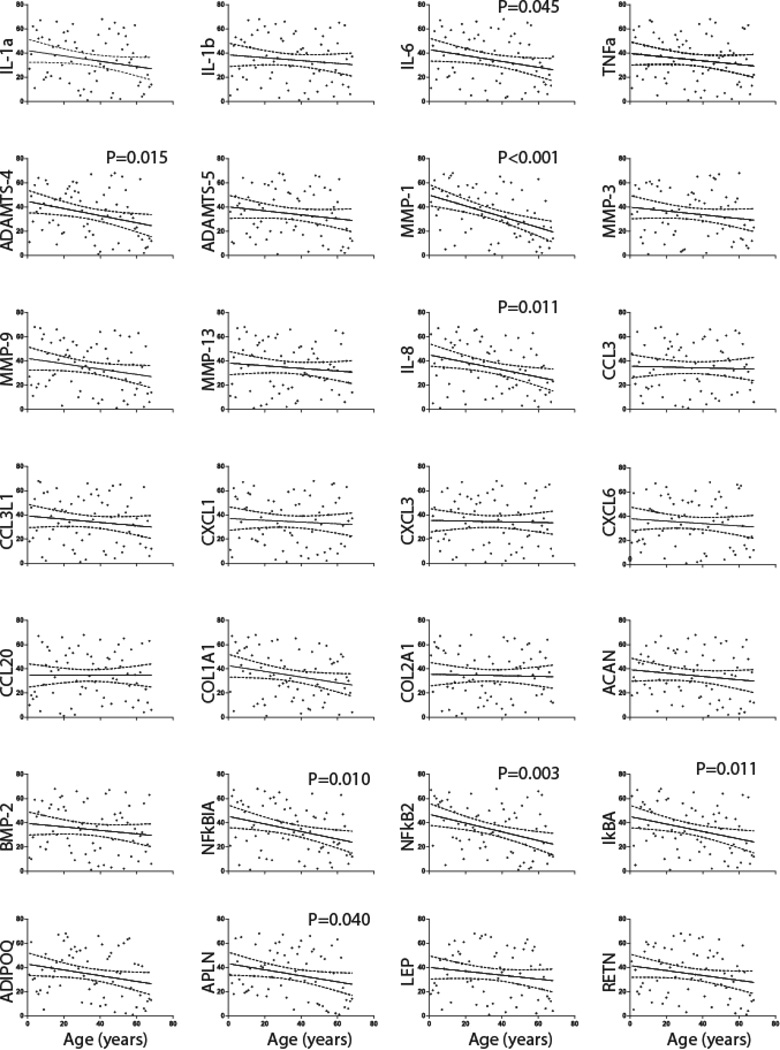
Fig. 2. Correlation of gene expression of candidate genes with age through qPCR studies in patients (N=68) from which meniscal tissues were resected.
The gene expression for each target gene was normalized to GAPDH. Spearman’s rank-order correlation for best fitted curve (solid line) with 95% confidence interval (dotted lines) from the curve is shown for all subjects (filled circles). The expression of MMP-1, NFκB2, NFκBIA, IκBA, IL-8, ADAMTS-4, APLN and IL-6 was significantly negatively correlated with age.
Proinflammatory cytokines
Although, the mRNA expression of various proinflammatory cytokines including IL-1α (r=−0.219), IL-1β (r=−0.119), IL-6 (r=−0.243) and TNFα (r=−0.151) were negatively correlated with age, only IL-6 expression was statistically significant (r=-0.244; P=0.045).
Chemokines
Chemokine expression levels are negatively correlated with age but the only statistically significant negative correlation was found to be between age and IL-8 expression (r=-0.31; P=0.011).
Matrix degrading enzymes
Both ADAMTS-4 (r=−0.294) and MMP-1 (r=−0.447) were strongly significantly negatively associated with age (P<0.05) while ADAMTS-5 and other MMPs levels were negatively correlated with age without being statistically significant.
Transcription factors
NFκB2 (r=−0.361), NFκBIA (r=−0.312) and IκBA (r=−0.308) were all significantly negatively correlated with age (P<0.05).
Cartilage/meniscus matrix genes
The quantification of extracellular matrix genes showed that COL1A1 (r=−0.234), COL2A1 (r=−0.030), ACAN (r=−0.139) and BMP-2 (r=−0.144) were negatively correlated with age at a sub-significant level. COL1A1 had a borderline significant level of correlation (P=0.054).
Obesity-related genes
The quantification of genes related to obesity showed that their expression was also negatively correlated with age. Gene expression for APLN (r=−0.250) was significantly negatively correlated with age (P=0.040) while that of ADIPOQ (r=−0.250) was at a borderline significance level (P=0.052) and LEP (r=−0.160) and RETN (r=−0.202) showed no statistical significance.
Gene expression is negatively associated with BMI
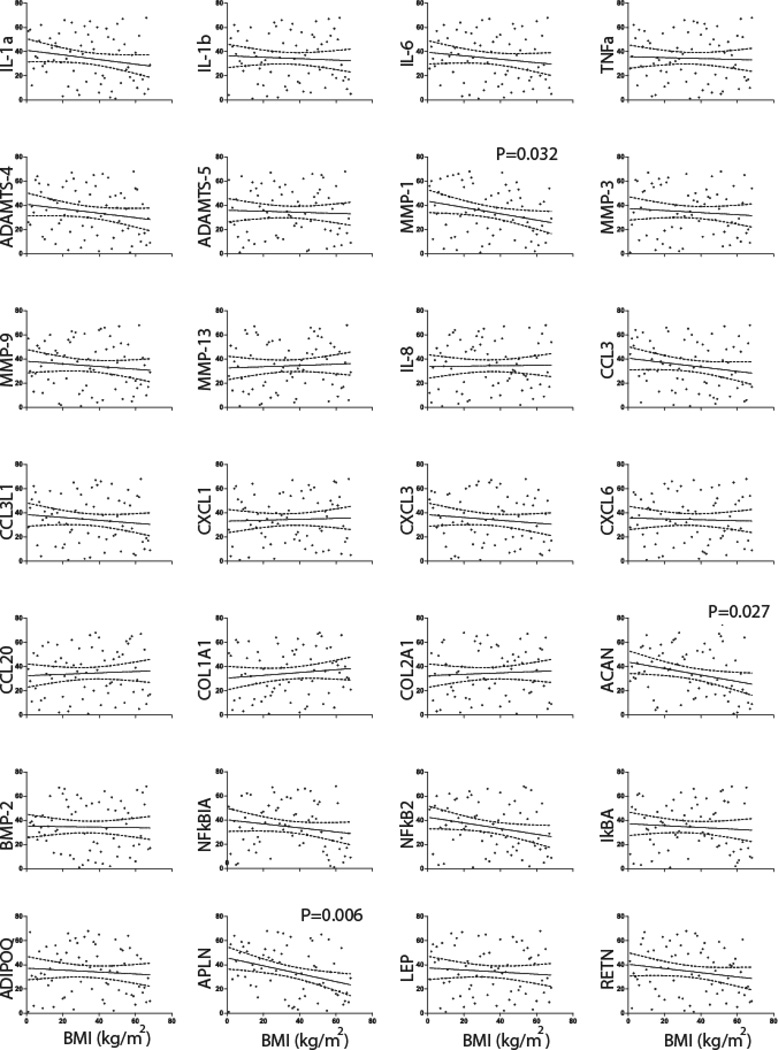
We found that a majority of the selected genes were also negatively correlated with BMI (Figure 3, Supplementary Table 2). There were no significant correlations for proinflammatory cytokines, chemokines, or aggrecanases. The expression of all MMPs except for MMP-13 was also found to be negatively correlated with BMI with only MMP-1 (r=−0.261) reaching statistical significant (P=0.032). For transcription factors, only NFκB2 (r=−0.183) was of borderline significance (P=0.053). The expression of ACAN (r=−0.267) was negatively correlated with BMI (P=0.027). Among obesity related genes studied here only APLN (r=−0.328) was found to be significantly negatively correlated (P=0.006) with BMI.
Fig. 3. Correlation of gene expression of candidate genes with BMI through qPCR studies in patients (N=68) from which meniscal tissues were resected.
The gene expression for each target gene was normalized to GAPDH. Spearman’s rank-order correlation for best fitted curve (solid line) with 95% confidence interval (dotted lines) from the curve is shown for all subjects (filled circles). The expression of MMP-1, ACAN and APLN and IL-6 was significantly negatively correlated with BMI.
Age and BMI were significantly positively correlated (r=0.310; P=0.008) in this cohort. This correlation indicated that majority of the older patients were obese (Supplementary Fig.1) and vice versa.
Age has more impact on gene expression pattern than does BMI
Rank-order partial correlation of gene expression level with age, controlling for BMI, was often significant and negative (Table 2). Among these are ADAMTS-4 (r=−0.253; P=0.038), MMP-1 (r=−0.399; P=0.0008), IL-8 (r=−0.326; P=0.007), COL1A1 (r=−0.287; P=0.018), NFκBIA (r=−0.278; P=0.023), NFκB2 (r=−0.312; P=0.01), IκBA (r=−0.299; P=0.014) and ADIPOQ (r=−0.224; P = 0.068) at borderline significance. However, when BMI is considered independently from age, only APLN (r=−0.224) remained significantly negatively correlated with BMI (P=0.027). Other gene expression levels that were originally negatively correlated with BMI are no longer significant after controlling for age. These results indicate that age effects on gene expression in the meniscus are stronger than the effects of BMI on gene expression. Most of the apparent negative correlation between gene expression levels and BMI can be accounted for by age differences.
Table 2.
Spearman’s Rank-order Partial Correlation
| Gene | t(Exp-Age) | P | t(Exp-BMI) | P | Exp-BMI(age)# | t | P | Exp-Age(BMI)$ | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| IL-1α | −1.823 | 0.073 | −1.585 | 0.118 | −0.133 | −1.084 | 0.282 | −0.171 | −1.400 | 0.166 |
| IL-1β | −0.965 | 0.338 | −0.456 | 0.650 | −0.021 | −0.166 | 0.869 | −0.106 | −0.860 | 0.393 |
| IL-6 | −2.035 | 0.046 | −1.158 | 0.251 | −0.071 | 0.577 | 0.566 | −0.212 | −1.746 | 0.085 |
| TNFα | −1.239 | 0.220 | −0.318 | 0.751 | 0.008 | 0.065 | 0.948 | −0.146 | −1.190 | 0.239 |
| ADAMST-4 | −2.495 | 0.015 | −1.510 | 0.136 | −0.101 | −0.818 | 0.416 | −0.253 | −2.113 | 0.038 |
| ADAMTS-5 | −1.348 | 0.182 | −0.335 | 0.739 | 0.010 | 0.082 | 0.935 | −0.159 | −1.298 | 0.199 |
| BMP-2 | −1.186 | 0.240 | −0.174 | 0.862 | 0.025 | 0.200 | 0.842 | −0.145 | −1.181 | 0.242 |
| MMP-1 | −4.060 | <0.001 | −2.196 | 0.032 | −0.144 | −1.172 | 0.245 | −0.399 | −3.507 | 0.001 |
| MMP-3 | −1.294 | 0.200 | −0.702 | 0.485 | −0.040 | −0.320 | 0.750 | −0.138 | −1.123 | 0.266 |
| MMP-9 | −1.843 | 0.070 | −0.899 | 0.372 | −0.045 | −0.361 | 0.720 | −0.198 | −1.629 | 0.108 |
| MMP-13 | −0.899 | 0.372 | 0.447 | 0.656 | 0.094 | 0.763 | 0.448 | −0.134 | −1.089 | 0.280 |
| IL-8 | −2.603 | 0.011 | 0.139 | 0.890 | 0.123 | 1.003 | 0.320 | −0.327 | −2.785 | 0.007 |
| CCL3 | −0.276 | 0.783 | −1.482 | 0.143 | −0.178 | −1.457 | 0.150 | 0.023 | 0.187 | 0.853 |
| CCL3L1 | −1.105 | 0.273 | −0.970 | 0.335 | −0.082 | −0.659 | 0.512 | −0.104 | −0.842 | 0.403 |
| CXCL1 | −0.598 | 0.552 | 0.319 | 0.750 | 0.065 | 0.529 | 0.599 | −0.090 | −0.729 | 0.469 |
| CXCL3 | −0.234 | 0.816 | −0.947 | 0.347 | −0.112 | −0.912 | 0.365 | 0.007 | 0.060 | 0.952 |
| CXCL6 | −0.805 | 0.424 | −0.292 | 0.771 | −0.006 | −0.046 | 0.964 | −0.092 | −0.745 | 0.459 |
| CCL20 | −0.004 | 0.997 | 0.475 | 0.637 | 0.061 | 0.497 | 0.621 | −0.020 | −0.157 | 0.875 |
| COL1A1 | −1.959 | 0.054 | 0.963 | 0.339 | 0.206 | 1.697 | 0.095 | −0.287 | −2.415 | 0.019 |
| COL2A1 | −0.244 | 0.808 | 0.461 | 0.646 | 0.069 | 0.561 | 0.577 | −0.050 | −0.404 | 0.687 |
| ACAN | −1.140 | 0.258 | −2.266 | 0.027 | −0.240 | −1.990 | 0.051 | −0.061 | −0.491 | 0.625 |
| NFκB1A | −2.668 | 0.010 | −1.377 | 0.173 | −0.078 | −0.631 | 0.530 | −0.278 | −2.329 | 0.023 |
| NFκB2 | −3.145 | 0.002 | −1.968 | 0.053 | −0.139 | −1.134 | 0.261 | −0.312 | −2.645 | 0.010 |
| IκBA | −2.628 | 0.011 | −0.633 | 0.529 | 0.020 | 0.158 | 0.875 | −0.299 | −2.529 | 0.014 |
| ADIPOQ | −1.983 | 0.052 | −0.656 | 0.514 | −0.008 | -0.061 | 0.952 | −0.224 | −1.852 | 0.069 |
| APLN | −2.098 | 0.040 | −2.816 | 0.006 | −0.272 | −2.276 | 0.026 | −0.165 | −1.351 | 0.181 |
| LEP | −1.313 | 0.194 | −0.701 | 0.486 | −0.039 | −0.314 | 0.755 | −0.140 | −1.143 | 0.257 |
| RETN | −1.677 | 0.098 | −1.424 | 0.159 | −0.118 | −0.959 | 0.341 | −0.159 | −1.297 | 0.199 |
= BMI effect independent of age effect;
= Age effect independent of BMI effect
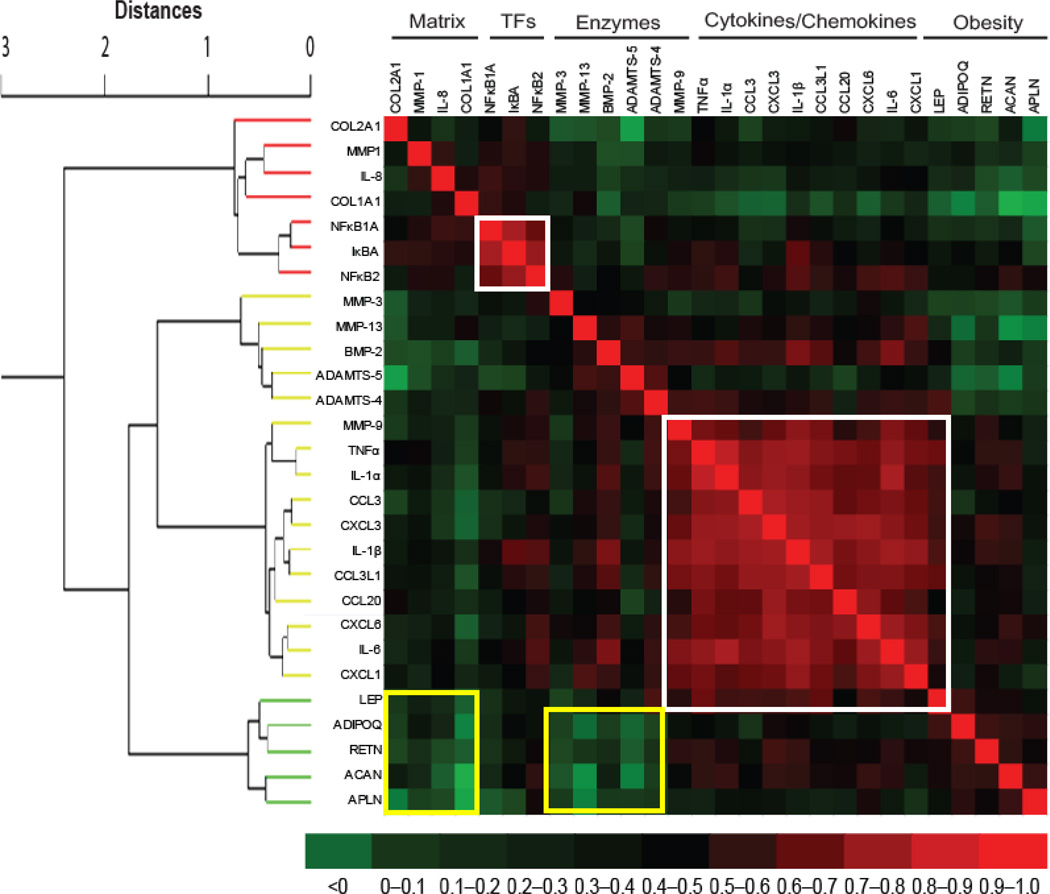
Genes of similar categories cluster together
Gene expression levels were correlated pairwise for all candidate genes to identify potentially common regulatory mechanisms based on concordant or discordant expression patterns (Fig. 4). We observed strong inter-gene correlations among a majority of cytokine/chemokine genes. There was also a strong inter-correlation among various transcription factors involved in most if not all cytokine/chemokine pathways in osteoarthritis pathophysiology. In addition, weak inter-gene correlations were found between matrix genes (collagens) and matrix degrading enzymes (metalloproteinases and aggrecanases).
Fig. 4. Inter-relations of gene expression levels.
The gene expression values were subjected to separate different categories for their inter-relationship. For this, hierarchical cluster dendrogram was generated using Ward Minimum Variance Method and it comprised of 28 modules with four categories. The Correlations were converted to distances between 0 and 2, where d = (1 – r).The adjacent modules are related closely in terms of expression profile and similarity in correlation fades away with the increase in distance. The heat map was then manually generated from correlation matrix to see the pairwise correlations of candidate genes. In the heat map, the dark red color shows a strong positive expression correlation while dark green color depicts weak expression correlation while the rest color codes fall in between. The two gene categories were strongly correlated (red areas surrounded by white squares) while two gene categories were weakly correlated (green areas surrounded by yellow squares). TFs = transcription factors.
DISCUSSION
In this study, we sought to determine the gene expression signatures in torn menisci (obtained from patients undergoing arthroscopic partial meniscectomy) and to study their correlation with patients’ age and BMI. Gene expression was generally negatively correlated with both age and BMI. Although most of the apparent negative correlation between gene expression levels and BMI can be accounted for by age differences, this is the first study we are aware of to identify differences in gene expression in the injured meniscus which relate to patient BMI.
The published evidence suggests a relationship between obesity and osteoarthritis36–38. Dysregulation of lipid homeostasis is one of the mechanisms potentially leading to osteoarthritis development 37. Among the twenty-eight genes analyzed in this study, six genes (IL-8, MMP-13, CXCL1, CCL20, COL1A1 and COL2A1) had an insignificant positive correlation with BMI while expression of the remaining twenty-two genes was negatively correlated with BMI, with only three genes (APLN, ACAN and MMP-1) significantly so. Only the expression of APLN was exclusively due to obesity status independent of age. As stated above, APLN is more closely related to obesity than osteoarthritis, but its precise role remains uncertain44. However, it is reported that APLN expression is regulated by insulin45 and might be involved in regulation of glucose homeostasis and obesity46. Since APLN is known to play a catabolic role in articular cartilage44 and bone47 metabolisms, APLN may have a catabolic role in meniscal homeostasis, particularly in obese patients, and may constitute a risk factor for the development of osteoarthritis. The expression of APLN in the injured menisci of normal weight individuals is presumably appropriate, although this cannot be established without comparison to uninjured menisci.
What is less clear is how APLN interacts with meniscal and other joint tissue, and how the magnitude of APLN expression in the torn meniscus relates to joint health. Perhaps paradoxically, the torn menisci of obese patients express less APLN than torn menisci of patients with a lower BMI. At least one study has shown worse long term clinical outcomes for patients with elevated BMI after partial lateral meniscectomy in otherwise normal knees48. The decreased expression of APLN in obese patients suggests a biological pathway by which obesity affects the knee joint and may influence the risk of developing osteoarthritis. Future investigation should look at how the expression of APLN affects meniscal and other joint tissue. Another important question is whether expression of APLN is plastic and could be modified by future weight loss (or gain).
Aging is known to affect the basal-level gene expression pattern in joint tissues including the meniscus49. We have also reported that gene expression in meniscus tears varies by age39, perhaps because of lower cellularity in torn meniscus in patients younger than forty years50. Here, we validate our results with increased sample size and with more statistical power to show that for most genes, expression levels are negatively associated with a patient’s age. A moderate significant negative correlation of MMP-1, NFκB2, NFκBIA, IκBA, IL-8, ADAMTS-4, IL-6 and APLN (apelin) expression with age was observed. Several of these genes were previously found to show less expression in older patients. Except for APLN, which is an obesity-related gene, all other genes are involved in osteoarthritis pathogenesis and their expression pattern is an important consideration in the molecular characterization of osteoarthritis51–53. The precise role of APLN in osteoarthritis is not well-studied, but it is suggested that it may be involved in cartilage metabolism and constitutes a risk factor in the pathophysiology of osteoarthritis44. In parallel, although not statistically significant, the expression of other genes (Fig. 2; Supplementary Table-1) was also negatively correlated with age. It was interesting to note that none of the osteoarthritis- or obesity-related genes were significantly positively correlated with age. Taken together, these findings demonstrate that an elevated intrinsic response exists in torn menisci from younger individuals compared to older individuals. These results also support our previous findings where it was reported that osteoarthritis-related proinflammatory and catabolic genes are downregulated in older patients39. In addition, it has been shown that there is a greater prevalence of traumatic tears in younger patients18 that are associated with an increased risk for initiation and perpetuation of osteoarthritis8, 17–19. Thus, our findings provide a molecular rationale for the increased risk of developing osteoarthritis in young individuals with a meniscus tear.
The gene expression pattern as it relates to aging and obesity has clinical relevance in several ways. For example, negative correlation of gene expression with age may relate to the reduced long-term clinical outcomes seen in older patients after meniscal repair54. Although short-term clinical success rates are good in older individuals55, these patients have reportedly lower rates of meniscal healing and higher long-term failure rates post-repair. As a lack of viable cells in the meniscus is associated with degeneration and repeat tearing56, one plausible physiologic reason for higher late failure rates in older patients may be the decreased intrinsic and perimeniscal cellularity found in the torn menisci of this patient group50. Differences in gene expression and intrinsic inflammation may contribute to this difference in healing potential as well. The fact that some of the genes are negatively correlated with obesity independent of age (Table-2) indicates that factors other than obesity and age likely play a role in differential gene expression patterns. Among these factors, physical activity level could be the most significant because it has been reported that a high percentage of obese individuals with knee osteoarthritis are sedentary suggesting that a lack of physical activity may increase the susceptibility to cellular inflammation57, 58.
Osteoarthritis is a sexually dimorphic trait more common among females than males40, 41, thus giving rise to the concept that sex is a contributing factor for osteoarthritis development. This might lead one to expect higher expression of osteoarthritis-related genes in women than men following a meniscal tear. Our previous findings provided weak evidence in support of this hypothesis where we detected that only CCL3L1 was significantly upregulated in women compared to men39. In the present study, however, we failed to appreciate any significant difference in the gene expression profile between the two sexes even with greater sample size and more statistical power. It is unclear what the clinical implications of these findings may be in this context but these differences are unlikely to contribute to the greater prevalence of knee osteoarthritis in women.
The co-expression analysis and its ability to define distinct physiological sets of genes are portrayed by hierarchical cluster dendrogram and heat map (Fig. 4). The genes with similar physiological role are clustered together with varying degree of correlation strengths. These findings implicate that common regulatory mechanism for genes or group of genes may exist. For instance, we found that cytokine and chemokines were strongly inter-correlated similar to transcription factors. In contrast, obesity-related genes were weakly correlated with matrix genes and matrix-degrading enzymes.
One limitation of this study is the inability to test for differences in gene expression between the inner and outer zones of the meniscus. The meniscus contains several different cell types that exhibit different gene expression patterns and are highly localized to the inner vs outer regions59, 60. While the majority of our samples are from the inner meniscus, potential regional variation could influence our results.
Another major limitation of this study is the lack of data on gene expression in normal menisci and its potential relationship to age and BMI. While it would be interesting to have data on expression from normal meniscal tissue, such information is not essential for a couple of reasons. First and foremost, this study focuses on the injured meniscus. The intention is not to compare the injured meniscus to the uninjured meniscus but rather to identify BMI and age related differences in gene expression of injured menisci. Patients with meniscus tears are the population of interest and the impact of BMI and age on gene expression is potentially very clinically relevant in terms of the prognosis for these patients. Given the heightened risk of developing osteoarthritis in patients with meniscus tears, this information may help stratify this risk independently of the potential relationship of gene expression in torn menisci to gene expression in normal menisci. Secondly, age and BMI may have similar or dissimilar effects on gene expression in the injured meniscus compared to the uninjured and that question is not the focus of our present investigation. Since sampling normal meniscal tissue is not entirely benign, we do not consider it an ethical approach in patients with intact menisci. Considering that measuring gene expression in normal meniscal tissue is not paramount or practical, the relationship, if any, between gene expression of normal meniscus tissue and torn meniscus tissue is interesting but not integral to the current investigation.
Conclusion and future directions
Since aging and obesity both contribute to development of knee osteoarthritis, we predicted that the expression pattern of genes of interest will help understand tissue-specific responses to age and obesity. We found that patients’ age and BMI were negatively correlated with the expression of several osteoarthritis- and obesity-related genes in the meniscus. While a bulk of these changes appear to be driven by age, expression of APLN in the meniscus is related to BMI. Further investigation into the role of APLN in meniscus and joint health is warranted. These findings also suggest that age-related changes are more relevant than obesity-related differences for the role of the meniscus in the pathogenesis of osteoarthritis. The potential effect of other factors such as physical activity, ethnicity, smoking, diabetes and inflammation on meniscal gene expression should also be investigated.
Supplementary Material
Spearman’s rank-order correlation was calculated to plot the relationship between age and BMI. Solid line show the best fitted correlation curve, while dotted lines show 95% confidence interval based on the curve. In this study, age and BMI (obesity) were found to be strongly positively correlated.
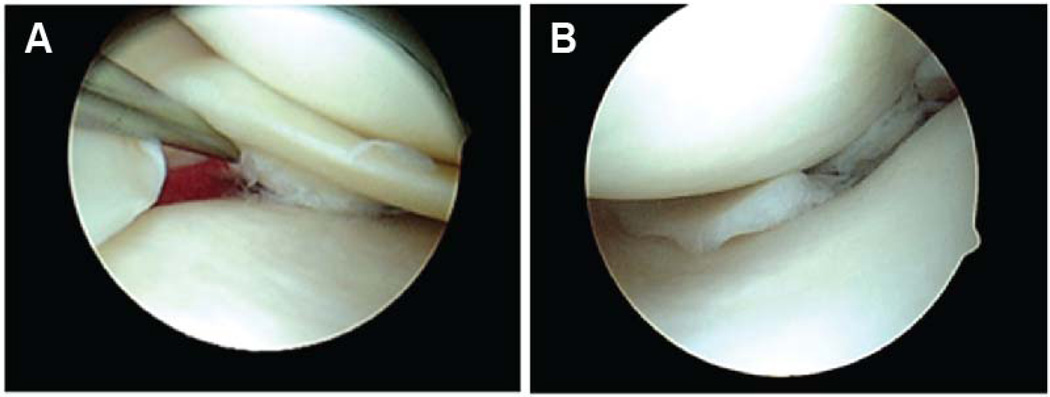
Fig. 1. Arthroscopy for partial meniscectomy.
Representative arthroscopic images of a traumatic meniscal tear (A) that was removed with partial meniscectomy. The rim of healthy meniscal tissue was preserved after removal of the damaged (torn) portion (B).
Acknowledgments
FUNDING SOURCE
Funding for this study was provided by an Orthopaedic Research and Education Foundation (OREF) grant (R.H.B.), National Institute of Arthritis and Musculoskeletal and Skin Diseases grant RO1-AR036994 (L.J.S.) and Musculoskeletal Research Center, grant P30-AR057235. M.F.R. is supported by Ruth L. Kirschstein National Research Service Award Fellowship (T32-AR060719) from National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis, Musculoskeletal and Skin Diseases or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
None
SUPPLEMENTARY INFORMATION
Supplementary information is available at IJO’s website.
REFERENCES
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: the relationship with articular, kinesiological, and psychological characteristics. J Rheumatol. 1998;25(1):125–133. [PubMed] [Google Scholar]
- 3.Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65(10):1261–1264. doi: 10.1136/ard.2006.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26(3):371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Masouros SD, McDermott ID, Amis AA, Bull AM. Biomechanics of the meniscus-meniscal ligament construct of the knee. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1121–1132. doi: 10.1007/s00167-008-0616-9. [DOI] [PubMed] [Google Scholar]
- 8.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD. Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res. 2009;27(9):1197–1203. doi: 10.1002/jor.20869. [DOI] [PubMed] [Google Scholar]
- 10.Macconaill MA. The Function of Intra-Articular Fibrocartilages, with Special Reference to the Knee and Inferior Radio-Ulnar Joints. J Anat. 1932;66(Pt 2):210–227. [PMC free article] [PubMed] [Google Scholar]
- 11.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161–178. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renstrom P, Johnson RJ. Anatomy and biomechanics of the menisci. Clinics in sports medicine. 1990;9(3):523–538. [PubMed] [Google Scholar]
- 13.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 14.Lim SY, Peh WC. Magnetic resonance imaging of sports injuries of the knee. Annals of the Academy of Medicine, Singapore. 2008;37(4):354–361. [PubMed] [Google Scholar]
- 15.Hayes CW, Coggins CA. Sports-related injuries of the knee: an approach to MRI interpretation. Clinics in sports medicine. 2006;25(4):659–679. doi: 10.1016/j.csm.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 16.McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88(12):1549–1556. doi: 10.1302/0301-620X.88B12.18140. [DOI] [PubMed] [Google Scholar]
- 17.Englund M. The role of the meniscus in osteoarthritis genesis. Rheum Dis Clin North Am. 2008;34(3):573–579. doi: 10.1016/j.rdc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiologic clinics of North America. 2009;47(4):703–712. doi: 10.1016/j.rcl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Englund M. Meniscal tear--a feature of osteoarthritis. Acta orthopaedica Scandinavica. Supplementum. 2004;75(312):1–45. backcover. [PubMed] [Google Scholar]
- 20.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34(4):776–784. [PubMed] [Google Scholar]
- 21.Zeichen J, Hankemeier S, Knobloch K, Jagodzinski M. [Arthroscopic partial meniscectomy] Oper Orthop Traumatol. 2006;18(5–6):380–392. doi: 10.1007/s00064-006-1184-0. [DOI] [PubMed] [Google Scholar]
- 22.Noble J, Hamblen DL. The pathology of the degenerate meniscus lesion. J Bone Joint Surg Br. 1975;57(2):180–186. [PubMed] [Google Scholar]
- 23.Casscells SW. The torn or degenerated meniscus and its relationship to degeneration of the weight-bearing areas of the femur and tibia. Clin Orthop Relat Res. 1978;132:196–200. [PubMed] [Google Scholar]
- 24.Lewandrowski KU, Muller J, Schollmeier G. Concomitant meniscal and articular cartilage lesions in the femorotibial joint. Am J Sports Med. 1997;25(4):486–494. doi: 10.1177/036354659702500411. [DOI] [PubMed] [Google Scholar]
- 25.Bennett LD, Buckland-Wright JC. Meniscal and articular cartilage changes in knee osteoarthritis: a cross-sectional double-contrast macroradiographic study. Rheumatology (Oxford) 2002;41(8):917–923. doi: 10.1093/rheumatology/41.8.917. [DOI] [PubMed] [Google Scholar]
- 26.Brophy RH, Matava MJ. Surgical options for meniscal replacement. J Am Acad Orthop Surg. 2012;20(5):265–272. doi: 10.5435/JAAOS-20-05-265. [DOI] [PubMed] [Google Scholar]
- 27.Howell JR, Handoll HH. Surgical treatment for meniscal injuries of the knee in adults. Cochrane Database Syst Rev. 2000;(2):CD001353. doi: 10.1002/14651858.CD001353. [DOI] [PubMed] [Google Scholar]
- 28.Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27(9):1275–1288. doi: 10.1016/j.arthro.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 29.Andersson-Molina H, Karlsson H, Rockborn P. Arthroscopic partial and total meniscectomy: A long-term follow-up study with matched controls. Arthroscopy. 2002;18(2):183–189. doi: 10.1053/jars.2002.30435. [DOI] [PubMed] [Google Scholar]
- 30.Hede A, Larsen E, Sandberg H. Partial versus total meniscectomy. A prospective, randomised study with long-term follow-up. J Bone Joint Surg Br. 1992;74(1):118–121. doi: 10.1302/0301-620X.74B1.1732238. [DOI] [PubMed] [Google Scholar]
- 31.Aigner T, Richter W. OA in 2011: Age-related OA--a concept emerging from infancy? Nat Rev Rheumatol. 2012;8(2):70–72. doi: 10.1038/nrrheum.2011.206. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Engstrom G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68(4):490–496. doi: 10.1136/ard.2008.089748. [DOI] [PubMed] [Google Scholar]
- 33.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop (Belle Mead NJ) 2008;37(3):148–151. [PubMed] [Google Scholar]
- 34.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012 doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–155. doi: 10.1016/j.jbspin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Critical reviews in eukaryotic gene expression. 2011;21(2):131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 37.Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65(11):1403–1405. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma L, Chang A. Overweight: advancing our understanding of its impact on the knee and the hip. Ann Rheum Dis. 2007;66(2):141–142. doi: 10.1136/ard.2006.059931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94(5):385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosner IA, Goldberg VM, Moskowitz RW. Estrogens and osteoarthritis. Clin Orthop Relat Res. 1986;(213):77–83. [PubMed] [Google Scholar]
- 41.Verbrugge LM. Women, men, and osteoarthritis. Arthritis Care Res. 1995;8(4):212–220. doi: 10.1002/art.1790080404. [DOI] [PubMed] [Google Scholar]
- 42.Geiger K, Muendlein A, Stark N, Saely CH, Wabitsch M, Fraunberger P, et al. Hypoxia induces apelin expression in human adipocytes. Horm Metab Res. 2011;43(6):380–385. doi: 10.1055/s-0031-1273767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nohira T, Nagao K, Kameyama K, Nakai H, Fukumine N, Okabe K, et al. Identification of an alternative splicing transcript for the resistin gene and distribution of its mRNA in human tissue. Eur J Endocrinol. 2004;151(1):151–154. doi: 10.1530/eje.0.1510151. [DOI] [PubMed] [Google Scholar]
- 44.Hu PF, Chen WP, Tang JL, Bao JP, Wu LD. Apelin plays a catabolic role on articular cartilage: in vivo and in vitro studies. Int J Mol Med. 2010;26(3):357–363. [PubMed] [Google Scholar]
- 45.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107(2):198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Rayalam S, Della-Fera MA, Krieg PA, Cox CM, Robins A, Baile CA. A putative role for apelin in the etiology of obesity. Biochem Biophys Res Commun. 2008;368(3):815–819. doi: 10.1016/j.bbrc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Guo LJ, et al. Apelin suppresses apoptosis of human osteoblasts. Apoptosis : an international journal on programmed cell death. 2007;12(1):247–254. doi: 10.1007/s10495-006-0489-7. [DOI] [PubMed] [Google Scholar]
- 48.Scheller G, Sobau C, Bulow JU. Arthroscopic partial lateral meniscectomy in an otherwise normal knee: Clinical, functional, and radiographic results of a long-term follow-up study. Arthroscopy. 2001;17(9):946–952. doi: 10.1053/jars.2001.28952. [DOI] [PubMed] [Google Scholar]
- 49.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705–717. doi: 10.1002/art.33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35(1):103–112. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, Jr, et al. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord. 2010;11:19. doi: 10.1186/1471-2474-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1ß. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellio Le Graverand MP, Vignon E, Otterness IG, Hart DA. Early changes in lapine menisci during osteoarthritis development: Part II: molecular alterations. Osteoarthritis Cartilage. 2001;9(1):65–72. doi: 10.1053/joca.2000.0351. [DOI] [PubMed] [Google Scholar]
- 54.Eggli S, Wegmuller H, Kosina J, Huckell C, Jakob RP. Long-term results of arthroscopic meniscal repair. An analysis of isolated tears. Am J Sports Med. 1995;23(6):715–720. doi: 10.1177/036354659502300614. [DOI] [PubMed] [Google Scholar]
- 55.Kotsovolos ES, Hantes ME, Mastrokalos DS, Lorbach O, Paessler HH. Results of all-inside meniscal repair with the FasT-Fix meniscal repair system. Arthroscopy. 2006;22(1):3–9. doi: 10.1016/j.arthro.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. Journal of athletic training. 2001;36(2):160–169. [PMC free article] [PubMed] [Google Scholar]
- 57.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiology of aging & age related diseases. 2012;2(2012) doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mork PJ, Holtermann A, Nilsen TI. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. J Epidemiol Community Health. 2012 doi: 10.1136/jech-2011-200834. [DOI] [PubMed] [Google Scholar]
- 59.Spindler KP, Miller RR, Andrish JT, McDevitt CA. Comparison of collagen synthesis in the peripheral and central region of the canine meniscus. Clin Orthop Relat Res. 1994;(303):256–263. [PubMed] [Google Scholar]
- 60.Fuller ES, Smith MM, Little CB, Melrose J. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthritis Cartilage. 2012;20(1):49–59. doi: 10.1016/j.joca.2011.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman’s rank-order correlation was calculated to plot the relationship between age and BMI. Solid line show the best fitted correlation curve, while dotted lines show 95% confidence interval based on the curve. In this study, age and BMI (obesity) were found to be strongly positively correlated.