Abstract
We report here the induction of specific protective cellular immunity against Mycobacterium tuberculosis by the employment of vaccination with recombinant attenuated Listeria monocytogenes strains. We constructed self-destructing attenuated L. monocytogenes Δ2 strains carrying eukaryotic expression plasmids for the antigen 85 complex (Ag85A and Ag85B) and for MPB/MPT51 (mycobacterial protein secreted by M. bovis BCG/mycobacterial protein secreted by M. tuberculosis) molecules. Infection of these recombinant bacteria allowed expression of the genes in the J774A.1 murine macrophage cell line. Intraperitoneal vaccination of C57BL/6 mice with these recombinant bacteria was capable of inducing purified protein derivative-specific cellular immune responses, such as foot pad reactions, proliferative responses of splenocytes, and gamma interferon production from splenocytes, suggesting the efficacy of vaccination against mycobacterial infection by use of these recombinant L. monocytogenes strains. Furthermore, intravenous vaccination with recombinant bacteria carrying expression plasmids for Ag85A, Ag85B, or MPB/MPT51 in BALB/c mice elicited significant protective responses, comparable to those evoked by a live Mycobacterium bovis BCG vaccine. Notably, this is the first report to show that MPB/MPT51 is a major protective antigen in addition to Ag85A and Ag85B, which have been reported to be major mycobacterial protective antigens.
Tuberculosis (TB) remains an urgent public health problem worldwide, resulting in 8 million new cases and 2 million deaths each year (14). Outbreaks of TB, especially in immunocompromised people, such as aged groups and AIDS patients, have also been reported. In addition, the appearance of multidrug-resistant Mycobacterium tuberculosis strains is also a serious issue in the world.
The only TB vaccine currently available is the attenuated Mycobacterium bovis strain bacillus Calmette-Guérin (BCG), which has been reported to have a variable protective efficacy, ranging from 0 to 85% in different controlled studies (6). Therefore, there remains an urgent need for an improved vaccine. A DNA vaccine is one of the most promising candidates for future TB vaccines. Many reports on DNA vaccination against TB have been accumulating. Secreted molecules have been known to be recognized by the protective immune response against TB. In these reports, various target antigens (Ags) for TB DNA vaccination have been reported, including the Ag85 complex molecules, Hsp65, Hsp70, the 38-kDa Ag, and ESAT-6 (reviewed in reference 28).
Ag85 complex molecules have been reported to be the dominant secreted Ags expressed by nearly all mycobacterial species analyzed so far (reviewed in reference 39). The complex consists of three structurally related components, namely Ag85A (p32A; 32-kDa Ag), Ag85B (p30; 30-kDa Ag, also termed α Ag), and Ag85C. Ag85 complex molecules are cross-reactive Ags and are highly conserved among Mycobacterium spp. The genes encode proteins with fibronectin-binding capacities (1) and mycolyltransferase activities, which are involved in the final stage of mycobacterial cell wall assembly (5). Ag85A and Ag85B have been reported to stimulate B- and T-cell responses in TB patients (24, 25), and immunization with Ag85A and Ag85B proteins induced protection against an aerosol challenge with M. tuberculosis in mice and guinea pigs, respectively (19). In addition, reports of successful naked DNA vaccines against TB, employing the Ag85A (3, 4, 9, 13, 21, 29, 36, 37) and Ag85B (22, 29, 37) genes, have also accumulated. According to these reports, the Ag85A and Ag85B molecules seem to be two of the most promising candidates for future subunit TB vaccines. Another molecule, MPB/MPT51 (mycobacterial protein secreted by M. bovis BCG/mycobacterial protein secreted by M. tuberculosis), has also been reported to be related to this family (31). The amino acid sequence deduced for MPB51 (GenBank/EMBL/DDBJ accession number D26486) is identical to the sequence deduced for MPT51 of M. tuberculosis strains H37Rv (AL022076) and CDC1551 (AE007185). So far, MPB/MPT51 has not been reported as a target Ag for vaccination against M. tuberculosis.
DNA vaccines offer many advantages over other methods of immunization: they have a relatively easy design and construction by recombinant DNA techniques, a strong induction of cellular immunity, chemical stability, a relatively low cost, and so on (reviewed in references 2 and 12). For successful results with DNA vaccination (for example, in the case of Ag85A), however, intramuscular immunization with large amounts (50 to 100 μg) of plasmid DNA was reported to be necessary (36), and the induction of immunity with intramuscular immunization of plasmid DNA is poor in terms of reproducibility (41). Recently, several investigators used attenuated intracellular bacteria as the carriers of DNA vaccines (reviewed in reference 11). These bacterial carrier systems have several special features, including direct delivery of the plasmid DNA to professional antigen-presenting cells and the possibility of oral administration. Bacteria utilized as this type of vaccine carrier include Salmonella (8) and Shigella (35) as well as Listeria (10). Gram-negative carriers such as Salmonella and Shigella have the disadvantage of containing abundant amounts of toxic lipopolysaccharide. Therefore, Listeria monocytogenes, a gram-positive bacterium, is a good candidate for a carrier. Furthermore, this bacterium is considered a possible effective recombinant vaccine vector based on its predilection for professional antigen-presenting cells such as macrophages and dendritic cells and its capacity to escape from phagolysosomes and to live in the cytoplasm of host cells (34, 38). In addition, this bacterium has been reported to have the ability to induce T-helper cell type 1 (Th1) immune responses (20). These features are favorable for eliciting effective cellular immunity against TB. Dietrich et al. (10) reported a DNA vaccination system using an attenuated self-destructing L. monocytogenes strain. They demonstrated the feasibility of the system in a cell culture system. They used a deletion mutant of L. monocytogenes Δ2 that lacks the entire lecithinase operon, including the virulence-associated genes actA, mpl, and plcB (17). This strain can infect macrophages and replicate in the cytoplasm but cannot spread to adjacent cells. This attenuated mutant was introduced with a plasmid containing the gene for lysis protein PLY118 of the listerial bacteriophage A118. PLY118 expression was controlled by the actA promoter, which is active when L. monocytogenes is in the host cell cytoplasm. Thus, this L. monocytogenes mutant escapes from the phagosome and then lyses when the PLY118 gene is expressed in the cytoplasm. Autolysis of the L. monocytogenes mutant apparently releases the plasmid DNA into the host cell cytoplasm, allowing expression of the transgene in the host cells. However, it was still unknown whether this DNA vaccine carrier system is capable of inducing specific immunity and protective immunity against infection in vivo.
For this study, we examined the inducibility of protective cellular immunity against M. tuberculosis by immunization of mice with this attenuated L. monocytogenes strain carrying a eukaryotic expression plasmid for Ag85A, Ag85B, or MPB51. The results showed that vaccination with the attenuated self-destructing L. monocytogenes strain could induce protective cellular immunity against M. tuberculosis infection. Furthermore, we show for the first time that MPB/MPT51, which is related to Ag85 family molecules, is a major protective Ag.
MATERIALS AND METHODS
Bacteria and plasmids.
M. bovis BCG (substrain Tokyo) was purchased from Japan BCG Inc. (Tokyo, Japan). The attenuated L. monocytogenes strain Δ2 (10, 17) and plasmids p3LOVA118 and pcDNA3L (10) were kindly provided by Werner Goebel, Guido Dietrich, and Ivaylo Gentschev (University of Würzburg, Germany). Attenuated L. monocytogenes Δ2 was cultured in brain heart infusion (BHI) broth (Becton Dickinson, Sparks, Md.) at 37°C. Escherichia coli DH5α was cultured in L broth. M. tuberculosis H37Rv was kindly donated by Isamu Sugawara (Research Institute of Tuberculosis, Tokyo, Japan).
Construction of recombinant plasmids p3L118R-Ag85A, p3L118R-Ag85B, and p3L118R-MPB51.
The NruI-NotI fragment of p3LOVA118, covering half of the cytomegalovirus (CMV) promoter and the ovalbumin epitope region, was removed and replaced with the corresponding region of pcDNA3L, resulting in p3L118R. This procedure removed the ovalbumin epitope region from p3LOVA118 and recreated a NotI site for future subcloning of genes of interest under control of the CMV promoter. The BCG Ag85A, Ag85B, and MPB51 genes were amplified from plasmids pMB49 (for Ag85A and MPB51) (31) and pαL-1 (30) (for Ag85B) by PCRs with the following primer pairs: 5′-ATAAGAATGCGGCCGCACCATGCAGCTTGTTGACAGG-3′ and 5′-ATAGTTTAGCGGCCGCTGTTCGGAGCTAGGCGC-3′ for Ag85A, 5′-ATAAGAATGCGGCCGCACCATGACAGACGTGAGCCGA-3′ and 5′-ATAGTTTAGCGGCCGCGGGCCCGTTGATCCCGTCAGCCGGC-3′ for Ag85B, and 5′-ATAAGAATGCGGCCGCTCGAGCACCATGAAGGGTCGGTCGGCG-3′ and 5′-ATAGTTTAGCGGCCGCGGGCCCGGCACCTGGCTTAGCGGA-3′ for MPB51 (underlined text indicates a NotI site). These PCR fragments were digested with NotI and inserted into a NotI site of p3L118R. The integrity of the nucleotide sequences was validated by automated DNA sequencing (ABI PRISM 310 genetic analyzer; Applied Biosystems, Foster City, Calif.) using a dye primer cycle sequencing kit (Applied Biosystems). The resultant plasmids were introduced into the attenuated L. monocytogenes Δ2 strain by electroporation, as described below.
Electroporation of plasmids into L. monocytogenes Δ2.
The electroporation procedure was basically in accordance with a previously described protocol (33). Briefly, L. monocytogenes Δ2 cells were shaken in 200 ml of BHI broth at 37°C until an optical density at 600 nm of 1.0. Next, 2,000 U of penicillin G was added and the culture was subjected to a 1-h incubation. The cells were harvested, washed twice with sucrose electroporation buffer (1 mM HEPES [pH 7.0], 0.5 M sucrose), and resuspended in 500 μl of the buffer. One hundred microliters of the cell suspension and 1 μg of one of the expression plasmids were then transferred to an electroporation cuvette and subjected to electroshock with a Gene-Pulser electroporation apparatus (Bio-Rad Laboratories, Hercules, Calif.). The electroporation conditions were as follows: cuvette gap, 0.4 cm; voltage, 2.5 kV; field strength, 6.25 kV/cm; capacitor, 25 μF; and resistor, 200 Ω. Next, the cell solution was incubated on ice for 10 min, added to 0.7 ml of BHI broth, and incubated at 37°C for 1 h. After centrifugation at 1,200 × g for 15 min at 4°C, 0.6 ml of the solution was removed. The remaining solution was plated onto a Trypticase soy agar plate (Becton Dickinson) containing 12.5 μg of tetracycline/ml and was incubated at 37°C for 18 h. Resultant tetracycline-resistant colonies were cultured and stored. These were named Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, and Δ2/p3L118R-MPB51 and harbored the recombinant plasmids p3L118R, p3L118R-Ag85A, p3L118R-Ag85B, and p3L118R-MPB51, respectively.
Mammalian cell culture.
The murine macrophage-like cell line J774A.1 (American Type Culture Collection, Manassas, Va.) and spleen cells of immunized mice were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 5% CO2 in an incubator.
Infection of J774A.1 cells with recombinant L. monocytogenes Δ2 strains.
J774A.1 cells (5 × 105 cells) were plated on 60-mm-diameter plates at the beginning of experiments. The medium was renewed 24 h before the experiments. Recombinant Δ2 strains (105 cells) were added to J774A.1 cells. After 5 h, 10 μg of gentamicin sulfate/ml was added to the medium to remove extracellular bacteria. After a 36-h incubation, the infected cells were harvested.
Reverse transcription (RT)-PCR analysis for Ag85A, Ag85B, or MPB51 gene detection.
Δ2/p3L118R-, Δ2/p3L118R-Ag85A-, Δ2/p3L118R-Ag85B-, or Δ2/p3L118R-MPB51-infected J774A.1 cells were harvested, and total RNAs were prepared from the cells by use of Isogen RNA extraction solution (Nippon Gene, Tokyo, Japan). Single-stranded cDNAs were synthesized with Molony murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, Md.) and then were used for PCR analysis. The primers used for Ag85A, Ag85B, and MPB51 gene detection were as follows: for Ag85A, 5′-AGGCCAACAGGCACGTCAA-3′ and 5′-ACATGTCGGAGGCCTTGTA-3′; for Ag85B, 5′-GAACAACTCACCTGCGGTT-3′ and 5′-CATCGACAAGCCGATTGC-3′; and for MPB51, 5′-GATGTCAGTAACTGGGTCAC-3′ and 5′-ACATTCCGTTGGTGTCCACA-3′. To refute the possibility of contamination of the plasmids in cDNA pools, we performed PCRs with combinations of a primer located in the CMV enhancer-promoter region of p3L118R, 5′-GGTGGGAGGTCTATATAAGC-3′, and the reverse primers for the Ag85A, Ag85B, and MPB51 genes.
Mice.
C57BL/6 and BALB/c mice (Japan SLC, Hamamatsu, Japan) were kept under specific-pathogen-free conditions and fed autoclaved food and water ad libitum at the Experimental Animal Institute of the Hamamatsu University School of Medicine. All animal experiments were performed according to the Guidelines for Animal Experimentation, Hamamatsu University School of Medicine.
Immunization procedures.
Mice were immunized intraperitoneally (i.p.) (C57BL/6; ∼107 CFU) or intravenously (i.v.) (BALB/c; ∼106 CFU) with a recombinant attenuated L. monocytogenes Δ2 strain three times at 2-week intervals. As a control, mice were also immunized with BCG i.p. once (C57BL/6, ∼107 CFU) or subcutaneously twice at a 2-week interval (BALB/c, ∼106 CFU).
Genomic DNA PCR.
Δ2/p3L118R-MPB51 or Δ2/p3L118R Listeria (∼108 CFU) was injected i.p. into C57BL/6 mice, and Δ2/p3L118R-MPB51 or L. monocytogenes EGD (a parental strain of Δ2; ∼107 CFU) was injected i.v. into BALB/c mice. One day after the injection, tissue cell suspensions from injected mice were prepared and washed three times after the lysis of erythrocytes with Tris-buffered 0.83% ammonium chloride solution. After a brief centrifugation, the cells were added to 10 volumes of proteinase K solution (1 mg/ml; Boehringer Mannheim GmbH, Mannheim, Germany) in 10 mM Tris (pH 7.4), 10 mM EDTA, 150 mM NaCl, and 0.4% sodium dodecyl sulfate and were incubated for 15 min at 65°C. The cells were further incubated in the same solution overnight at 37°C. Genomic DNA was prepared from the cells after phenol extraction and ethanol precipitation. A nested PCR was performed with 1 μg of genomic DNA for MPB51 gene detection. The first-round PCR was performed with the same primer pairs that were used for RT-PCR analysis, and the second-round PCR was performed with 1 μl of the first-round PCR solution (20-μl total volume) and the following primer pairs located just inside of the first-round PCR primers: 5′-CGCGGGTAACGCGATGAACAC-3′ and 5′-CACACCGCCGAATTGCTGCAT-3′. For both PCRs, the conditions were 25 cycles of 94°C for 30 s, 62°C for 50 s, and 72°C for 30 s. The expected size of the MPB51 PCR product was 341 bp.
Delayed-type hypersensitivity (DTH) reaction.
Purified protein derivative (PPD) was purchased from Japan BCG Inc. C57BL/6 mice were injected with 5 μg of PPD in 50 μl of phosphate-buffered saline (PBS) in the left hind foot pad. As controls, mice were injected with 50 μl of PBS alone in the right hind foot pad. The swelling of foot pads was measured with a caliper meter (Mitsutoyo Corp., Osaka, Japan) 48 h after injection. Naïve mice were treated in the same way as the controls for nonspecific swelling.
Lymphocyte proliferation assay.
Spleen cells (105/well) from the immunized C57BL/6 mice were incubated for 48 h at 37°C in 96-well round-bottom tissue culture plates in the presence or absence of 5 μg of PPD/ml. After 48 h of culturing, de novo DNA synthesis was assessed by the addition of 0.5 μCi of [methyl-3H]thymidine (10 Ci/mmol; ICN Biochemicals, Irvine, Calif.)/well for the last 12 h of culture. Quintuplicate cultures were harvested onto glass fiber filters, and the radioactivity was counted by liquid scintillation. The [methyl-3H]thymidine incorporation was calculated in counts per minute per 104 cells.
Cytokine ELISA.
Spleen cells were harvested from the immunized C57BL/6 mice. Recovered cells were incubated for 4 days in 24-well plates at 2 × 106 cells/well in RPMI-10% fetal bovine serum in the presence or absence of 5 μg of PPD solution/ml. Concentrations of gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-5 in the culture supernatants were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) as described elsewhere (40). For the sandwich ELISA, the following combinations of coating and biotinylated monoclonal antibodies were used: R4-6A2 and XMG1.2 for IFN-γ, 11B11 and BVD6-24G2 for IL-4, and TRFK5 and TRFK4 for IL-5. All monoclonal antibodies were purchased from BD PharMingen (San Diego, Calif.). The amounts of cytokines were calculated by using standard murine recombinant cytokine curves run on the same immunoplate.
Semiquantitative RT-PCR for IFN-γ gene.
Immune spleen cells (C57BL/6 mice) were cultured for 48 h at 107 cells/ml in the presence or absence of 5 μg of PPD solution/ml. Total RNAs were extracted from cells by use of Isogen RNA extraction solution (Nippon Gene). Single-stranded cDNAs were synthesized with Molony murine leukemia virus reverse transcriptase (Life Technologies) and then used in PCRs for IFN-γ gene detection as described elsewhere (40).
In vivo protection assay.
Immunized BALB/c mice were infected with 5 × 105 CFU of M. tuberculosis H37Rv i.v. 2 months after the last immunization. Mice were sacrificed 10 weeks later, and the bacterial numbers in the spleens, livers, and lungs were counted in CFU on Middlebrook 7H11 medium (Becton Dickinson).
Statistics.
Data from multiple experiments were expressed as means ± standard deviations (SD). Statistical analyses were performed with the StatView-J 4.02 statistics program (Abacus Concepts, Berkeley, Calif.). Data were analyzed by Fisher's protected least significant difference.
RESULTS
Infection of recombinant L. monocytogenes allowed expression of genes in J774A.1 murine macrophage cell line.
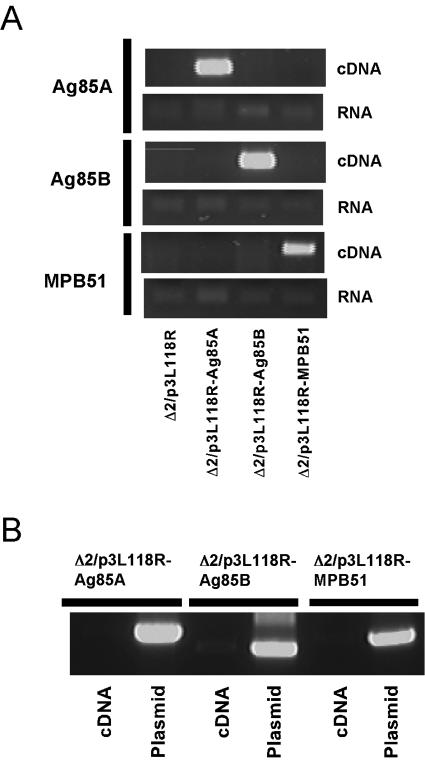
J774A.1 murine macrophage-like cells were infected with an L. monocytogenes Δ2 mutant carrying the plasmid p3L118R, p3L118R-Ag85A, p3L118R-Ag85B, or p3L118R-MPB51. Thirty-six hours after infection, the infected cells were harvested for the isolation of total RNA. RT-PCR was then performed to confirm the expression of Ag85A, Ag85B, or MPB51 mRNA in the cells. As shown in Fig. 1A, clear bands for these mRNAs were detected after RT of total RNA solutions but not before reverse transcriptase treatment. In addition, to refute the possibility of contamination of plasmids p3L118R-Ag85A, p3L118R-Ag85B, and p3L118R-MPB51 in the cDNA pools used, we subjected the cDNA pools to PCRs with relevant primer sets, by which only the plasmid DNAs, but not the transcripts, were detected (see Materials and Methods for details). The results showed that no bands were detected with the cDNA pools by PCR, while p3L118R-Ag85A, p3L118R-Ag85B, and p3L118R-MPB51 controls gave specific bands, indicating no contamination of plasmids in the cDNA pools. These data indicate that the Ag85A, Ag85B, and MPB51 genes were expressed in J774A.1 cells by this attenuated L. monocytogenes system.
FIG. 1.
Expression of Ag85A, Ag85B, and MPB51 mRNAs in murine macrophage cells infected with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. (A) Murine macrophage cell line J774A.1 was infected with Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. Total RNAs from these infected cells were recovered and reverse transcribed with random hexamers to make cDNA pools. PCRs were then performed with Ag85A, Ag85B, and MPB51 gene-specific primers (cDNA panels). After the recovery of total RNAs, the same PCRs were performed for Ag85A, Ag85B, or MPB51 gene detection without RT (RNA panels). (B) To refute the possibility of contamination of p3L118R-Ag85A, p3L118R-Ag85B, or p3L118R-MPB51 in the cDNA pools used for panel A, we subjected the cDNA pools to PCRs with primer sets by which only the plasmid DNAs, not the transcripts, were detected. See Materials and Methods for details.
Detection of injected plasmid DNA in tissues of mice infected with recombinant attenuated L. monocytogenes.
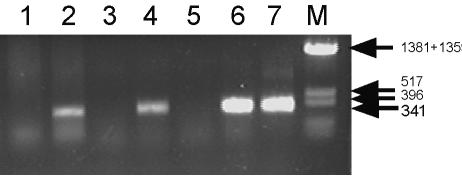
Δ2/p3L118R-MPB51 recombinant Listeria was injected i.p. (C57BL/6 mice) or i.v. (BALB/c mice). In order to check for p3L118R-MPB51 plasmid transfer to tissues of the injected mice, we prepared genomic DNAs from cells of the tissues and performed PCR analysis for MPB51 gene detection. As shown in Fig. 2, we observed an MPB51-specific band only for DNAs derived from mice injected with Δ2/p3L118R-MPB51 Listeria. The PCR was performed with tissue cells washed with PBS, suggesting that the p3L118R-MPB51 plasmid was transferred into host cells after recombinant Listeria injection. It is noteworthy that we observed no colonies of carrier L. monocytogenes in the spleens of the i.p. immunized C57BL/6 mice by plating of the tissue homogenates on Trypticase soy agar (data not shown).
FIG. 2.
Detection of p3L118R-MPB51 plasmid in tissues of Δ2/p3L118R-MPB51-injected mice. Mice were injected with Δ2/p3L118R-MPB51 or control Listeria i.p. (C57BL/6) or i.v. (BALB/c). Genomic DNA was prepared from tissues of the injected mice 1 day after injection, and a nested PCR was performed for MPB51 DNA detection. Lane 1, spleen of C57BL/6 mouse injected with Δ2/p3L118R control; lane 2, spleen of C57BL/6 mouse injected with Δ2/p3L118R-MPB51; lane 3, spleen of BALB/c mouse injected with L. monocytogenes EGD; lane 4, spleen of BALB/c mouse injected with Δ2/p3L118R-MPB51; lane 5, liver of BALB/c mouse injected with L. monocytogenes EGD; lane 6, liver of BALB/c mouse injected with Δ2/p3L118R-MPB51; lane 7, control p3L118R-MPB51 plasmid. A size marker was also loaded (lane M). DNA fragment sizes are shown to the right.
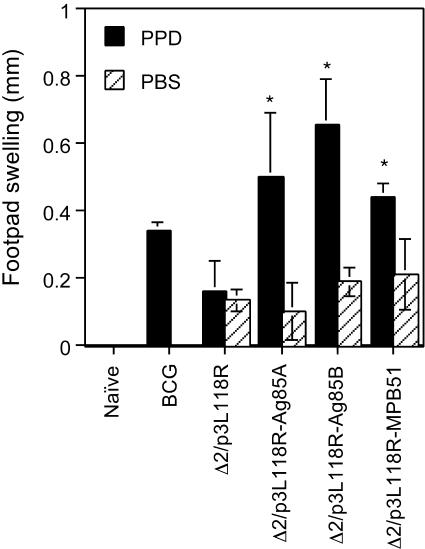
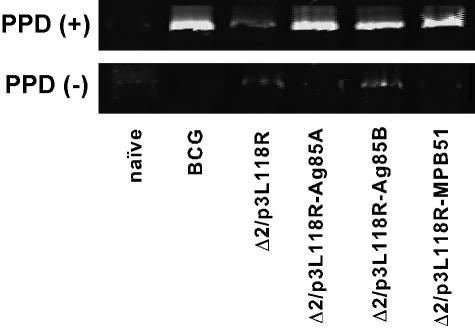
PPD-specific DTH reaction with recombinant attenuated L. monocytogenes vaccination.
For effective protective immunity against M. tuberculosis, specific cellular immunity against the bacterium plays a critical role. We first examined DTH responses of C57BL/6 mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. As shown in Fig. 3, mice immunized with these recombinant Listeria strains significantly responded to PPD, but not to PBS alone. Similar, but lower, responses were obtained for mice immunized with M. bovis BCG. Mice immunized with the Δ2/p3L118R control strain failed to show specific DTH reactions to PPD. These results indicate that mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 successfully elicited cellular immunity against M. tuberculosis.
FIG. 3.
Foot pad swelling responses of mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. C57BL/6 mice were immunized with Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 three times at 2-week intervals. The data for mice immunized once with M. bovis BCG are also shown as a control. One month after the last immunization, foot pad swelling responses directed against PPD were examined. Black bars, footpad swelling after in vivo PPD stimulation; hatched bars, foot pad swelling with PBS alone. The means ± SD of four to five mice per group are shown. Asterisks indicate statistically significant (P ≤ 0.001) differences with the value for a control (Δ2/p3L118R) immunization.
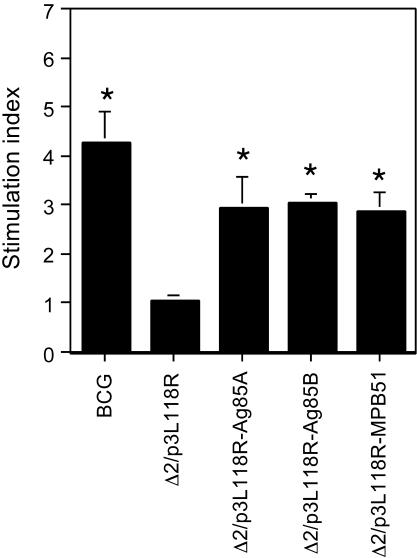
PPD-specific lymphocyte proliferation after recombinant attenuated L. monocytogenes vaccination.
We next examined proliferative responses of splenocytes derived from the immunized C57BL/6 mice in response to in vitro PPD stimulation. As shown in Fig. 4, a strong proliferative response was observed in control BCG-immunized mice. Immunization with recombinant Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 also caused significant proliferative responses, but the levels of specific proliferation were lower than that evoked by immunization with M. bovis BCG. Splenocytes of mice immunized with the Δ2/p3L118R control recombinant L. monocytogenes strain did not have a significant response.
FIG. 4.
PPD-specific splenocyte proliferation of mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. C57BL/6 mice were immunized with Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 three times at 2-week intervals. The data for mice immunized once with M. bovis BCG are also shown as a control. Spleen cells from the immunized mice were harvested 1 month after the last immunization, cultured in vitro in the presence or absence of 5 μg of PPD/ml for 48 h, and pulsed with 0.5 μCi of [methyl-3H]thymidine/ml for the last 12 h. The values represent stimulation indexes (the values after in vitro stimulation in the presence of PPD divided by the values in the absence of PPD). The means ± SD of quintuplicate determinations from a representative experiment of three independent experiments are shown. Asterisks indicate statistically significant (P < 0.0001) differences with the value for a control (Δ2/p3L118R) immunization.
PPD-specific cytokine production with recombinant attenuated L. monocytogenes vaccination.
IFN-γ is known to be a key factor for the elicitation of effective protection against M. tuberculosis. Therefore, employing RT-PCR analysis, we semiquantitatively assessed IFN-γ mRNA expression from splenocytes of immunized C57BL/6 mice upon PPD stimulation. As shown in Fig. 5, IFN-γ mRNA-specific bands were clearly detected in PPD-stimulated splenocytes of C57BL/6 mice immunized with recombinant Listeria strain Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. The strengths of the bands were comparable to that of mice immunized with M. bovis BCG. Again, splenocytes of mice immunized with the Δ2/p3L118R control gave only a faint IFN-γ mRNA-specific band.
FIG. 5.
IFN-γ mRNA expression by spleen cells of mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51. C57BL/6 mice were immunized with Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 three times at 2-week intervals. The data for mice immunized once with M. bovis BCG are also shown as a control. Spleen cells from the immunized mice were harvested 1 month after the last immunization and cultured in vitro in the presence [PPD (+)] or absence [PPD (−)] of 5 μg of PPD/ml for 48 h. IFN-γ mRNA expression was evaluated by semiquantitative RT-PCR with IFN-γ-specific primers.
In addition, we examined the cytokine production of splenocytes from immunized C57BL/6 mice by a sandwich ELISA for IFN-γ, IL-4, and IL-5 (Table 1). Correlating with the results of RT-PCRs, splenocytes from mice immunized with M. bovis BCG or a recombinant Listeria strain harboring the Ag85A, Ag85B, or MPB51 gene produced high amounts of IFN-γ after in vitro stimulation with PPD. We observed the production of moderate levels of IFN-γ from spleen cells of naïve mice and control Listeria (Δ2/p3L118R)-immunized mice upon PPD stimulation. We did not detect significantly enhanced production of IL-4 or IL-5 for any of the mice examined.
TABLE 1.
Cytokine production by spleen cells from mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51
| Mouse group | Stimulation with PPDa | Cytokine production (pg/ml)b
|
||
|---|---|---|---|---|
| IFN-γ | IL-4 | IL-5 | ||
| Naïve | − | 131 | 122 | 71 |
| + | 868 | 54 | 0 | |
| BCG immunized | − | 186 | 92 | 52 |
| + | 4,103 | 70 | 6 | |
| Δ2/p3L118R immunized | − | 51 | 44 | 10 |
| + | 740 | 94 | 31 | |
| Δ2/p3L118R-Ag85A immunized | − | 218 | 74 | 43 |
| + | 1,728 | 35 | 0 | |
| Δ2/p3L118R-Ag85B immunized | − | 210 | 44 | 18 |
| + | 3,432 | 66 | 14 | |
| Δ2/p3L118R-MPB51 immunized | − | 191 | 19 | 0 |
| + | 4,093 | 31 | 0 | |
Spleen cells from immunized C57BL/6 mice (2 × 106 cells per well) were cultured in the presence (+) or absence (−) of 5 μg of PPD/ml.
After 4 days, cytokine concentrations in culture supernatants were quantified by IFN-γ-, IL-4-, and IL-5-specific ELISA, as described in Materials and Methods. The values for naïve and BCG-immunized mice are also shown as controls. Averages of duplicate representative data from several similar experiments are shown.
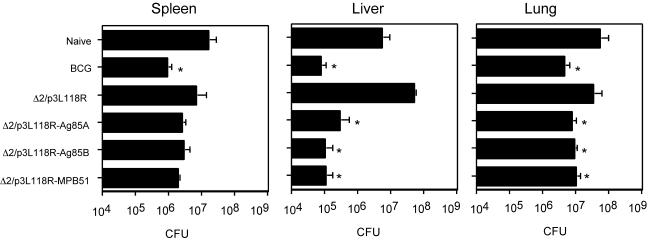
Recombinant attenuated L. monocytogenes vaccination conferred protective immunity against M. tuberculosis infection comparable to M. bovis BCG immunization in BALB/c mice.
We evaluated the effects of recombinant attenuated Listeria vaccination on protective immunity against M. tuberculosis H37Rv infection and compared them with those of M. bovis BCG vaccination. At first, we used C57BL/6 mice for the experiments, but the relative resistance against M. tuberculosis infection of the strain hampered the evaluation of the vaccination effects. Therefore, we used BALB/c mice for the evaluation. Ten weeks after i.v. injection with M. tuberculosis H37Rv, spleens, livers, and lungs were prepared from the immunized mice and the numbers of CFU of M. tuberculosis H37Rv in these tissues were evaluated. Figure 6 shows viable colony counts for tissues from mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 Listeria compared with those from naïve mice, mice immunized with the Δ2/p3L118R control, and BCG-vaccinated mice. The protective effects of these recombinant Listeria immunizations were obvious in all tissues examined and were comparable to those of live BCG vaccination. In the liver, particularly, we detected an approximately 2-orders-of-magnitude reduction in CFU for Ag85A, Ag85B, and MPB51 DNA vaccine- and live BCG-immunized mice.
FIG. 6.
In vivo protective activity of mice immunized with Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 against virulent M. tuberculosis. BALB/c mice were immunized with Δ2/p3L118R, Δ2/p3L118R-Ag85A, Δ2/p3L118R-Ag85B, or Δ2/p3L118R-MPB51 three times at 2-week intervals. The data for mice immunized once with M. bovis BCG are also shown as a control. The mice were challenged i.v. with live M. tuberculosis H37Rv. Numbers of CFU in the spleens, livers, and lungs were determined 10 weeks later. The means ± SD of six mice are shown. Asterisks indicate statistically significant (P < 0.05) differences with the value for a control (Δ2/p3L118R) immunization.
DISCUSSION
From the findings described in this paper, we drew the following conclusions concerning the attenuated self-destructing L. monocytogenes-harboring DNA vaccine against M. tuberculosis. (i) Inoculation with recombinant L. monocytogenes-harboring plasmid DNA vaccines for Ag85 complex and MPB/MPT51 molecules is able to induce specific type 1 cellular immune responses in spleen cells of mice. (ii) Inoculation with these vaccines can confer protective immunity against TB. (iii) The MPB/MPT51 molecule, which is related to the Ag85 family, appears to be a major protective Ag, in addition to Ag85A and Ag85B.
We detected a significant level of PPD-specific IFN-γ secretion, which is a hallmark of type 1 immune responses and is considered an important factor in the protective immunity against M. tuberculosis (7, 15, 23), in splenocytes of mice immunized with attenuated recombinant Listeria harboring an Ag85A, Ag85B, or MPB/MPT51 DNA vaccine (Table 1). The production of moderate levels of IFN-γ from splenocytes of naïve mice and control Listeria (Δ2/p3L118R)-immunized mice may be caused by nonspecific responses of these mice against PPD.
Cellular immunity, including CD8+ cytotoxic T lymphocytes and CD4+ Th1 cells, has been reported to play critical roles in effective protective immunity against M. tuberculosis (reviewed in references 16 and 32). In this context, the attenuated Listeria immunization system shown here should be a favorable immunization method, as it is able to elicit effective type 1 cellular immune responses against M. tuberculosis. Furthermore, an attenuated Listeria strain harboring the suicide gene ply118 was revealed to be almost nontoxic, since inoculation with ∼108 CFU of the attenuated Listeria, but not virulent L. monocytogenes, failed to kill even IFN-γ receptor knockout mice as well as C57BL/6 wild-type mice (data not shown). Also, we could not detect carrier L. monocytogenes, although the plasmid DNA vaccines were detected in the spleens of i.p. immunized C57BL/6 mice (data not shown).
Several heterologous carrier systems for mycobacterial Ags have been reported. Zhu et al. (42) showed that the recombinant vaccinia virus system for M. tuberculosis-derived 19- and 38-kDa glycolipoproteins is effective for protection against murine M. tuberculosis infection. Hess et al. (18) reported that a recombinant Salmonella enterica serovar Typhimurium vaccine which secretes Ag85B is effective for the induction of pathogen-specific IFN-γ and tumor necrosis factor and also for protection against murine TB. It will be interesting to compare the system shown here with these systems in terms of the induction of protective immunity against M. tuberculosis.
As a general rule, the determination of a target Ag is very important for the development of effective DNA vaccines against bacterial infection. Many reports have already shown the effectiveness of Ag85A and Ag85B for eliciting protective immunity against M. tuberculosis. We also confirmed with our system that Ag85A and Ag85B are capable of inducing cellular and protective immunity. In addition, we evaluated the effectiveness of MPB/MPT51 as a target Ag for an anti-TB vaccine. Our results indicate that MPB/MPT51 is also a protective Ag and is comparable to Ag85A and Ag85B. In particular, immunization with Δ2/p3L118R-MPB51 induced enhanced PPD-specific IFN-γ production from splenocytes, the expression level of which was comparable to that by BCG immunization. So far, MPB/MPT51 has not been reported as a target Ag for vaccination. Therefore, it is interesting and important to examine the antigenicity of the molecule in detail to study, for example, the capacity to induce specific CD4+- and CD8+-T-cell effectors, and to identify the T-cell epitopes in the molecule. We identified T-cell epitopes in C57BL/6 and BALB/c mice (M. Suzuki, T. Aoshi, T. Nagata, and Y. Koide, submitted for publication). The spleen cells derived from Δ2/p3L118R-MPB51-immunized mice were able to induce IFN-γ in response to these epitope peptides, indicating that the responses are MPB51 specific (data not shown).
For the induction of effective immunity, the route of vaccination is an important factor. The Listeria carrier system is suitable for the induction of mucosal immunity. Particularly, intranasal inoculation of our recombinant Listeria strains may be capable of inducing protective T-cell immune responses in the lung. That study is now in progress. For the present study, however, we immunized mice with Listeria i.p. (C57BL/6 mice) or i.v. (BALB/c mice). The main reason for not choosing the oral route for immunization is that the mouse was reported not to be a good model for the entry of L. monocytogenes into intestinal epithelium due to a Glu-to-Pro substitution in mouse E-cadherin, which serves as a receptor for internalin A of L. monocytogenes (26, 27). In humans, however, oral immunization with L. monocytogenes-harboring plasmid DNA vaccines seems to be a possible choice for DNA vaccine delivery. Although mice are devoid of the E-cadherin molecule, L. monocytogenes may have a capacity to enter into M cells located in the intestinal epithelium. Therefore, oral administration of attenuated L. monocytogenes strains is also worthwhile to try in mice.
Taken together, we show here that DNA vaccines with the attenuated self-destructing L. monocytogenes carrier system may be favorable DNA vaccination systems in vivo when accompanied with the adjuvanticity to induce Th1-type immune responses and the predilection of the bacterium to interact with macrophages.
Acknowledgments
We are very grateful to Werner Goebel, Guido Dietrich, and Ivaylo Gentschev (University of Würzburg, Germany) for their kindness in providing the p3LOVA118 and pcDNA3L plasmids and L. monocytogenes strain Δ2. We also thank Isamu Sugawara (The Research Institute of Tuberculosis, Tokyo, Japan) for providing the M. tuberculosis H37Rv strain.
This work was supported by a grant-in-aid for scientific research from the Japan Society for the Promotion of Science; a grant-in-aid for the Centers of Excellence (COE) research program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Health and Labour Sciences research grant from the Ministry of Health, Labour and Welfare of Japan; and the United States-Japan Cooperative Medical Science Program.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abou-Zeid, C., T. L. Ratliff, H. G. Wiker, M. Harboe, J. Bennedsen, and G. A. W. Rook. 1988. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 56:3046-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon, J. B., G. W. Waine, and D. P. McManus. 1999. DNA vaccines: technology and application as anti-parasite and anti-microbial agents. Adv. Parasitol. 42:343-410. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Liu, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin, S. L., C. D. D'Souza, I. M. Orme, M. A. Liu, K. Huygen, O. Dennis, A. Tang, L. Zhu, D. Montgomery, and J. B. Ulmer. 1999. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tuber. Lung Dis. 79:251-259. [DOI] [PubMed] [Google Scholar]
- 5.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Bersa. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 6.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA 271:698-702. [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darji, A., C. A. Guzmán, B. Gerstel, P. Wachholz, K. N. Timmis, J. Wehland, T. Chakraborty, and S. Weiss. 1997. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 91:765-775. [DOI] [PubMed] [Google Scholar]
- 9.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T.-P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich, G., A. Bubert, I. Gentchev, Z. Sokolovic, A. Simm, A. Catic, S. H. E. Kaufmann, J. Hess, A. A. Szaley, and W. Goebel. 1998. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat. Biotechnol. 16:181-185. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich, G., I. Gentchev, J. Hess, J. B. Ulmer, S. H. E. Kaufmann, and W. Goebel. 1999. Delivery of DNA vaccines by attenuated intracellular bacteria. Immunol. Today 20:251-253. [DOI] [PubMed] [Google Scholar]
- 12.Donnelley, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 13.D'Souza, S., O. Denis, T. Scorza, F. Nzabintwali, H. Verschueren, and K. Huygen. 2000. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur. J. Immunol. 30:2455-2459. [DOI] [PubMed] [Google Scholar]
- 14.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 17.Hauf, N., W. Goebel, F. Fiedler, Z. Sokolovic, and M. Kuhn. 1997. Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-κB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IκBα and IκBβ degradation. Proc. Natl. Acad. Sci. USA 94:9394-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess, J., L. Grode, J. Hellwig, P. Conradt, I. Gentschev, W. Goebel, C. Ladel, and S. H. E. Kaufmann. 2000. Protection against murine tuberculosis by an attenuated recombinant Salmonella typhimurium vaccine strain that secretes the 30-kDa antigen of Mycobacterium bovis BCG. FEMS Immunol. Med. Microbiol. 27:283-289. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz, M. A., B.-W. E. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh, C.-S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 21.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J.-P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 22.Kamath, A. T., C. G. Feng, M. MacDonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura, I., H. Tsukada, H. Yoshikawa, M. Fujita, K. Nomoto, and M. Mitsuyama. 1992. IFN-γ-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J. Immunol. 148:2887-2893. [PubMed] [Google Scholar]
- 24.Launois, P., M. N. Niang, J. De Bruyn, J.-L. Sarthou, F. Rivier, A. Drowart, J.-P. Van Vooren, J. Millan, and K. Huygen. 1993. The major secreted antigen complex (Ag85) from Mycobacterium bovis bacille Calmette-Guérin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J. Infect. Dis. 167:1160-1167. [DOI] [PubMed] [Google Scholar]
- 25.Launois, P., R. DeLeys, M. N. Niang, A. Drowart, M. Andrien, P. Dierckx, J.-L. Cartel, J.-L. Sarthou, J.-P. Van Vooren, and K. Huygen. 1994. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect. Immun. 62:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cassart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 28.Lowrie, D. B., C. L. Silva, and R. E. Tascon. 1998. Genetic vaccination against tuberculosis, p. 59-71. In E. Raz, (ed.), Gene vaccination: theory and practice. Springer-Verlag, Berlin, Germany.
- 29.Lozes, E., K. Huygen, J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, P. Vandenbussche, J.-P. Van Vooren, A. Drowart, J. B. Ulmer, and M. A. Liu. 1997. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine 15:830-833. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo, K., R. Yamaguchi, A. Yamazaki, H. Tasaka, and T. Yamada. 1998. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular α antigen. J. Bacteriol. 170:3847-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohara, N., H. Kitaura, H. Hotokezaka, T. Nishiyama, N. Wada, S. Matsumoto, T. Matsuo, M. Naito, and T. Yamada. 1995. Characterization of the gene encoding the MPB51, one of the major secreted protein antigens of Mycobacterium bovis BCG, and identification of the secreted protein closely related to the fibronectin binding 85 complex. Scand. J. Immunol. 41:433-442. [DOI] [PubMed] [Google Scholar]
- 32.Orme, I. M., P. Andersen, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 33.Park, S. F., and G. S. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 34.Paterson, Y., and G. Ikonomidis. 1996. Recombinant Listeria monocytogenes cancer vaccines. Curr. Opin. Immunol. 8:664-669. [DOI] [PubMed] [Google Scholar]
- 35.Sizmore, D. R., A. A. Branstrom, and J. C. Sadoff. 1995. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science 270:299-302. [DOI] [PubMed] [Google Scholar]
- 36.Tanghe, A., O. Denis, B. Lambrecht, V. Motte, T. van den Berg, and K. Huygen. 2000. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect. Immun. 68:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulmer, J. B., M. A. Liu, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, J. Content, and K. Huygen. 1997. Expression and immunogenicity of Mycobacterium tuberculosis antigen 85 by DNA vaccination. Vaccine 15:792-794. [DOI] [PubMed] [Google Scholar]
- 38.Weiskirch, L. M., and Y. Paterson. 1997. Listeria monocytogenes: a potent vaccine vector for neoplastic and infectious disease. Immunol. Rev. 158:159-169. [DOI] [PubMed] [Google Scholar]
- 39.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida, A., Y. Koide, M. Uchijima, and T. O. Yoshida. 1995. Dissection of strain difference in acquired protective immunity against Mycobacterium bovis bacillus Calmette-Guérin (BCG). J. Immunol. 155:2057-2066. [PubMed] [Google Scholar]
- 41.Yoshida, A., T. Nagata, M. Uchijima, T. Higashi, and Y. Koide. 2000. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 18:1725-1729. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, X., N. Venkataprasad, J. Ivanyi, and H. M. Vordermeier. 1997. Vaccination with recombinant vaccinia viruses protects mice against Mycobacterium tuberculosis infection. Immunology 92:6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]