Abstract
Background
Pain is common in patients with liver disease, difficult to treat, and poorly understood.
Aims
The aim of this study was to determine factors associated with pain and prescription opioid use in a large cohort of patients with confirmed chronic liver.
Methods
This was a retrospective cohort study of consecutive patients with chronic liver disease visiting a tertiary-care hepatology clinic. Pain was determined by self-report and rated numerically from 0–10. Symptoms of mood and sleep disorders and emotional distress were based on a symptom checklist. Etiology and stage of liver disease and use of prescribed opioids were abstracted from the electronic medical record. Logistic regression was used to establish factors associated with pain and prescription opioid use.
Results
Among 1286 patients with chronic liver disease, 34% had pain and 25% used opioids. The strongest predictor of pain in multivariate modeling was emotional distress (OR=3.66, CI=2.40,5.64), followed by non-white race (OR=1.87, CI=1.24,2.79), mood symptoms (OR=1.47, CI=1.04,2.07), sleep disturbance/fatigue (OR=1.70, CI=1.24,2.32), and advanced liver disease (Child class B: OR=1.73, CI=1.15,2.60; Child class C: OR=2.78 CI=1.49,5.24) compared to no cirrhosis. Emotional distress, mood-related symptoms, and advanced liver disease were also significant predictors of prescription opioid use, as were age, nicotine use, and etiology of liver disease.
Conclusions
This large cohort study demonstrates the high prevalence of pain and opioid use in chronic liver disease. While disease variables contribute to pain, psychological symptoms were most strongly associated with pain and opioid use, providing rationale and target for therapeutic interventions.
Keywords: narcotics, cirrhosis, pain management, psychosocial
Introduction
Pain is called the fifth vital sign because it is at the crux of the experience of disease. Pain relates to disability, distress, and health outcomes, and yet it has been under-studied in the area of chronic liver disease. Estimates of pain prevalence in chronic liver diseases range from 17%–24% [1] in cirrhosis up to 67% in chronic hepatitis C virus (HCV)[2]. For affected patients, pain constitutes a significant burden with poor health-related quality of life [1]. For healthcare providers, pain in patients with chronic liver disease is difficult to treat as available medical management is associated with a higher complication rate due to bleeding or hepatotoxicity or comes with concerns about relapsing drug abuse, and worsening cognitive function. Moreover, the more widespread use of prescribed opioids for benign disorders has been linked to a significant increase in the number of death caused by unintentional drug overdoses [3] and worsened outcomes [4]. Additionally, preliminary work has shown that opioids may increase the progression of liver fibrosis in animal models [5].
The first step in developing evidence-based treatments for pain in patients with chronic liver disease is to understand the risk factors for pain in this population. Relatively little work has been done in this regard. It is unclear whether pain is associated with severity of liver disease, though a small study of patients with cirrhosis suggests that this may be the case [6]. It is also unclear whether particular etiologies of liver disease are associated with more pain, though one study demonstrated increased dyspepsia in patients with HCV compared to other liver diseases. [7] Fibromyalgia and functional dyspepsia, the two pain syndrome examined in patients with liver disease, are both associated with depression [8, 9], and psychiatric comorbidities are found in up to two thirds of patients with liver disease.[10, 11] However, it is unknown how psychiatric symptoms relate to pain in chronic liver disease.
Considering the importance of pain as a burden in terms of prevalence, impact on quality of life and difficult to manage problem for physicians, the purpose of this investigation was to understand factors associated with pain and its treatment in patients with chronic liver disease in order to find potentially modifiable targets for intervention. We hypothesized that pain would be related to advanced stage of liver disease and to psychological symptoms.
Methods
This retrospective chart review study was conducted at the University of Pittsburgh after obtaining permission from the Institutional Review Board. A consecutive sample of outpatients seen in the Center for Liver Diseases over a 2-month period between 12/1/10 and 1/31/11 was assessed. Patients were excluded if they did not have confirmed chronic liver disease, were not seen by a hepatology physician or physician extender (e.g. seen for vaccinations, teaching, or lab draws), were pregnant, had previously undergone liver transplantation, or were seen for acute viral or acute alcoholic hepatitis or abnormal liver function tests without defined underlying chronic liver disease.
Pain
Pain and prescription opioid use were the main outcomes of interest. The presence and location of pain were based on patient report. This information is routinely obtained by the triaging clinic nurse at each visit who asked the patients if they had pain on that day. These data are entered in the electronic medical record. In addition, presence and severity of pain were defined by numeric pain rating (range 0–10). Patients were categorized as having pain if they endorsed pain at any visit over a 6-month period of follow-up. Consistent with prior reports [12], “moderate pain” was defined as any pain rating of ≥6. A detailed self-assessment form for review of systems was reviewed for the time of the index visit to abstract painful symptoms, including chest pain, headache, stomach discomfort, joint aches, leg cramps. The use of opioid medications and other analgesics was determined based on the list of prescription medications charted by the clinic nurse at the time of the index visit. Methadone was not placed in the “opioid” category because the overwhelming majority of patients were on methadone for addiction management rather than for pain management and the goal of this study was to determine the factors associated with opioid-based pain management.
Disease Classification
Chronic liver disease was defined as any liver condition lasting or expected to last for ≥6 months. Cirrhosis and etiology, stage, and complications of disease were defined by physician notation in the chart or by the most recent blood tests, imaging, and physical exam if not stated. The etiologies were collapsed as follows: autoimmune hepatitis/primary biliary cirrhosis/primary sclerosing cholangitis (AIH/PBC/PSC), alcohol alone, non-alcoholic fatty liver disease (NAFLD), HCV, hepatitis B virus (HBV), and other metabolic and structural causes of chronic liver disease (e.g. hereditary hemochromatosis, alpha-1-antitrypsin deficiency, nodular regenerative hyperplasia). NAFLD included those with “cryptogenic” cirrhosis thought to be most likely NAFLD-related. Hepatocellular carcinoma (HCC) status was obtained by reviewing the physician notes and radiology reports.
Neuro-psychological symptoms
The presence of mood and sleep symptoms was based on a series of questions contained in the routinely-administered self-assessment form and recorded at the index visit. Mood symptoms were defined as a positive response to any of the following items: “feeling overwhelmed”, “anxious/nervous”, “lonely/depressed”, or “thoughts of hurting yourself in the past month”. Sleep symptoms included a positive response to either “tire easily,” “trouble sleeping/nightmares”. Emotional distress was defined by a positive response to a question asked by a nurse as follows: “During the past 4 weeks have you experienced any emotional difficulties that have affected your ability to complete your activities of daily living?” at the index visit.
Other Variables
Other variables were collected from the electronic medical record as close to the index visit as possible. Race and marital status were self-reported. BMI was calculated from computerized data extraction of the height and weight. Nicotine, alcohol, and drug use were coded as past or ongoing based on the detailed review of the electronic medical record with the closest proximity to the visit of interest. Nicotine use was used rather than smoking, and included those with use of nicotine in other forms. Because very few patients (<1%) endorsed active heavy use of alcohol or drugs, the variables included in modeling were any history alcohol use beyond that considered by the physician to be social drinking and any illicit drug use. Marijuana use was excluded as it was not routinely assessed and recorded.
Statistical Analysis
Analyses were completed using the R statistical package, version 2.14.0. Data are given as mean ± standard deviation or medians with interquartile ranges (IQR). The Kruskal-Wallis test was used to compare the median number of pain-related symptoms on review of systems forms to the stage of liver disease. Univariate analyses were completed for those with pain vs. no pain and covariates using t-tests, chi-square, and Fisher’s exact test where appropriate. Covariates with p-values ≤ 0.2 on univariate testing were included in the initial logistic regression models based on an a priori decision rule. Multivariable logistic regression models were made, adjusting first for Child’s class and then for ascites and encephalopathy in order to explore whether specific complications of advanced disease were driving pain and opioid use. In these models the dependent variables were pain defined as any pain and opioid use. Secondary analyses were run using moderate pain as the dependent variable.
The StepAIC function was used in R to create logistic regression models to predict pain. Overall p-values for multi-level variables were reported in the tables using a Wald Test. Where multiple pair-wise comparisons were made, a Holm-Bonferroni correction was used. Continuous variables including age and BMI were standardized before performing logistic regression. Models were checked for multicollinearity using variance inflation factor scores with a pre-specified limit of 5.0.
Results
Population
A total of 1437 individuals were seen for evaluation of liver disease within the timeframe of the study. Out of this group 151 patients were excluded (prior liver transplant: n=20; no evidence of chronic liver disease: n=98; acute hepatitis: n=24; pregnancy: n=3; no assessment of pain over a 6 month period: n=6), leaving a cohort of 1286 patients. The sample was predominantly Caucasian (87%), 51% male, with a mean age of 54±13 years and 48% of the patients carrying a diagnosis of cirrhosis. The most common etiologies of liver disease were HCV±alcohol (46%), NAFLD (19%), cholestatic and autoimmune etiologies (12%), alcohol alone (10%), and HBV (5%).
Pain and Medication Use
Twenty-five percent were prescribed opioids at the index visit, and 34% reported pain over a 6-month period. Among 436 patients with pain, detailed pain ratings were missing in 24%. Moderate pain levels (≥6 on the rating scale) were reported by 45%. Interestingly, 38% of the patients with pain continued to describe pain despite being prescribed opioids. Consistent with this finding, the mean pain levels for patients with pain on opioids was 6.2±2.1 compared to 5.4±2.3 for those not taking opioids (p=0.0004). Conversely, 18% of patients without pain were using opioids (Table 1). Many patients were taking a wide variety of medications, such as antidepressants (33%), benzodiazepines (19%), sleep medications (9%), and analgesic drugs (44%). Reporting pain correlated with a higher likelihood of taking analgesic medications but also with all other agents examined other than aspirin, which was typically used in the cardioprotective dosing range (Table 1). As liver disease advanced, significantly more opioids and less non-steroidal anti-inflammatory drugs (NSAIDs) were prescribed without differences in other medications across the stages of liver disease (Table 1).
Table 1.
Prescription medication use by pain status and stage of liver disease+
| Medication | No pain (N=850) | Pain (N=436) | P | No cirrhosis (N=674) | Child’s A (N=342) | Child’s B (N=207) | Child’s C (N=63) | P |
|---|---|---|---|---|---|---|---|---|
| Antidepressants (SSRI/SNRI) | 238 (28) | 182 (42) | <0.0001* | 234 (35) | 105 (31) | 57 (28) | 24 (38) | 0.15 |
| Benzodiazepines | 138 (16) | 101 (23) | 0.003* | 132 (20) | 61 (18) | 30 (14) | 16 (25) | 0.19 |
| Sleep aids | 60 (7) | 55 (13) | 0.001* | 60 (9) | 33 (10) | 14 (7) | 8 (13) | 0.47 |
| Mood stabilizers | 134 (16) | 132 (30) | <0.0001* | 146 (22) | 67 (20) | 41(20) | 12 (19) | 0.84 |
| Opioids | 155 (18) | 166 (38) | <0.0001* | 135 (20) | 81 (24) | 70 (34) | 35 (56) | <0.0001* |
| Methadone | 18 (2) | 25 (6) | 0.001* | 26 (4) | 6 (2) | 6 (3) | 5 (8) | 0.06 |
| TCA | 41 (5) | 38 (9) | 0.01* | 53 (8) | 16 (5) | 7 (3) | 3 (5) | 0.05 |
| Trazodone | 34 (4) | 57 (13) | <0.0001* | 50 (7) | 15 (4) | 19 (9) | 7 (11) | 0.08 |
| Acetaminophen | 126 (15) | 105 (24) | <0.0001* | 133 (20) | 58 (17) | 28 (14) | 12 (19) | 0.21 |
| NSAIDs | 140 (16) | 111 (25) | 0.0002* | 168 (25) | 61 (18) | 18 (9) | 4 (6) | <0.0001* |
| Aspirin | 163 (19) | 77 (18) | 0.56 | 118 (18) | 85 (25) | 27 (13) | 10 (16) | 0.003* |
Numbers in () are column percentages
statistical significance (p<0.05)
SSRI=serotonin reuptake inhibitor, SNRI=serotonin-norepinephrine reuptake inhibitor
TCA=tricyclic antidepressant
NSAIDs=non-steroidal anti-inflammatory drugs
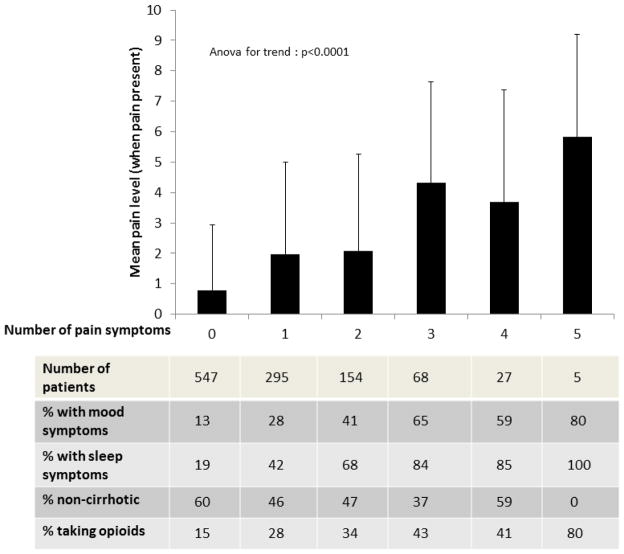
Among the 331 out of 436 patients with pain who completed pain scales, the mean pain severity score (when pain was reported) was 5.7±2.3 out of 10. The most common pain locations were abdominal (n=308) and joint/extremity and/or back (n=75) and 57 patients described more than one painful area. In 15 patients, no information about the location of their pain was available. Figure 1 demonstrates that as the number of pain-related symptoms endorsed on the review of systems form increased, the patients’ mean pain ratings (p<0.0001) and likelihood of sleep or mood symptoms and opioid use rose(p<0.0001). There was a similar relationship between more advanced stages of liver disease and increased number of symptoms (p<0.0001).
Figure 1.
Relationship between the number of pain-related symptoms on the review of systems form and other variables
Univariate Analysis
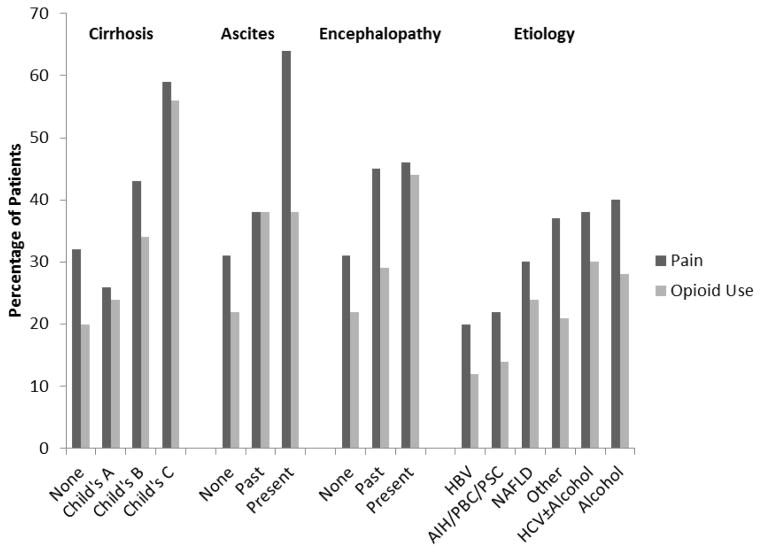
Table 3 shows the factors associated with pain in univariate analyses. Patients complaining of pain (N=428) were significantly more likely to be younger, non-white, and unmarried with more nicotine, alcohol, and drug use and a higher likelihood of mood and sleep symptoms and emotional distress. The positive associations between disease factors such and pain are highlighted by Figure 2.
Table 3.
Final logistic regression model for predictors of pain+
| Any Pain | Moderate Pain | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR | CI | P | OR | CI | P* |
| Age | 0.89 | 0.76,1.04 | 0.15 | |||
| Non-white race | 1.87 | 1.24,2.79 | 0.002* | 2.46 | 1.42,4.21 | 0.001* |
| Married vs. not | 0.79 | 0.59,1.05 | 0.11 | 0.66 | 0.45,0.98 | 0.04* |
| BMI | 1.13 | 0.98,1.29 | 0.09 | 1.20 | 1.00,1.44 | 0.049* |
| Mood symptoms | 1.47 | 1.04,2.07 | 0.03* | 1.46 | 0.93,2.29 | 0.10 |
| Sleep symptoms | 1.70 | 1.24,2.32 | 0.0008* | 2.35 | 1.53,3.61 | <0.0001* |
| Emotional distress | 3.66 | 2.40,5.64 | <0.0001* | 5.07 | 3.04,8.50 | <0.0001* |
| Nicotine use | 1.29 | 0.97,1.72 | 0.08 | 1.65 | 1.09,2.53 | 0.02* |
| Child’s class (vs. no cirrhosis)Ŧ | 0.0002* | <0.0001* | ||||
| Child’s A | 0.86 | 0.60,1.24 | 0.42 | 1.05 | 0.64,1.70 | 0.85 |
| Child’s B | 1.73 | 1.15,2.60 | 0.02* | 2.24 | 1.33,3.77 | 0.004* |
| Child’s C | 2.78 | 1.49,5.24 | 0.003* | 5.14 | 2.47,10.68 | <0.0001* |
N=1062 for any pain, N=873 for moderate pain (those with lower pain levels or no pain scores were excluded)
statistical significance (p<0.05)
overall p value based on Wald test for the overall variable significance. Each level p value was adjusted with Holm-Bonferroni correction
Figure 2.
Percentage of patients with pain and opioid use by baseline characteristics
Multivariate Analyses
Table 3 shows that the strongest predictor of pain in multivariate modeling was emotional distress (OR=3.66, CI=2.40,5.64), with other independently-associated factors including non-white race (OR=1.87, CI=1.24,2.79), symptoms of anxiety and depression (OR=1.47, CI=1.04,2.07), sleep disturbance or fatigue (OR=1.70, CI=1.24,2.32), and advanced liver disease including Child’s class B (OR=1.73, CI=1.15,2.60) and C (OR=2.78 CI=1.49,5.24) compared to no cirrhosis. Once controlling for these factors, etiology of liver disease was no longer a significant predictor of pain. Changing the definition of pain to moderate pain on a pain scale produced similar results, though unmarried marital status, higher BMI, and nicotine use reached significance in the final model.
When symptoms of advanced cirrhosis including encephalopathy and ascites were substituted for Child’s class, the results were similar. Encephalopathy was associated with pain in the final model (OR=1.81, CI=1.12,2.91), as was the presence of ascites on the day of the index visit (OR=2.47, CI=1.37,4.49). In contrast, a history of ascites that had since resolved did not correlate with pain. Similar results were found for those with moderate pain (Table 3).
Prescription Opioid Use
In univariate analysis, older age, BMI, advanced cirrhosis, HCC, HE, ascites, mood symptoms, sleep symptoms, emotional distress, and substance use were significantly associated with increased prevalence of opioid use (Table 2). The rates of opioid use varied by etiology and stage of liver disease (Figure 2). Opioid use was significantly associated with older age, mood symptoms, emotional distress, nicotine use, and advanced cirrhosis in the final model (Table 5). Etiology overall significantly correlated with opioid use in the final model, with those with HCV±alcohol and NAFLD having the most opioid use and those with AIH/PBC/PSC and alcohol having the least. Opioid use for the HCV±alcohol group was no different from the NAFLD group. Substituting ascites and encephalopathy for Child’s class did not change the above results significantly, and encephalopathy remained in the final model (OR=2.33, CI=1.59,3.41).
Table 2.
Characteristics associated with pain and narcotic use+
| Characteristic | No pain (N=850) | Any pain (N=436) | P | No narcotic (N=965) | Narcotic use (N=321) | P |
|---|---|---|---|---|---|---|
| Age | 53.2±13.6 | 51.4±11.7 | 0.01* | 52.1+13.8 | 54.1+10.4 | 0.008* |
| Non-white race | 99 (12) | 71 (16) | 0.03* | 123 (12) | 47 (15) | 0.52 |
| Female gender | 409 (48) | 227 (52) | 0.20 | 486 (50) | 164 (51) | 0.87 |
| Married | 465 (55) | 194 (44) | 0.0007* | 493 (51) | 166 (51) | 0.90 |
| BMI | 28.7±6.3 | 29.4±6.5 | 0.08 | 28.7±6.1 | 29.7±7.0 | 0.03* |
| Cirrhosis | <0.0001* | <0.0001* | ||||
| None | 454 (53) | 220 (50) | 539 (56) | 135 (42) | ||
| Child’s A | 252 (30) | 90 (21) | 261 (27) | 81 (25) | ||
| Child’s B | 118 (14) | 89 (20) | 137 (14) | 70 (22) | ||
| Child’s C | 26 (3) | 37 (8) | 28 (3) | 35 (11) | ||
| Nicotine exposure | 0.0008* | 0.001* | ||||
| None | 367 (43) | 144 (33) | 412 (43) | 99 (31) | ||
| Past | 235 (28) | 132 (30) | 263 (27) | 104 (32) | ||
| Current | 243 (29) | 160 (37) | 285 (30) | 118 (37) | ||
| Alcohol use (more than social) | 244 (29) | 165 (38) | 0.001* | 279 (29) | 130 (40) | 0.0001* |
| Drug use | 252 (30) | 165 (38) | 0.004* | 292 (30) | 125 (39) | 0.005* |
| Etiology | 0.0003* | 0.0002* | ||||
| AIH/PBC/PSC | 121 (14) | 34(8) | 133 (14) | 22 (7) | ||
| ALCOHOL | 78 (9) | 52 (12) | 93 (10) | 37 (12) | ||
| HCV±ALCOHOL | 363 (43) | 223 (51) | 413 (43) | 173 (54) | ||
| NAFLD | 167 (20) | 73 (17) | 182 (18) | 58 (18) | ||
| HBV | 48 (6) | 12 (3) | 53 (5) | 7 (2) | ||
| Other | 73 (9) | 42 (10) | 91 (9) | 24 (7) | ||
| Mood symptoms | 142 (17) | 140 (32) | <0.0001* | 182 (19) | 100 (31) | <0.0001* |
| Sleep symptoms | 225 (26) | 195 (45) | <0.0001* | 286 (30) | 134 (42) | <0.0001* |
| Emotional distress | 59(7) | 107 (25) | <0.0001* | 104 (11) | 62 (19) | <0.0001* |
| HCC | 19 (2) | 12 (3) | 0.70 | 17 (2) | 14 (4) | 0.02* |
| Ascites | <0.0001* | <0.0001* | ||||
| None | 701 (82) | 308 (71) | 792 (82) | 217 (68) | ||
| Past | 116 (14) | 70 (13) | 54 (6) | 33 (10) | ||
| Present | 31 (4) | 56 (16) | 116 (12) | 70 (22) | ||
| Encephalopathy | 0.0003* | <0.0001* | ||||
| None | 729 (86) | 334 (77) | 831 (86) | 232 (72) | ||
| Past | 93 (11) | 77 (18) | 35 (4) | 14 (4) | ||
| Present | 26 (3) | 23 (5) | 96 (10) | 74 (23) |
Categorical variables are reported as N(%), continuous are mean±sd
p value statistically significant (<0.05)
Discussion
Our investigation is the first cohort study to determine prevalence of pain and opioid use and associated risk factors in a large population of outpatients with chronic liver disease of all etiologies. The data clearly confirm the importance of pain, which affected one third of the patients and was rated as moderate or severe in more than half of the cases. Published case series on pain prevalence in patients with liver disease vary significantly, from 20–96% depending on study design, patient selection, and focus on different pain syndromes [13] [14]. Our results are consistent with most studies with larger and less skewed patient populations, describing pain as a problem in about 30–40% of patients with liver disease [15–17] [1, 2], though these studies have predominantly focused on HCV. Pain is not only common, but also constitutes a significant burden for patients, the healthcare system and society, as it is associated with poor quality of life, impaired role functioning, higher resource utilization, and absenteeism or disability [15, 16, 18].
Consistent with prior studies, the majority of patients complained about abdominal pain [7, 16, 17, 19]. Hepatocytes do not have sensory innervation, with afferent nerves instead tracking along the vasculature and biliary tree [20]. While hepatic capsular distension, splenomegaly, and ascites with abdominal distension potentially explain some of the discomfort with advancing disease, the majority of patients presented with less advanced stages of liver disease in this study. In fact, only 13% of patients with pain had ongoing ascites, and 71% had no history of ascites. Additionally, the rise in pain prevalence with advancing disease included pain at any site (not just the abdomen) with painful symptoms at an increasing number of sites as liver disease advanced, which implies systemic mechanisms or changes in central pain processing. Indeed, patients with HCV, who have been the best-studied regarding painful systemic conditions, have been found to have a high prevalence of functional gastrointestinal disorders and fibromyalgia [21, 22]. The link between these painful systemic conditions and liver disease may be underlying psychopathology.
Psychiatric symptoms were strongly associated with pain, highlighting the potential role of central pain mechanisms in pain in chronic liver diseases. Mood disorders have commonly been described in patients with liver disease [11, 23] with a prevalence of up to 50%. In other disease models psychiatric disease has been associated with increased pain reporting [24, 25]. In one study of HCV, depression was the best predictor of pain and pain functioning[26]. This supports a central process rather than peripherally mediated sensitization of nociceptive pathways as the likely underlying mechanism for the high prevalence of pain in patients with liver disease. This association may have therapeutic implications, as treatment of mood disorders may ameliorate pain or at least lessen the impact of pain on overall function, which may in turn lower the need for other interventions, including opioid use.
We did not find a strong correlation between pain and the etiology of liver disease, once we controlled for disease stage and confounding psychiatric symptoms. Since some categories had very few patients and HCV is thought to be associated with pain, we collapsed the etiologies into HCV vs. other and confirmed that etiology remained a non-significant contributor to pain. These results differ from prior research [7, 19, 27], which demonstrated a higher prevalence of functional gastrointestinal disorders and musculoskeletal pain in HCV than in patients with other liver diseases. However, this discrepancy likely arises from the fact that prior studies did not control for psychiatric disease. Mood symptoms and/or reported emotional distress were very common in our cohort of HCV-infected patients. All of these factors have previously been reported as risk factors for pain and opioid use and controlling for these may account for this apparent discrepancy [28, 29].
Pain was significantly associated with non-white race in this study. Such racial differences in pain have been previously reported and attributed to implicit provider biases leading to decreased opioid prescriptions for African-American patients [30]. However, a secondary analysis looking at African-American race specifically, found a significant association with pain but not with opioid use after controlling for pain, arguing against such a bias as an underlying factor in our population. We were unable to assess socioeconomic status (SES) in this retrospective cohort, which is a known confounder of race. However, in one study, African-Americans had increased chronic pain and pain-related disability even after carefully controlling for SES [31]. While our data do not allow firm conclusions about the impact of race on pain and pain management, they point towards racial disparities in healthcare, which have been previously described as related to subtle biases in the medical system, the stress of discrimination, and differential access to healthcare [32].
Our results also demonstrate a 25% prevalence of prescription opioid use among patients with chronic liver disease. While higher than in the general population [33] [2], the findings fall within the range of opiate use in patients with other benign disorders of the gastrointestinal tract [4] [34] [35]. Interestingly, the correlation between pain and opioid use was quite low. Among patients with pain, opioid use was associated with higher pain ratings, and 18% of pain-free patients were prescribed opioids. While we could not determine the impact of opioids on pain severity, our data correspond to prior studies demonstrating that chronic opioid use may only slightly improve pain ratings and has a limited impact on overall functional status in patients with benign disorders [36, 37].
Opioid use increased with progression of liver disease, psychiatric symptoms, and etiology of liver disease. The association with progression of disease may in part reflect the higher prevalence of pain or the difficulty of using other agents such as NSAIDS and acetaminophen for pain management in cirrhosis. However, opioids have deleterious effects in cirrhosis, and this finding illustrates a need to explore other methods of pain control. Beyond concerns about the potential of recurrent drug abuse and addiction, encephalopathy is associated with opioid use. In fact, those patients without pain who were on opioids had the highest rates of active encephalopathy (7% vs. 3–4% in other groups of patients), highlighting the need to closely monitor and/or limit opioid use in individuals at risk for encephalopathy. Opioid use also correlated with mood and emotional distress. While such affective symptoms potentially contribute to central sensitization and pain, they are also strong predictors of non-medical use of opioids [29, 38, 39]. Consistent with non-medical use of prescription opioids, patients with HCV had the highest rates of prior drug abuse and also the highest rates of prescription opioid use. However, a history of substance abuse does not account for all differences in opioid use by etiology. NAFLD and HCV patients had surprisingly similar rates of prescription opioid use. This may in part relate to the association of NAFLD with obesity, which in turn is associated with osteoarthritis. These findings illustrate the need for alternative strategies for pain management in cirrhosis of all etiologies.
There were several notable limitations of this study. Given that retrospective design precludes causal inferences, it is difficult to say whether the psychiatric symptoms were caused by having pain or, alternatively, if they sensitized to or even mediated pain through somatization or catastrophizing. However, the results fit into the larger body of existing literature, demonstrating the important role of affect in the manifestations of disorders with chronic pain [8]. Furthermore, we relied on non-validated screening questions rather than standardized questionnaires to assess psychiatric symptoms. While this approach does not allow us to conclude about the presence, nature, and severity of defined affective spectrum disorders, the use of a priori symptom clustering and a question regarding emotional distress affecting activities of daily living focused on manifestations of psychiatric disorders and were very strong predictors of pain and opioid use. Similarly, the definition of pain was based on a yes/no response to a triage question, with the more detailed numerical pain severity rating missing in some patients. While the missing response could potentially skew our data, there were no significant differences of baseline characteristics between patients with and without pain ratings. Minor pain may lead to an affirmative response to this screening question, but may not be clinically relevant. We therefore operationally defined boundaries for a likely relevant pain by a value that exceeded the midpoint of the 11 point numeric rating scale. While somewhat arbitrary, this value coincided with the median pain intensity of those reporting pain in this cohort. The qualitatively similar results of both approaches support our conclusions. Pain severity ratings do not reliably reflect pain-related dysfunction or disability, which was not assessed. Additionally, substance abuse was not prospectively or systematically assessed, and very few patients admitted to ongoing substance abuse. Finally, we did not account for frequency and duration of use or differences in opioid dosing, which will be important in order to relate opioid use to disease mechanisms or patient outcomes in the future. Though this study was completed in a single tertiary care center, the use of a large population of unselected patients with different etiologies and stages of liver disease make it likely that our results highlight important mechanisms that contribute to pain and opioid use in these patients.
In conclusion, this first large study of pain in unselected patients with chronic liver disease demonstrates that pain is a common symptom and is strongly associated with psychiatric symptoms, race, and progression of disease, rather than etiology of liver disease, as was previously thought. Beyond providing potential mechanistic insight, the important correlation between psychiatric symptoms with pain and prescription opioid use suggests a potentially modifiable target for therapeutic interventions.
Table 4.
Final logistic regression model for narcotic use, N=1087
| Covariate | OR+ | 95% CI | p* |
|---|---|---|---|
| Age | 1.21 | 1.02,1.44 | 0.03* |
| Mood sxs | 1.63 | 1.13,2.34 | 0.009* |
| Sleep sxs | 1.34 | 0.96,1.87 | 0.08 |
| Emotional Distress | 1.60 | 1.04,2.44 | 0.03* |
| Nicotine use | 1.45 | 1.05,2.04 | 0.03* |
| Cirrhosis (vs. none)+ | <0.0001* | ||
| Child’s A | 1.16 | 0.79,1.69 | 0.43 |
| Child’s B | 1.75 | 1.13,2.70 | 0.02* |
| Child’s C | 4.17 | 2.19,8.00 | <0.0001* |
| Etiology of liver disease (vs. HCV±alcohol)+ | 0.01* | ||
| Alcohol | 0.49 | 0.30,0.88 | 0.04* |
| NAFLD | 0.85 | 0.58,1.34 | 0.44 |
| AIH/PBC/PSC | 0.43 | 0.24,0.77 | 0.03* |
| HBV | 0.50 | 0.19,1.21 | 0.39 |
| Other | 0.66 | 0.38,1.20 | 0.30 |
denotes statistical significance (p<0.05)
p from Wald test for the overall variable, p values for each level were adjusted with a Holm-Bonferroni correction
Acknowledgments
Grant Support: Shari Rogal was supported by NIH-T32 grant number DK063922. Eva Szigethy has funding from the NIMH. The project described was supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005.
Footnotes
Disclosures: Eva Szigethy has received an honorarium and travel expenses as speaker Merck for an international pediatiatric IBD symposium in Holland. The other authors have nothing to disclose.
Writing Assistance: None
Contributor Information
Shari S. Rogal, Email: rogalss@upmc.edu.
Daniel Winger, Email: dgw14@pitt.edu.
Klaus Bielefeldt, Email: bielefeldtk@upmc.edu.
Eva Szigethy, Email: szigethye@upmc.edu.
References
- 1.Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–8. doi: 10.1053/gast.2001.21193. S0016508501044213 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Whitehead AJ, Dobscha SK, Morasco BJ, Ruimy S, Bussell C, Hauser P. Pain, substance use disorders and opioid analgesic prescription patterns in veterans with hepatitis C. J Pain Symptom Manage. 2008;36(1):39–45. doi: 10.1016/j.jpainsymman.2007.08.013. S0885-3924(08)00063-8 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. 152/2/85 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621–30. doi: 10.1016/j.cgh.2006.03.002. S1542-3565(06)00228-X [pii] [DOI] [PubMed] [Google Scholar]
- 5.Bekheet SH. Morphine sulphate induced histopathological and histochemical changes in the rat liver. Tissue Cell. 2010;42(4):266–72. doi: 10.1016/j.tice.2010.06.001. S0040-8166(10)00048-0 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Thacker LR, Wade JB, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Aliment Pharmacol Ther. 2011;34(9):1123–32. doi: 10.1111/j.1365-2036.2011.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley TR, 3rd, Koch K. Characteristics of upper abdominal pain in those with chronic liver disease. Dig Dis Sci. 2003;48(10):1914–8. doi: 10.1023/a:1026149732756. [DOI] [PubMed] [Google Scholar]
- 8.Van Oudenhove L, Vandenberghe J, Geeraerts B, et al. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57(12):1666–73. doi: 10.1136/gut.2008.158162. gut.2008.158162 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Alok R, Das SK, Agarwal GG, Salwahan L, Srivastava R. Relationship of severity of depression, anxiety and stress with severity of fibromyalgia. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S70–2. 4818 [pii] [PubMed] [Google Scholar]
- 10.Kravitz HM, Janssen I, Lotrich FE, Kado DM, Bromberger JT. Sex steroid hormone gene polymorphisms and depressive symptoms in women at midlife. Am J Med. 2006;119(9 Suppl 1):S87–93. doi: 10.1016/j.amjmed.2006.07.010. S0002-9343(06)00832-1 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Ewusi-Mensah I, Saunders JB, Wodak AD, Murray RM, Williams R. Psychiatric morbidity in patients with alcoholic liver disease. Br Med J (Clin Res Ed) 1983;287(6403):1417–9. doi: 10.1136/bmj.287.6403.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–84. doi: 10.1016/0304-3959(94)00178-H. 0304-3959(94)00178-H [pii] [DOI] [PubMed] [Google Scholar]
- 13.Laurin JM, DeSotel CK, Jorgensen RA, Dickson ER, Lindor KD. The natural history of abdominal pain associated with primary biliary cirrhosis. Am J Gastroenterol. 1994;89(10):1840–3. [PubMed] [Google Scholar]
- 14.McKenna O, Cunningham C, Blake C. Socio-demographic and clinical features of Irish iatrogenic hepatitis C patients: a cross-sectional survey. BMC Public Health. 2009;9:232. doi: 10.1186/1471-2458-9-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.daCosta DiBonaventura M, Yuan Y, Wagner J, L’Italien G, Lescrauwaet B, Langley P. The burden of viral hepatitis C in Europe: a propensity analysis of patient outcomes. Eur J Gastroenterol Hepatol. 2012;24:869–77. doi: 10.1097/MEG.0b013e3283551dee. [DOI] [PubMed] [Google Scholar]
- 16.Louie K, St Laurent S, Forssen U, Mundy L, Pimenta J. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis. 2012;12:86. doi: 10.1186/1471-2334-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oettinger R, Brunnberg A, Gerner P, Wintermeyer P, Jenke A, Wirth S. Clinical features and biochemical data of Caucasian children at diagnosis of autoimmune hepatitis. Journal of Autoimmunity. 2005;24(1):79–84. doi: 10.1016/j.jaut.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, DiBonaventura M, Yuan Y, et al. The burden of illness for patients with viral hepatitis C: evidence from a national survey in Japan. Value Health. 2012;15:S65–71. doi: 10.1016/j.jval.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Fouad YM, Makhlouf MM, Khalaf H, Mostafa Z, Raheem EA, Meneasi W. Is irritable bowel syndrome associated with chronic hepatitis C? Journal of Gastroenterology and Hepatology. 2010;25(7):1285–8. doi: 10.1111/j.1440-1746.2010.06311.x. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):827–35. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 21.Bondini S, Kallman J, Dan A, et al. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27(8):1119–25. doi: 10.1111/j.1478-3231.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 22.Fouad YM, Makhlouf MM, Khalaf H, Mostafa Z, Abdel Raheem E, Meneasi W. Is irritable bowel syndrome associated with chronic hepatitis C? J Gastroenterol Hepatol. 2010;25(7):1285–8. doi: 10.1111/j.1440-1746.2010.06311.x. JGH6311 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosom Med. 2006;68(4):563–9. doi: 10.1097/01.psy.0000221276.17823.df. 68/4/563 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Kojima T, Suzuki S, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(8):1018–24. doi: 10.1002/art.24647. [DOI] [PubMed] [Google Scholar]
- 25.Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42(1):1–11. doi: 10.1016/j.jpainsymman.2010.10.261. S0885-3924(11)00018-2 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Morasco BJ, Huckans M, Loftis JM, et al. Predictors of pain intensity and pain functioning in patients with the hepatitis C virus. Gen Hosp Psychiatry. 2010;32(4):413–8. doi: 10.1016/j.genhosppsych.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkhuizen A, Rosen HR, Wolf S, Flora K, Benner K, Bennett RM. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology clinic patients. Am J Gastroenterol. 1999;94(5):1355–60. doi: 10.1111/j.1572-0241.1999.01087.x. S0002-9270(99)00143-4 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Dhingra L, Masson C, Perlman DC, et al. Epidemiology of pain among outpatients in methadone maintenance treatment programs. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2012.08.003. (0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: Psychiatric, medical and substance use correlates. Drug and Alcohol Dependence. 2008;94(1–3):38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Sabin JA, Greenwald AG. The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am J Public Health. 2012;102(5):988–95. doi: 10.2105/AJPH.2011.300621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green CR, Hart-Johnson T. The association between race and neighborhood socioeconomic status in younger black and white adults with chronic pain. J Pain. 2012;13(2):176–86. doi: 10.1016/j.jpain.2011.10.008. S1526-5900(11)00862-5 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–88. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–9. doi: 10.1016/j.pain.2008.04.027. S0304-3959(08)00235-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nusrat S, Yadav D, Bielefeldt K. Pain and opioid use in chronic pancreatitis. Pancreas. 2012;41(2):264–70. doi: 10.1097/MPA.0b013e318224056f. [DOI] [PubMed] [Google Scholar]
- 35.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9(12):1056–64. doi: 10.1016/j.cgh.2011.08.013. quiz e133–4. S1542-3565(11)00890-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusrat S, Yadav D, Bielefeldt K. Pain and Opioid Use in Chronic Pancreatitis. Pancreas. 2012:264–267. doi: 10.1097/MPA.0b013e318224056f. [DOI] [PubMed] [Google Scholar]
- 38.Barry DT, Goulet JL, Kerns RK, et al. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain. 2011;152(5):1133–8. doi: 10.1016/j.pain.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE. Gender and non-medical use of prescription opioids: results from a national US survey*. Addiction. 2008;103(2):258–68. doi: 10.1111/j.1360-0443.2007.02056.x. [DOI] [PubMed] [Google Scholar]