Abstract
Brucella spp. are facultative intracellular bacteria that can establish themselves and cause chronic disease in humans and animals. NK cells play a key role in host defense. They are implicated in an early immune response to a variety of pathogens. However, it was shown that they do not control Brucella infection in mice. On the other hand, NK cell activity is impaired in patients with acute brucellosis, and recently it was demonstrated that human NK cells mediate the killing of intramacrophagic Mycobacterium tuberculosis in in vitro infection. Therefore, we have analyzed the behavior of Brucella suis infecting isolated human macrophages in the presence of syngeneic NK cells. We show that (i) NK cells impair the intramacrophagic development of B. suis, a phenomenon enhanced by NK cell activators, such as interleukin-2; (ii) NK cells cultured in the presence of infected macrophages are highly activated and secrete gamma interferon and tumor necrosis factor alpha; (iii) impairment of bacterial multiplication inside infected cells is marginally associated with the cytokines produced during the early phase of macrophage-NK cell cocultures; (iv) direct cell-to-cell contact is required for NK cells to mediate the inhibition of B. suis development; and (v) inhibition of B. suis development results from an induction of NK cell cytotoxicity against infected macrophages. Altogether, these findings show that NK cells could participate early in controlling the intramacrophagic development of B. suis in humans. It seems thus reasonable to hypothesize a role for NK cells in the control of human brucellosis. However, by impairing the activity of these cells in the acute phase of the illness, the pathogen should avoid this control.
Brucellosis is a worldwide zoonosis that affects domestic animals and humans. The pathogenesis exhibited by this disease differs remarkably between humans and animals (35, 43). In domestic animals, brucellosis is mainly an abortive disease that results from a long-lasting often-unapparent infection. On the other hand, human brucellosis, also named Malta fever, is a complex disease that begins with a systemic infection that can be followed either by localized infection or chronic disease (15, 29, 43). Murine models are commonly used to study Brucella infection. Many strains of mice are sensitive to Brucella spp. but develop a disease quite different from that observed in domestic animals or in humans. Infection with Brucella does not modify pregnancy in mice, and acute infection results in a long-lasting presence of bacteria in the spleen and liver, predominantly. In humans, chronic infection is characterized by a specific bacterial localization within the body (spleen, liver, heart, bones, and brain) and a deleterious effect of the bacteria on these organs (43). The difference in pathogeny results most likely from differences in the immune responses in humans, domestic animals, and animal models (14). Some important differences between the immune responses of mice and humans have been reported. Brucella suis inhibits macrophage tumor necrosis factor alpha (TNF-α) production in humans but not in mice (7, 8) and this property is due to the outer membrane protein Omp25 (23). Furthermore, humans possess a specific type of circulating lymphocytes, called Tγ9δ2, which is absent in mice. These lymphocytes proliferate importantly in human blood after Brucella infection (2) and impair intramacrophagic multiplication (36, 37).
The mouse immune response has been widely studied (1, 19) although the reasons for the persistence of Brucella are not understood (22). The immune response in mammals can be divided into innate and adaptive immunity. Although the adaptive immunity is the most efficient response to eliminate infectious agents, it is widely accepted that innate response determines the behavior of specific response particularly in defining Th1 versus Th2 polarized response. Thus, the innate response is very important for the evolution of the infection. The importance of the Th1 cytokine gamma interferon (IFN-γ) in controlling infection in mice has been emphasized (1, 33). Among the cells involved in IFN-γ production, NK cells play a particular role; they are the most important IFN-γ producers in the beginning of infection. However, it has been clearly demonstrated that NK cells do not play a major role in the early control of Brucella abortus infection in mice (18). On the other hand, it was formally established that in the acute phase of the illness, the activity of the NK cells is impaired in humans developing brucellosis (40). Moreover, human NK cells were recently shown to mediate the intracellular killing of Mycobacterium tuberculosis (4). Therefore, in order to better understand the role of NK cells in bacterial development, we have analyzed the behavior of Brucella suis in human macrophages infected in the presence of syngeneic NK cells. Our results show (i) that NK cells are activated by B. suis-infected macrophages and (ii) that they inhibit the intracellular multiplication of the bacteria by lysing the infected cells, thus suggesting that NK cells could be one actor of the control of Brucella development in humans.
MATERIALS AND METHODS
Bacteria.
B. suis 1330 (ATCC 23444) was obtained from the American Type Culture Collection (Manassas, Va.). Some experiments were performed with B. suis producing green fluorescent protein (GFP-B. suis) (38). Bacteria were grown at 37°C with vigorous shaking to stationary phase.
Cell preparations.
Peripheral blood mononuclear cells from healthy donors were prepared by density centrifugation on Ficoll-Paque (Eurobio, Les Ulis, France). Monocytes were purified from peripheral blood mononuclear cells by adherence (21). Based on the binding of fluorescein isothiocyanate (FITC)-labeled anti-CD14 monoclonal antibodies (MAb), blood monocyte preparations had a purity of 95%. Cells were cultured in complete medium (RPMI 1640-glutamate [Life Technologies, Paisley, United Kingdom], supplemented with 10% heat-inactivated fetal calf serum [FCS], 0.075% sodium bicarbonate, and gentamicin [20 μg/ml]).
Autologous NK cells were prepared from blood lymphocytes using a MACS magnetic column separation system (Miltenyi Biotech, Auburn, Calif.), where CD56 cells were positively selected using anti-CD56 antibodies. Each portion of 108 peripheral blood lymphocytes, depleted from monocytes, was resuspended in 800 μl of MACS buffer (phosphate-buffered saline with 0.5% bovine serum albumin and 2 mM EDTA) and incubated with 200 μl of MACS CD56 MicroBeads for 15 min at 4°C. Cells were washed with 10 to 20 ml of MACS buffer and resuspended in 1 ml of buffer. Blood lymphocytes were then added to a MACS-MS separation column (catalog no. 422-01) on a MiniMACS magnet that had been precooled for 20 min at 4°C and primed with 500 μl of buffer. CD56-negative cells were allowed to run through the column, which was then washed three times with 500 ml of buffer. The column was then removed from the magnet, and the labeled cells were recovered by elution, according to the supplier's protocol (Miltenyi Biotec), and put into culture in RPMI 1640-10% FCS. The cell purity was assessed by flow cytometry analysis (FACScan; Becton Dickinson) using FITC-anti-CD56 MAbs and phycoerythrin anti-CD3 MAbs (Becton Dickinson). The cells were also tested for the absence of CD14 and CD19 antigens with FITC-anti-CD14 and FITC-anti-CD19 (Becton Dickinson).
Infection.
Purified monocytes were seeded in 24-well Falcon plates at a density of 8 × 105 cells/ml in complete culture medium supplemented with 10−7 M 1.25 dihydroxyvitamin D3 (VD) (generous gift of Hoffman La Roche, Basel, Switzerland) for 72 h. VD-differentiated monocytes display macrophagic cell properties and can be readily infected with Brucella. They are hereafter referred to as macrophages. The culture medium was then removed, and the adherent cells were washed twice and infected with B. suis 1330, at a multiplicity of infection (MOI) of 20, in 200 μl of complete medium without gentamicin (30 μg/ml). After 30 min of infection, the cells were washed with phosphate-buffered saline and incubated in 1 ml of complete medium alone. At different time points postinfection (p.i.), supernatants were harvested and intracellular viable bacteria were estimated by CFU counting after cell lysis with 0.2% Triton X-100. Serial 10-fold dilutions of lysates were plated on tryptic soy agar and CFU were counted after a 48-h incubation at 37°C (7). Each CFU determination was performed in triplicate.
Cocultures of purified NK cells with infected macrophages.
The effect of NK cells on macrophage infection by Brucella was studied by adding NK cells to infected macrophages after phagocytosis, i.e., concomitantly with gentamicin, and coculturing both cell populations. To maximize the NK cell effect, studies were performed with ratios of NK cells to autologous infected macrophages varying from 0.1 to 2. Intramacrophagic Brucella levels were determined at chosen time points as described above. When indicated, dual-chamber studies were performed using 24-well Transwell plates (Costar, Cambridge, Mass.). In these experiments, infected macrophages in the lower chamber were separated from NK cells placed in the upper chamber by a 0.4-μm-pore-size filter. Intramacrophagic Brucella levels were compared to those within cultures in which NK cells and infected macrophages were in direct contact or in which macrophages alone were infected. In some experiments, 100 U of rhIL-2 (Chiron, Emeryville, Calif.)/ml was added to cell cocultures concomitantly with NK cells, in order to potentiate the activity of these cells.
Cocultures were also performed in eight-chamber culture slides (Lab-Tek, Nunc, Naperville, Ill.). In these experiments, macrophages (2 × 105) were infected with GFP-B. suis (MOI = 20) and cultured in the presence of syngeneic NK cells (2 × 105) in 400 μl of RPMI-FCS-gentamicin. At different times p.i., the infection was assayed by consecutive visualization of the GFP-B. suis-infected cells by phase-contrast microscopy and UV fluorescent microscopy. Observations were generally performed at 24 h p.i., because after that period, Brucella did not develop within the host cells in the presence of NK cells.
Cytokine determination.
Supernatants of Brucella-infected macrophages cultured in the presence or absence of NK cells were collected at different times p.i. IFN-γ and TNF-α concentrations in the different cell supernatants were measured using enzyme-linked immunosorbent assay kits, OPTEIA human IFN-γ set and OPTEIA human TNF-α set (Pharmingen), following the supplier's protocol.
Analysis of soluble factors.
NK cells and infected macrophages were cultured for different periods: 7, 24, or 48 h. Cell supernatants were collected and filtered through a 0.2-μm-pore-size filter. Macrophages were then infected and after phagocytosis, cultured in the presence of gentamicin and different dilutions of cell supernatants. Levels of intramacrophagic Brucella were then evaluated at different time points p.i. To assess the effect of IFN-γ and TNF-α, 10-μg/ml concentrations of commercial anti-TNF-α and anti-IFN-γ blocking monoclonal antibodies (R&D Systems Inc., Minneapolis, Minn.) were added to cocultures of infected macrophages and NK cells. They remained present in the culture medium during the 48 h of infection. Isotypically matched control mouse immunoglobulin G1 (IgG1) and IgG2 purchased from Beckman Coulter (Brea, Calif.) were used as controls. Although the antibodies were claimed to be blocking antibodies by the manufacturer, their ability to impair the activity of the respective cytokine was first tested by measuring (i) the inhibition of TNF-α-induced apoptosis of L929 cells and (ii) the inhibition of IFN-γ-induced expression of major histocompatibility complex (MHC) class II antigens on THP-1 cells as previously described (36).
Treatment of NK cells with Sr2+.
To prevent the calcium-dependent release of cytotoxic granules, NK cells were degranulated by Sr2+ treatment before being added to infected macrophages. Briefly, NK cells (3 × 106 cells/ml of RPMI-FCS) were treated overnight at 37°C with 25 mM strontium chloride (SrCl2; Sigma Chemicals) as indicated previously (34). They were then washed twice, counted, and resuspended in fresh medium before being used in coculture experiments. Control experiments were performed to verify (i) that the viability of NK cells was not affected by 25 mM Sr2+ and (ii) that the NK cell lytic activity was completely abolished during the 48 h which followed Sr2+ treatment. These controls were performed exactly as indicated previously (34), and K562 cells were used as target cells.
Cytotoxicity assay.
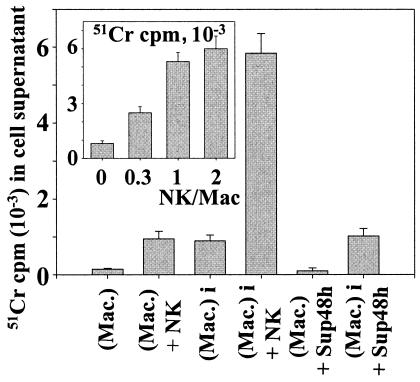
Macrophages were first labeled as previously reported (36). Briefly, 8 × 105 VD-differentiated monocytes were incubated for 2 h at 37°C with 25 μCi of radioactive 51Cr from ICN Biomedicals (Orsay, France) in 500 μl of RPMI in 24-well plates. They were then extensively washed with RPMI medium and infected or not with Brucella (MOI = 20) in the presence or absence of 8 × 105 NK cells (ratio of macrophages to NK cells = 1) or in the presence of supernatants from B. suis-infected macrophages cultured in the presence of autologous NK cells for 48 h. After 48 h of culture in 1 ml of RPMI-FCS-gentamicin, cell supernatants were harvested and filtered and NK cell cytotoxicity was estimated by quantification of 51Cr release (γ-Counter [model 5500B]; Beckman Instruments). Specific 51Cr released represents the mean from triplicate wells. Noninfected cells were used as a control to measure the amount of 51Cr incorporated in macrophages. 51Cr-labeled control cells were cultured in RPMI-FCS medium during the 48 h of infection. They were then washed and lysed with 0.2% Triton X-100, the lysate was filtered, and the radioactive count of an aliquot was determined. In four experiments reported in this work (see Fig. 7), macrophages incorporated 8.23 × 105 ± 1.11 × 105 cpm of 51Cr/well.
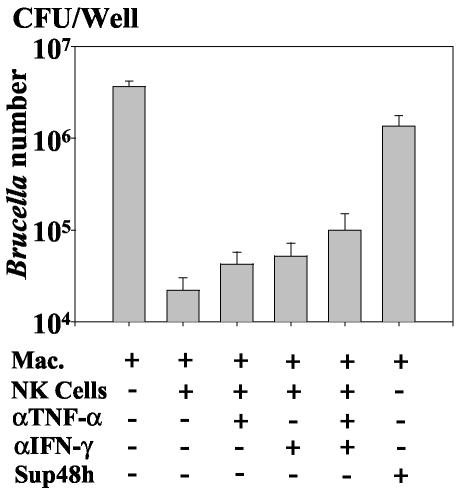
FIG. 7.
Cytotoxicity of NK cells against infected macrophages. A total of 8 × 105 macrophages in 24-well plates were first labeled with 25 μCi of radioactive 51Cr as indicated in Materials and Methods. They were then infected (Mac-i) or not (Mac) with B. suis (MOI = 20) and cultured in the presence (Mac-i/NK and Mac/NK, respectively) or absence (Mac and Mac-i, respectively) of 8 × 105 NK cells (macrophage/NK cell ratio = 1) for a further 48 h in 1 ml of RPMI-FCS-gentamicin. 51Cr-labeled macrophages were also cultured in the presence of 500 μl of Sup48h (supernatants of 48 -h cocultures of B. suis-infected macrophages with autologous NK cells [Fig. 4]) (Mac-i/Sup48h and Mac/Sup48h, respectively) in the presence of gentamicin (conditions of Fig. 4). The cell supernatants were then harvested and filtered. Macrophage lysis was estimated by measuring the radioactivity (51Cr released) present in cell supernatants. For the insert, 8 × 105 infected 51Cr-labeled macrophages were cultured for 48 h in the presence of various amounts of syngeneic NK cells, and radioactivity in cell supernatants was then measured. The y axis shows radioactivity in cell supernatant; the x axis shows number of NK cells per number of macrophages in the assay. Control measurement revealed that macrophages incorporated 8.23 × 105 ± 1.11 × 105 cpm of 51Cr/well. For each determination, 51Cr release was the mean from triplicate wells, four experiments being performed. Error bars, SEMs for four different experiments.
Statistical analysis.
Comparisons between groups in each experiment were performed using unpaired Student t tests. All data are expressed as the mean ± standard error of the mean (SEM).
RESULTS
Macrophage infection with B. suis is impaired in the presence of purified autologous NK cells.
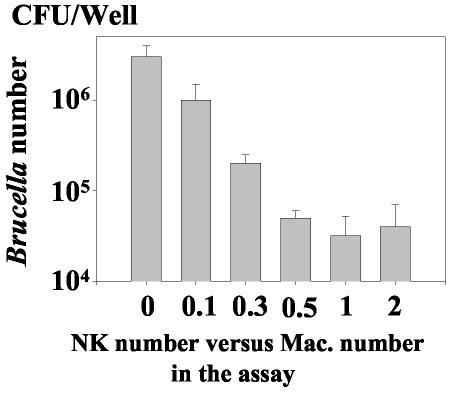
To evaluate the effect of NK cells on macrophage infection with B. suis, NK cells were isolated from peripheral blood lymphocytes using anti-CD56 antibodies in a MACS magnetic column separation system. This cell enrichment procedure consistently yielded a cell population composed of 90% CD56+ CD3− lymphocytes, which were referred to as NK cells and were used throughout the study. Macrophages purified from the same donor were then infected in vitro with B. suis, as previously described (21). After phagocytosis (i.e., 30 min p.i.), extracellular bacteria were washed out and NK cells were added or not to infected cells concomitantly with gentamicin. The intramacrophagic development of the bacteria was quantified by determining the CFU of intracellular Brucella at different time points. Growth of Brucella within macrophages alone and that in macrophages cocultured with NK cells were then compared. In preliminary studies, as previously performed with γδ T cells (36), the NK cell effect was assessed by varying their ratio to infected macrophages from 0.1 to 2 and measuring the CFU of intramacrophagic Brucella at 48 h p.i. Figure 1 indicates that the addition of NK cells resulted in a dramatic decrease in the number of intracellular Brucella organisms. It also shows that the NK cell-induced inhibition increased when the ratio of NK cells to infected macrophages increased. The inhibition was optimal when there was 0.5 to 1 NK cell per macrophage, depending on the donor. In any case, the inhibition of Brucella growth was always significant for the two values of this ratio (P < 0.01 and P < 0.005, respectively), and there were at least 100-fold fewer bacteria in infected macrophages cocultured with NK cells than in infected macrophages alone. Therefore, an optimal NK cell/macrophage ratio of 1 was chosen in the subsequent studies.
FIG. 1.
The intramacrophagic development of B. suis is reduced in the presence of syngeneic NK cells. A total of 8 × 105 human macrophages were infected with B. suis in 24-well plates as described in Materials and Methods. After extensive washing, different amounts of syngeneic NK cells were added (or not) to the infected cells. Both cell populations were cocultured in 1 ml of culture medium (RPMI-10% FCS) supplemented with gentamicin. The ratio of the number of NK cells to the number of B. suis-infected cells varied from 0.1 to 2. After 48 h of incubation, the number of intramacrophagic viable B. suis cells was determined and expressed as CFU per well. For each experimental condition, experiments were performed in triplicate. Values are the means + SEMs (error bars) of four similar experiments performed separately.
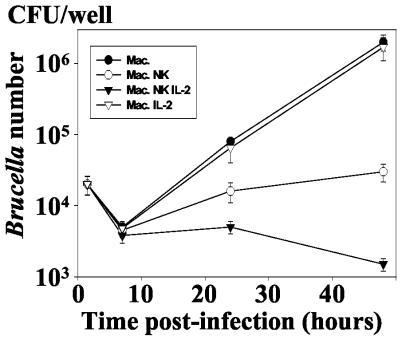
To better analyze the NK cell effect, their ability to inhibit Brucella development was measured at different times p.i. (Fig. 2). There was no relative reduction or only a slight relative reduction in intracellular Brucella level within the first 7 h p.i., a period during which a consequent number of internalized bacteria are killed (9). The magnitude of the reduction then became substantial at 24 h p.i., with a 5- to 10-fold (P < 0.005) decrease compared to controls (infection without NK cells). Also, this reduction was more than 100-fold at 48 h p.i. This was observed in five experiments with different donors.
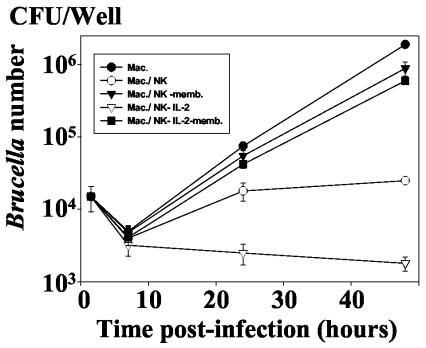
FIG. 2.
Kinetics of the intramacrophagic development of B. suis in the presence of autologous NK cells. Infected macrophages (8 × 105/well) were cultured in 1 ml of culture medium in the presence of NK cells (ratio of NK cells to B. suis-infected cells = 1) under the same conditions as for Fig. 1. In order to assess the role of NK cell activation, rIL-2 was added to the cell culture concomitantly with NK cells at a concentration of 100 U/ml. The number of viable B. suis CFU was then determined at different times p.i., as described in Materials and Methods. Experiments were performed in triplicates and means ± SEMs (error bars) of four different experiments are shown. Symbols: •, infected macrophage alone; ○, infected macrophage incubated with NK cells; ▿, infected macrophages incubated with rIL-2 (100 U/ml); ▾, infected macrophages incubated with NK cells and rIL-2.
Interleukin-2 (IL-2) is a potent mediator of the events mediated by NK cells, such as cell cytotoxicity or cytokine secretion (41). Therefore, we have measured the intracellular development of Brucella in infected macrophages cocultured with recombinant IL-2 (rIL-2)-activated NK cells. We observed that rIL-2 strongly potentiated the reduction in the number of intramacrophagic Brucella mediated by NK cells (Fig. 2). On the contrary, this cytokine had no significant effect on macrophage infection in the absence NK cells.
Together, the data provide evidence that in an in vitro model of Brucella infection, human NK cells are able to mediate protective functions against intracellular development of Brucella within infected syngeneic macrophages.
Production of TNF-α and IFN-γ in cocultures of Brucella-infected macrophages and NK cells.
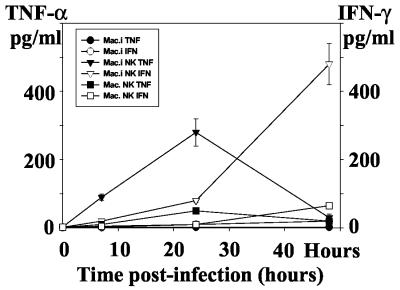
Human macrophages infected with Brucella do not produce any TNF-α, and exogenous TNF-α potentiates the host cell microbicidal activity against the pathogen (7, 8). On the other hand, IFN-γ is produced by NK cells and is essential for NK cell-mediated protection against several intracellular pathogens. Therefore, we have measured TNF-α and IFN-γ levels in supernatants of B. suis-infected macrophages alone and of infected macrophages in the presence of NK cells. None of the cell populations analyzed produced any detectable amounts of TNF-α or IFN-γ. Moreover, only a minimal production of TNF-α and IFN-γ was measured when noninfected macrophages were cultured in the presence of NK cells. In contrast, a strong production of both cytokines was observed when the infected macrophages were cocultured with NK cells (Fig. 3). The production of TNF-α was transient and peaked at 24 h (250 ± 50 pg/ml), whereas the concentration of IFN-γ increased slightly during the first 24 h and faster thereafter to reach 550 ± 100 pg/ml at 48 h p.i. This demonstrates a cross talk between infected macrophages and NK cells and, in all cases, an activation of at least one cell population or perhaps of both, resulting in cytokine production.
FIG. 3.
TNF-α and IFN-γ production in cocultures of B. suis-infected macrophages and autologous NK cells. Infected macrophages (8 × 105/well) were cultured in 1 ml of culture medium in the presence of NK cells for different periods of time (ratio of NK cells to B. suis-infected cells = 1). TNF-α and IFN-γ secreted in cell culture supernatants were measured in triplicates as described in Materials and Methods, and means of four different experiments are shown. First, we confirmed that B. suis-infected macrophages did not secrete TNF-α or IFN-γ (•). Addition of NK cells to the infected macrophages resulted in strong production of TNF-α (▾) and IFN-γ (▿). When noninfected macrophages were mixed with NK cells, only a marginal production of TNF-α (▪) or IFN-γ (□) was observed. Error bars, SEMs for four different experiments.
Production of TNF-α, IFN-γ, and/or other soluble factors cannot account for NK cell-induced inhibition in CFU of intramacrophagic Brucella.
We then analyzed the possibility that the NK cell-mediated reduction of intramacrophagic Brucella resulted from host cell activation by TNF-α, IFN-γ, or a combination of these cytokines. Experiments were conducted in the presence of antibodies directed against TNF-α or/and IFN-γ, after analyzing the capacity of these antibodies to block the activities of TNF-α (300 pg/ml) or IFN-γ (500 pg/ml). Figure 4 indicates that the addition of antibodies separately or together significantly reversed the NK cell action to a relatively low extent. This effect was not observed when isotypically matched control IgG1 (or IgG2) was substituted for anti-TNF-α (or IFN-γ) antibodies (data not shown). The maximal reversion was observed in the presence of both antibodies. These results revealed that TNF-α and IFN-γ released during the course of macrophage infection in the presence of NK cells played only a minor role in the observed phenomenon.
FIG. 4.
The impairment of B. suis development in infected macrophages by autologous NK cells is not reversed by antibodies to block the TNF-α or IFN-γ action. Macrophages were infected with B. suis and cultured in the presence of autologous NK cells (ratio = 1) under the same conditions as for Fig. 2. When indicated, antibodies that inhibit the TNF-α (αTNF-α) or IFN-γ (αIFN-γ) action were added to infected macrophages concomitantly with NK cells and gentamicin. (The capacity of the antibodies to block the cytokine effect was previously evaluated as described previously [36].) For each experiment, the number of intramacrophagic viable bacteria (CFU per well) was measured at 48 h p.i. The right slot shows the effect of secreted factors on the intramacrophagic development of B. suis. Supernatants of B. suis-infected macrophages cultured in the presence of autologous NK cells for 48 h (conditions of Fig. 2) were harvested (Sup48h). Then, 8 × 105 fresh macrophages were infected and cultured for 48 h in 1 ml of medium containing 500 μl of Sup48h, 500 μl of RPMI-10% FCS, and gentamicin. The intracellular number of viable Brucella was measured and compared to that observed in infected macrophages cultured in 1 ml of RPMI-10% FCS supplemented with gentamicin. Results are means ± SEMs (error bars) of triplicates from four different experiments.
We also investigated whether cytokines other than TNF-α and IFN-γ were involved in the impairment of intramacrophagic Brucella number. For that, we tested the ability of supernatants from cocultures of infected macrophages and NK cells to affect the intramacrophagic multiplication of B. suis. The CFU levels of intramacrophagic B. suis were not affected by supernatants from cocultures harvested at 7 h and 24 h p.i (data not shown). In contrast, Fig. 4 shows that supernatants collected at 48 h resulted in a slight (two- to threefold) but significant reduction of the number of intramacrophagic bacteria compared to that observed in infected macrophages alone (Student t test, P < 0.007) (Fig. 4). The optimal effect was observed with a one-half dilution of the supernatant.
Together, these results indicate that the decrease in the number of intramacrophagic Brucella in macrophages cultured in the presence of human NK cells was not principally associated with the production of soluble factors. Even though TNF-α and IFN-γ play a significant role in this phenomenon, their role seems to be of minor importance.
Maximum impairment of Brucella multiplication by NK cells required NK cell contact with infected macrophages.
Because of the modest role of soluble factors, we then assessed whether the NK cell-induced inhibition in the CFU of intramacrophagic Brucella required direct contact with infected macrophages. Macrophage infection with Brucella was therefore analyzed in the presence of NK cells cultured either in contact with or separated from infected cells by a semipermeable membrane (dual-chamber culture system). Figure 5 confirms that at 48 h p.i., NK cells that were directly cocultured with infected macrophages promoted an important decrease in the CFU of intramacrophagic Brucella compared to the bacterial development within macrophages alone. This potent inhibitory effect (2 log) was not observed when NK cells were separated from macrophages by a semipermeable membrane. In this situation, even if the CFU of Brucella within macrophages was altered, the NK cell-induced effect was found to be relatively low (fivefold), although significant (P < 0.005, in four experiments involving different donors). Therefore, the optimal NK effect on Brucella proliferation required a cell-to-cell contact. This was confirmed in experiments involving IL-2-activated NK cells. The IL-2-mediated effect was not observed when NK cells were separated from infected macrophages by a semipermeable membrane, indicating that it did not result from release of cytosolic effectors (Fig. 5).
FIG. 5.
The impairment of B. suis intramacrophagic development requires cell-to-cell contact between infected macrophages and NK cells. B. suis-infected macrophages (8 × 105/ml) were kept in cultures with syngeneic NK cells (8 × 105/ml) in the absence (○, ▾) or presence (▿, ▪) of rIL-2 (100 U/ml) (conditions of Fig. 2). NK cells and macrophages were either in direct contact (○, ▿) or separated by a semipermeable membrane (▾, ▪), where the NK cells were on the upper part of the membrane. The control represented the development of B. suis in infected macrophages cultured without any NK cells (•). At different times p.i., the intramacrophagic number of viable Brucella was measured and expressed in CFU/well. Results are means ± SEMs (error bars) of four different experiments.
NK cells cultured with Brucella-infected macrophages develop contact-dependent cytotoxicity.
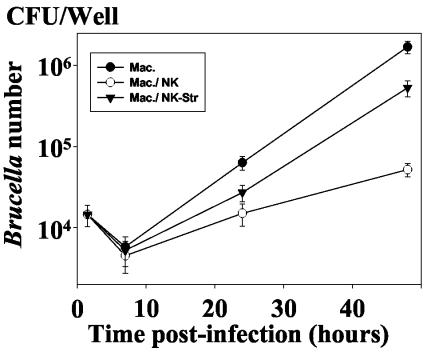
Granule-mediated cytotoxicity is the major effector mechanism of NK cells (39). It requires cell-to-cell contact. Therefore, we assessed whether this pathway could be involved in the inhibition of the intramacrophagic development of Brucella. It is well established that NK cell treatment by strontium chloride prevents the calcium-dependent granule release and activity. This method is commonly used to investigate the role of granule formation in NK cytotoxicity (12, 13). In accordance with others (34), we first confirmed that pretreatment of NK cells for 18 h with 25 mM strontium chloride in RPMI-FCS impaired granule-mediated lysis of K562 target cells (data not shown). Then, in parallel experiments, we demonstrated that NK cells pretreated under similar conditions before being added to infected macrophages had a reduced capacity to inhibit the intramacrophagic development of Brucella compared to the untreated NK cells (Fig. 6). This finding strongly indicated that the NK cell-mediated effect on Brucella intramacrophagic growth was associated with the release of cytotoxic granules.
FIG. 6.
After degranulation by Sr2+, NK cells lost their property to reduce the intramacrophagic multiplication of B. suis. NK cells were treated or not with 25 mM Sr2+ overnight at 37°C (see Materials and Methods). They were then washed twice and cocultured in the presence of B. suis-infected autologous macrophages for different periods of time (conditions of Fig. 2). The number of viable intramacrophagic bacteria (CFU per well) was then determined as previously. Symbols: •, control, B. suis-infected macrophages alone; ○, B. suis-infected macrophages cultured with NK cells; ▾, B. suis-infected macrophages cultured with Sr 2+-treated NK cells. Results are means ± SEMs (error bars) of four different experiments.
Therefore, when NK cells are cultured with infected macrophages, they could develop granule-dependent cytotoxicity against host cells. This supports the requirement of cell-to-cell contact. The apparent inhibition of the bacterial development was due to this cytotoxicity, since bacteria were released in gentamicin-supplemented medium. To test this hypothesis, a technique previously used to analyze the interaction between infected macrophages and γδ T cells was applied to our model (36). Macrophages were loaded with 51Cr before being infected with Brucella and cultured in the presence or absence of NK cells. As controls, noninfected 51Cr-labeled macrophages were kept in culture in the presence or absence of NK cells. For each condition, macrophage death was estimated by measuring the release of 51Cr in cell supernatants 48 h after the onset of the cultures. When NK cells were cultured in the presence of Brucella-infected macrophages (the NK cell/total macrophage ratio being equal to 1), they became highly cytotoxic and were capable of killing macrophages as observed by the high level of 51Cr in cell supernatants (Fig. 7). The macrophage lysis was dependent on the NK cell number. When the NK cell/total macrophage ratio was decreased at 0.3, there was threefold less 51Cr released in the medium of macrophage supernatants. Increasing this ratio to 2 did not significantly modify the amount of 51Cr released. By contrast, only a low level of 51Cr was observed in supernatants of noninfected macrophages cocultured with NK cells or in supernatants of Brucella-infected macrophages alone (Fig. 7). These findings demonstrated that in cocultures, NK cells were able to lyse Brucella-infected macrophages. Such an effect likely accounted for the decrease of the intramacrophagic CFU of Brucella described above, as the bacteria were released in gentamicin-supplemented medium. The data in Fig. 7 also show that less than 10% of the total macrophages were lysed by NK cells; this percentage agreed with the percentage of infected macrophages observed by microscopy at 48 h p.i., when GFP-B. suis was the infectious agent. It means that only infected macrophages were lysed by NK cells.
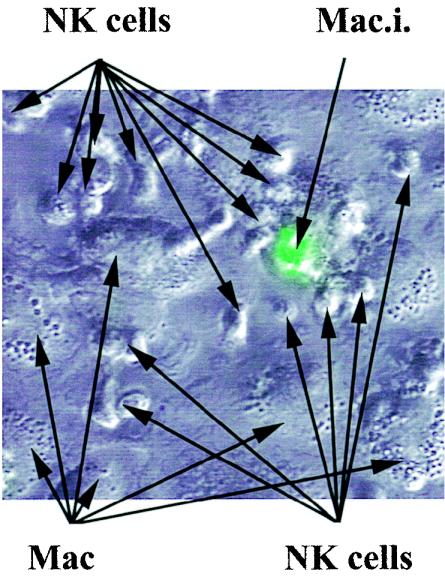
At 24 h p.i., microscopy observations of cocultures of GFP-B. suis-infected macrophages and NK cells were in agreement with this result. They showed that NK cells were in close contact with the Brucella-infected cells, while they bound poorly or not at all to noninfected macrophages (Fig. 8).
FIG. 8.
NK cells associate tightly with infected macrophages. Macrophages (2 × 105) were infected with GFP-B. suis (MOI = 20) in eight-chamber culture slides and cultured in the presence of syngeneic NK cells (2 × 105) in 400 μl of RPMI-FCS-gentamicin. At 24 h p.i., infection was assayed by consecutive visualization of the GFP-B. suis-infected cells by phase-contrast microscopy and UV fluorescent microscopy. The merged images of both observations showed a tight association between an infected macrophage (Mac.i.) and the NK cells, whereas noninfected macrophages (Mac.) poorly interact with NK cells.
In order to better analyze the negative effect on Brucella development of supernatants from infected macrophages cocultured with NK cells, we postulated that these supernatants also promoted the death of Brucella-infected macrophages. However, results shown in Fig. 7 (last bar) firmly ruled out such a possibility.
Together, these findings indicate that in the presence of infected macrophages, NK cells become highly cytotoxic for the Brucella host cells and that a granule-mediated mechanism regulates this phenomenon. In addition, they showed that the effectors released in cocultures of infected macrophages and NK cells exerted their microbicidal activity through other mechanisms, likely by activating macrophages.
DISCUSSION
The present study demonstrates that human NK cells were activated when they were cocultured with autologous macrophages infected by B. suis. This activation resulted in the production of soluble mediators, including TNF-α and IFN-γ, and in a significant reduction in the intramacrophagic development of the bacteria. This finding firmly demonstrated a cross talk between both cell populations when macrophages are infected. Studies involving transfer of supernatants of cocultured NK cells with infected macrophages, use of blocking antibodies, and assessment of NK cell activity in Transwell cultures indicated that the soluble mediators played only a minor role in the impairment of intracellular Brucella growth. This minor role was likely due to the ability of the mediators to induce the host cell microbicidal activity against Brucella as already described for TNF-α (8) or IFN-γ (1). In fact, the most important result obtained in our experiments was that the observed inhibition of Brucella development resulted from the triggering of the lytic machinery of NK cells against autologous Brucella-infected macrophages through a cell-to-cell contact between NK cells and infected macrophages. NK cell cytotoxicity is principally associated with the release of granules containing perforin and other effector molecules (24). By pretreating NK cells with SrCl2, a technique commonly used to analyze such a phenomenon (12, 13, 34), we confirmed that NK cells mediated the killing of Brucella-infected cells by a granule release mechanism. This observation was totally in accordance with the requirement of a contact between NK cells and pathogen-infected macrophages.
This is the first report demonstrating that NK cells may be capable of modulating the development of Brucella in humans, by lysing infected host cells. This activity is innate since no prior exposure to the pathogen was required, as freshly purified NK cells were used to lyse Brucella-infected macrophages. Limited studies had already suggested that NK cells can destroy cells infected with intracellular bacteria such as Listeria, Salmonella, and Legionella spp., but these studies used lymphokine-activated killer cells rather than purified NK cells (3, 20, 26). However, recent reports indicated that highly purified NK cells from healthy subjects lyse M. tuberculosis-infected monocytes (4, 42) by mechanisms which also require cell-to-cell contact.
NK cell lytic activity is controlled by a balance between inhibitory and activating receptors (27, 31). This cytotoxicity is not exerted against normal autologous nucleated cells, because surface MHC class I antigens recognize the inhibitory receptors of NK cells. On the other hand, NK cell cytotoxicity against mononuclear phagocytes infected with viruses or intracellular bacteria may reflect the fact that the pathogens lowered or modified MHC class I expression on their host cells (5, 9, 25). Therefore, a modification of MHC class I receptor at the surface of Brucella-infected macrophages could account for the cytolytic activation of NK cells. This was not confirmed experimentally. Indeed, we did not observe any qualitative and quantitative modifications of MHC class I at the surface of B. suis-infected macrophages, in spite of the use of GFP-B. suis (38) to firmly distinguish Brucella-infected macrophages from noninfected ones (unpublished result). This finding concerning human macrophages is in agreement with a recent report showing that Brucella failed to modify the expression of MHC class I at the surface of infected mouse macrophages (32). NK cells also recognize nonclassical MHC class I molecules, such as HLA-E, or MHC-like molecules, such as CD1a, -b, and -c (6). Therefore, mechanisms involving a modification of these receptors whose expression is induced by Brucella could be involved. However, the modification of these receptors favors the inhibition or target cell lysis rather than promoting its induction (6). Moreover, as in the case of the MHC class I molecules, infection with Brucella did not modify the level of CD1a, -b, or -c expression at the surface of the infected macrophages. Together, these observations suggest that the MHC class I antigens of the Brucella host cells are not involved in NK cell activation. In this sense, the NK cell cytolytic activity directed against Brucella-infected macrophages should differ from that induced against syngeneic dendritic cells (DC). In NK cell-DC cocultures, NK cells which have been exposed to bacterium-infected DC become highly cytolytic against syngeneic immature DC which express a low level of MHC class I molecules (16, 17). On the contrary, these NK cells do not display any cytolytic activity against infected mature DC, which display an up-regulated expression of HLA class 1 molecules on their surface (16, 17). These findings award a crucial role to HLA class 1 molecules in the cross talk between NK cells and DC and to the regulation which results from this interaction, important for the development of both innate and adaptive immunity against the bacteria (30).
NK cell-mediated lysis of M. tuberculosis-infected macrophages which did not require the engagement of MHC class I molecules (4, 42) is associated with an increased expression of the NK cell receptor NKp46 (42). The triggering of this receptor activates the NK cell-mediated lytic activity of M. tuberculosis-infected cells. Indeed, it was speculated in this study that one of the mechanism by which NK cells contribute to innate immunity is through recognition by the NKp46 of conformational molecular structures of intracellular pathogens that are expressed at the surface of infected cells, in a manner analogous to that of Toll-like receptors that trigger macrophage activation. This feature could be shared by several intracellular pathogens (42). The lysis of Brucella-infected macrophages could be induced through a similar pathway, since the pathogen processing and the time required for the overexpression of the NKp46 are in agreement with the kinetics of the NK cell effects.
Other activation pathways are also possible. The discovery of new receptors involved in the regulation of NK cell functions (10) considerably enhances the number hypothetical mechanisms which could be responsible for the NK cell lytic activity against Brucella-infected cells. These receptors could be, for instance, the NKp30 natural cytotoxicity receptor, which is engaged in the cytotoxicity of activated NK cells against immature DC (17), or the NKp44 natural cytotoxicity receptor (11), or even coreceptors like the CD59 molecule, which associated with NKp46 and NKp30 to participate in NK cell activation (28). Moreover, besides the recognition step requiring cell-to-cell contact, activation of NK cells can result from a molecular signal produced by infected cells. Such a signal was described by Zaitseva et al., who demonstrated that IL-12 produced by macrophages phagocytosing killed Brucella stimulated the cytotoxicity of NK cells against allogeneic cells (44). We also observed an induction of IL-12 p40 transcripts in human macrophages infected with B. suis (data not shown).
The antimicrobial functions of NK cells have been studied in murine models of infection with a wide variety of intracellular pathogens where production of IFN-γ by NK cells plays an essential role in the containment of murine infection with listeriae, viruses, or parasites. In contrast, our data on B. suis and those of Brill et al. (4) on M. tuberculosis showed that human NK cells killed intracellular bacteria by mechanisms mostly independent of IFN-γ. Such mechanisms can be specific for human immunity against intracellular bacteria. Nevertheless, our results differ from those of Brill et al. (4) in the sense that those authors did not demonstrate any participation of NK cell granules to the function of NK cells directed against M. tuberculosis-infected macrophages. In their experiments, those authors used EGTA as a degranulation agent and the conclusion was drawn from the inefficiency of this compound at impairing the killing activity of NK cells. When we tested EGTA, it was associated with a high mortality of NK cells and macrophages. This finding explains that, as proposed by different authors (12, 13, 24), SrCl2 was used to induce NK cell degranulation, the cell viability being carefully controlled during the experiment. Definitive analysis of the cytotoxicity mechanism, both in Brucella and Mycobacterium infections, could be obtained with granule-negative NK cells, but to our knowledge such a mutant is not yet available for human studies.
A major observation was made concerning the role of NK cells in mice (1, 18). Despite an increase in activity within the NK cell population in response to Brucella infection, depletion of NK cells using lytic antibodies does not affect the development of the pathogen in infected C57BL/10 mice. Therefore, NK cells could contribute to early resistance, but their presence is not essential in the evolving cellular response. As elimination of NK cells is impossible in humans, we cannot analyze whether such a phenomenon occurs in humans. Analysis of Brucella-infected patients has revealed a dysfunction of NK cells during the acute phase of the disease (40). The inability of NK cells to exert a normal function could allow Brucella to avoid the deleterious effect triggered by these cells. The NK cell dysfunction could indeed reflect an anergy resulting from a sustained activation occurring at the onset of infection. Therefore, Brucella infection can avoid NK cell-mediated effects both in mice and humans.
On the other hand, some differences have been observed in the host cell response (i.e., macrophages) during Brucella infection in mice and humans. For instance, it has been shown that human macrophages do not respond to Brucella infection by producing TNF-α, while mouse macrophages do (7). Differences concerning the expression of surface antigen could also exist between mice and humans. A specific membrane antigen could be modified at the surface of infected macrophages and be recognized by NK cells only in humans. Furthermore, the mechanism of NK cell activation differs in mice and humans (10). Therefore, it remains possible that NK cells do not affect Brucella infection in the same way in humans and mice and that a specific impairment of NK cell activity induced by Brucella is required for its development in humans.
Evolution of clinical brucellosis in humans varies strongly between individuals. Some patients exhibit a classical undulant fever while others remain asymptomatic. Chronicity is also unpredictable. The innate immune response could account for such differences. In our experiments, we observed that the efficiency of the NK cell response against infected macrophages differs between donors, although it is always present. The efficiency of this response could be important for the evolution of the disease in humans.
Finally, even if the pathway by which NK cells recognize their target is not identified, our data firmly establish that in vitro NK cells control Brucella development by triggering a direct cytotoxicity against infected macrophages. Therefore, it seems reasonable to hypothesize a role for NK cells in the early control of intramacrophagic infection. This could be true in subjects with acute brucellosis; the impairment of NK cell activity in these patients should counteract this role (40). The reason for this impairment should be addressed.
Acknowledgments
This work was supported by grants from INSERM, from the European Community QLK2-1999-0014, and from an exchange program from ECOS Nord-CONACYT.
We thank S. Dudal for correcting and improving the English in the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Baldwin, C. L., and M. Parent. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90:367-382. [DOI] [PubMed] [Google Scholar]
- 2.Bertotto, A., R. Gerli, F. Spinozzi, C. Muscat, F. Scalise, G. Castellucci, M. Sposito, F. Candio, and R. Vaccaro. 1993. Lymphocytes bearing the gamma delta T cell receptor in acute Brucella melitensis infection. Eur. J. Immunol. 23:1177-1180. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, D. K., W. E. Stewart II, T. W. Klein, H. Friedman, and J. Y. Djeu. 1987. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J. Immunol. 139:551-556. [PubMed] [Google Scholar]
- 4.Brill, K. J., Q. Li, R. Larkin, D. H. Canaday, D. R. Kaplan, W. H. Boom, and R. F. Silver. 2001. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect. Immun. 69:1755-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brutkiewicz, R. R., and R. M. Welsh. 1995. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. Virol. 69:3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone, E., G. Terrazzano, A. Melian, D. Zanzi, L. Moretta, S. Porcelli, K. Karre, and S. Zappacosta. 2000. Inhibition of human NK cell-mediated killing by CD1 molecules. J. Immunol. 164:6130-6137. [DOI] [PubMed] [Google Scholar]
- 7.Caron, E., A. Gross, J. P. Liautard, and J. Dornand. 1996. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J. Immunol. 156:2885-2893. [PubMed] [Google Scholar]
- 8.Caron, E., T. Peyrard, S. Kohler, S. Cabane, J. P. Liautard, and J. Dornand. 1994. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect. Immun. 62:5267-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspar-Bauguil, S., B. Puissant, D. Nazzal, J. C. Lefevre, M. Thomsen, R. Salvayre, and H. Benoist. 2000. Chlamydia pneumoniae induces interleukin-10 production that down-regulates major histocompatibility complex class I expression. J. Infect. Dis. 182:1394-1401. [DOI] [PubMed] [Google Scholar]
- 10.Colucci, F., J. P. Di Santo, and P. J. Leibson. 2002. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat. Immunol. 3:807-813. [DOI] [PubMed] [Google Scholar]
- 11.De Maria, A., M. Fogli, P. Costa, G. Murdaca, F. Puppo, D. Mavilio, A. Moretta, and L. Moretta. 2003. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol. 33:2410-2418. [DOI] [PubMed] [Google Scholar]
- 12.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 13.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, A. M. Krensky, M. Bonneville, M. A. Peyrat, N. Caccamo, G. Sireci, and A. Salerno. 2001. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J. Infect. Dis. 184:1082-1085. [DOI] [PubMed] [Google Scholar]
- 14.Dornand, J., A. Gross, V. Lafont, J. Liautard, J. Oliaro, and J. P. Liautard. 2002. The innate immune response against Brucella in humans. Vet. Microbiol. 90:383-394. [DOI] [PubMed] [Google Scholar]
- 15.Evans, A. C. 1934. Chronic brucellosis. JAMA 103:665-667. [Google Scholar]
- 16.Ferlazzo, G., B. Morandi, A. D'Agostino, R. Meazza, G. Melioli, A. Moretta, and L. Moretta. 2003. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur. J. Immunol. 33:306-313. [DOI] [PubMed] [Google Scholar]
- 17.Ferlazzo, G., M. L. Tsang, L. Moretta, G. Melioli, R. M. Steinman, and C. Munz. 2002. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes, D. M., R. Benson, and C. L. Baldwin. 1995. Lack of a role for natural killer cells in early control of Brucella abortus 2308 infections in mice. Infect. Immun. 63:4029-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes, D. M., X. Jiang, J. H. Jung, and C. L. Baldwin. 1996. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol. Med. Microbiol. 16:193-203. [DOI] [PubMed] [Google Scholar]
- 20.Gregory, S. H., X. Jiang, and E. J. Wing. 1996. Lymphokine-activated killer cells lyse Listeria-infected hepatocytes and produce elevated quantities of interferon-gamma. J. Infect. Dis. 174:1073-1079. [DOI] [PubMed] [Google Scholar]
- 21.Gross, A., A. Terraza, S. Ouahrani-Bettache, J. P. Liautard, and J. Dornand. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hort, G. M., J. Weisenburger, B. Borsdorf, C. Peters, M. Banai, H. Hahn, J. Jacob, and M. E. Mielke. 2003. Delayed type hypersensitivity-associated disruption of splenic periarteriolar lymphatic sheaths coincides with temporary loss of IFN-gamma production and impaired eradication of bacteria in Brucella abortus-infected mice. Microbes Infect. 5:95-106. [DOI] [PubMed] [Google Scholar]
- 23.Jubier-Maurin, V., R. A. Boigegrain, A. Cloeckaert, A. Gross, M. T. Alvarez-Martinez, A. Terraza, J. Liautard, S. Kohler, B. Rouot, J. Dornand, and J. P. Liautard. 2001. Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages. Infect. Immun. 69:4823-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-332. [DOI] [PubMed] [Google Scholar]
- 25.Kirveskari, J., Q. He, M. Leirisalo-Repo, O. Maki-Ikola, M. Wuorela, A. Putto-Laurila, and K. Granfors. 1999. Enterobacterial infection modulates major histocompatibility complex class I expression on mononuclear cells. Immunology 97:420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimpel, G. R., D. W. Niesel, and K. D. Klimpel. 1986. Natural cytotoxic effector cell activity against Shigella flexneri-infected HeLa cells. J. Immunol. 136:1081-1086. [PubMed] [Google Scholar]
- 27.Lanier, L. L. 2001. On guard—activating NK cell receptors. Nat. Immunol. 2:23-27. [DOI] [PubMed] [Google Scholar]
- 28.Marcenaro, E., R. Augugliaro, M. Falco, R. Castriconi, S. Parolini, S. Sivori, E. Romeo, R. Millo, L. Moretta, C. Bottino, and A. Moretta. 2003. CD59 is physically and functionally associated with natural cytotoxicity receptors and activates human NK cell-mediated cytotoxicity. Eur. J. Immunol. 33:3367-3376. [DOI] [PubMed] [Google Scholar]
- 29.McDevitt, D. G. 1973. Symptomatology of chronic brucellosis. Br. J. Ind. Med. 30:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta, A., R. Biassoni, C. Bottino, and L. Moretta. 2000. Surface receptors delivering opposite signals regulate the function of human NK cells. Semin. Immunol. 12:129-138. [DOI] [PubMed] [Google Scholar]
- 31.Moretta, A. 2002. Natural killer cells and dendritic cells: rendez-vous in abused tissues. Nat. Rev. Immunol. 2:957-964. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, E., G. T. Robertson, M. Parent, S. D. Hagius, R. M. Roop, II, P. H. Elzer, and C. L. Baldwin. 2002. Major histocompatibility complex class I and II expression on macrophages containing a virulent strain of Brucella abortus measured using green fluorescent protein-expressing brucellae and flow cytometry. FEMS Immunol. Med. Microbiol. 33:191-200. [DOI] [PubMed] [Google Scholar]
- 33.Murphy, E. A., J. Sathiyaseelan, M. A. Parent, B. Zou, and C. L. Baldwin. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neighbour, P. A., and H. S. Huberman. 1982. Sr++-induced inhibition of human natural killer (NK) cell-mediated cytotoxicity. J. Immunol. 128:1236-1240. [PubMed] [Google Scholar]
- 35.Nielsen, K., and J. R. Duncan. 1990. Animal brucellosis. CRC Press, Boca Raton, Fla.
- 36.Ottones, F., J. Dornand, A. Naroeni, J. P. Liautard, and J. Favero. 2000. V γ 9V δ 2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J. Immunol. 165:7133-7139. [DOI] [PubMed] [Google Scholar]
- 37.Ottones, F., J. Liautard, A. Gross, F. Rabenoelina, J. P. Liautard, and J. Favero. 2000. Activation of human Vγ9Vδ2 T cells by a Brucella suis non-peptidic fraction impairs bacterial intracellular multiplication in monocytic infected cells. Immunology 100:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouahrani-Bettache, S., F. Porte, J. Teyssier, J. P. Liautard, and S. Kohler. 1999. pBBR1-GFP: a broad-host-range vector for prokaryotic promoter studies. BioTechniques 26:620-622. [DOI] [PubMed] [Google Scholar]
- 39.Robertson, M. J., and J. Ritz. 1990. Biology and clinical relevance of human natural killer cells. Blood 76:2421-24238. [PubMed] [Google Scholar]
- 40.Salmeron, I., M. Rodriguez-Zapata, O. Salmeron, L. Manzano, S. Vaquer, and M. Alvarez-Mon. 1992. Impaired activity of natural killer cells in patients with acute brucellosis. Clin. Infect. Dis. 15:764-770. [DOI] [PubMed] [Google Scholar]
- 41.Tarkkanen, J., T. U. Kosunen, and E. Saksela. 1993. Contact of lymphocytes with Helicobacter pylori augments natural killer cell activity and induces production of gamma interferon. Infect. Immun. 61:3012-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vankayalapati, R., B. Wizel, S. E. Weis, H. Safi, D. L. Lakey, O. Mandelboim, B. Samten, A. Porgador, and P. F. Barnes. 2002. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 168:3451-3457. [DOI] [PubMed] [Google Scholar]
- 43.Young, E. J. 1989. Clinical manifestations of human brucellosis, p. 97-126. In E. J. Young and M. J. Corbel (ed.), Brucellosis: clinical and laboratory aspects. CRC Press, Inc., Boca Raton, Fla.
- 44.Zaitseva, M., H. Golding, J. Manischewitz, D. Webb, and B. Golding. 1996. Brucella abortus as a potential vaccine candidate: induction of interleukin-12 secretion and enhanced B7.1 and B7.2 and intercellular adhesion molecule 1 surface expression in elutriated human monocytes stimulated by heat-inactivated B. abortus. Infect. Immun. 64:3109-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]