Abstract
We have reported that Mycobacterium tuberculosis residing within the phagosomes of human monocyte-derived macrophages (MDM) can acquire Fe from extracellular transferrin (TF) and sources within the MDM. In the lung, Fe is also bound to lactoferrin (LF) and low-molecular-weight chelates. We therefore investigated the ability of intraphagosomal M. tuberculosis to acquire Fe from these sources. M. tuberculosis acquired 30-fold and 3-fold more Fe from LF and citrate, respectively, compared to TF, in spite of similar MDM-associated Fe. M. tuberculosis infection decreased MDM-associated Fe relative to uninfected MDM as follows: TF (38.7%), citrate (21.1%), and LF (15.3%). M. tuberculosis Fe acquisition from extracellular chelates (exogenous source) and from endogenous MDM Fe initially acquired from the three chelates (endogenous source) was compared. M. tuberculosis Fe acquisition was similar from exogenous and endogenous sources supplied as Fe-TF. In contrast, there was much greater intracellular M. tuberculosis Fe uptake from LF and citrate from the exogenous than endogenous source. Gamma interferon (IFN-γ) reduced MDM Fe uptake from each chelate by ∼50% and augmented the M. tuberculosis-induced decrease in MDM Fe uptake from exogenous TF, but not from LF or citrate. IFN-γ minimally decreased intracellular M. tuberculosis Fe acquisition from exogenous Fe-TF but significantly increased Fe uptake from LF and citrate. Intraphagosomal M. tuberculosis Fe acquisition from both exogenous and endogenous MDM sources, and the effect of IFN-γ on this process, is influenced by the nature of the extracellular Fe chelate. M. tuberculosis has developed efficient mechanisms of acquiring Fe from a variety of Fe chelates that it likely encounters within the human lung.
Iron (Fe) acquisition is critical to the metabolism and growth of most microbes. Limiting Fe availability is a strategy of host defense (20, 31). In vivo, nearly all extracellular Fe is chelated to the serum and mucosal proteins transferrin (TF) and lactoferrin (LF). Such binding markedly decreases microbial access to Fe (20). Furthermore, during infections Fe shifts from the serum to reticuloendothelial system macrophages. Essentially all successful pathogens have evolved strategies to acquire host Fe (35). Whereas most bacteria have access to extracellular Fe chelates, pathogens that live predominantly within intracellular environments face unique challenges in that they must acquire Fe from within their intracellular locale.
Mycobacterium tuberculosis is a major human intracellular pathogen. It enters and multiplies within human macrophages in a unique phagosomal compartment. Fe is needed for M. tuberculosis growth in in vitro culture media and in macrophages, and microbial siderophore production is critical to this process (14, 46, 60). However, little is known about how M. tuberculosis acquires Fe while sequestered within the macrophage phagosome.
Extracellular TF cycles to M. tuberculosis-containing phagosomes through plasma membrane TF receptor (TFR) trafficking to early endosomes (12, 50), and it has been proposed that this provides M. tuberculosis with Fe via phagosome-endosome fusion (12). We have shown that M. tuberculosis residing within phagosomes of human macrophages can acquire Fe from extracellular TF (37, 39). More recently, we demonstrated that intraphagosomal M. tuberculosis can acquire Fe from sources within the macrophage (endogenous), as well as from extracellular TF (exogenous) (39). Our data raise the possibility that a portion of the Fe uptake from extracellular TF by M. tuberculosis may involve initial Fe transfer from endocytosed TF, presumably via DMT-1 (21), to the monocyte-derived macrophage (MDM) cytoplasm or another internal site, where M. tuberculosis then gains access to it.
Gamma interferon (IFN-γ) plays a key role in host defense against M. tuberculosis, as it helps convert macrophages from a quiescent to activated state (22). At least some of the antimicrobial effects of IFN-γ have been attributed to alterations of macrophage Fe stores. Even though IFN-γ lowered the total Fe content of MDM, we found that the ability of M. tuberculosis to acquire intracellular Fe from IFN-γ-treated macrophages was only impaired to a small extent (39). This suggests that IFN-γ does not effectively alter the amount of Fe in the intracellular macrophage pools that can be accessed by intraphagosomal M. tuberculosis and may explain in part why IFN-γ does not slow the growth of M. tuberculosis in human MDM (17, 37).
Our studies to date and those of other investigators have focused on Fe bound initially to TF. However, within the human lung, there are greater or similar levels of LF relative to TF (55). As such, Fe bound to LF is particularly relevant to pulmonary infection with M. tuberculosis and may in fact be more important to the pathogenesis of pulmonary tuberculosis than Fe chelated to TF.
In spite of their similarities, TF and LF differ in a number of physicochemical properties and in their interaction with macrophages. LF does not bind to the TFR on the surface of human cells (4, 5, 58). Macrophages can acquire Fe from LF, although the mechanism(s) remains ill-defined. LF may bind to variably characterized surface receptors distinct from the TFR (4) or, alternatively, via cell surface glycans (47). There is no consensus as to how Fe is taken up once LF binding occurs.
Many cells, including macrophages, can also take up Fe bound to various low-molecular-weight chelates, such as citrate (11, 29, 32, 42, 44, 51). Much less Fe exists in this form in vivo than is bound to TF or LF. However, these levels rise with cell damage that results in Fe leakage from intracellular sites (45) or from exogenous sources such as inhaled cigarette smoke (54). The mechanism used for cellular uptake of these Fe forms remains unclear, in spite of a variety of proposed mechanisms (13, 32, 40, 41, 45, 51).
Given the known differences in the cellular metabolism of Fe bound to LF and low-molecular-weight forms of Fe by macrophages and their presence in the airway of M. tuberculosis-infected individuals, we examined the hypothesis that the ability of intraphagosomal M. tuberculosis to acquire Fe from the extracellular environment differs with the nature of the Fe chelate to which the macrophage was exposed. We provide the first definitive evidence of the ability of M. tuberculosis to acquire Fe from LF and citrate and demonstrate features that are distinct from that using TF. Furthermore, we examined whether the ability of IFN-γ to modulate Fe acquisition of intraphagosomal M. tuberculosis is Fe chelate specific.
MATERIALS AND METHODS
M. tuberculosis.
All experiments were performed with the virulent Erdman M. tuberculosis strain (ATCC 35801). M. tuberculosis was cultivated for 10 days and then harvested in RPMI containing 10 mM HEPES to form predominantly single-cell suspensions using previously described methods (49). The bacterial suspension was used within 1 h of preparation in all experiments.
Macrophage culture.
Peripheral blood mononuclear cells were obtained from healthy adult volunteers as previously described (24, 39). All subjects were purified protein derivative negative and had no known previous history of infection with any mycobacterial species. The mononuclear cells were cultured for 5 days in RPMI supplemented with 20% autologous serum using Teflon wells (Savillex, Minnetonka, Minn.). The resultant MDM fraction was isolated by adherence to tissue culture wells: mononuclear cells were placed in four-well tissue culture plates (ICN Biomedicals, Aurora, Ohio) at 1 × 106 to 2 × 106 MDM/well or into 96-well culture plates (Falcon, Lincoln Park, N.J.) at 105 MDM/well for 2 h with 10% autologous serum and washed, and then the cells were maintained for 7 days at 37°C in RPMI (Gibco, Grand Island, N.Y.) supplemented with 20% autologous serum (24, 37, 39).
Fe uptake by intraphagosomal M. tuberculosis: exogenous source.
M. tuberculosis was added to 12-day-old MDM monolayers as previously reported (37, 39). Briefly, the MDM monolayers were washed three times in RPMI and incubated with single suspensions of M. tuberculosis at a bacteria/MDM ratio of 5 (multiplicity of infection [MOI] = 5) for 2 h in RPMI containing 10 mM HEPES and 1 mg of human serum albumin/ml. Monolayers were washed in RPMI and repleted with RPMI supplemented with 1% autologous serum. After 24 h, 10 μM 59Fe2 chelated to TF, LF, or citrate (38) (each from Sigma Chemical, St. Louis, Mo.) was added to the monolayers and incubated for another 24 h. Both TF and LF were loaded with 59Fe to achieve saturation of >95% of the proteins' two Fe-binding sites as previously described (38). Citrate was complexed with 59Fe at an Fe/citrate ratio of 1:5. Intracellular M. tuberculosis cells were harvested as described previously (37, 39). Briefly, MDM were washed with medium containing ascorbate (5 mM) at pH 5.0 to remove any 59Fe associated with the MDM plasma membrane. Bacilli were recovered by lysing the MDM using 0.1% sodium dodecyl sulfate in the presence of 1,000 U of DNase (Invitrogen, Carlsbad, Calif.)/ml and an EDTA-free protease inhibitor cocktail tablet (Boehringer Mannheim/Roche, Indianapolis, Ind.). 59Fe present in duplicate (30-μl) aliquots of the cell lysate was measured by gamma counter. The released bacilli were recovered by centrifuging the supernatant at 10,000 × g for 10 min at 4°C in conjunction with washing the resultant bacterial pellet three times with 0.01% sodium dodecyl sulfate in RPMI. The bacilli were finally resuspended and filtered using a 0.22-μm-pore-size filter, and 59Fe associated with the filter containing the trapped bacilli was determined by gamma counter.
Fe uptake by intraphagosomal M. tuberculosis: endogenous source.
MDM monolayers were incubated with the desired 59Fe2 chelate for 24 h and washed. After an additional 24-h period (chase), the M. tuberculosis cells were added to the monolayer at an MOI of 5 for 2 h, following which M. tuberculosis cells not associated with the MDM were removed by washing of the monolayer. Intracellular M. tuberculosis bacilli were harvested from MDM, and their 59Fe content was determined as above at various time periods.
Analysis of Fe acquisition by IFN-γ-treated MDM and intraphagosomal M. tuberculosis harvested from these cells.
Twelve-day-old MDM monolayers were treated with 1,000 U of IFN-γ/ml (or medium only for control) for 5 days prior to the addition of M. tuberculosis and subsequent addition (24 h later) of the desired 59Fe chelate or with the 59Fe chelate alone (no M. tuberculosis). MDM and M. tuberculosis were separated from one another as described above, and the amounts of 59Fe acquired by MDM and M. tuberculosis were determined by gamma counter. The integrity of the IFN-γ-treated MDM monolayers was maintained, as viewed by light microscopy. Treatment of the monolayers with IFN-γ led to some cells forming aggregates, but they remained intact and attached throughout the experiments.
Phagocytosis.
In order to determine the relative number of M. tuberculosis cells phagocytosed by MDM, M. tuberculosis was incubated with MDM (MOI = 5) for 2 h and washed. Cells were lysed to release intracellular bacilli. The lysate was centrifuged (10,000 × g, 10 min, 4°C), and the medium was removed. The pellet was resuspended in 200 μl of 7H9 and injected into a BACTEC bottle for growth index assessment, as previously described (37). Growth index is a measure of metabolic activity of bacteria in culture. There is a very good correlation with increasing numbers of bacteria up to a growth index of 500.
Statistics.
Results obtained under different experimental conditions were compared by Student's paired t test when independent variables were being assessed or by analysis of variance when trends were being determined. For both types of analyses, results were considered significant at a P value of ≤0.05. Because absolute results vary from MDM donor to donor, each experiment was analyzed relative to its own control group(s).
RESULTS
Intraphagosomal M. tuberculosis acquires Fe from different extracellular chelates.
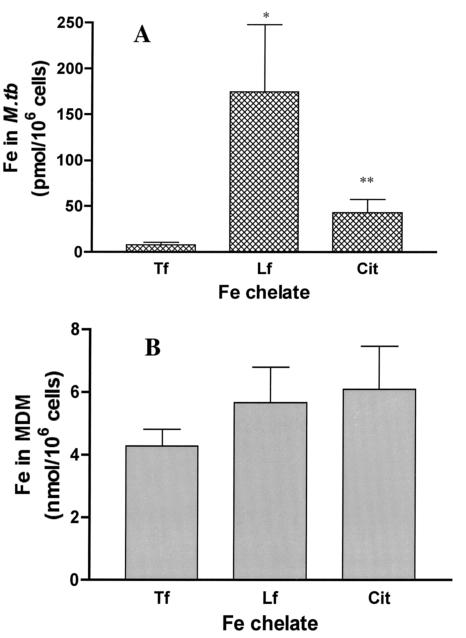
M. tuberculosis-infected macrophages would be potentially exposed to several different forms of Fe chelates in the lung in vivo. Therefore, we examined to what extent M. tuberculosis residing within the phagosome of human MDM could acquire Fe bound initially to extracellular TF, LF, or citrate in vitro. Virulent Erdman M. tuberculosis cells were added to MDM monolayers for 2 h to allow for phagocytosis. Nonphagocytosed organisms were then removed by washing, and the monolayer was incubated an additional 24 h. At that time, [59Fe]TF, [59Fe]LF, or 59Fe-citrate was added to the M. tuberculosis-infected monolayers. At defined time points, the monolayers were lysed, and both total MDM- and M. tuberculosis-associated 59Fe levels were determined using our previously developed methodology (37, 39). Consistent with our previous work, intraphagosomal M. tuberculosis demonstrated the ability to acquire 59Fe from extracellular TF (Fig. 1A). M. tuberculosis also acquired Fe from both citrate and LF, with M. tuberculosis Fe acquisition from LF being the largest (Fig. 1A). In comparison to that from TF, 59Fe acquisition from LF and citrate was 27.9-fold (± 12.7) and 8.8-fold (± 4.0) greater, respectively, than that observed from TF. This could not be explained by the extent to which MDM acquired Fe from each of the chelates, as this was quite similar (Fig. 1B). Consistent with our previous work, infection with M. tuberculosis led to a 38.7% ± 12.3% (mean ± standard error of the mean [SEM]; n = 6; P < 0.002) decrease in 59Fe acquisition from TF by the MDM. M. tuberculosis infection had a lesser effect on the magnitude of 59Fe acquired by MDM from either LF or citrate, decreasing it by 15.3% ± 3.1% (n = 11; P < 0.002) and 21.3% ± 8.8% (n = 7; P < 0.02), respectively.
FIG. 1.
M. tuberculosis differentially acquires Fe from various exogenous Fe chelates. Twelve-day-old MDM monolayers (1 × 106 to 2 × 106 cells) were incubated with M. tuberculosis (Erdman; MOI = 5) for 2 h and washed to remove nonphagocytosed bacteria, and then fresh medium was added. The desired 59Fe chelate (TF, LF, or citrate; 10 μM) was then added. After 24 h, the monolayer was washed to remove cell-free 59Fe (see Materials and Methods). The cells were lysed, and MDM- and M. tuberculosis-associated 59Fe levels were determined. Shown is the mean ± SEM (n = 6 to 12 independent experiments) of M. tuberculosis-associated 59Fe from macrophages incubated with TF, LF, and citrate (A) or of MDM-associated 59Fe from TF, LF, and citrate (B). Although there were differences in the amounts of M. tuberculosis-associated Fe with respect to chelate (*, P = 0.013; **, P = 0.02 relative to TF), there was no significant difference in the amount of Fe taken up by the corresponding MDM from which the bacilli were harvested.
Intraphagosomal M. tuberculosis acquisition of Fe from endogenous macrophage pools is influenced by the nature of the Fe chelate.
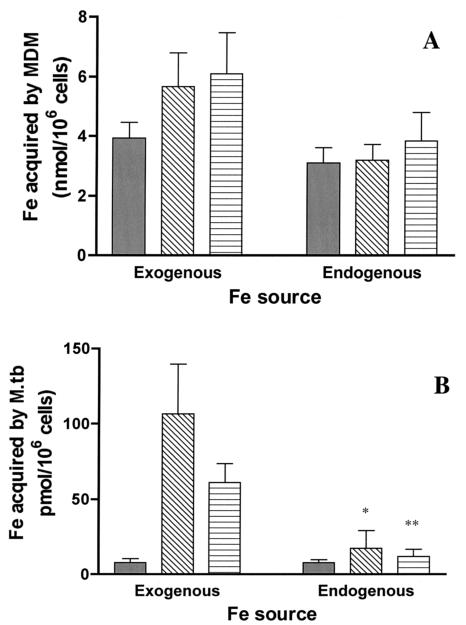
Recent work demonstrated that intraphagosomal M. tuberculosis can acquire Fe from a location(s) within the MDM when that Fe is initially provided to the MDM bound to TF (39). Fe is potentially handled differently by the macrophage when it is presented bound to LF or to a low-molecular-weight Fe chelate such as citrate. Whether this would influence the extent to which the Fe was available from intracellular macrophage locations is unknown and was therefore tested. MDM were incubated with [59Fe]TF, [59Fe]LF, or 59Fe-citrate for 24 h at 37°C, followed by extensive washing and an additional incubation for 24 h (chase period) prior to adding M. tuberculosis cells, to allow the macrophages adequate time to internalize any surface-bound 59Fe. Since no extracellular 59Fe chelate was present during M. tuberculosis infection, only Fe located within the MDM could serve as the 59Fe source (endogenous sources). Culture supernatant showed negligible release of 59Fe during the 24-h chase (data not shown). This indicates that once internalized by the MDM, no significant amount of 59Fe was returned to the extracellular environment over the time period of the experiment, a process that could have confounded data interpretation.
As shown in Fig. 2A, MDM-associated 59Fe was similar 24 h after the exogenous 59Fe had been removed regardless of the exogenous 59Fe chelate to which the MDM had been exposed (labeled endogenous). Acquisition of endogenous MDM 59Fe by intraphagosomal M. tuberculosis was similar when the MDM were loaded with 59Fe from TF and citrate. Although there was a trend toward 59Fe acquisition by intraphagosomal M. tuberculosis cells from endogenous MDM sources being greater when the MDM were loaded with 59Fe bound to LF relative to that with TF or citrate (Fig. 2B), this did not reach statistical significance (P = 0.3; n = 3).
FIG. 2.
M. tuberculosis acquires Fe from a variety of exogenous and endogenous Fe sources. For the exogenous paradigm, M. tuberculosis (Erdman; MOI = 5) was added to 12-day-old MDM monolayers for 2 h in RPMI containing 10 mM HEPES and 1 mg of human serum albumin/ml. Monolayers were washed and incubated with RPMI supplemented with 1% autologous serum plus 10 μM 59Fe chelated to either TF, LF, or citrate for 24 h. For the endogenous paradigm, MDM monolayers were incubated with the desired 10 μM 59Fe2-chelate for 24 h and washed. After an additional 24-h period (chase), M. tuberculosis cells were added to the monolayer at an MOI of 5 for 2 h, following which M. tuberculosis cells not associated with the MDM were removed by washing of the monolayer. At 24 h later, intracellular M. tuberculosis bacilli were harvested. For both paradigms, M. tuberculosis- and MDM-associated 59Fe was determined at the end of 24 h of M. tuberculosis infection. Shown are the mean ± SEM of MDM-associated 59Fe in nanomoles (n = 4 to 7) (A) and intracellular M. tuberculosis-associated 59Fe in picomoles (n = 5 to 12) (B) when 59Fe was initially chelated to TF (solid bar), LF (open bar with diagonal lines), or citrate (open bar with horizontal lines). Fe uptake from TF by M. tuberculosis was similar irrespective of the source (exogenous versus endogenous; P > 0.05). Uptake from LF and citrate by M. tuberculosis was much greater from the exogenous compared to the endogenous sources (*, P = 0.032; **, P = 0.002). However, there was no significant difference in Fe uptake by MDM from which the M. tuberculosis cells were isolated.
When M. tuberculosis acquisition from endogenous Fe sources (Fig. 2B) was compared to that observed with the addition of exogenous 59Fe chelates, there was little difference observed with TF. However, Fe acquisition was much greater using the exogenous versus the endogenous protocol when the initial source of 59Fe was LF or citrate (Fig. 2B).
These results could not be explained on the basis of differences in the number of organisms ingested by the MDM, as phagocytosis of M. tuberculosis by the MDM monolayers (see Materials and Methods) was unaffected by exposure to any of the Fe chelates employed (data not shown).
The effect of IFN-γ on Fe acquisition by MDM and intracellular M. tuberculosis differs depending on the nature of the Fe chelate.
IFN-γ plays a major role in host defense against M. tuberculosis (22), as well as other intracellular pathogens (23). Part of the antimicrobial mechanism of IFN-γ against some pathogens has been linked to its ability to decrease the availability of macrophage Fe for use by the pathogen (2, 9). Most studies have focused on Fe acquisition from TF, since IFN-γ decreases macrophage TFR expression (10, 62). However, studies with Legionella pneumophila provide evidence that IFN-γ also impacts Fe acquisition from LF by that organism (9).
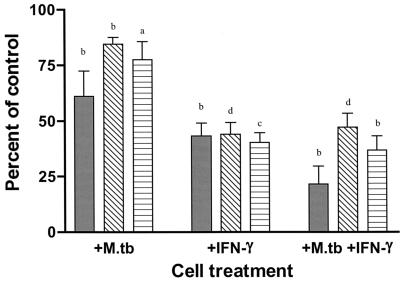
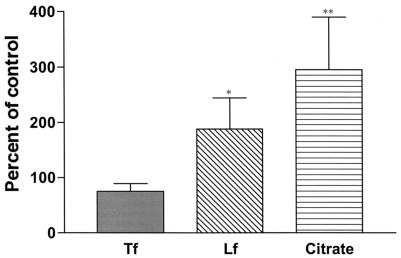
In contrast to the above, we recently showed that, although IFN-γ does decrease MDM Fe acquisition from TF, it has little effect on the ability of intraphagosomal M. tuberculosis to acquire Fe from TF (39). Whether IFN-γ impacts on intraphagosomal M. tuberculosis Fe acquisition from LF or citrate has not been evaluated. Therefore, we examined whether IFN-γ treatment of MDM limits intraphagosomal M. tuberculosis acquisition of Fe from TF, LF, or citrate. We found that treatment of MDM with IFN-γ (1,000 U/ml) for 5 days significantly decreased MDM Fe acquisition from each of the three extracellular Fe chelates to a similar extent (50 to 60%) (Fig. 3, +IFN-γ). The decrease in MDM Fe acquisition from TF was greater with the combination of M. tuberculosis infection and IFN-γ treatment than that seen with either treatment alone. However, M. tuberculosis infection did not add to the IFN-γ-mediated decrease in MDM Fe acquisition from LF or citrate that was observed (Fig. 3). With regard to Fe acquisition by intraphagosomal M. tuberculosis, IFN-γ treatment of the MDM had only a minimal effect on the ability of the organism to acquire Fe from TF (Fig. 4). Surprisingly, IFN-γ treatment resulted in a two- and threefold increase in M. tuberculosis Fe acquisition from LF and citrate, respectively, relative to that seen in the absence of IFN-γ treatment (Fig. 4). Control experiments showed no effect of IFN-γ on initial phagocytosis of M. tuberculosis by the MDM monolayers (data not shown).
FIG. 3.
IFN-γ reduces Fe uptake by MDM. MDM were treated with 1,000 U of IFN-γ/ml for 5 days. Controls received medium alone. Cells were then incubated with M. tuberculosis (Erdman; MOI = 5) for 2 h, washed, and repleted with 1% autologous serum in RPMI. After 24 h, a 10 μM concentration of the desired 59Fe chelate was added for an additional 24 h. IFN-γ treatment continued throughout the experiment. The cells were lysed, and MDM-associated 59Fe was determined. Shown is the mean ± SEM of MDM-associated 59Fe of 6 to 11 independent experiments. Results were expressed as the percentage of control (non-IFN-γ, non-M. tuberculosis-infected MDM). There was a similar (>50%) decrease in 59Fe uptake from each of the three chelates (TF [solid bar], LF [open bar with diagonal lines], or citrate [open bar with horizontal lines]) by MDM after treatment with IFN-γ. There was an additional decrease in Fe acquisition from TF (but not from LF or citrate) when the cells were also infected with M. tuberculosis. a, P < 0.02; b, P < 0.002; c, P < 0.0002; d, P < 0.00002.
FIG. 4.
Effect of IFN-γ on Fe uptake by intraphagosomal M. tuberculosis. Cells were treated as described in the legend for Fig. 3, and M. tuberculosis-associated 59Fe was determined with a gamma counter. Results were expressed as the percentage of control (no IFN-γ treatment); n = 4 to 8. There was no significant decrease in M. tuberculosis-associated Fe from TF and a significant increase from LF and citrate (*, P = 0.044; **, P = 0.019).
DISCUSSION
Bacteria that replicate within macrophage phagosomes, such as M. tuberculosis, face a greater challenge to obtain adequate Fe to meet their metabolic needs than extracellular pathogens that have direct access to host extracellular Fe chelates. We have previously demonstrated that intraphagosomal M. tuberculosis can acquire Fe from extracellular TF, as well as from intracellular sites within the macrophage initially supplied by extracellular Fe-TF (39). We have now extended these observations to demonstrate the novel findings that intraphagosomal M. tuberculosis can acquire Fe from two other extracellular Fe chelates, LF and citrate. Observations with these chelates are particularly important, because LF is a major Fe chelator within the airway (55), where primary M. tuberculosis infection occurs. Fe also exists in the airway in the form of low-molecular-weight chelates in vivo. Levels of such forms of Fe rise with cell damage that results in Fe leakage from intracellular sites (45) or from deposition of exogenous Fe from inhaled cigarette smoke (54). In the present study, potentially important differences in M. tuberculosis and/or to a lesser extent MDM Fe acquisition and/or storage from these chelates were noted. To our knowledge, these studies are the first that directly assess the acquisition of Fe from LF and citrate by intraphagosomal M. tuberculosis.
MDM exhibited a similar capacity to acquire Fe from TF, LF, or citrate under our experimental conditions. We previously showed that MDM infection with M. tuberculosis led to a decrease in subsequent Fe acquisition by MDM from extracellular TF by a mechanism that remains unknown (39). Interestingly, M. tuberculosis infection had less of an impact on MDM Fe acquisition from either LF or citrate. These results suggest that the effect of M. tuberculosis infection on MDM Fe acquisition may involve alterations specific to the acquisition of Fe from TF and not to Fe acquisition or storage in general. Infection of murine peritoneal macrophages with Mycobacterium avium complex decreases macrophage TFR and ferritin mRNA levels (62). It has also been shown that M. tuberculosis infection alters expression of a number of MDM genes (43), although regulation of TFR expression largely occurs posttranscriptionally (18, 19). We are currently examining the impact of MDM infection on cellular factors known to contribute to cellular Fe acquisition from TF (e.g., TFR expression, iron response protein activity, etc.).
Levels of MDM-associated 59Fe that resulted from the incubation of MDM with 59Fe bound to TF, LF, or citrate were not statistically different. In contrast to the MDM data, the magnitude of Fe acquired by intraphagosomal M. tuberculosis from the various chelates varied considerably. The organism most effectively acquired Fe from LF, with acquisition from TF being the smallest. Fe acquisition from LF was 30-fold greater compared to that from TF and 3- to 4-fold greater than from citrate. These data suggest that the nature of the Fe chelate, and perhaps how it interfaces with MDM Fe acquisition mechanisms, affects its subsequent availability to the intracellular pathogen.
Our recent study demonstrated that intraphagosomal M. tuberculosis can also access one or more intracellular Fe pools within the MDM supplied by Fe acquired from extracellular TF (39). Our present work indicates that intraphagosomal M. tuberculosis may also acquire Fe from internal macrophage sites supplied from either LF or citrate. However, in contrast to results with TF, endogenous sources of Fe derived from citrate or LF appeared to be much less accessible to M. tuberculosis than when the Fe was available as an extracellular chelate.
The specific routes by which extracellular Fe reaches intraphagosomal M. tuberculosis remain to be defined. It has been proposed that a portion of Fe chelated to TF traffics directly to the phagosome bound to TF via receptor-mediated endocytosis and early endosome fusion (12). A similar process could be involved with Fe acquisition from LF, although the nature of the LF receptor(s) on MDM and its role in Fe acquisition from this protein are much less well-defined than for TF. Furthermore, it is unclear whether receptor-mediated endocytosis contributes to Fe acquisition from LF by macrophages. The large difference between the magnitude of Fe acquired from exogenous and endogenous sources of LF-derived Fe raises the possibility that there may be a mechanism that directly transports Fe bound initially to LF to the phagosome, bypassing the intracellular macrophage Fe pool.
Previous work has shown that M. tuberculosis relies on the secretion of exochelins to remove Fe from TF or LF when the organism is grown in broth medium (25) and that siderophore production is both tightly regulated (46) and required for optimal growth of M. tuberculosis in macrophages (15). Thus, Fe removal from TF or LF that reaches the phagosome could occur through the combined action of M. tuberculosis exochelins (25), the lowered pH of the phagosome, and phagosomal Fe reductase activity. Interestingly, Fe release from LF does not occur until a pH below that of the M. tuberculosis phagosome is reached. Yet, in our studies M. tuberculosis Fe acquisition appears to be greater from LF than for TF. A recent study reported that application of LF to the nasal mucosa of β-2-microglobulin knockout mice, which exhibit evidence of systemic Fe overload, decreases the susceptibility of these animals to tuberculosis to the level of wild-type mice (48). Those authors attributed their finding to Fe chelation by LF, but LF also enhanced macrophage nitric oxide production. Regardless, it is unlikely that the M. tuberculosis organisms in this study came into contact with Fe bound to LF. Thus, these results are not directly applicable to our data.
Siderophore production appears to be very important to M. tuberculosis's growth within macrophages, as illustrated by the poor growth of a mutant M. tuberculosis strain, unable to synthesize siderophores, within the human macrophage THP-1 cell line (15). At what step siderophore production is required for the acquisition of Fe by intraphagosomal M. tuberculosis remains unclear, as is its relative importance in the acquisition of Fe from different Fe sources. M. tuberculosis siderophores may leave the phagosome, bind cytoplasmic Fe within the MDM, and carry it back to the microbe. Movement of M. tuberculosis products from the phagosome into the macrophage cytoplasm with subsequent extracellular secretion has been reported (3, 53, 61). However, to our knowledge there is no evidence for or against movement of M. tuberculosis siderophores out of the macrophage phagosome.
If M. tuberculosis siderophores do not leave the phagosome, Fe must be delivered to them, perhaps through a phagosome-associated Fe transporter. DMT-1 (Nramp2) moves Fe out of the endosome, not into it (28). The related protein Nramp1 is an integral membrane protein expressed in macrophage late endosomes and lysosomes that is related to DMT-1 (27). Nramp1 has been linked to resistance to infection with BCG and other intracellular pathogens in mice (57), but not virulent M. tuberculosis (7, 36, 56). Whether Nramp1 can transport Fe, as well as the direction of that transport (into or out of the phagosome), remains an area of controversy (26, 30, 33, 63).
M. tuberculosis itself expresses a pH-dependent divalent cation transporter that is an orthologue of eukaryotic Nramp proteins (1, 6, 16), termed Mramp. Overall amino acid identities between Mramp and eukaryotic Nramps are 21 to 24% (1). It has been shown that Mramp can transport Fe2+ (1), and its deletion results in a slowing of extracellular growth of the organism under Fe-limited conditions (6). However, the absence of Mramp does not alter growth of M. tuberculosis in murine macrophages or bacterial virulence in mice (6, 16). Clearly, further studies will be required to define the route whereby cytoplasmic MDM Fe reaches the M. tuberculosis phagosome.
IFN-γ plays a key role in host defense against M. tuberculosis through its role as an activator of macrophage antimicrobial mechanisms (22). At least some of the antimicrobial effects of IFN-γ appear to be related to effects on macrophage Fe metabolism. Previous data from a variety of investigators (62), as well as our own laboratory (39), have shown that IFN-γ decreases macrophage ferritin, TFR expression, and MDM Fe acquisition from TF (8, 10, 34, 52, 59). The ability of IFN-γ to decrease MDM Fe acquisition cannot be attributed solely to its effects on TFR expression, as we observed that IFN-γ treatment also decreased MDM Fe acquisition from LF and citrate to a similar extent to that which occurred from TF.
Even though IFN-γ lowered the total Fe content of MDM following incubation with TF, LF, or citrate, the ability of M. tuberculosis to acquire Fe from these chelates was only minimally impaired for TF and in fact resulted in increased Fe acquisition from LF and citrate. The mechanism whereby IFN-γ allowed greater access of intraphagosomal M. tuberculosis to Fe remains unclear. Nevertheless, these data suggest that IFN-γ does not effectively alter the amount of Fe in the intracellular macrophage pools that can be accessed by intraphagosomal M. tuberculosis and may explain in part why IFN-γ does not slow the growth of M. tuberculosis in human MDM (17, 37). Furthermore, these data provide additional evidence that selective macrophage pools, rather than the total Fe content of the macrophage, may be the key determinant in the ability of intraphagosomal M. tuberculosis to acquire Fe.
We have made novel observations regarding the ability of virulent M. tuberculosis residing within human MDM phagosomes to acquire Fe from extracellular TF, LF, or citrate, as well as endogenous MDM Fe stores supplied by Fe from these chelates. Additional efforts to define the intracellular Fe pools accessible to M. tuberculosis, the mechanism of delivery of extracellular and cytoplasmic Fe to the phagosome from these biologically relevant extracellular Fe chelates, and the applicability of these observations to other intracellular pathogens are the focus of current studies in our laboratories.
Acknowledgments
We thank Thomas Kaufman for his expert technical assistance.
This work was supported in part by VA Merit Review grants (B.E.B. and L.S.S.) and National Institutes of Health grants (AI24954 [B.E.B.], AI33004 [L.S.S.], and AI43870 [L.S.S.]).
Editor: F. C. Fang
REFERENCES
- 1.Agranoff, D., I. M. Monahan, J. A. Mangan, P. D. Butcher, and S. Krishna. 1999. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J. Exp. Med. 190:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnewall, R. E., and Y. Rikihisa. 1994. Abrogation of gamma interferon-induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron transferrin. Infect. Immun. 62:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty, W. L., and D. G. Russell. 2000. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 68:6997-7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birgens, H. S. 1991. The interaction of lactoferrin with human monocytes. Dan. Med. Bull. 38:244-252. [PubMed] [Google Scholar]
- 5.Birgens, H. S., N. E. Hansen, H. Karle, and L. O. Kristensen. 1983. Receptor binding of lactoferrin to human monocytes. Br. J. Haematol. 54:383-391. [DOI] [PubMed] [Google Scholar]
- 6.Boechat, N., B. Lagier-Roger, S. Petit, Y. Bordat, J. Rauzier, A. J. Hance, B. Gicquel, and J.-M. Reyrat. 2002. Disruption of the gene homologous to mammalian Nramp1 in Mycobacterium tuberculosis does not affect virulence in mice. Infect. Immun. 70:4124-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buu, N., F. Sánchez, and E. Schurr. 2000. The Bcg host-resistance gene. Clin. Infect. Dis. 31(Suppl.):S81-S85. [DOI] [PubMed] [Google Scholar]
- 8.Byrd, T. F., and M. A. Horwitz. 1989. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Investig. 83:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, T. F., and M. A. Horwitz. 1991. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J. Clin. Investig. 88:1103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd, T. F., and M. A. Horwitz. 1993. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J. Clin. Investig. 91:969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitambar, C. R., and D. Sax. 1992. Regulatory effects of gallium on transferrin-independent iron uptake by human leukemic HL60 cells. Blood 80:505-511. [PubMed] [Google Scholar]
- 12.Clemens, D. L., and M. A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Silva, D. M., C. C. Askwith, and J. Kaplan. 1996. Molecular mechanisms of iron uptake in eukaryotes. Physiol. Rev. 76:31-47. [DOI] [PubMed] [Google Scholar]
- 14.De Voss, J. J., K. Rutter, B. G. Schroeder, and C. E. Barry III. 1999. Iron acquisition and metabolism by mycobacteria. J. Bacteriol. 181:4443-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Voss, J. J., K. Rutter, B. G. Schroeder, H. Su, Y. Q. Zhu, and C. E. Barry III. 2000. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 97:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domenech, P., A. S. Pym, M. Cellier, C. E. Barry III, and S. T. Cole. 2002. Inactivation of the Mycobacterium tuberculosis Nramp orthologue (mntH) does not affect virulence in a mouse model of tuberculosis. FEMS Microbiol. Lett. 207:81-86. [DOI] [PubMed] [Google Scholar]
- 17.Douvas, G. S., D. L. Looker, A. E. Vatter, and A. J. Crowle. 1985. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect. Immun. 50:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. P. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenstein, R. S. 2000. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu. Rev. Nutr. 20:627-662. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein, R. A., C. V. Sciortino, and M. A. McIntosh. 1983. Role of iron in microbe-host interactions. Rev. Infect. Dis. 5:5759-5777. [DOI] [PubMed] [Google Scholar]
- 21.Fleming, R. E., and W. S. Sly. 2002. Mechanisms of iron accumulation in hereditary hemochromatosis. Annu. Rev. Physiol. 64:663-680. [DOI] [PubMed] [Google Scholar]
- 22.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 23.Gallin, J. I., J. M. Farber, S. M. Holland, and T. B. Nutman. 1995. Interferon-gamma in the management of infectious diseases. Ann. Intern. Med. 123:216-224. [DOI] [PubMed] [Google Scholar]
- 24.Gaynor, C. D., F. X. McCormack, D. R. Voelker, S. E. McGowan, and L. S. Schlesinger. 1995. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 155:5343-5351. [PubMed] [Google Scholar]
- 25.Gobin, J., and M. A. Horwitz. 1996. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell wall. J. Exp. Med. 183:1527-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes, M. S., and R. Appelberg. 1998. Evidence for a link between iron metabolism and Nramp1 gene function in innate resistance against Mycobacterium avium. Immunology 95:165-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruenheid, S., F. Canonne-Hergaux, S. Gauthier, D. J. Hackam, S. Grinstein, and P. Gros. 1999. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J. Exp. Med. 189:831-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunshin, H., B. Mackenzie, U. V. Berger, Y. Gunshin, M. F. Romero, W. F. Boron, S. Nussberger, J. L. Gollan, and M. A. Hediger. 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482-488. [DOI] [PubMed] [Google Scholar]
- 29.Hamazaki, S., and J. Glass. 1992. Non-transferrin dependent 59Fe uptake in phytohemagglutinin-stimulated human peripheral lymphocytes. Exp. Hematol. 20:436-441. [PubMed] [Google Scholar]
- 30.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (NRAMP1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurado, R. L. 1997. Iron, infections, and anemia of inflammation. Clin. Infect. Dis. 25:888-895. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan, J., I. Jordan, and A. Sturrock. 1991. Regulation of the transferrin-independent iron transport system in cultured cells. J. Biol. Chem. 266:2997-3004. [PubMed] [Google Scholar]
- 33.Kuhn, D. E., W. P. Lafuse, and B. S. Zwilling. 2001. Iron transport into Mycobacterium avium-containing phagosomes from an Nramp1Gly169-transfected RAW264.7 macrophage cell line. J. Leukoc. Biol. 69:43-49. [PubMed] [Google Scholar]
- 34.Mulero, V., and J. H. Brock. 1999. Regulation of iron metabolism in murine J774 macrophages: role of nitric oxide-dependent and independent pathways following activation with gamma interferon and lipopolysaccharide. Blood 94:2383-2389. [PubMed] [Google Scholar]
- 35.Nielands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 36.North, R. J., R. LaCourse, L. Ryan, and P. Gros. 1999. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect. Immun. 67:5811-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olakanmi, O., B. E. Britigan, and L. S. Schlesinger. 2000. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68:5619-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olakanmi, O., G. T. Rasmussen, T. S. Lewis, J. B. Stokes, J. D. Kemp, and B. E. Britigan. 2002. Multivalent metal-induced iron acquisition from transferrin and lactoferrin by myeloid cells. J. Immunol. 169:2076-2084. [DOI] [PubMed] [Google Scholar]
- 39.Olakanmi, O., L. S. Schlesinger, A. Ahmed, and B. E. Britigan. 2002. Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular iron and intracellular iron pools: impact of interferon-γ and hemochromatosis. J. Biol. Chem. 277:49727-49734. [DOI] [PubMed] [Google Scholar]
- 40.Olakanmi, O., J. B. Stokes, and B. E. Britigan. 1994. Acquisition of iron bound to low molecular weight chelates by human monocyte-derived macrophages. J. Immunol. 153:2691-2703. [PubMed] [Google Scholar]
- 41.Olakanmi, O., J. B. Stokes, S. Pathan, and B. E. Britigan. 1997. Polyvalent cationic metals induce the rate of transferrin- independent iron acquisition by HL-60 cells. J. Biol. Chem. 272:2599-2606. [DOI] [PubMed] [Google Scholar]
- 42.Qian, Z. M., and E. H. Morgan. 1991. Effect of metabolic inhibitors on uptake of non-transferrin-bound iron by reticulocytes. Biochim. Biophys. Acta 1073:456-462. [DOI] [PubMed] [Google Scholar]
- 43.Ragno, S., M. Romano, S. Howell, D. J. C. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson, D., and E. Baker. 1991. The uptake of inorganic iron complexes by human melanoma cells. Biochim. Biophys. Acta 1093:20-28. [DOI] [PubMed] [Google Scholar]
- 45.Richardson, D. R., and P. Ponka. 1997. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331:1-40. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez, G. M., and I. Smith. 2003. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 47:1485-1494. [DOI] [PubMed] [Google Scholar]
- 47.Roseanu, A., F. Chelu, M. Trif, C. Motas, and J. H. Brock. 2000. Inhibition of binding of lactoferrin to the human promonocyte cell line THP-1 by heparin: the role of cell surface sulphated molecules. Biochim. Biophys. Acta 1475:35-38. [DOI] [PubMed] [Google Scholar]
- 48.Schaible, U. E., H. L. Collins, F. Priem, and S. H. E. Kaufmann. 2002. Correction of the iron overload defect in β-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 196:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 50.Sturgill-Koszycki, S., U. E. Schaible, and D. G. Russell. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960-6968. [PMC free article] [PubMed] [Google Scholar]
- 51.Sturrock, A., J. Alexander, J. Lamb, C. M. Craven, and J. Kaplan. 1990. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J. Biol. Chem. 265:3139-3145. [PubMed] [Google Scholar]
- 52.Taetle, R., and J. M. Honeysett. 1988. γ-Interferon modulates human monocyte/macrophage transferrin receptor expression. Blood 71:1590-1595. [PubMed] [Google Scholar]
- 53.Teitelbaum, R., M. Cammer, M. L. Maitland, N. E. Freitag, J. Condeelis, and B. R. Bloom. 1999. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. USA 96:15190-15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, A. B., T. Bohling, A. Heires, J. Linder, and S. I. Rennard. 1991. Lower respiratory tract iron burden is increased in association with cigarette smoking. J. Lab. Clin. Med. 117:493-499. [PubMed] [Google Scholar]
- 55.Thompson, A. B., T. Bohling, F. Payvandi, and S. I. Rennard. 1990. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J. Lab. Clin. Med. 115:148-158. [PubMed] [Google Scholar]
- 56.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Bcg locus: natural reistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]
- 58.Volpp, B. D., W. M. Nauseef, J. E. Donelson, D. R. Moser, and R. A. Clark. 1989. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc. Natl. Acad. Sci. USA 86:7195-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wardrop, S. L., and D. R. Richardson. 2000. Interferon-gamma and lipopolysaccharide regulate the expression of Nramp2 and increase the uptake of iron from low relative molecular mass complexes by macrophages. Eur. J. Biochem. 267:6586-6593. [DOI] [PubMed] [Google Scholar]
- 60.Wheeler, P. R., and C. Ratledge. 1994. Metabolism of Mycobacterium tuberculosis, p. 353-388. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 61.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]
- 62.Zhong, W. J., W. P. Lafuse, and B. S. Zwilling. 2001. Infection with Mycobacterium avium differentially regulates the expression of iron transport protein mRNA in murine peritoneal macrophages. Infect. Immun. 69:6618-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zwilling, B. S., D. E. Kuhn, L. Wikoff, D. Brown, and W. Lafuse. 1999. Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect. Immun. 67:1386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]