Abstract
A number of new positive allosteric modulators (PAMs) have been reported that enhance responses of neuronal alpha7 and alpha4beta2 nicotinic acetylcholine receptor subtypes to orthosteric ligands. PAMs represent promising new leads for the development of therapeutic agents for disorders involving alterations in nicotinic neurotransmission including Autism, Alzheimer's and Parkinson's disease. During our recent studies of alpha4beta2 PAMs, we identified a novel effect of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The effects of HEPES were evaluated in a phosphate buffered recording solution using two-electrode voltage clamp techniques and alpha4beta2 and alpha7 nicotinic acetylcholine receptor subtypes expressed in Xenopus laevis oocytes. Acetylcholine induced responses of high-sensitivity alpha4beta2 receptors were potentiated 190% by co-exposure to HEPES. Responses were inhibited at higher concentrations (bell-shaped concentration/response curve). Coincidentally, at concentrations of HEPES typically used in oocyte recording (5–10 mM), the potentiating effects of HEPES are matched by its inhibitory effects, thus producing no net effect. Mutagenesis results suggest HEPES potentiates the high-sensitivity stoichiometry of the alpha4beta2 receptors through action at the beta2+/beta2− interface and is dependent on residue beta2D218. HEPES did not potentiate low-sensitivity alpha4beta2 receptors and did not produce any observable effect on acetylcholine induced responses on alpha7 nicotinic acetylcholine receptors.
Keywords: Alpha4beta2, Alpha7, Nicotinic acetylcholine receptor, Ligand gated ion channels, Positive allosteric modulation, HEPES, desformylflustrabromine, dFbr, Two-electrode voltage clamp, Mutagenesis
1. Introduction
Alterations in expression of nicotinic acetylcholine receptors have been implicated in the etiology of several neurological disorders including Alzheimer's disease (Court et al., 2001), Parkinson's disease (Aubert et al., 1992), Autism (Martin-Ruiz et al., 2004), Schizophrenia (Adams and Stevens, 2007) and nicotine addiction (Picciotto et al., 2001). Selective targeting of specific nicotinic acetylcholine receptor subtypes by drugs that act as positive allosteric modulators (PAMs) may provide treatment options for these disorders.
PAMs are ligands that affect (1) the peak current response (Type I profile) or (2) both the peak current response and time course of an agonist-evoked response (Type II profile) (Bertrand and Gopalakrishnan, 2007). Both positive and negative allosteric modulators have been identified that bind at distinct locations from the endogenous neurotransmitter. The study of PAMS has become an active research area over the past several years and a number of modulators have been discovered including several small molecules, metal ions and anesthetics (Kim et al., 2007; Moroni et al., 2008; Nury et al., 2011; Pavlovicz et al., 2011). PAMs are of particular interest since they lack the ability to activate the receptors in the absence of an agonist and thus may preserve the spatial and temporal parameters of cholinergic signaling. Elucidation of PAM binding sites will aid the development of more potent, efficacious and selective ligands.
During our recent studies of α4β2 PAMs, we identified a novel potentiating effect of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), suggesting that it might also be a nicotinic acetylcholine receptor PAM. HEPES is a buffering agent developed by Normon Good and colleagues with a pKa value around physiological pH and thought to be physiologically inert (Good et al., 1966). Accumulating evidence suggests that HEPES is a bioactive molecule in vitro. HEPES has been shown to stimulate the production of ATP, decrease the uptake of P-glycoprotein, affect cell membranes and block chloride ion channels and mammalian 5-hydroxytryptamine transporters (Li et al., 2002). The potential for HEPES to interact with nicotinic acetylcholine receptors is suggested by its presence in the acetylcholine binding site of the 2.7 Å crystal structure of the acetylcholine Binding Protein (Brejc et al., 2001).
This study investigates the effect of HEPES on α4β2 and α7 nicotinic acetylcholine receptors including effects on acetylcholine concentration/response profiles, potentiation of acetylcholine induced responses and the effect of HEPES on potentiation of α4β2 receptors by the β2 selective PAM desformylflustrabromine (dFBr) previously described by our laboratory (Kim et al., 2007). In the case of α4β2 receptors, we have also utilized receptor preparations previously developed by Moroni et al. to investigate selectivity of these effects on high sensitivity (HS) and low sensitivity (LS) receptors (Moroni and Bermudez, 2006). LS α4β2 receptors possess a lower acetylcholine sensitivity, reduced responsiveness to up-regulation by agonists, desensitize more rapidly and display a higher Ca2+ permeability compared to HS receptors (Nelson et al., 2003; Tapia et al., 2007). Studies have suggested that both HS and LS α4β2 nicotinic acetylcholine receptors are expressed in the mammalian brain and are located presynaptically and preterminally (Butt et al., 2002; Gotti et al., 2008; Marks et al., 2000; Wonnacott, 1997).
2. Materials and methods
2.1. Receptors and mRNA
The cDNA sequences for human α4 (NCBI Reference Sequence: NM_000744.5), β2 (NCBI Reference Sequence: NM_000748.2) and α7 (NCBI Reference Sequence: NM_000746.3) nicotinic acetylcholine receptor subunits were used to synthesize a full length cDNA for each subunit. cDNA synthesis was conducted by GeneArt Inc. (Burlingame, CA). The β2 cDNA was inserted into the pcDNA3.1/ Zeo(+) mammalian expression vector with restriction enzymes Not I and Xho I and the α4 cDNA was inserted into the pcDNA3.1/ hygromyocin mammalian expression vector with restriction enzymes Hind III and BamHI (vectors procured from Invitrogen, Carlsbad, CA; restriction enzymes purchased from New England Biolabs). The constructs were transformed into AG1 super-competent cells (Stratagene) for production of cDNA The cDNA for the α7 nicotinic acetylcholine receptor subunit was inserted with restriction enzymes Sal I and Xba into the pBudCE4.1 expression vector (Invitrogen). Synthetic cRNA transcripts for the α4, β2 and α7 subunits were prepared using the T7 mMESSAGE mMACHINE™ High Yield Capped RNA Transcription Kit (Ambion, Austin, TX). All constructs were fully sequenced and confirmed to be identical to the published sequences for each subunit.
The β2D218A mutation was created using the QuickChange® mutagenesis kit (Agilent Technologies, Inc. Santa Clara, CA). The resulting DNA was used to transform AG1 super-competent cells and individual colonies were screened to identify those producing mutant β2 cDNA. To facilitate screening of mutant receptors, a silent Sac II restriction site was engineered into the mutant cDNA The mutation was confirmed by commercial DNA sequencing (Sequetech, Mountain View, CA).
2.2. Test compounds
4-(2-hydroxyethyl)-1-piperazineethanesulfonic (HEPES) sodium salt, cell culture tested, HEPES acid, ≥ 99.5% (titration), acetylcholine chloride, ≥ 99% (TLC), Tris (hydroxymethyl)-aminomethane hydrochloride, 99–101%, ACS reagent; sodium phosphate monobasic, minimum 99.0%, potassium phosphate dibasic, premium, and other salts and buffering agents were obtained from Sigma–Aldrich, Inc. (MO). Desformylflustrabromine · HCl (dFBr) was synthesized by Dr. Richard Glennon (Virginia Commonwealth University) according to a previously published procedure (Kim et al., 2007).
2.3. Xenopus laevis oocytes and receptor expression
Xenopus laevis oocytes were prepared as previously described (Weltzin and Schulte, 2010). Isolated oocytes were washed twice in Ca2+-free Barth's buffer (82.5 mM NaCl; 2.5 mM KCl; 1 mM MgCl2; 5 mM HEPES, pH 7.4) then gently shaken with 1.5 mg/ml collagenase (Sigma type II, Sigma–Aldrich Inc., MO) for 20 min at 20–25 °C. Stage V and VI oocytes were selected for microinjection.
For expression of high sensitivity (HS) and low sensitivity (LS) subtypes, oocytes were injected with 50 nl cRNA Injected oocytes were incubated at 19 °C for 24–72 h prior to their use in voltage clamp experiments. For expression of primarily HS receptors 50 nl of a mixture containing 50 ng/μl of α4 cRNA and 250 ng/μl of β2 cRNA was injected (1:5 ratio of α4 to β2). Expression of LS receptors was achieved by injecting 50 nl of a mixture of 250 ng/μl α4 cRNA and 50 ng/μl β2 cRNA (5:1 ratio of α4 to β2). Expression of α7 nicotinic acetylcholine receptors was achieved by injecting 50 nl of 250 ng/μl of α7 cRNA The EC50 values obtained for acetylcholine-induced currents on the HS and LS subtypes obtained from these injection ratios were verified by electrophysiology assays as described below and found to compare well with published values for the HS and LS receptors (Moroni et al., 2006; Zwart and Vijverberg, 1998). EC50 values and response profiles indicated the expression of predominantly the HS or LS receptor subtypes although it is likely that both are present in each experiment (see the Results section).
2.4. Two-electrode voltage clamp
Two electrode voltage clamp was performed as indicated in previous studies from our laboratory (Weltzin and Schulte, 2010). Recording and current electrodes with resistance 1–4 MΩ were filled with 3 M KCl. Oocytes were held in a vertical flow chamber of 200 μl volume, clamped at a holding potential of –60 mV and perfused with various ND-96 recording buffers. Three different ND-96 recording buffers were used in these experiments: HEPES-ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES); Tris ND-96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2,1 mM MgCl2, 5 mM Tris–HCl); phosphate ND-96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2 mM phosphate). The pH of the Tris and phosphate ND-96 buffers was adjusted slightly to 7.4 with the addition of 1 M NaOH (< 200 μl/l). The final pH for all three buffers was 7.4. Experiments were performed at room temperatures of 25–26 °C. Osmolarity differences between the three recording buffers were minimal based on calculated osmolarities (HEPES buffer: 214.4 mOsm, phosphate buffer: 212.4 mOsm, Tris buffer: 219.4 mOsm). Since osmolarity differences can affect ion channel function, all running buffers, wash solutions and test solutions containing agonists or modulators (including HEPES) were made from common stock solutions to minimize any effects due to slight osmolality differences. At the start of an experiment or on exchanging oocytes, oocytes were equilibrated in the appropriate buffer for at least 7 min prior to the initiation or continuation of an experiment. To assure that observed effects were not a result of slight pH changes on addition of test compounds, the pH of every buffer and test solution was verified using a calibrated pH meter. Addition of HEPES to phosphate and Tris buffers did not alter the pH for HEPES concentrations less than 10 mM, thus pH changes were not observed for the solutions described in this study. Oocytes were perfused with the different recording buffers at a rate of 20 ml/min. Test compounds were dissolved in buffer and injected into the chamber at 20 ml/min using a Gilson auto-sampler injection system (Joshi et al., 2004).
2.5. Electrophysiology concentration/response experiments
Concentration/response curves for the endogenous nicotinic acetylcholine receptor agonist acetylcholine · Cl, (Sigma–Aldrich) were determined for HS and LS α4β2 preparations and α7 receptors as indicated in the Results section. Concentration/response curves for HEPES and Tris-HCl were determined by co-application of HEPES or Tris–HCl with either 10 μM acetylcholine (EC75) for HS receptors or 100 μM acetylcholine (EC75) for LS receptors. Similarly, the α7 nicotinic acetylcholine receptor concentration/responses curves for HEPES were determined by co-application with 1 mM acetylcholine for nicotinic acetylcholine receptor at increasing HEPES concentrations. HEPES and Tris concentration/response curves were performed with phosphate ND-96 as the recording buffer.
In order to compare responses from different oocytes, individual responses to drug application were normalized to control responses elicited using 10 μM acetylcholine for HS receptors or 100 μM acetylcholine for LS receptors (evaluated after every three responses to test compounds). After application of a sample, the oocyte was bathed in buffer for 6 min to allow for complete wash out of drug and to allow the receptors to return to an inactive (but not desensitized) state. All experiments were repeated a minimum of four times using four different oocytes from at least two different frogs.
2.6. Data analysis and statistics
Concentration/response curves were fit using non-linear curve fitting and GraphPad Prism Software (San Diego, CA) with standard built-in algorithms as described previously (Weltzin and Schulte, 2010). pEC50 (−log EC50) and EC50 values for HEPES potentiation of acetylcholine-induced responses (see Fig. 1C) and acetylcholine concentration/response data (see Fig. 2 and Fig. 4A) were determined by fitting concentration/response data to a single site model.
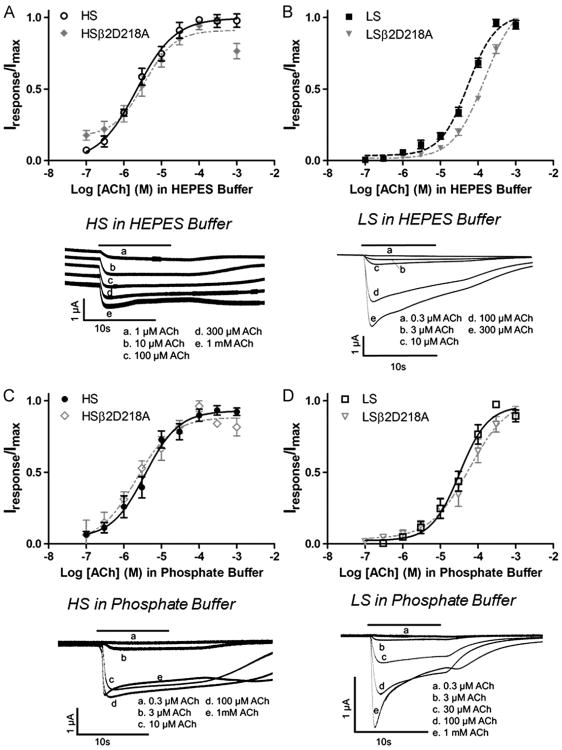
Fig. 1.
Acetylcholine concentration/response curves obtained in HEPES and phosphate ND-96 recording buffer. Xenopus oocytes expressing high-sensitivity α4β2 nicotinic acetylcholine receptors (HS) (mRNA injected at a ratio of 1α:5β) or low-sensitivity receptors (LS) (mRNA injected at a ratio of 5α:1β) were exposed to increasing concentrations of acetylcholine (ACh) in HEPES (A) and (B) or phosphate (C) and (D) recording buffer. Individual peak amplitudes were normalized to the Imax on the same oocyte. Response traces were recorded from a single oocyte in either the HS or LS preparation. The solid bar above the response trace indicates the time the oocyte was exposed to acetylcholine. In Fig. 1A, individual response traces were offset for clarity although all five traces had identical baseline currents. pEC50 and nH values (see Table 1) were determined using non-linear curve fitting as described in the methods. Data points represent at least four replicate values obtained from four different oocytes harvested from at least two different frogs. Error bars indicate ± S.E.M.
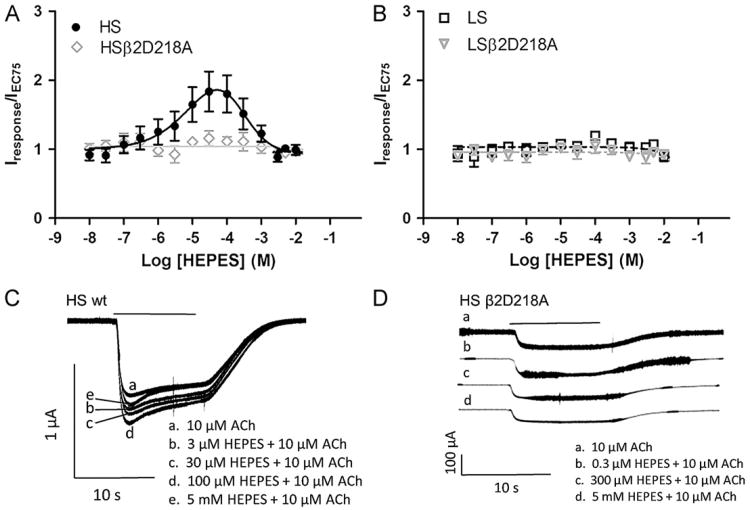
Fig. 2.
HEPES concentration/response curves obtained using high sensitivity (HS) and low sensitivity (LS) receptor preparations. HEPES and the appropriate concentration of acetylcholine (ACh) were co-applied to Xenopus oocytes expressing HS (mRNA injected at a ratio of 1α:5β) or LS receptors (mRNA injected at a ratio of 5α:1β). Responses were obtained from Xenopus oocytes under voltage clamp conditions (Vm= −60 mV). The peak currents were measured and responses normalized to currents elicited by acetylcholine applied alone to the same oocyte. Data points represent at least four replicate values obtained from four different oocytes harvested from at least two different frogs. Error bars indicate ± S.E.M. Response traces shown in (C) and (D) were each recorded from a single oocyte using a HS or HS β2D218A preparation. The solid bar above the response trace indicates the time the oocyte was exposed to HEPES and acetylcholine (10 s). (A) Concentration/response curves obtained on co-application of HEPES and 10 μM acetylcholine on HS and HS β2D218A receptors. (B) Concentration/response curves obtained on co-application of HEPES and 100 μM acetylcholine on LS and LSβ2D218A receptors. (C) Responses obtained on co-application of 3 μM, 30 μM, 100 μM and 5 mM HEPES with 10 μM acetylcholine on an oocyte using the wildtype HS receptor preparation. (D) Responses obtained on co-application of 0.3 μM, 300 μM and 5 mM HEPES with 10 μM acetylcholine on an oocyte using the HS β2D218A receptor preparation. Response traces in (D) were offset for clarity although baseline responses for all four traces were identical.
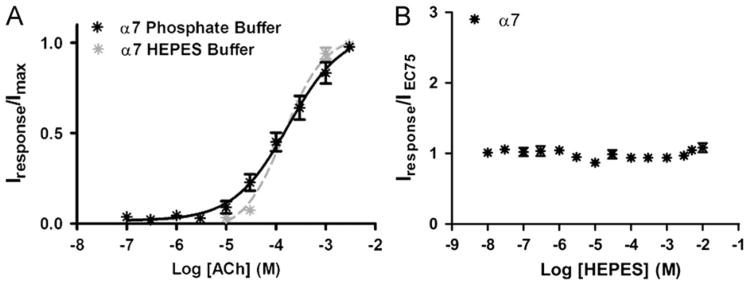
Fig. 4.
Acetylcholine concentration/response curves for α7 nicotinic acetylcholine receptors obtained using either HEPES or phosphate ND-96 recording buffers. (A) Concentration/response curve for acetylcholine (ACh) stimulation of α7 receptors in phosphate or HEPES ND-96 buffers. (B) Effect of HEPES on responses of α7 nicotinic acetylcholine receptors to 1 mM acetylcholine. Increasing concentrations of HEPES were co-applied with 1 mM acetylcholine. Individual peak amplitudes in both (A) and (B) were normalized to the Imax on the same oocyte. pEC50 and nH values were determined using non-linear curve fitting as described in the methods. Data points represent at least four replicate values obtained from four different oocytes harvested from at least two different frogs. Error bars indicate ± S.E.M.
PAM concentration/response curves were fit using a non-linear curve fitting algorithm and GraphPad Prism Software (San Diego, CA). PAMs often produce bell-shaped concentration/response curves with both potentiating and inhibiting phases. HEPES and dFBr displayed this typical concentration/response profile (see Figs. 1A, 5 and 6B–D). pEC50 (−log EC50) and pIC50 (−log IC50) values were determined in these cases by simultaneously fitting both the potentiation and inhibition phases using a bell shaped concentration/response model integral to the GraphPad Prism Sofware. Similar equations have been used previously to examine bell-shaped PAM data (Harvey et al., 1999; Hsiao et al., 2001; Kim et al., 2007; Weltzin and Schulte, 2010).
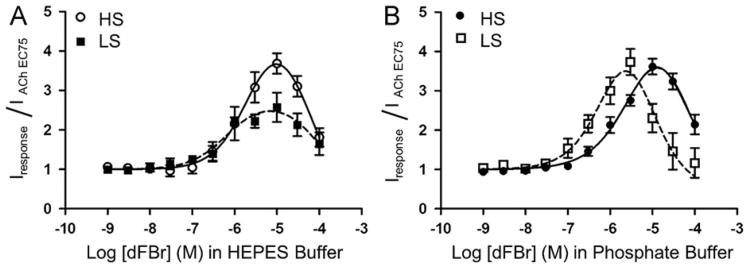
Fig. 5.
Effect of HEPES on potentiation of high sensitivity (HS) and low sensitivity (LS) α4β2 nicotinic acetylcholine receptors by the PAM dFBr. (A) Concentration/response curves obtained from co-application of dFBr and acetylcholine (concentration equal to the acetylcholine EC75) on HS and LS α4β2 nicotinic acetylcholine receptors in HEPES buffer. (B) Concentration/response curves obtained from co-application of dFBr and acetylcholine (concentration equal to the acetylcholine EC75) on HS and LS α4β2 nicotinic acetylcholine receptors in phosphate recording buffer. For all experiments, responses were obtained from Xenopus oocytes under voltage clamp conditions (Vm= −60 mV). Individual peak amplitudes were normalized to the response obtained using an identical concentration of acetylcholine alone (equal to the acetylcholine EC75). pEC50, pIC50 and Imax (%) values (see Table 2) were determined using non-linear curve fitting as described in the methods. Data points represent at least four replicate values obtained from four different oocytes harvested from at least two different frogs. Error bars indicate ± S.E.M.
Fig. 6.
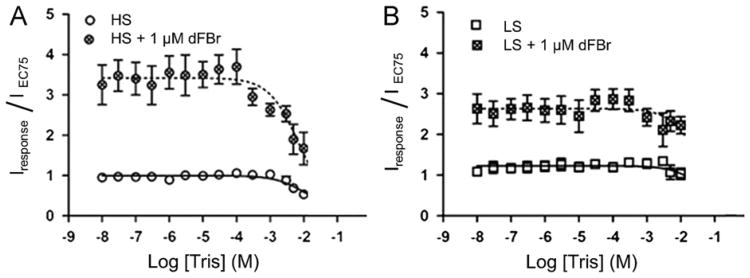
Effect of Tris buffer on potentiation of high sensitivity (HS) and low sensitivity (LS) α4β2 nicotinic acetylcholine receptors by the PAM dFBr. Xenopus oocytes expressing (A) HS or (B) LS receptors were exposed to increasing concentrations of Tris and acetylcholine (at concentrations equal to the EC75) in phosphate ND-96 buffer. Responses were potentiated using 1 μM of the PAM dFBR. Individual peak amplitudes were normalized to those elicited by the identical concentration of acetylcholine alone on the same oocyte. pEC50, pIC50 and nH values were determined using non-linear curve fitting as described in the methods. Data points represent at least four replicate values obtained from four different oocytes harvested from at least two different frogs. Error bars indicate ± S.E.M.
Simultaneous fitting of two Hill equations can prove difficult if the EC50 and IC50 values are close together due to the inability to collect data near the value of Imax. In these cases, some constants must be approximated for the fit to converge and the appropriate EC50 and IC50 values determined. In order to overcome these limitations, Hill slopes for potentiation and inhibition were typically fixed at +1 and −1, respectively unless otherwise indicated.
The GraphPad bell shaped curve fitting model describes the sum of two Hill equations and is considered an appropriate model for two interacting binding sites (Kim et al., 2007; Pandya and Yakel, 2011; Weltzin and Schulte, 2010). This is in contrast to use of the product of Hill Equations described by Kasai et al. which is more appropriate for two independent binding sites (Kasai, 1998). We compared curve fits using the summation model to the multiplicative model. Comparison of the two models showed that the fits were significantly different (p=0.0192; F=5.679). The summation model fits the data better and hence we used this model, consistent with our hypothesis of dependent sites, to calculate the data provided in the results section (see Results section for statistical analysis of these curve fits).
Statistical comparisons of pEC50 or pIC50 values used an unpaired t-test with p values calculated based on the null hypothesis. One way ANOVA was used to compare Imax values in different ND-96 recording buffers for both high and low-sensitivity receptors.
3. Results
3.1. Similar acetylcholine concentration/response curves are produced using either HEPES or phosphate ND-96 recording buffers
To examine the effect of HEPES on acetylcholine induced responses of α4β2 nicotinic acetylcholine receptors, we performed concentration/response experiments using both HEPES and Phosphate based ND-96 recording buffers. HS and LS receptors were expressed in oocytes using either a 1:5 or 5:1 α4:β2 mRNA injection ratio as described in the methods. Previous studies have shown that a predominance of the HS and LS α4β2 stoichiometries can be expressed in Xenopus laevis oocytes using these injection ratios (Moroni and Bermudez, 2006; Moroni et al., 2006; Tapia et al., 2007; Zwart and Vijverberg, 1998). However, since some heterogeneity of the receptor populations is likely we will refer to these as HS and LS preparations to indicate the predominance of one stoichiometry over the other.
Acetylcholine concentration/response curves were compared for HS and LS α4β2 receptors in both 5 mM HEPES and 2 mM phosphate containing buffers (Fig. 1). Responses to acetylcholine on the HS oocyte preparation appeared similar in both phosphate and HEPES buffers. Different amplitudes can be observed in the responses shown in Fig. 1(A and C) but these can be attributed to different expression levels in the different oocytes tested. For LS preparations, slight variations in response characteristics were observed mostly with respect to the sharpness of the peak response at higher acetylcholine concentrations (Fig. 1, B and D).
Acetylcholine concentration/response curves determined from peak currents showed no effect of buffer composition on acetylcholine pEC50 (p=0.2365) and Hill slope (p=0.7478) for HS receptors (Fig. 1A and C, and Table 1). A slight, but significant change in pEC50 for acetylcholine stimulation was observed for LS receptors (p=0.0277) although there was no significant change in Hill slopes (p=0.9162) (Fig. 1B and D, and Table 1).
Table 1.
Summary data for ACh concentration/response curves obtained using HEPES and phosphate ND-96 recording buffer (Fig. 1).
| Receptor | Buffer | pEC50 ± S.E.M. (EC50 μM) | nH ± S.E.M. |
|---|---|---|---|
| HS | HEPES | 5.7 ± 0.1 (2.2) | 0.9 ± 0.2 |
| HSβ2D218A | HEPES | 5.5 ± 0.1 (3.5) | 1.1 ± 0.3 |
| LS | HEPES | 4.3 ± 0.1 (54) | 1.2 ± 0.1 |
| LSβ2D218A | HEPES | 3.83 ± 0.04 (150) | 1.0 ± 0.1 |
| HS | Phosphate | 5.4 ± 0.1 (3.7) | 1.0 ± 0.2 |
| HSβ2D218A | Phosphate | 5.7 ± 0.2 (2.1) | 0.9 ± 0.3 |
| LS | Phosphate | 4.5 ± 0.1 (32) | 1.2 ± 0.2 |
| LSβ2D218A | Phosphate | 4.2 ± 0.2 (58) | 0.8 ± 0.2 |
In HEPES exposure experiments discussed below, we examined the effects of a mutation on the β+ face (forming the non-acetylcholine binding subunit interfaces) using an alanine mutation of β2D218. As an important control, we evaluated the effects of this mutation on acetylcholine concentration/response curves and response profiles. The mutant mRNA was injected into oocytes with a 1:5 or 5:1 ratio of a4: β2D218A mRNA (Fig. 1 A and B). This mutation had no effect on acetylcholine pEC50 values for HS receptors in either phosphate or HEPES buffers (Fig. 1A and C, and Table 1). Similar potencies and response characteristics for the 1:5 and the 5:1 injection ratios using the β2D218A mRNA compared to wildtype receptors produced under the same conditions indicates that mutant receptors are likely assembling similar HS and LS receptor subtypes.
As seen in Fig. 1, the phosphate buffered ND-96 had minimal or no effect on acetylcholine EC50 values for either receptor preparation. To further evaluate possible effects of the phosphate buffer, Imax values obtained at saturating concentrations of acetylcholine in different concentrations of phosphate buffer were determined. Varying concentrations of phosphate buffer ranging from 0.5 mM to 2 mM were used to prepare phosphate ND-96 recording solutions. The pH for each recording buffer was 7.4. The Imax currents induced by acetylcholine for each stoichiometry (300 μM and 1 mM for HS and LS receptors, respectively) were measured for each phosphate buffer. Each phosphate concentration course was run on the same oocyte. Alterations in the acetylcholine-induced current in different buffers containing different concentrations of phosphate would indicate interactions of phosphate with the receptors and/or ligands. We saw no change in the acetylcholine-induced Imax current for any concentration of phosphate buffer for the HS (ANOVA p=0.6751) and LS (ANOVA p=0.8921) α4β2 receptors (results not shown). No induced responses were seen with application of different phosphate solutions when applied alone (without agonist). These data show no apparent effect of the phosphate buffer on α4β2 receptors, making it a good choice for evaluation of α4β2 receptor function.
3.2. HEPES is an allosteric modulator of the high-sensitivity α4β2 nicotinic acetylcholine receptor
To explore the effects of HEPES on HS and LS preparations of α4β2 receptors, increasing concentrations of HEPES (0.01 μM–300 mM) were co-applied with acetylcholine at a concentration equal to the acetylcholine EC75 (10 μM for HS receptors or 100 μM for LS receptors) in a 2 mM phosphate buffered ND-96 recording solution (pH 7.4) on HS and LS preparations (Fig. 2). Test solutions were monitored for any changes in pH. No changes in pH were observed at HEPES concentrations ≤ 10.0 mM.
Concentration/response curves show increased peak responses to 10 μM acetylcholine at increasing HEPES concentrations for the HS preparation with no increased peak response in the LS preparation (Fig. 2A and B). A bell shaped concentration/response curve was observed for HS receptor preparations with a maximum potentiation of 190% (the calculated Imax was 2.7 ± 3.1). The pEC50 value for HEPES potentiation of HS receptors was 5.2 ± 0.9 (EC50=7.1 μM). HEPES concentrations >300 μM produced inhibition of the acetylcholine-induced responses. This inhibition appears to plateau at 1.0 (the normalized, unpotentiated response amplitude using 10 μM acetylcholine). The pIC50 value for inhibition was 3.4 ± 0.4 (IC50=430 μM) (Fig. 2A). On LS preparations, HEPES produced only a slight inhibition of the response with no observable potentiation (Fig. 2B). Coincidentally, minimal net effects of HEPES on HS receptors are seen at concentrations between 5 mM and 10 mM (typical concentration in HEPES recording buffers). This may be one reason why the functional effects of HEPES potentiation have gone unobserved. Comparison of data from oocytes expressing predominantly LS and HS receptors suggest that HEPES selectively potentiates the HS population compared to the LS stoichiometry. The application of HEPES alone to α4β2 nicotinic acetylcholine receptor expressing oocytes produced no induced current (data not shown).
With co-application of HEPES concentrations <300 μM (potentiating concentrations) and 10 μM acetylcholine on HS receptors, the response profile showed a slight sharpening of the response peak and larger overall responses compared to those obtained in the absence of HEPES (Fig. 2A, C responses b–d). Co-application of HEPES at concentrations from 300 μM to 5 mM with 10 μM acetylcholine further sharpened the response peak but also decreased the overall amplitude of the response compared to peak potentiated responses (Fig. 2A, C response e). On LS receptors, increasing concentrations of HEPES co-applied with 100 μM acetylcholine produced only a very slight inhibition of responses at HEPES concentrations ≤ 10 mM (Fig. 2B, response traces not shown). No significant potentiation was evident when HEPES was co-applied with acetylcholine on LS receptors.
We also evaluated the effects of HEPES on α4β2 receptors containing a β2D218A mutation. Mutation of this residue has been previously shown to alter potentiation of α4β2 receptors by Zn+ and possibly comprise one region of a novel binding domain for some α4β2 PAMs (Moroni et al., 2008). This amino acid is located at a position homologous to the vicinal cysteines in the identical C-loop region on the α4 subunit that forms the orthosteric acetylcholine binding site. HS or LS receptor preparations were prepared using wildtype α4 and β2D218A mutant subunits as described above for wildtype receptors. Receptors containing the β2D218A mutation were not potentiated by HEPES (Fig. 2A and B). As indicated in section 3.1 (Fig. 1A) the EC50 values for acetylcholine activation of HS and LS receptors was unaffected by the β2D218A mutation. Thus the β2D218A containing receptors appeared to represent HS receptors that are insensitive to HEPES potentiation. LS receptors containing the β2D218A mutation remained insensitive to HEPES.
While acetylcholine pEC50 and Hill slopes remained similar for wildtype and mutant HS and LS receptors, response profiles were slightly different. For the HS preparation, β2D218A responses obtained by application of acetylcholine alone showed similar response kinetics compared to wild-type receptors but the induced current in mutated receptors was much smaller than wild-type receptors (Fig. 2D). This observation may suggest that some alteration in receptor function, conductance and/or expression may be occurring with β2D218A receptors (Fig. 2D, bottom. right and left set of responses, trace a). For oocytes injected with a 5:1 ratio of α4:β2D218A, response amplitudes to acetylcholine alone appeared to decrease with a loss of the sharp response peak and altered desensitization (Fig. 2D, trace a). The differences in response profiles on both the HS and LS β2D218A receptors compared to wild-type receptors suggest that the β2D218 residue may play a role in receptor function. The complete eradication of HEPES potentiation by this mutation suggests that the β2D218 may be located in the HEPES binding site or play a key role in the mechanism of HEPES potentiation.
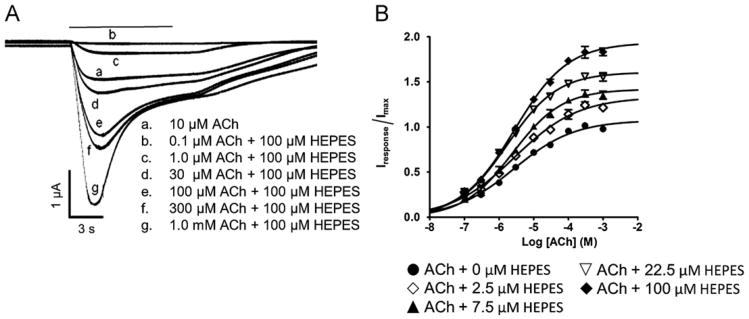
Positive modulation by HEPES was further investigated by exposure of oocytes expressing predominantly HS receptors to potentiating concentrations of HEPES (2.5, 7.5, 22.5 and 100 μM) over a range of acetylcholine concentrations (0.1 μM–1 mM) (Fig. 3A and B). Larger responses and sharper peaks were observed in the presence of HEPES compared to those obtained by exposure to acetylcholine alone (Fig. 3A). In the absence of HEPES, the pEC50 for acetylcholine stimulation was 5.4 ± 0.1 (EC50=3.7 μM). In the presence of HEPES, the pEC50 values were not significantly changed with co-application of 2.5 μM, 7.5 μM, or 100 μM HEPES (Fig. 3B). A small but statistically significant increase in the pEC50 value (5.8 ± 0.04; EC50=1.7 μM) was seen with application of 22.5 μM of HEPES (p=0.0433). Application of 100 μM HEPES produced a 194% potentiation of the acetylcholine-induced Imax currents. The Hill slope for acetylcholine stimulation was identical for all concentrations of HEPES tested (2.5 μM, 7.5 μM, 22.5 μM or 100 μM). HEPES potentiation observed in the HS preparation was not surmountable by high concentrations of acetylcholine.
Fig. 3.
Effect of HEPES on acetylcholine concentration/response curves. Acetylcholine concentration/response kinetics were determined in Phosphate ND-96 buffer containing five different concentrations of HEPES. (A) Response traces obtained from a single oocytes expressing the HS α4β2 preparation in phosphate ND-96 buffer at increasing acetylcholine (ACh) concentrations (0.1 μM–1 mM) either in the absence of HEPES (trace a) or co-applied with 100 μM HEPES (traces b–g). (B) Acetylcholine concentration/response curves obtained using the HS receptor preparation in the presence of 0 μM, 2.5 μM, 7.5 μM, 22.5 μM or 100 μM HEPES (potentiating concentrations). Individual response amplitudes were normalized to the peak response obtained on exposure to 10 μM acetylcholine alone. Each point represents the composite data obtained from four different experiments on four different oocytes from at least 2 different frogs. Individual pEC50 values are given in the text.
3.3. The α7 nicotinic acetylcholine receptor is unaffected by HEPES
The α7 nicotinic acetylcholine receptor is one of the most common nicotinic acetylcholine receptors found in the central nervous system. The expression of α7 nicotinic acetylcholine receptors is altered in many neurological disorders including Alzheimer's disease and Schizophrenia (Levin and Rezvani, 2007). The effects we observed on α4β2 receptors led us to investigate possible interactions of HEPES with the α7 nicotinic acetylcholine receptor subtype. Acetylcholine concentration/ response curves were determined from peak currents obtained from oocytes expressing α7 receptors in either HEPES or phosphate ND-96 recording buffers (Fig. 4A). For acetylcholine stimulation, pEC50 values were identical in both HEPES and phosphate buffers (HEPES: pEC50=3.8 ± 0.1; EC50=146 μM; Phosphate buffer: pEC50=3.8 ± 0.1; EC50=177 μM) (p=0.5893). The Hill slopes were 1.1 ± 0.2 and 0.79 ± 0.13 for HEPES and phosphate recording buffers, respectively (p=0.2061).
To investigate the effects of HEPES on the α7 nicotinic acetylcholine receptor, we co-applied varying concentrations of HEPES (0.01 μM–10 mM) with 1 mM acetylcholine (acetylcholine EC75) to oocytes expressing α7 receptors (Fig. 4B). At concentrations ≤ 10 mM, HEPES produced little observable effect on responses of α7 receptors to acetylcholine.
3.4. Desformylflustrabromine modulation of high- and low-sensitivity α4β2 receptors in HEPES ND-96 recording buffer
In light of our data demonstrating potentiation of α4β2 nicotinic acetylcholine receptors by HEPES, we hypothesized that HEPES may have altered the results of previous studies using other modulators. We investigated the possible differences in the effects observed for the previously tested α4β2 PAM desformylflustrabromine using both HEPES and phosphate ND-96 recording buffers. Desformylflustrabromine (dFBr) is a novel PAM that potentiates acetylcholine-induced currents of α4β2 nicotinic acetylcholine receptor by 265% at concentrations <10 μM and inhibits at concentrations >10 μM (Kim et al., 2007; Sala et al., 2005; Weltzin and Schulte, 2010). Previous experiments were conducted using a 5 mM HEPES ND-96 recording buffer. Given the current findings it is possible that the dFBr data may have been altered by the presence of HEPES.
We duplicated our previous studies using HS and LS α4β2 nicotinic acetylcholine receptors and compared the results obtained using both a HEPES and phosphate buffered ND-96 recording solution (Fig. 5). A range of dFBr concentrations (0.001–100 μM) were co-applied with either 10 μM or 100 μM acetylcholine on HS and LS α4β2 nicotinic acetylcholine receptor preparations. Both receptor types were potentiated by dFBr.
In HEPES ND-96 recording buffer, the pEC50 values for dFBr on the two receptor types were not significantly different (p=0.4040) (Fig. 5A and Table 2). dFBr produced greater potentiation using the HS preparation compared to the LS preparation in HEPES ND-96 recording buffer. The LS preparation was potentiated 260% while the HS receptors were potentiated by 370%. These findings suggest that either; (1) dFBr has a higher efficacy on the HS receptor; (2) The potentiating effects of HEPES and dFBr produce an additive effect on the HS stoichiometry; or (3) competition between HEPES and dFBr is causing a reduction in the apparent efficacy on the LS stoichiometry.
Table 2.
Summary data for dFBr concentration/responses curves obtained for HS and LS α4β2 nicotinic acetylcholine receptor preparations using HEPES or phosphate ND-96 buffers.
| Receptor | Buffer | pEC50 ± S.E.M. (EC50 μM) | Imax (%) | pIC50 ± S.E.M. (IC50 μM) |
|---|---|---|---|---|
| HS | HEPES | 5.6 ± 0.5 (2.5) | 370 | 4.3 ± 0.8 (50) |
| LS | HEPES | 6.2 ± 0.4 (0.63) | 260 | 4.0 ± 1.3 (1 0 0) |
| HS | Phosphate | 5.5 ± 0.3 (3.2) | 360 | 4.1 ± 0.1 (79) |
| LS | Phosphate | 5.9 ± 1.1 (1.3) | 370 | 5.2 ± 0.6 (6.3) |
To evaluate possible differences of dFBr-receptor interactions without the confounding actions of HEPES, dFBr concentration/ response curves were performed in a phosphate buffered ND-96 recording solution (Fig. 5B). Varying concentrations of dFBr (0.001– 100 μM) were co-applied with either 10 μM or 100 μM acetylcholine on oocytes expressing HS or LS receptor preparations. The amount of potentiation of acetylcholine-induced responses produced by dFBr in Phosphate ND-96 was similar for HS (360%) and LS (370%) receptors (Fig. 5B and Table 2). The pEC50 values for dFBr potentiation were not significantly different between HS and LS receptors tested in phosphate buffered ND-96 (p=0.6362). The pEC50 values for dFBr potentiation of HS (p=0.7459) and LS (p=0.8377) nicotinic acetylcholine receptors were also not significantly different from those determined in HEPES ND-96 recording buffer although the degree of potentiation of HS receptors by dFBr was decreased in HEPES relative to phosphate buffer (Fig. 5 and Table 2).
No significant difference in pIC50 values were observed for inhibition of acetylcholine-induced currents by application of high (> 10 μM) dFBr concentrations using either HEPES or phosphate buffer on HS or LS receptors (HS, p=0.7683); LS, p=0.4040) (Fig. 5A and B, and Table 2). In addition no change was observed in dFBr pIC50 values for HS or LS receptors in HEPES buffer (p=0.8485). An increase in the pIC50 value on the LS receptors was seen compared to the HS receptor in phosphate buffered ND-96 (p=0.0435) (Fig. 5B and Table 2).
3.5. Effects of Tris buffer on potentiation of HS and LS α4β2 receptors by the PAM dFBr
As a result of our discovery that HEPES modulates α4β2 nicotinic acetylcholine receptor function, we also considered whether or not the commonly used Tris buffer had any effect on α4β2 receptors. Like HEPES, Tris is a polar molecule that is a member of the family of Good's buffers and is used as a common physiological buffering agent, We examined the effect of Tris on acetylcholine-induced responses on HS and LS subtypes of the α4β2 nicotinic acetylcholine receptor. Increasing concentrations ofTris (0.01 μM–10mM) were co-applied with either 10 μM or 100 μM acetylcholine to HS and LS oocytes preparations using a phosphate ND-96 recording buffer. Tris inhibited HS receptors by 50% at a Tris concentration of 10mM (pIC50=2.0 ± 0.1; IC50=10mM) (Fig. 6A). LS α4β2 nicotinic acetylcholine receptor preparations were only slightly inhibited at Tris concentrations ≤ 10 mM (Fig. 5B).
We also examined the effects of Tris buffer on responses of HS and LS receptors potentiated by the PAM desformylflustrabromine (dFBr) (Kim et al., 2007; Sala et al., 2005). dFBr was co-applied with acetylcholine and Tris in phosphate buffered ND-96 recording solution. As was observed in the absence of dFBr, the potentiated acetylcholine induced response was inhibited by about 50% at 10 mM Tris on the HS preparation. The potentiated response on the LS receptor preparation was only slightly inhibited at Tris concentrations ≤ 10 mM (also similar to non dFBr potentiated responses). dFBr produced potentiation of ∼360% when co-applied with 10 μM acetylcholine and Tris (Tris concentrations < 300 μM; non inhibiting) on HS receptors and 280% on LS preparations when compared to Tris and acetylcholine alone. Application of Tris in phosphate buffer in the absence of acetylcholine did not induce currents.
4. Discussion
4.1. HEPES modulation of the α4β2 nicotinic acetylcholine receptor
We investigated the effects of HEPES on HS and LS α4β2 and α7 nicotinic acetylcholine receptors. Our results demonstrate HEPES ability to potentiate (at HEPES concentrations < 100 μM) acetylcholine-induced currents on α4β2 nicotinic acetylcholine receptors expressed using the HS preparation. At concentrations ≤ 10 mM, HEPES produced little to no effect on preparations of LS α4β2 or α7 nicotinic acetylcholine receptors.
It's possible that HEPES is a nicotinic antagonist that potentiates currents induced by low-acetylcholine concentrations, as seen with other cholinergic ligands (Smulders et al., 2005). Fig. 3 clearly demonstrates that potentiation by HEPES is present at all acetylcholine concentrations (0.1–1000 μM). In addition, HEPES effects are not surmountable by high concentrations of acetylcholine as was reported by Smulders et al., (2005) for potentiation by competitive antagonists. Our data suggest that acetylcholine and HEPES are acting at independent binding sites and that potentiation is via alteration in acetylcholine efficacy.
The HS receptor is thought to contain an (α4)2(β2)3 stoichiometry containing a unique β2+/β2− subunit interface. The LS receptor is thought to have a (α4)3(β2)2 stoichiometry and does not contain a β2 + /β2− interface although both HS and LS receptors contain a β2 + /α4− interface. Mutagenesis data show that mutation of the β2D218 residue in the β2 + C-loop region abolishes HEPES potentiation. Both the selectivity of HEPES for the HS preparation and the effect of the β2D218A mutation support a putative HEPES potentiation site located at the β2 + /β2−interface of the HS α4β2 receptor site.
Inhibition of the HEPES potentiated response occurs at concentrations greater than 100 μM HEPES. This inhibition appears to plateau at a normalized response of 1, suggesting HEPES can only inhibit the potentiated response at these concentrations (100 μM–10 mM). These data do not support channel block as the mechanism of inhibition as has been observed for dFBr and other nicotinic acetylcholine receptor ligands but may be due to another mechanism since channel block by HEPES should inhibit the non-potentiated response as well. (Weltzin and Schulte, 2010). In addition, hump currents, also known as rebound or tail currents, are inward currents which occur during the desensitized phase of the response on washout of the ligand and have been previously linked to open-channel block (Liu et al., 2008). At the HEPES concentrations tested, no tail currents were observed, suggesting that open-channel block may not be an inhibition mechanism (Fig. 1A and B).
4.2. HEPES effects on α7 nicotinic acetylcholine receptors
We investigated the effects of HEPES on α7 nicotinic acetylcholine receptors to determine if similar modulatory effects of HEPES were present. The α7 acetylcholine pEC50 and nH values found using the HEPES and phosphate buffer systems were not different from one another. In addition α7 receptors were not affected by application of HEPES. The lack of observable effects of buffer composition on α7 nicotinic acetylcholine receptors indicates that both HEPES and phosphate buffer systems are likely appropriate for investigating α7 nicotinic acetylcholine receptor function.
4.3. HEPES as a lead compound for the development of novel, selective nicotinic receptor ligands
Several ligands have been shown to have different affinities and efficacies for the α4β2 subtypes including cytisine, acetylcholine, nicotine and epibatidine (Moroni et al., 2006). To our knowledge, the Senantiomer of mecamylamine (Targacept® TC-5213) is the only ligand to that selectively potentiates the HS α4β2 receptor while inhibiting the LS receptor (Taly et al., 2009). HEPES also appears to be a HS selective PAM relative to the LS α4β2 and α7 nicotinic acetylcholine receptors. While HEPES itself is unlikely to prove a useful PAM for CNS receptors, improved understanding of its mechanism and structure activity relationships could be used to develop compounds beneficial to elucidating the role of specific stoichiometries of α4β2 receptors in the CNS. Such ligands could also prove useful in the diagnosis and treatment of CNS disorders.
4.4. Mounting evidence that Good's Buffers interact with physiological systems
Over the years, Good's buffers have been shown to alter physiological systems. HEPES has been shown to stimulate the production of ATP and decrease the uptake of P-glycoprotein in Caco-2 and MDCK-MDR1 cells (Luo et al., 2010). The hyperpolarization-activated transient currents of human and rat 5-hydro-xytryptamine transporters (SERT) expressed in Xenopus oocytes were blocked by HEPES with alterations in SERT kinetics (Li et al., 2002). The SERT currents decreased 10–50% by HEPES at concentrations greater than 1 mM. Additionally, HEPES has been shown to affect cell membrane (Poole et al., 1982) and block chloride ion channels (Yamamoto and Suzuki, 1987).
Tris buffer and similar compounds have also been shown to alter physiological responses. Studies suggest that Tris inhibits the function of enzymes such as aminopeptidase and alpha-amylase (Desmarais et al., 2002; Ghalanbor et al., 2008). In addition, Tris, TES and related buffer compounds have been shown to react with nerve agents to form new products (Gab et al., 2010).
To add to this growing list of the physiological and biochemical effects of Good's buffer's, the current study demonstrates alteration of nicotinic acetylcholine receptor function by HEPES and Tris. HEPES potentiates and inhibits acetylcholine-induced responses on α4β2 nicotinic acetylcholine receptors in a stoichiometric dependent manner.
4.5. HEPES competition with dFBr
Positive allosteric modulators (PAMs) have recently become alluring targets in the search for potential therapeutic agents. PAMs generally function to increase the sensitive and/or efficacy of endogenous ligands such as acetylcholine. Since many electrophysiological studies use a HEPES recording buffer, the discovery that HEPES is an allosteric modulator of the α4β2 nicotinic acetylcholine receptor presents a concern regarding data interpretation. HEPES potentiation or inhibition may alter the responses of the compound in question. These concerns are especially pertinent with the study and development of other modulators.
We investigated the effects of buffer composition on the sensitive and/or efficacy of the PAM dFBr. The affinities and efficacies of dFBr were altered by Tris and HEPES buffers. A previous study comparing Tris and HEPES buffers showed that the gamma aminobutyric acid type A receptor (GABAA receptor) PAM, thymol, displayed biphasic behavior in a HEPES buffer while only inhibition was seen when using a Tris buffer (Garcia et al., 2008). Similarly in our laboratory, dFBr appears to be more efficacious for the HS versus LS α4β2 receptors when using a HEPES buffer. But in the absence of HEPES no difference is evident. The apparent selectivity of HEPES for the HS subtype suggests that it may interact at the β+ /β− cleft unique to that subtype of the receptor. This is further supported by the ability of the β2D218A mutation to obliterate HEPES potentiation. The lower efficacy of dFBr in the HS subtype may be the result of competition between HEPES and dFBr. The interaction of HEPES with HS receptors clearly alters the apparent efficacy and potency of dFBr on α4β2 receptor subtypes. The effects of using HEPES buffer is likely not unique to the PAM dFBr and similar effects are likely to be observed with other α4β2 PAMS as well. Such effects might be particularly pronounced in mutagenesis studies since removal of HEPES potentiation by mutations such as β2 D218A could produce effects that might indicate interaction of a PAM under investigation when the effect is actually due to changes in HEPES interactions. For example, removal of HEPES binding by mutation of a residue such as β2D218 could produce enhancement of potentiation of a PAM interacting at another binding site by removing inhibition by HEPES. Such a result might lead to the erroneous conclusion that the PAM interacts with β2 D218.
5. Conclusions
Our data have shown that HEPES is capable of potentiating HS α4β2 receptors at micromolar concentrations. This molecule could serve as a potentially valuable lead for a new class of stoichiometric selective ligands of α4β2 receptors. Continued work in this direction will likely elucidate features of both ligand and receptor responsible for this selectivity. In addition we have shown that HEPES buffer can interfere in physiological systems involving nicotinic acetylcholine receptors, a finding that would preclude its use in many studies. This is particularly true with respect to α4β2 receptor PAMs. Our data indicate that Tris would be unsuitable as a replacement buffer in these studies. At this point, Phosphate buffers appear to be useful but may be limited in some cases, particularly in those involving divalent metals such as Zn2+ which have low solubility in Phosphate containing buffers. Since many Good's buffers share common structural features with HEPES and they may also exert effects on nicotinic acetylcholine receptors. A though characterization of these buffers is currently being performed in our laboratory to identify suitable replacements.
Acknowledgments
We would like to thank Dr. Richard Glennon and his laboratory (Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University) for the synthesis and generous donation of the desformylflustrabromine used in these studies, Yeganeh Ataian for her assistance in Xenopus laevis surgeries and Mary van Muelken for editorial assistance.
Footnotes
This research was supported by grants from the National Center for Research Resources (5P20RR016466, Alaska INBRE Program) and The National Institutes of Neurological Disorders and Stroke [1R01NS066059 and 1R01NS057366], Components of the National Institutes of Health (NIH).
References
- Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- Aubert I, Araujo DM, Cecyre D, Robitaille Y, Gauthier S, Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem. 1992;58:529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an acetylcholine-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Marks MJ, Collins AC. Bovine serum albumin enhances nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2002;83:48–56. doi: 10.1046/j.1471-4159.2002.01135.x. [DOI] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer's disease. Biol Psychiatry. 2001;49:175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Desmarais WT, Bienvenue DL, Bzymek KP, Holz RC, Petsko GA, Ringe D. The 1.20 A resolution crystal structure of the aminopeptidase from Aeromonas proteolytica complexed with tris: a tale of buffer inhibition. Structure. 2002;10:1063–1072. doi: 10.1016/s0969-2126(02)00810-9. [DOI] [PubMed] [Google Scholar]
- Gab J, John H, Melzer M, Blum MM. Stable adducts of nerve agents sarin, soman and cyclosarin with TRIS, TES and related buffer compounds—characterization by LC-ESI-MS/MS and NMR and implications for analytical chemistry. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878:1382–1390. doi: 10.1016/j.jchromb.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Garcia DA, Vendrell I, Galofre M, Sunol C. GABA released from cultured cortical neurons influences the modulation of t-[(35)S]butylbicyclophosphorothionate binding at the GABAA receptor Effects of thymol. Eur J Pharmacol. 2008;600:26–31. doi: 10.1016/j.ejphar.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Ghalanbor Z, Ghaemi N, Marashi SA, Amanlou M, Habibi-Rezaei M, Khajeh K, Ranjbar B. Binding of Tris to Bacillus licheniformis alpha-amylase can affect its starch hydrolysis activity. Protein Pept Lett. 2008;15:212–214. doi: 10.2174/092986608783489616. [DOI] [PubMed] [Google Scholar]
- Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RM. Hydrogen ion buffers for biological research. Biochemistry. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor alpha4 or beta2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha4 and beta2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520(Pt 1):53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao B, Dweck D, Luetje CW. Subunit-dependent modulation of neuronal nicotinic receptors by zinc. J Neurosci. 2001;21:1848–1856. doi: 10.1523/JNEUROSCI.21-06-01848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PR, Suryanarayanan A, Schulte MK. A vertical flow chamber for Xenopus oocyte electrophysiology and automated drug screening. J Neurosci Methods. 2004;132:69–79. doi: 10.1016/j.jneumeth.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Kasai M. A comment on the analysis of bell-shaped dose-response curves. Jpn J Physiol. 1998;48:91–93. doi: 10.2170/jjphysiol.48.91. [DOI] [PubMed] [Google Scholar]
- Kim JS, Padnya A, Weltzin M, Edmonds BW, Schulte MK, Glennon RA. Synthesis of desformylflustrabromine and its evaluation as an alpha4beta2 and alpha7 nACh receptor modulator. Bioorg Med Chem Lett. 2007;17:4855–4860. doi: 10.1016/j.bmcl.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Farley RA, Lester HA. Voltage-dependent transient currents of human and rat 5-HT transporters (SERT) are blocked by HEPES and ion channel ligands. FEBS Lett. 2002;513:247–252. doi: 10.1016/s0014-5793(02)02322-0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yu KW, Chang YC, Lukas RJ, Wu J. Agonist-induced hump current production in heterologously-expressed human alpha4beta2-nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2008;29:305–319. doi: 10.1111/j.1745-7254.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Luo S, Pal D, Shah SJ, Kwatra D, Paturi KD, Mitra AK. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Mol Pharmacol. 2010;7:412–420. doi: 10.1021/mp900193e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Grady SR, Picciotto MR, Changeux JP, Collins AC. Nicotinic-agonist stimulated (86)Rb(+) efflux and [(3)H]epibatidine binding of mice differing in beta2 genotype. Neuropharmacology. 2000;39:2632–2645. doi: 10.1016/s0028-3908(00)00115-5. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Lee M, Perry RH, Baumann M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Moroni M, Bermudez I. Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J Mol Neurosci. 2006;30:95–96. doi: 10.1385/JMN:30:1:95. [DOI] [PubMed] [Google Scholar]
- Moroni M, Vijayan R, Carbone A, Zwart R, Biggin PC, Bermudez I. Non-agonist-binding subunit interfaces confer distinct functional signatures to the alternate stoichiometries of the alpha4beta2 nicotinic receptor: an alpha4– alpha4 interface is required for Zn2+ potentiation. J Neurosci. 2008;28:6884–6894. doi: 10.1523/JNEUROSCI.1228-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- Pandya A, Yakel JL. Allosteric modulator desformylflustrabromine relieves the inhibition of alpha2beta2 and alpha4beta2 nicotinic acetylcholine receptors by beta-Amyloid(1–) peptide. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovicz RE, Henderson BJ, Bonnell AB, Boyd RT, McKay DB, Li C. Identification of a negative allosteric site on human alpha4beta2 and alpha3beta4 neuronal nicotinic acetylcholine receptors. PLoS One. 2011;6:e24949. doi: 10.1371/journal.pone.0024949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Poole CA, Reilly HC, Flint MH. The adverse effects of HEPES, TES, and BES zwitterion buffers on the ultrastructure of cultured chick embryo epiphyseal chondrocytes. In Vitro. 1982;18:755–765. doi: 10.1007/BF02796499. [DOI] [PubMed] [Google Scholar]
- Sala F, Mulet J, Reddy KP, Bernal JA, Wikman P, Valor LM, Peters L, Konig GM, Criado M, Sala S. Potentiation of human alpha4beta2 neuronal nicotinic receptors by a flustra foliacea metabolite. Neurosci Lett. 2005;373:144–149. doi: 10.1016/j.neulet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Smulders CJ, Zwart R, Bermudez I, van Kleef RG, Groot-Kormelink PJ, Vijverberg HP. Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol. 2005;509:97–108. doi: 10.1016/j.ejphar.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discovery. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(−beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther. 2010;334:917–926. doi: 10.1124/jpet.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic acetylcholine receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Suzuki N. Blockage of chloride channels by HEPES buffer. Proc R Soc London, Ser B Biol Sci. 1987;230:93–100. doi: 10.1098/rspb.1987.0011. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]