Abstract
Objectives
The objective of this study was to report thoracic impedance cardiography (ICG) measurements and compare them to echocardiography (echo) measurements throughout pregnancy and in varied maternal positions.
Methods
A prospective cohort study involving 28 healthy parturients was performed using ICG and echo at three time points and in two maternal positions. Pearson correlations, Bland-Altman plots and paired t-tests were used for statistical analysis.
Results
Significant agreements between many but not all ICG and echo contractility, flow and resistance measurements were demonstrated. Differences in stroke volume due to maternal position were also detected by ICG in the antepartum period. Significant trends were observed by ICG for cardiac output and thoracic fluid content (TFC) (p < 0.025) with advancing pregnancy stages.
Conclusions
ICG and echo demonstrate significant correlations in some but not all measurements of cardiac function. ICG has the ability to detect small changes in SV associated with maternal position change. ICG measurements reflected maximal cardiac contractility in the antepartum period yet reflected a decrease in contractility and an increase in TFC in the postpartum period.
Keywords: Thoracic Impedance Cardiography, echocardiography, Pregnancy, labor
Introduction
Cardiac disease has become one of the most common causes of pregnancy-related mortality and a potentially preventable cause of mortality [1–5]. With the increasing impact of cardiac disease on maternal mortality, non-invasive, portable and less labor intensive cardiovascular monitoring might improve maternal outcomes. Women undergo significant structural and cardiovascular changes during pregnancy that put them at risk for increased morbidities due to pre-existing or new onset of cardiac disease. These changes have traditionally been documented using invasive monitoring (indirect Fick method and dye dilution indicator) and labor-intensive echocardiography with and without doppler [6–12]. During pregnancy, cardiac function also changes with position. Cardiac output (CO) increases with uterine displacement in the left lateral recumbent position, as increased venous return occurs with decompression of the great vessels [13, 14].
Currently echocardiography (echo) is the most commonly used assessment of cardiac function in the pregnant woman. With echo, left ventricular function and contractility can be measured by way of the ejection fraction (EF), left ventricle end-diastolic diameter (LEVDD), fractional shortening (FS) and mean velocity of circumferential fiber shortening (MVCF) [15]. Echo has its limitations in the assessment of contractility, for example the assessment of left ventricular ejection fraction has significant inter- and intra-observer variability and subtle changes in FS and EF may not able to be detected. Whereas echo measures heart function using changes in heart structure and flow, impedance cardiography (ICG) directly measures electrical impedance and thereby contractility and SV.
ICG is easily obtained using four electrode sensors placed on the patient’s neck and thorax, ICG measurements are based on changes in thoracic electrical impedance during the cardiac cycle and produce hemodynamic parameters that are highly producible and strongly correlate with invasive measures such as thermodilution in the non-pregnant state [16–18].
ICG directly measures parameters of left ventricular contractility, including the left ventricular ejection time (LVET), velocity index (VI), pre-ejection period (PEP) and directly measures SV, a measurement of flow, using the Z MARC algorithm. Indirect, or derived, measures of systolic function include the systolic time ratio (STR) defined as the pre-ejection period (PEP) or electrical systole divided by the left ventricular ejection time (LVET) or mechanical systole; and acceleration index (ACI) [19].. Calculations of systemic vascular resistance rely on estimates of the central venous pressure, which are potentially less reliable in the pregnant patient. Other assumptions made when using ICG include that there are no valvular defects, and no respiratory diseases [20].
ICG has been proposed as an alternative method for monitoring cardiac function that is easy to use, cost-effective, reproducible, and less operator and interpreter dependent than echo [21]. ICG, unlike echo, has the ability to directly assess cardiac contractility, stroke volume (SV) and to measure thoracic fluid content. Although there may be instances in which echo and ICG may substitute for one another in the care of a pregnant woman, it is more likely that that these fundamentally different cardiac monitoring technologies will complement one another.
This study describes and compares ICG and echo measures of contractility, flow and resistance. ICG and echo measurements were obtained at three different time points during pregnancy, and ICG measurements were obtained in two physiologically different maternal positions. We hypothesize that ICG measurements of contractility, flow and resistance will correlate with similar echocardiography (echo) measurements and will also be able to detect anticipated changes in maternal physiology with changes in maternal position and stages of pregnancy.
Patients and Methods
Prior to initiating this prospective cohort study, IRB approval was obtained and all study participants were consented prior to enrollment. Recruitment took place at the Queen’s Medical Center and Kapiolani Medical Center for Women and Children in Honolulu, Hawaii, between April 2009 and April 2010. Inclusion criteria included women 18–45 years with an uncomplicated pregnancy and gestational age of at least 32 weeks. Exclusion criteria included any pre-existing heart disease, diagnosed hypertension or pulmonary edema, height < 48 inches or > 90 inches, weight < 67 Lbs. or > 341 Lbs., and multiple gestations.
The three time points for data collection were antepartum with a minimum of 32 weeks gestation (AP), intrapartum (IP), and postpartum within 48 hours of delivery (PP). To be included in analysis, each participant had to have at least two of three visits completed. ICG values were recorded twice consecutively, once in the left lateral recumbent supine position (LLRSP) and once in a semi-recumbent position at an angle of about 60 degrees position (SR60P). Echo values were only recorded in the LLRSP immediately before or after the ICG measurements using the SonoSite CardioDynamics BioZ Dx device (ICG). Blood pressure was measured separately for each ICG reading. An estimated standard central venous pressure (CVP) of 6 mmHg was used.
A certified cardiac sonographer using a General Electric Vivid e (echo), with images obtained in the LLRSP, performed transthoracic echo exams. Standard 2-dimensional images and Doppler parameters were obtained from the parasternal and apical windows according to standard methods [22]. Left ventricular end-systolic and end-diastolic chamber dimensions were obtained from M-mode images in the parasternal long-axis view, with 2-dimensional images used if necessary. FS was calculated by dividing the change in left ventricular chamber size from diastole to systole, divided by the left ventricular end-diastolic chamber size. EF was assessed by visual estimate by a single physician board-certified and Level III trained in adult echo. CO was estimated using the velocity time integral of flow through the LV outflow tract multiplied by the area of the outflow tract based on measured diameter [22]. Both ICG and echo measurements were performed within 30 minutes of each other.
We used Pearson correlations to compare ICG and echo measurements of flow and resistance parameters (CO, SV, and SVR). Bland-Altman plots were used to study the agreement between ICG (STR, LVET, PEP, VI, ACI) and echo (EF and FS) measurements of contractility. Paired t-tests were used to compare ICG measurements in two different maternal positions. All analyses were performed using IBM SPSS Statistics (version 19.0.1). Continuous data are reported as means with standard deviations.
Results
We enrolled 28 parturients (age 30.8±5.2 years, BMI 30.6±6.3, 34.0±1.2 weeks gestation, gestational age at delivery 38.8±1.2 weeks), with 35.7% (n=10) of subjects pregnant for the first time. A total of 64 ICG and echo paired measurements were carried out: 26 antepartum, 18 intrapartum during different stages of labor and 20 postpartum. 13 participants were evaluated at all three time points. Ten of the 28 participants were delivered by cesarean section.
When comparing the same flow and resistance parameters (CO, SV, and SVR) between ICG and echo, Pearson correlations were statistically significant in the LLRSP for SV and SVR and antepartum; SV, CO and SVR intrapartum; and SVR postpartum; and in the SR60P for SV and SVR antepartum; and SVR postpartum. Means with standard deviations and correlation coefficients for both echo and ICG are described in Table 1.
Table 1.
Means and standard deviations for ECHO and ICG parameters at three stages of pregnancy in the left later recumbent supine position with Pearson correlations (r) and p values
| Parameter | ECHO | ICG | ECHO | ICG | ECHO | ICG |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Time period | Antepartum (n =26) | Intrapartum (n = 18) | Postpartum (n = 20) | |||
| Stroke Volume (ml) | 74 ±21 | 84 ± 24 | 78 ± 23 | 81 ± 24 | 76 ± 19 | 67 ± 22 |
| r = 0.44 (p = .023)* | r = 0.77 (p = .000)** | r = 0.19 (p = .423) | ||||
| Cardiac Output (l/min) | 5.8 ± 1.6 | 6.4 ± 1.9 | 5.6 ± 1.3 | 5.8 ± 1.5 | 5.6 ± 1.1 | 5.0 ± 1.6 |
| r = 0.25 (p = .223) | r = 0.71 (p = .001)** | r = 0.23 (p = .329) | ||||
| Systemic Vascular Resistance (dynes sec/cm5) | 993 ± 392 | 921 ± 319 | 1,116 ± 242 | 1,093 ± 314 | 1,079 ± 184 | 1,324 ± 473 |
| r = 0.56 (p = .004)** | r = 0.66 (p = .003)** | r = 0.34 (p = .146) | ||||
| Acceleration Index (per 100 sec2) | 100 ± 38 | 109 ± 23 | 101 ± 30 | |||
| Velocity Index (per 1000 sec) | 63 ± 18 | 65 ± 11 | 58 ± 14 | |||
| Systolic Time Ratio | 0.25 ± 0.03 | 0.25 ± 0.05 | 0.32 ± 0.12 | |||
| Pre-Ejection Period (msec) | 76 ± 8 | 74 ± 6 | 83 ± 16 | |||
| Left Ventricular Ejection Time (msec) | 303 ± 23 | 292 ± 42 | 266 ± 61 | |||
p < .005;
p < .05
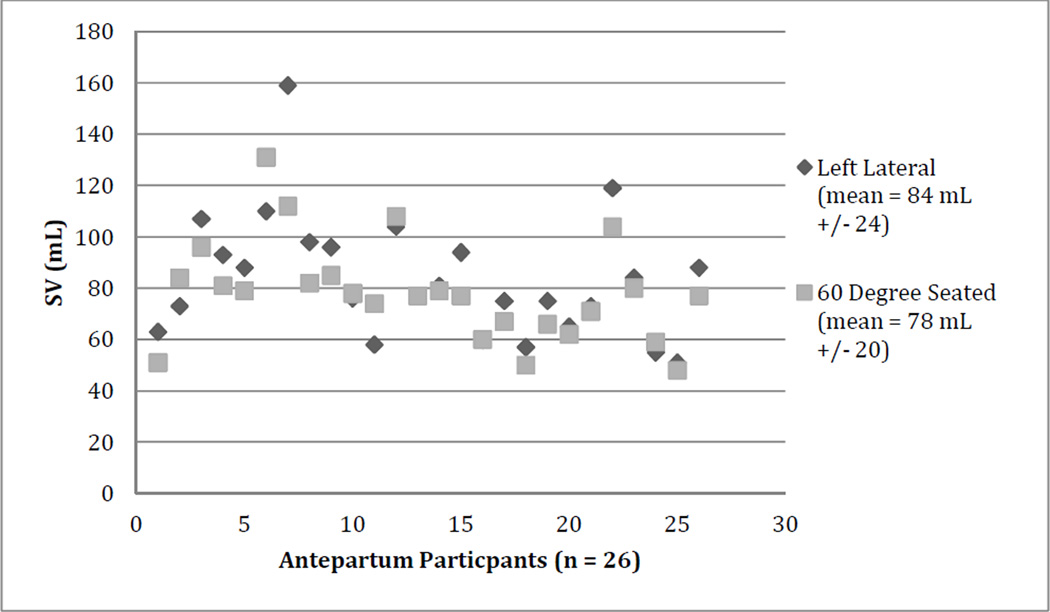
The comparison of the ICG measurements between the two positions did not show any consistent differences. Other than changes detected in antepartum SV, there was little effect of maternal position on ICG readings as described in Table 2. Individual participant’s changes in SV during the antepartum period are shown in Figure 1.
Table 2.
Affect of position on ICG measurements with paired t test mean differences +/− standard deviations and (95% confidence intervals)
| Parameter | 60 degree seated |
Left lateral | 60 degree seated |
Left lateral | 60 degree seated |
Left lateral |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Pregnancy Stage | Antepartum (n = 26) | Intrapartum (n = 18) | Postpartum (n = 20) | |||
| Stroke Volume (ml) | 78 ± 20 | 84 ± 24 | 76 ± 24 | 81 ± 24 | 70 ± 29 | 67 ± 22 |
| 5.42 ± 12.71 (.29, 10.56)* | 4.83 ± 22.94 (−6.57, 16.24) | −2.70 ± 21.14 (−12.13, 6.73) | ||||
| Cardiac Output (l/min) | 6.1 ± 1.5 | 6.4 ± 1.9 | 5.7 ± 1.6 | 5.8 ± 1.5 | 5.1 ± 2.0 | 5.0 ± 1.6 |
| 0.28 ± 0.80 (−.0.43, 0.60) | 0.14 ± 1.69 (−7.0, 0.98) | −0.13 ± (−0.73, 0.48) | ||||
| Systemic Vascular Resistance (dynes sec/cm5) | 1050 ± 313 | 921 ± 319 | 1198 ± 478 | 1,093 ± 314 | 1430 ± 617 | 1,324 ± 473 |
| −130.00 ± 123.53 (−180.99, −79.01)** | −105.33 ± 442.53 (−325.40, 114.73) | −105.70 ± 491.53 (−335.74, 124.34) | ||||
| Acceleration Index (per 100 sec2) | 101 ± 31 | 100 ± 38 | 101 ± 27 | 109 ± 23 | 103 ± 34 | 101 ± 30 |
| −1.15 ± 21.28 (−9.75, 7.44) | 8.22 ± 20.29 (−1.87, 18.31) | −1.55 ± 23.62 (−12.44, 9.34) | ||||
| Velocity Index (per 1000 sec) | 61 ± 14 | 63 ± 18 | 64 ± 11 | 65 ± 11 | 61 ± 20 | 58 ± 14 |
| 1.69 ± 8.41 (−1.70, 5.09) | 1.06 ± 8.63 (−3.24, 5.35) | −3.15 ± 11.34 (−8.46, 2.16) | ||||
| Systolic Time Ratio | 0.26 ± 0.06 | 0.25 ± 0.03 | 0.27 ± 0.06 | 0.25 ± 0.05 | 0.30 ± 0.06 | 0.32 ± 0.12 |
| −0.01 ± 0.07 (−0.04, 0.01) | −0.02 ± 0.07 (0.02, −0.06) | 0.03 ± 0.13 (−0.03, 0.09) | ||||
| Pre-Ejection Period (msec) | 76 ± 11 | 76 ± 8 | 75 ± 6 | 74 ± 6 | 77 ± 11 | 83 ± 16 |
| 0.00 ± 13.49 (−5.45, 5.45) | −1.22 ± 7.70 (−5.10, 2.61) | 6.00 ± 13.73 (−0.42, 12.42) | ||||
| Left Ventricular Ejection Time (msec) | 292 ± 31 | 303 ± 23 | 278 ± 48 | 292 ± 42 | 264 ± 56 | 266 ± 61 |
| 11.92 ± 30.14 (−0.25, 24.10) | 14.17 ± 59.94 (−15.64, 43.97) | 1.25 ± 59.84 (−26.76, 29.26) | ||||
| Thoracic Fluid Content (per kohm) | 32.2 ± 3.6 | 32.6 ± 3.9 | 35.9 ± 4.1 | 37.0 ± 3.9 | 38.7 ± 4.6 | 39.4 ± 4.7 |
| 0.40 ± 2.14 (−0.46, 1.26) | 1.16 ± 1.24 (0.29, 0.54)** | 0.66 ± 1.43 (−0.01, 1.32) | ||||
p value < .005;
p value < .05
Figure 1.
Individual changes in stroke volume (SV) as measured by ICG with position change in the antepartum period
While correlations measure the data’s linearity, Bland-Altman plots show the data’s agreement using mean differences (how much higher or lower one measure is than the other) and confidence intervals (what is the variability of the differences and is it small enough to warrant the use of one device in place of the other). When comparing measurements made by ICG and echo, Bland-Altman plots showed acceptable (at least 95%) but modest agreement between ICG contractility parameters (STR, LVET, PEP, VI, ACI) and echo contractility parameters (EF and FS) most consistently during the antepartum period (Table 3). Bland-Altman plots also showed acceptable agreement for both SV, CO and SVR in the antepartum and intrapartum periods; and for SVR in the postpartum period (Table 4).
Table 3.
Comparison of ECHO and ICG contractility measurements of the effect of pregnancy stage in the left lateral recumbent supine position (LLRSP) with Bland-Altman percentages of points within the confidence intervals
| Antepartum N=26 |
Intrapartum N=18 |
Postpartum N=20 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | STR | LVET | PEP | VI | ACI | STR | LVET | PEP | VI | ACI | STR | LVET | PEP | VI | ACI |
| EF | 100 | 91 | 86 | 95 | 91 | 100 | 93 | 93 | 100 | 100 | 94 | 100 | 94 | 94 | 94 |
| FS | 91 | 95 | 91 | 95 | 95 | 100 | 93 | 93 | 100 | 93 | 94 | 100 | 94 | 94 | 100 |
Table 4.
Comparison of ECHO and ICG flow and resistance measurements at different pregnancy stages in the left lateral recumbent supine position (LLRSP) with Bland-Altman mean differences, confidence intervals and percentages of points within the confidence intervals
| Parameter | Antepartum | Intrapartum | Postpartum |
|---|---|---|---|
| SV (ml) |
15.1 (−29.1,59.3) 100% |
1.5 (−28.7, 31.8) 100% |
−4.0 (−51.6, 43.6) 94% |
| CO (l/min) |
1.0 (−2.9, 4.9) 95% |
0.2 (−2.2, 2.5) 100% |
−0.3 (−3.3, 2.8) 88% |
| SVR (dyne-sec/cm5) |
−141 (−782, 500) 100% |
−2 (−504, 500) 100% |
143 (−483, 770) 100% |
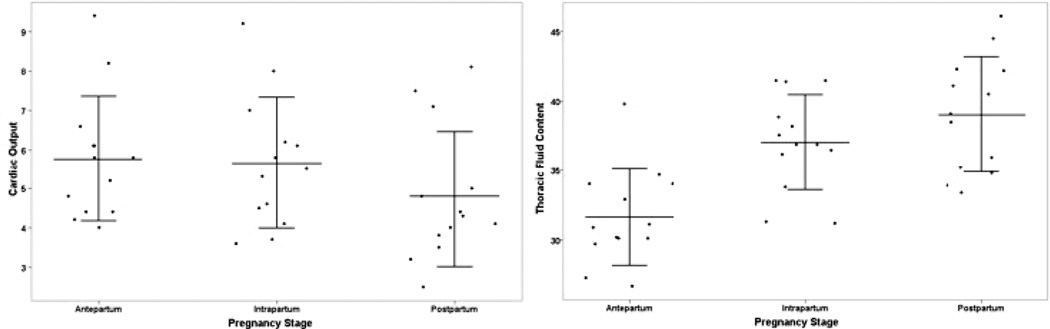
ICG demonstrated statistically significant different measurements across and between the different stages of pregnancy using ANOVA with both multiple comparisons using a Bonferoni adjustment and with pairwise comparisons (Table 5). CO and TFC demonstrated statistically significantly different physiology at all three stages (AP, IP and PP) of pregnancy (Figure 2).
Table 5.
P-values from repeated measures ANOVA and pairwise comparisons of different pregnancy stages
| Variable |
Repeated measures ANOVA p-value n = 13 |
Intrapartum vs. Antepartum p-value |
Pair wise comparisons Postpartum vs. Antepartum p-value |
Postpartum vs. Intrapartum p-value |
|---|---|---|---|---|
| Stroke volume | 0.08 | 0.58 | 0.03** | 0.11 |
| Cardiac output | 0.01* | 0.83 | 0.007* | 0.055 |
| Vascular resistance | 0.15 | 0.81 | 0.06 | 0.26 |
| Acceleration index | 0.54 | 0.33 | 0.59 | 0.73 |
| Velocity index | 0.46 | 0.92 | 0.23 | 0.32 |
| Systolic time ratio | 0.07 | 0.56 | 0.03* | 0.08 |
| Pre-ejection period | 0.15 | 0.95 | 0.06 | 0.11 |
| Left ventricular ejection time | 0.12 | 0.69 | 0.053 | 0.22 |
| Thoracic fluid content | < 0.001* | < 0.001* | < 0.001* | 0.04** |
p < 0.025 using Bonferoni adjustment for multiple comparisons;
p < 0.05
Figure 2.
Discussion
ICG has already been validated as a reliable and convenient modality for the evaluation of heart function in the non-pregnant patient using invasive techniques and echo [23–25]. However, our study fills in some gaps left by previous observations of ICG use in pregnancy. Whereas the initial research on ICG use in pregnancy was inconsistent and relied on older equations [26–30], recent updates in the ICG signal analysis software have been made to improve accuracy with changes in breathing and allow for measurements over fewer cardiac cycles. To date little data exist on ICG use in pregnancy since the recently updated ICG software was updated in 1998.[19] What does exist is either retrospective, has only a single time point, or lacks comparison with other cardiovascular assessments such as thermography or echo. Moreover, there are little comparative data on maternal hemodynamic parameters during labor using ICG and echo.[24, 30–45] Our study is both prospective and longitudinal, and it provides a comparison with echo evaluations. Furthermore, few ICG studies even report on cardiac function in more than one position and only one involves pregnancy.[44, 46, 47]
Comparisons between our ICG and echo data as they pertain to contractility (STR, LVET, PEP, VI and ACI for ICG, and EF and FS for echo) before, during and after labor yielded a number of statistically significant correlations as well as acceptable agreement between these two devices. The lack of consistent agreement at all time points may in part be attributed to echo’s lack of sensitivity in detecting small changes in EF and FS especially in healthy participants. Comparisons between our ICG and echo data as they pertain to flow (SV and CO) and resistance (SVR) before, during and after labor also yielded a number of statistically significant correlations as well as acceptable agreement between these two methods of measuring hemodynamic data.
Interestingly ICG was able to detect a subtle increase in SV with change in maternal position in the antepartum period. This is possibly due to the increased venous return the LLRP. However STR, PEP and LVET demonstrated no change in contractility with position change. This could be explained by the increasing blood volume in the third trimester and increasing preload with stroke volume increasing according to the Frank Starling phenomena. The increased volume should stretch the myocardium and increase contractility. If the myocardium is already at the apex of the Frank Starling curve further stretching would not increase contractility but with more preload and fixed contractility, stroke volume should increase. The fact that measurements of contractility such as STR, PEP and LVET demonstrated no change with position may also represent too small a sample size or more likely demonstrates the hearts fixed contractility at the peak of the Frank Starling curve as preload and therefore SV increases. It was somewhat unexpected that CO as measured by ICG did not change with maternal position. This may be due to lack of precision as a derived measurement and assumptions made it the calculation of CO. Other possible reasons for the lack of change typically seen by position change may in part be attributed to the fluid shifts including positioning of breast tissue with position change.
We also demonstrated that ICG was able to detect changes reflecting physiologic adaptations occurring over three stages of pregnancy from the late antepartum to the immediate postpartum period. For example, CO measurements significantly decreased over the late stages of pregnancy and TFC significantly increased, whereas trends in PEP appeared to nadir during labor and increased within 48 hours postpartum while trends in LVET appeared to do the opposite. LVET is thought to represents mechanical systole while PEP represents electrical systole. TFC is the inverse of base impedance, which measures all the fluid and soft tissue in the thorax. The observed increase in TFC likely reflects an increase in intravascular volume, which would be anticipated in late pregnancy and during labor. This includes the pulmonary venous circulation or left ventricular preload. If left ventricular preload were increased with a normal left ventricle, you would expect an increase in CO and a decrease in PEP because of the Frank-Starling effect. The increased PEP and the decreased LVET are consistent with a decline in left ventricular contractility during the intrapartum and immediate postpartum periods. If there is in fact a temporary or subclinical decrease in left ventricular contractility, this may be a reason why cardiomyopathy and clinical heart failure present most commonly in the immediate postpartum period.
A major limitation of this study was the small numbers of participants. Using the Bland-Altman analysis with a sample size of 13 you can only detect differences in the SV measured by echo versus ICG with 95% limits of agreement of 0.96 times the standard deviation of the differences between measurements by the two methods. With larger numbers subtle differences between echo and ICG may have been detected. Another limitation is that echo measurements were only taken in the LLRP, and therefore we were unable to test for echos ability to detect changes cardiac function brought on by position change. It is intriguing that ICG was able to detect differences in antenatal stroke volume with change in position, however we are unable to claim that echo cannot detect the same changes. Finally limitations also included the assumption that the CVP was 6, and the inability to control for the stage of labor during which the measurements were obtained. The strengths of this study include a prospective longitudinal cohort design with serial pregnancy time points, two maternal positions, and blood pressure values with each ICG measurement.
In summary, this study demonstrated significant correlations between ICG and echo in some but not all measurements of cardiac function. ICG also demonstrated the ability to detect small antepartum changes in SV associated with maternal position change. ICG antepartum measurements reflected maximal cardiac contractility and postpartum ICG measurements reflected a subclinical decrease in contractility and an increase in TFC. The future clinical utility of ICG may lie in following cardiac function trends in women with or at risk for heart disease during pregnancy. In addition ICG may do what echo cannot such as to detect subtle changes in SV and to measure thoracic fluid content. ICG also has advantages over echo, namely allowing continuous monitoring and not requiring expert interpretation of ultrasound images. Future research should evaluate the accuracy of TFC and with larger numbers corroborate the difference seen by ICG with maternal position change. Although our intent was to compare two technologies during pregnancy, it is important to remember that echo and ICG measure different aspects of heart function and are more likely to complement each other than replace each other in patient care.
Acknowledgements
The project described was supported by Award No. U54RR026136 from the National Center for Research Resources (NCRR), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR, NIH or The Queen’s Medical Center.
References
- 1.Burlingame J, Horiuchi B, Ohana P, Onaka A, Sauvage LM. The contribution of heart disease to pregnancy-related mortality according to the pregnancy mortality surveillance system. Journal of perinatology : official journal of the California Perinatal Association. 2012;32(3):163–169. doi: 10.1038/jp.2011.74. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991–1997. Obstetrics and gynecology. 2003;102(6):1326–1331. doi: 10.1016/j.obstetgynecol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Fett JD. Pregnancy-related mortality due to cardiomyopathy: United States, 1991–1997. Obstetrics and gynecology. 2004;103(6):1342. doi: 10.1097/01.AOG.0000128117.91548.ba. author reply 1343. [DOI] [PubMed] [Google Scholar]
- 4.Berg CJ, Harper MA, Atkinson SM, Bell EA, Brown HL, Hage ML, Mitra AG, Moise KJ, Jr, Callaghan WM. Preventability of pregnancy-related deaths: results of a state-wide review. Obstetrics and gynecology. 2005;106(6):1228–1234. doi: 10.1097/01.AOG.0000187894.71913.e8. [DOI] [PubMed] [Google Scholar]
- 5.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstetrics and gynecology. 2003;101(2):289–296. doi: 10.1016/s0029-7844(02)02587-5. [DOI] [PubMed] [Google Scholar]
- 6.van Oppen AC, Stigter RH, Bruinse HW. Cardiac output in normal pregnancy: a critical review. Obstetrics and gynecology. 1996;87(2):310–318. doi: 10.1016/0029-7844(95)00348-7. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy TG, MacDonald D, Hennessy MS, Maguire M, Blake S, McCann HA, Sugrue DD. Serial changes in cardiac output during normal pregnancy: a Doppler ultrasound study. European journal of obstetrics, gynecology, and reproductive biology. 1996;70(2):117–122. doi: 10.1016/s0301-2115(95)02582-0. [DOI] [PubMed] [Google Scholar]
- 8.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J et al. Central hemodynamic assessment of normal term pregnancy. American journal of obstetrics and gynecology. 1989;161(6 Pt 1):1439–1442. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 9.Clapp JF, 3rd, Seaward BL, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. American journal of obstetrics and gynecology. 1988;159(6):1456–1460. doi: 10.1016/0002-9378(88)90574-1. [DOI] [PubMed] [Google Scholar]
- 10.Atkins AF, Watt JM, Milan P, Davies P, Crawford JS. A longitudinal study of cardiovascular dynamic changes throughout pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 1981;12(4):215–224. doi: 10.1016/0028-2243(81)90012-5. [DOI] [PubMed] [Google Scholar]
- 11.Ueland K, Metcalfe J. Circulatory changes in pregnancy. Clinical obstetrics and gynecology. 1975;18(3):41–50. doi: 10.1097/00003081-197509000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Lund CJ, Donovan JC. Blood volume during pregnancy. Significance of plasma and red cell volumes. American journal of obstetrics and gynecology. 1967;98(3):394–403. [PubMed] [Google Scholar]
- 13.Hankins GD, Harvey CJ, Clark SL, Uckan EM, Van Hook JW. The effects of maternal position and cardiac output on intrapulmonary shunt in normal third-trimester pregnancy. Obstetrics and gynecology. 1996;88(3):327–330. doi: 10.1016/0029-7844(96)00212-8. [DOI] [PubMed] [Google Scholar]
- 14.Atkins AJ, Watt JM, Milan P, Davies P, Crawford JS. The influence of posture upon cardiovascular dynamics throughout pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 1981;12(6):357–372. doi: 10.1016/0028-2243(81)90081-2. [DOI] [PubMed] [Google Scholar]
- 15.Kerkkamp HJ, Heethaar RM. A comparison of bioimpedance and echocardiography in measuring systolic heart function in cardiac patients. Annals of the New York Academy of Sciences. 1999;873:149–154. doi: 10.1111/j.1749-6632.1999.tb09462.x. [DOI] [PubMed] [Google Scholar]
- 16.Albert NM, Hail MD, Li J, Young JB. Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2004;13(6):469–479. [PubMed] [Google Scholar]
- 17.Drazner MH, Thompson B, Rosenberg PB, Kaiser PA, Boehrer JD, Baldwin BJ, Dries DL, Yancy CW. Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. The American journal of cardiology. 2002;89(8):993–995. doi: 10.1016/s0002-9149(02)02257-9. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg BH, Hermann DD, Pranulis MF, Lazio L, Cloutier D. Reproducibility of impedance cardiography hemodynamic measures in clinically stable heart failure patients. Congest Heart Fail. 2000;6(2):74–80. doi: 10.1111/j.1527-5299.2000.80140.x. [DOI] [PubMed] [Google Scholar]
- 19.Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography: the next vital sign technology? Chest. 2003;123(6):2028–2033. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 20.Tang WH, Tong W. Measuring impedance in congestive heart failure: current options and clinical applications. American heart journal. 2009;157(3):402–411. doi: 10.1016/j.ahj.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail. 2003;9(5):241–250. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong W, Ryan T. Feigenbaum's Echocardiography. 7th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 23.Boudoulas H. Systolic time intervals. European heart journal. 1990;11(Suppl I):93–104. doi: 10.1093/eurheartj/11.suppl_i.93. [DOI] [PubMed] [Google Scholar]
- 24.Mattar JA, Shoemaker WC, Diament D, Lomar A, Lopes AC, De Freitas E, Stella FP, Factore LA. Systolic and diastolic time intervals in the critically ill patient. Critical care medicine. 1991;19(11):1382–1386. doi: 10.1097/00003246-199111000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Ranaei R, Heywood J, W E. Assessment of contractility and total arterial compliance by impedance cardiography determined parameters. J Card Fail. 2002;8(4) suppl:S97. [Google Scholar]
- 26.Myhrman P, Granerus G, Karlsson K, Lundgren Y. Cardiac output in normal pregnancy measured by impedance cardiography. Scandinavian journal of clinical and laboratory investigation. 1982;42(6):513–520. [PubMed] [Google Scholar]
- 27.Milsom I, Forssman L, Biber B, Dottori O, Sivertsson R. Measurement of cardiac stroke volume during cesarean section: a comparison between impedance cardiography and the dye dilution technique. Acta anaesthesiologica Scandinavica. 1983;27(5):421–426. doi: 10.1111/j.1399-6576.1983.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 28.Milsom I, Forssman L, Sivertsson R, Dottori O. Measurement of cardiac stroke volume by impedance cardiography in the last trimester of pregnancy. Acta obstetricia et gynecologica Scandinavica. 1983;62(5):473–479. doi: 10.3109/00016348309154222. [DOI] [PubMed] [Google Scholar]
- 29.de Swiet M, Talbert DG. The measurement of cardiac output by electrical impedance plethysmography in pregnancy. Are the assumptions valid? British journal of obstetrics and gynaecology. 1986;93(7):721–726. [PubMed] [Google Scholar]
- 30.Heethaar RM, van Oppen AC, Ottenhoff FA, Brouwer FA, Bruinse HW. Thoracic electrical bioimpedance: suitable for monitoring stroke volume during pregnancy? Eur J Obstet Gynecol Reprod Biol. 1995;58(2):183–190. doi: 10.1016/0028-2243(94)01991-6. [DOI] [PubMed] [Google Scholar]
- 31.San-Frutos LM, Fernandez R, Almagro J, Barbancho C, Salazar F, Perez-Medina T, Bueno B, Bajo J. Measure of hemodynamic patterns by thoracic electrical bioimpedance in normal pregnancy and in preeclampsia. European journal of obstetrics, gynecology, and reproductive biology. 2005;121(2):149–153. doi: 10.1016/j.ejogrb.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Scardo JA, Ellings J, Vermillion ST, Chauhan SP. Validation of bioimpedance estimates of cardiac output in preeclampsia. Am J Obstet Gynecol. 2000;183(4):911–913. doi: 10.1067/mob.2000.108892. [DOI] [PubMed] [Google Scholar]
- 33.Masaki DI, Greenspoon JS, Ouzounian JG. Measurement of cardiac output in pregnancy by thoracic electrical bioimpedance and thermodilution. A preliminary report. Am J Obstet Gynecol. 1989;161(3):680–684. doi: 10.1016/0002-9378(89)90379-7. [DOI] [PubMed] [Google Scholar]
- 34.Tihtonen KM, Koobi T, Vuolteenaho O, Huhtala HS, Uotila JT. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol. 2007;196(4):328 e321–327. doi: 10.1016/j.ajog.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Tihtonen KM, Koobi T, Uotila JT. Arterial stiffness in preeclamptic and chronic hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):180–186. doi: 10.1016/j.ejogrb.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Tihtonen K, Koobi T, Yli-Hankala A, Huhtala H, Uotila J. Maternal haemodynamics in pre-eclampsia compared with normal pregnancy during caesarean delivery. Bjog. 2006;113(6):657–663. doi: 10.1111/j.1471-0528.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 37.Tihtonen K, Koobi T, Yli-Hankala A, Uotila J. Maternal hemodynamics during cesarean delivery assessed by whole-body impedance cardiography. Acta Obstet Gynecol Scand. 2005;84(4):355–361. doi: 10.1111/j.0001-6349.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- 38.Tihtonen K, Koobi T, Huhtala H, Uotila J. Hemodynamic adaptation during pregnancy in chronic hypertension. Hypertens Pregnancy. 2007;26(3):315–328. doi: 10.1080/10641950701436016. [DOI] [PubMed] [Google Scholar]
- 39.Volman MN, Rep A, Kadzinska I, Berkhof J, van Geijn HP, Heethaar RM, de Vries JI. Haemodynamic changes in the second half of pregnancy: a longitudinal, noninvasive study with thoracic electrical bioimpedance. Bjog. 2007;114(5):576–581. doi: 10.1111/j.1471-0528.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 40.Newman RB, Pierre H, Scardo J. Thoracic-fluid conductivity in peripartum women with pulmonary edema. Obstet Gynecol. 1999;94(1):48–51. doi: 10.1016/s0029-7844(99)00232-x. [DOI] [PubMed] [Google Scholar]
- 41.Scardo JA, Vermillion ST, Hogg BB, Newman RB. Hemodynamic effects of oral nifedipine in preeclamptic hypertensive emergencies. Am J Obstet Gynecol. 1996;175(2):336–338. doi: 10.1016/s0002-9378(96)70143-6. discussion 338–340. [DOI] [PubMed] [Google Scholar]
- 42.Scardo J, Kiser R, Dillon A, Brost B, Newman R. Hemodynamic comparison of mild and severe preeclampsia: concept of stroke systemic vascular resistance index. J Matern Fetal Med. 1996;5(5):268–272. doi: 10.3109/14767059609025433. [DOI] [PubMed] [Google Scholar]
- 43.Scardo JA, Hogg BB, Newman RB. Favorable hemodynamic effects of magnesium sulfate in preeclampsia. Am J Obstet Gynecol. 1995;173(4):1249–1253. doi: 10.1016/0002-9378(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 44.Clark SL, Southwick J, Pivarnik JM, Cotton DB, Hankins GD, Phelan JP. A comparison of cardiac index in normal term pregnancy using thoracic electrical bio-impedance and oxygen extraction (Fick) techniques. Obstetrics and gynecology. 1994;83(5 Pt 1):669–672. [PubMed] [Google Scholar]
- 45.van Oppen AC, van der Tweel I, Alsbach GP, Heethaar RM, Bruinse HW. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstetrics and gynecology. 1996;88(1):40–46. doi: 10.1016/0029-7844(96)00069-5. [DOI] [PubMed] [Google Scholar]
- 46.Parrott CW, Burnham KM, Quale C, Lewis DL. Comparison of changes in ejection fraction to changes in impedance cardiography cardiac index and systolic time ratio. Congest Heart Fail. 2004;10(2) Suppl 2:11–13. doi: 10.1111/j.1527-5299.2004.03407.x. [DOI] [PubMed] [Google Scholar]
- 47.Cybulski G, Michalak E, Kozluk E, Piatkowska A, Niewiadomski W. Stroke volume and systolic time intervals: beat-to-beat comparison between echocardiography and ambulatory impedance cardiography in supine and tilted positions. Medical & biological engineering & computing. 2004;42(5):707–711. doi: 10.1007/BF02347554. [DOI] [PubMed] [Google Scholar]