Abstract
Toll-like receptor 4 (TLR4) has been identified as a transmembrane protein involved in the host innate immune response to gram-negative bacterial lipopolysaccharide (LPS). Upon activation by LPS recognition, the TIR domain of TLR4 signals through MyD88 to activate the nuclear factor κB (NF-κB) pathway, a critical regulator of many proinflammatory genes, including interleukin-8 (IL-8). Emerging evidence suggests that reactive oxygen species (ROS) can contribute to diverse signaling pathways, including the LPS-induced cascade. In the present study we investigated the role of ROS in TLR-mediated signaling. Purified Escherichia coli LPS, a highly specific TLR4 agonist, elicited an oxidative burst in the monocyte-like cell line THP-1 in a time- and dose-dependent manner. This oxidative burst was shown to be dependent on the presence of TLR4 through transfection studies in HEK cells, which do not normally express this protein, and with bone marrow-derived macrophages from C3H/HeJ mice, which express a mutated TLR4 protein. LPS-stimulated IL-8 expression could be blocked by the antioxidants N-acetyl-l-cysteine and dimethyl sulfoxide at both the protein and mRNA levels. These antioxidants also blocked LPS-induced IL-8 promoter transactivation as well as the nuclear translocation of NF-κB. These data provide evidence that ROS regulate immune signaling through TLR4 via their effects on NF-κB activation.
Toll-like receptors (TLRs) are a family of recently recognized receptors of the innate immune system (21, 25). These proteins are capable of directing an immune response to diverse pathogen components that have been collectively called pathogen-associated molecular patterns (23). Pathogen-associated molecular patterns are unique in that they are characterized by being essential for the survival of a pathogen. In turn, TLRs are part of a wider family of germ line-encoded immune receptors called pathogen recognition receptors that can recognize and bind pathogen-associated molecular patterns (23). To date, 10 members of the TLR family have been identified, although not all have been assigned a specific ligand (6, 23, 41). Currently the best understood are TLR2, which recognizes bacterial lipoteichoic acid and peptidoglycan (36); TLR4, which binds lipopolysaccharide (31); TLR5, which binds bacterial flagellin (16); and TLR9, which recognizes unmethylated bacterial CpG DNA (17).
Similar to the other TLRs, the TLR4 protein contains an extracellular leucine-rich repeat domain and an intracellular Toll/interleukin-1 (IL-1) receptor signaling domain (25, 15). The presence of secreted MD-2 and soluble or membrane-bound CD14 is also essential for lipopolysaccharide (LPS) signaling through TLR4 (1, 42). Upon activation by LPS recognition, the Toll/IL-1 receptor domain of TLR4 signals through the accessory protein MyD88 to activate the nuclear factor κB (NF-κB) pathway, a critical regulator of many proinflammatory genes, including IL-8 (26). Under normal cellular conditions, NF-κB is maintained in an inactive state through association with the inhibitor of NF-κB (IκB). Following stimulation, IκB kinases phosphorylate IκB, marking it for ubiquitination and subsequent degradation. The NF-κB protein complex is then free to translocate to the nucleus and bind to target DNA sequences. More recently, a second MyD88-independent, NF-κB-dependent TLR4 pathway has been described in MyD88-deficient mice (3). It has been hypothesized that the presence of more than one TLR-mediated signaling pathway allows greater specificity in the response to a particular TLR ligand (23).
An emerging body of evidence supports a role for reactive oxygen species (ROS) and reactive nitrogen species in intracellular signaling (12, 13, 33). In particular, it has been shown that both tumor necrosis factor alpha and LPS-induced IL-8 secretion can be inhibited by antioxidants although the role of various species involved appears to be complicated (7, 8, 11, 14, 38). It has been proposed that the mechanism by which antioxidants block cytokine transcription is by preventing transcription factor migration to the nucleus (35, 37, 38), although this finding has not been observed by others (40). In the present study we aimed to investigate a role for ROS and to dissect which specific species may be important in the TLR4-mediated signaling response to its ligand.
MATERIALS AND METHODS
Materials and reagents.
LPS (E. coli serotype O127:B8) was obtained from Sigma (St. Louis, Mo.). Prior to use, this LPS was subjected to an additional purification to remove contaminating protein, as described by Hirschfeld and colleagues (18). This procedure was carried out to ensure the LPS was a specific TLR4 agonist. The following reagents were purchased from Sigma and were used at the indicated concentrations unless otherwise specified: catalase (1,000 U), N-acetyl-l-cysteine (NAC) (50 mM), dimethyl sulfoxide (DMSO) (2.5%), mercaptoethyl guanidine (MEG) (30 μM), Nω-nitro-l-arginine methyl ester (L-NAME) (100 μM), hypoxanthine (100 μM), xanthine oxidase (2 mU) and sodium nitroprusside (10 μM). Manganese(III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) (used at 50 μM) was purchased from Oxis (Portland, Oreg.). Functional-grade anti-human TLR4 antibody was purchased from eBioscience (San Diego, Calif.), and purified mouse immunoglobulin G2a immunoglobulin isotype control was purchased from BD PharMingen (Cockeysville, Md.). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, Iowa).
DNA constructs.
Expression plasmids encoding human TLR4 and MD2 were described previously (4). For the generation of TLR4-expressing stable HEK293 cell lines, the coding region of TLR4 was excised from the pcDNA3.1Zeo plasmid and subcloned into the pcDNA5/FRT/TO vector (Invitrogen, Carlsbad, Calif.) to provide tetracycline-inducible expression.
Full-length IL-8-luciferase and truncated −99 IL-8-luciferase (lacking the AP-1 binding site) expression vectors were a gift of A. Casola (University of Texas Medical Branch, Galveston). The human CD14 expression vector was a gift of R. Ulevitch (Scripps Research Institute, La Jolla, Calif.). The thymidine kinase-Renilla luciferase control plasmid was purchased from Promega (Madison, Wis.).
Cell lines.
The human monocyte THP-1 cell line was purchased from the American Type Culture Collection (Rockville, Md.) and maintained in RPMI 1640 (HyClone, Logan, Utah) containing 10% heat-inactivated fetal bovine serum (Sigma) at 37°C in 5% CO2.
Flp-In T-REx-293 cells, containing an integrated Flp recombination target (FRT) and the pcDNA6/TR tetracycline repressor expression plasmid, were purchased from Invitrogen. To generate the HEK-TLR4 cell line, Flp-In T-Rex-293 cells were cotransfected with pcDNA5/FRT/TO-TLR4 and the expression vector for the Flp recombinase, pOG44. Stable integrants were selected by culture in hygromycin. Individual clones were analyzed for the ability to respond to LPS by the activation of an NF-κB-luciferase reporter in the presence of cotransfected MD2 and CD14 expression plasmids. All clones tested responded equally well, while the parental Flp-In T-Rex-293 cells were unresponsive. These cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (HyClone).
All cell lines were grown in the absence of serum for 24 h before use in any experiment, as it has been shown that serum may augment ROS induction following LPS treatment, possibly through the presence of LPS-binding protein in serum (32).
Animals.
C57BL/6 and C3H/HeJ mice were purchased from the Jackson Laboratory, Bar Harbor, Maine. Mice were maintained in the Association for Assessment and Accreditation of Laboratory Animal Care-approved animal core facility at the University of Virginia. Housing and handling of mice followed National Institutes of Health guidelines for the performance of animal experiments and were approved by the University of Virginia Institutional Animal Care and Use Committee.
Bone marrow-derived macrophages.
Bone marrow was collected from the femurs of mice ranging from 8 to 24 weeks of age with a 25-gauge needle and unlabeled DMEM. Cells were then centrifuged and resuspended in a macrophage differentiation medium. This medium consisted of DMEM, 10% heat-inactivated fetal bovine serum, 4 mM l-glutamine (Gibco, Grand Island, N.Y.), 100 U of penicillin (Gibco) per ml, 0.1 mg of streptomycin (Gibco) per ml, and 10% conditioned medium from L929 mouse fibroblasts (conditioned L929 medium contains granulocyte macrophage-colony-stimulating factor, necessary for macrophage differentiation and maturation). L929 cells were cultivated in DMEM with 1% penicillin-streptomycin, 4 mM l-glutamine, and 10% FBS; 105 cells were seeded in 150-cm2 flasks and cultured for 7 days at 37°C and 5% CO2. Without disturbing the cell layer, the medium was then collected, centrifuged to remove excess cellular debris, and stored at −20°C. Fresh medium was then added to the cells, cultured for an additional 7 days, and removed in a similar manner to combine with the prior week's collection. The bone marrow cells were seeded on 100-mm petri dishes and incubated at 37°C and 5% CO2. After 5 to 7 days, macrophage precursors divide and differentiate into mature macrophages. Cells were then harvested with a cell scraper, and 106 cells were seeded in each well of a 24-well plate in preparation for LPS stimulation and dye oxidation assays described below.
H2DCFDA and DHE assays.
The cell-permeant dye 2′,7′-dichlorofluoresceindiacetate (H2DCFDA) is oxidized by hydrogen peroxide, peroxinitrite (ONOO−), and hydroxyl radicals (OH•) to yield the fluorescent molecule 2′7′-dichlorofluorescein. Similarly, dihydroethidium (DHE) becomes oxidized in the presence of superoxide (O2−) to yield fluorescent ethidium. Thus, dye oxidation is an indirect measure of the presence of these reactive oxygen intermediates, calculated by dividing the mean channel fluorescence of a treated sample by that of the untreated one and multiplying by 100 to obtain the relative change, expressed as a percentage.
For LPS dose experiments, 106 cells were treated with 0, 1, 5, 10, 20, and 40 μg of LPS per ml for 15 min. For time course experiments, 106 cells were treated with 10 μg of LPS per ml every 15 min for 75 min. Following LPS stimulation, cells were incubated with either 10 μM H2DCFDA or 2 μM DHE (Molecular Probes, Eugene, Oreg.) for 15 min at 37°C and 5% CO2. Cells were collected and washed once with RPMI containing 10 mM HEPES (Gibco). For continuous DHE experiments, cells were incubated first for 15 min with 2 μM DHE prior to LPS treatment. Data for cells with or without LPS were collected every 2 min for up to 25 min. A change in fluorescence was assessed with a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, N.J.).
IL-8 ELISA.
Cells were seeded into a 96-well plate at a concentration of 2 × 105/well and stimulated for 4 h. Cell supernatants were tested for IL-8 protein with the commercially available DuoSet enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn.). In blocking experiments, cells were incubated with 1 μg of antibody for 1 h prior to stimulation.
Real-time reverse transcription-PCR.
Cells were seeded into 12-well plates at a concentration of 106/well. Following stimulation for 4 h, cells were collected and washed once in phosphate-buffered saline. Total RNA was extracted according to the manufacturer's instructions with the RNeasy kit (Qiagen, Valencia, Calif.), and yield was estimated spectrophotometrically. RNA was reverse transcribed with the Superscript kit (Invitrogen) random hexamer protocol as per the manufacturer's instructions.
Real-time dual-labeled probe PCR for IL-8 was carried out on a SmartCycler machine (Cepheid, Sunnyvale, Calif.) with a primer and TET-labeled probe set designed in our laboratory (IL-8-F, 5′-CCACCCCAAATTTATCAAAGAA-3′; IL-8-R, 5′-CAGACAGAGCTCTCTTCCATCA-3′; IL-8-probe, 5′-5TET-ATTGAGAGTGGACCACACTGCGC-BHQ-1-3′). The PCR contained 0.3 μM primer, 0.2 μM probe, 3 mM MgCl2 and 0.75 U of Platinum Taq polymerase (Invitrogen). PCR cycling conditions consisted of 95°C for 300 s, followed by 45 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Critical threshold values were compared against a standard curve to estimate starting amounts of mRNA, and the relative expression of IL-8 mRNA between samples was estimated by normalizing these values against 18s rRNA critical threshold values generated with an optimized 18s rRNA primer and probe set (Applied Biosystems, Foster City, Calif.).
IL-8-luciferase reporter gene assay.
Stable HEK293 cell lines transfected with either pcDNA5/FRT alone or this vector integrated with a TLR4-containing vector (as described above) were transiently transfected with MD2- and CD14-containing plasmids, along with an internal control plasmid, thymidine kinase-Renilla, and an IL-8-luciferase reporter plasmid (either full-length or truncated). Briefly, 105 cells were seeded on a 12-well plate overnight. The following day, these cells were transfected with 100 ng each of the MD2 and CD14 plasmids and 125 ng of reporter and control plasmid with Fugene reagent (Roche Diagnostics, Indianapolis, Ind.) at a 3:1 ratio (Fugene to DNA). Cells were incubated overnight and then placed in fresh serum-free medium 24 h prior to stimulation. Results, expressed as relative light units, were calculated by dividing the value for firefly luciferase (IL-8) by that of Renilla luciferase (thymidine kinase).
Electrophoretic mobility shift assay.
We treated 9 × 106 THP-1 cells for 15, 30, 45, 60, and 120 min with 10 μg of LPS or LPS in combination with NAC per ml. Nuclear extracts were prepared as previously described (39). Electrophoretic mobility shift assays were performed with the Gel Shift assay system protocol (Promega). Briefly, consensus NF-κB oligonucleotide probe was end labeled with [γ-32P]ATP (Amersham Biosciences, Buckinghamshire, United Kingdom). Reaction mixtures consisted of 10 μg of nuclear extract and binding buffer (2.5 mM dithiothreitol, 2.5 mM EDTA, 5 mM MgCl2, 20% glycerol, 250 mM NaCl, 50 mM Tris-HCl, 0.25 mg of poly[di-dC] per ml). Unlabeled NF-κB and AP-1 (3.5 pmol) consensus probes were added to the control samples as specific and nonspecific competitors. The reaction mixtures were incubated at room temperature for 10 min, 0.06 pmol of γ-32P-labeled probe was added and incubated for 20 min at room temperature, and the reaction mixtures were immediately subjected to nondenaturing 4% polyacrylamide gel electrophoresis. The gels were dried and autoradiographed at −80°C for 18 h. Gels were analyzed by densitometry to assess differences between treatment groups.
For supershift analysis, either polyclonal p50, p65, or c-rel antibody (Santa Cruz), or normal rabbit serum (Vector Laboratories, Burlingame, Calif.) was added to the relevant binding reaction immediately before addition of the 32P-labeled probe. The binding reaction incubation time was increased to 30 min at room temperature prior to gel electrophoresis.
Statistical analyses.
For parametric data, results are expressed as the mean ± standard error of the mean and were compared with the two-tailed Student's t test for paired samples. For nonparametric data, results are expressed as the median ± quartiles and were compared with the Wilcoxon signed rank test. Where indicated, an adjusted Bonferroni correction for multiple comparisons was used to reach an overall P value of ≤0.05 (30).
RESULTS
LPS induces ROS and reactive nitrogen species.
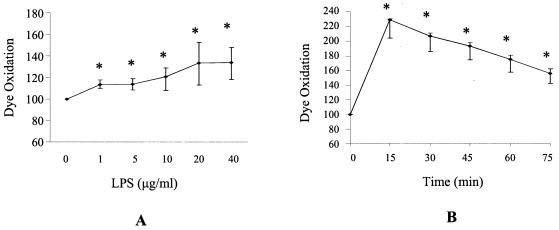
Purified E. coli LPS was found to induce increased oxidation of the dye H2DCFDA, indicating the presence of H2O2, ONOO−, or OH• (Fig. 1). This dye does not distinguish between these species; thus, it was not possible to deduce if LPS induced only one or combinations of these species. Oxidation of H2DCFDA was time and LPS dose dependent (Fig. 1). A minimum of 1 μg of LPS per ml for 15 min was sufficient to induce ROS and reactive nitrogen species in THP-1 cells. Dye oxidation was found to peak after 15 min, after which intracellular ROS and reactive nitrogen species levels were still elevated after 90 min. In contrast, LPS did not increase oxidation of DHE, indicating that LPS did not induce O2− (data not shown). In an attempt to confirm that we were not missing a brief transient burst of O2−, an assay was carried out in which dye oxidation was measured every minute following LPS stimulation of cells. An increase in O2− was not observed in this experiment either. A superoxide-generating system consisting of hypoxanthine and xanthine oxidase was used as a positive control to confirm dye integrity.
FIG. 1.
LPS elicits a time- and dose-dependent oxidative burst. (A) THP-1 cells were incubated with the H2DCFDA dye, which is oxidized by hydroxyl radicals, peroxinitrite, and hydrogen peroxide. Subsequently, THP-1 cells were treated with various doses of LPS for 15 min. Dye oxidation was calculated by dividing the mean channel fluorescence of a treated sample by that of the untreated sample and multiplying by 100 to obtain the relative change expressed as a percentage. (B) H2DCFDA dye oxidation in THP-1 cells treated with 10 μg of LPS per ml for various times. Data shown are the medians of duplicates ± the upper and lower quartiles from five separate experiments. *, statistical differences between dye oxidation of LPS-treated and control cells were significant to an overall P value of ≤0.05 as determined with the Wilcoxon test with an adjusted Bonferroni correction.
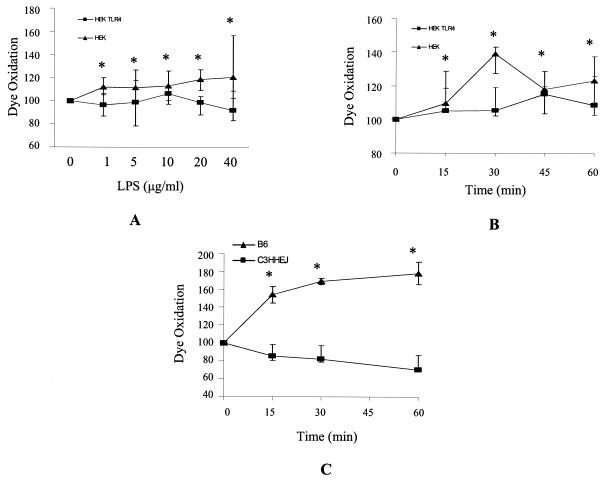
To further investigate the importance of TLR4 in facilitating an LPS-induced ROS and reactive nitrogen species burst in THP-1 cells, a TLR4-blocking antibody was used to try to inhibit ligand binding. The antibody alone, however, was found to induce an oxidative burst in these cells, possibly through interaction with Fc receptors on the cell surface, which could not be blocked by nonimmune serum, which also occupies the Fc receptors and induces ROS. An alternative method of investigation was then employed in which the H2DCFDA and DHE assays were carried out in TLR4 stably transfected HEK293 cells. Compared to HEK293 cells transfected with empty vector alone, cells containing TLR4 (in addition, both cell populations were simultaneously transiently transfected with MD2 and CD14) showed an increase in H2DCFDA dye oxidation (Fig. 2). An increased oxidative burst was apparent at LPS concentrations of 1 μg/ml and up to 40 μg/ml (Fig. 2A). To confirm these experiments, bone marrow-derived macrophages were isolated from control mice (C57BL/6) and mice with a missense mutation in TLR4 that impedes LPS signal transduction (C3H/HeJ) (31). In macrophage cells from C57BL/6 mice, which express a functional TLR4 receptor, LPS induced ROS generation, as detected by H2DCFDA dye oxidation. However, in cells from C3H/HeJ mice, which lack a functional TLR4 receptor, LPS did not induce the generation of free radicals (Fig. 2C).
FIG. 2.
LPS-induced oxidative burst was higher in cells expressing TLR4 compared to non-TLR4-expressing controls. The LPS had been purified to ensure that it was a highly specific TLR4 agonist and did not signal in the absence of TLR4. (A) Cells were incubated with H2DCFDA dye and then treated with various doses of LPS for 15 min. (B) Cells were incubated with H2DCFDA dye and then treated with 10 μg of LPS per ml for various times. •, TLR4-transfected HEK293 cells; ▴, empty vector-transfected HEK293cells. Data shown are from four separate experiments carried out in duplicate. (C) H2DCFDA dye oxidation in C57BL/6 and C3H/HeJ bone marrow-derived macrophages treated with 5 μg of LPS per ml for various times. •, C57BL/6; ▴, C3H/HeJ. Data shown are from three independent experiments. All data are expressed as the medians ± the upper and lower quartiles from three independent experiments. *, statistical differences between dye oxidation of LPS-treated and control cells were significant to an overall P value of ≤0.05, as determined with the Wilcoxon test with an adjusted Bonferroni correction.
Antioxidants repress LPS-induced IL-8 secretion.
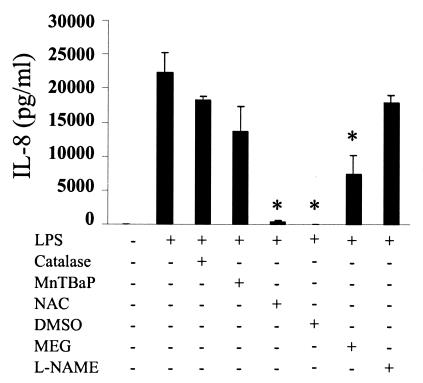
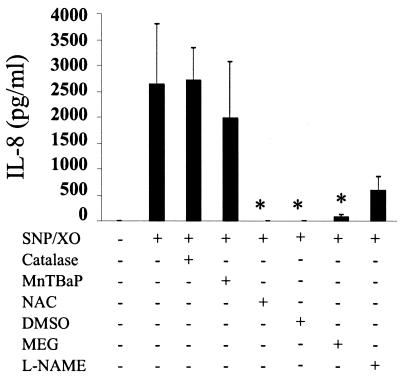
We have shown that LPS induces a TLR4-dependent oxidative burst. To investigate the relevance of this phenomenon for cellular signaling, we first examined the effect of a panel of antioxidants on LPS-induced IL-8 secretion. It has been known for some time that LPS induces the chemokine IL-8 via the NF-κB pathway (26). Using a specific anti-TLR4 blocking antibody, we confirmed that LPS was signaling through TLR4 (data not shown). In addition to the TLR4-blocking antibody, LPS-mediated IL-8 secretion was also eliminated by the antioxidants NAC, DMSO, and MEG but not catalase, MnTBAP, or L-NAME (Fig. 3). The finding that MEG, which is a scavenger of peroxinitrite, inhibited LPS-induced IL-8 led us to investigate whether a peroxinitrite-generating system alone could induce IL-8. Stimulation of THP-1 cells with xanthine oxidase, which generates superoxide, and sodium nitroprusside, which generates nitric oxide, did indeed induce IL-8, although at much lower levels than LPS (Fig. 3 and Fig. 4). Peroxinitrite-induced IL-8 was blocked by NAC, DMSO, and MEG (Fig. 4).
FIG. 3.
LPS-induced IL-8 secretion is inhibited by the antioxidants NAC, DMSO, and MEG. THP-1 cells were incubated with various antioxidants and then treated with 10 μg of LPS per ml for 4 h. Supernatants were collected and tested for IL-8 protein by ELISA. Data are expressed as the means ± standard error of the mean of three independent experiments carried out in duplicate. Statistical differences between LPS alone and LPS plus antioxidant samples are indicated; *, overall P value of ≤0.05, as determined with Student's t test and an adjusted Bonferroni correction.
FIG. 4.
IL-8 secretion induced by sodium nitroprusside and xanthine oxidase is eliminated by NAC, DMSO, and MEG. THP-1 cells were incubated with various antioxidants and then treated with a combination of sodium nitroprusside and xanthine oxidase for 4 h. Supernatants were collected and tested for IL-8 protein by ELISA. Data are expressed as the means ± standard error of the mean of three independent experiments carried out in duplicate. Statistical differences between samples treated with sodium nitroprusside and xanthine oxidase alone and sodium nitroprusside and xanthine oxidase plus antioxidant are indicated; *, overall P value of ≤0.05, as determined with Student's t test and an adjusted Bonferroni correction.
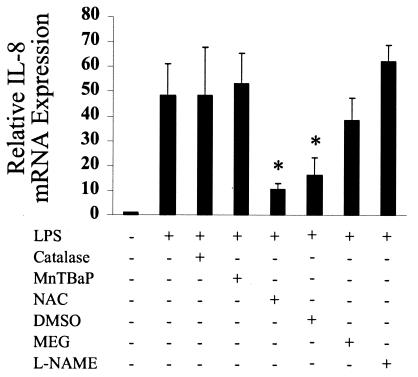
Antioxidants inhibited IL-8 mRNA expression.
Although IL-8 protein could not be detected in untreated THP-1 cells with an ELISA system (Fig. 3 and 4), a basal level of IL-8 mRNA was detected (Fig. 5). LPS was found to increase the levels of IL-8 mRNA, and this effect was eliminated by the antioxidants NAC and DMSO (Fig. 5). IL-8 mRNA levels were not significantly higher in peroxinitrite-stimulated cells compared to untreated controls (results not shown).
FIG. 5.
IL-8 mRNA expression induced by LPS is inhibited by NAC and DMSO. THP-1 cells were incubated with various antioxidants and then treated with 10 μg of LPS per ml for 4 h. Total RNA was extracted and reverse transcribed. IL-8 mRNA expression was assessed by real-time PCR. Data are expressed as the means ± standard error of the mean of four (NAC and DMSO) or three (all other samples) independent experiments carried out in duplicate. Statistical differences between LPS alone and LPS plus antioxidant samples are indicated; *, overall P value of ≤0.05, as determined with the Wilcoxon test with an adjusted Bonferroni correction.
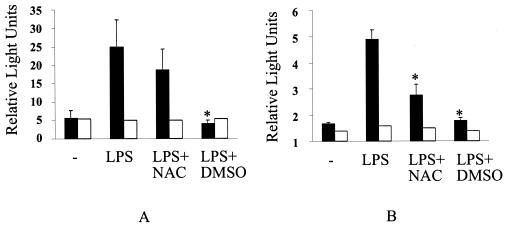
LPS induces IL-8 promoter transactivation, an effect eliminated by antioxidants.
IL-8 promoter transactivation was observed in HEK293 TLR4-transfected cells treated with LPS, and this effect was not seen in HEK293 cells containing the empty vector only (Fig. 6). The full-length IL-8 promoter used in this study contains binding sites for the transcription factors AP-1, NF-κB, and NFIL6 (5). Treatment of cells with the antioxidant DMSO reduced full-length IL-8 promoter transactivation to basal levels (Fig. 6A).
FIG. 6.
LPS-induced IL-8 promoter transactivation was eliminated by NAC and DMSO. To assess if antioxidants could block transactivation of the IL-8 gene, IL-8 promoter reporter constructs were transfected into HEK293 cells expressing TLR4 or not. Cells were incubated with various antioxidants and then treated with 10 μg of LPS per ml for 4 h. The IL-8 promoter construct was not transactivated by LPS in cells lacking TLR4 (open bars). (A) Full-length IL-8 promoter transactivation in TLR4-expressing HEK293 cells (solid bars) or non-TLR4-expressing cells (open bars). (B) Truncated IL-8 promoter (lacking an AP-1 binding site) transactivation in TLR4-expressing HEK293 cells (solid bars) or non-TLR4-expressing cells (open bars). Transactivation was not dependent on the presence of an AP-1 binding site. Data are expressed as the means ± standard error of the mean of three independent experiments carried out in triplicate. Statistical differences between LPS alone and LPS plus antioxidant samples are indicated; *, an overall P value of ≤0.05, as determined with the Wilcoxon test with an adjusted Bonferroni correction.
We repeated the above experiment with a plasmid that contains only 99 nucleotides of a truncated IL-8 promoter and lacks the AP-1 but not the NF-κB binding site (5). Consistent with our earlier experiments, we observed a similar level of LPS-induced transactivation, which was again blocked by DMSO but also this time by NAC (Fig. 6B). The observation that LPS-induced IL-8 promoter transactivation was not decreased in the absence of an AP-1 binding site indicates that NF-κB is more important in inducible IL-8 promoter binding. This is consistent with the idea that AP-1 is preferentially involved in constitutive IL-8 promoter binding (43).
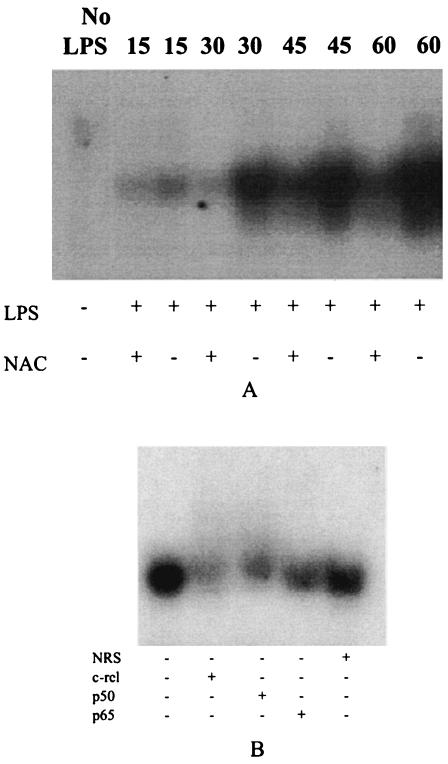
Antioxidants block nuclear translocation of NF-κB.
LPS was found to induce nuclear translocation of NFκB within 15 min (Fig. 7A). Total NF-κB protein in the nucleus continued to increase until 60 min, and NAC was found to decrease NF-κB translocation up until this time. Supershift experiments showed that LPS was definitively inducing NF-κB complexes consisting of c-rel and p50 (Fig. 7B). Although it appeared that a p65 complex was also present, this supershift was less apparent (Fig. 7B).
FIG. 7.
Nuclear translocation of NF-κB is sensitive to antioxidant treatment with NAC in THP-1 cells. (A) LPS induced the nuclear translocation of NF-κB protein after 15 min, which continued until 60 min, and NAC decreased NF-κB translocation at all time points. (B) Supershift analysis of nuclear protein from LPS-treated cells showed that NF-κB complexes contain c-rel, p50, and possibly p65 protein. The gels shown are representative of three independent experiments.
DISCUSSION
It has long been known that ROS play a role in intracellular signaling, but the various mechanisms by which the cellular redox state can influence signaling pathways are extremely complex (2, 14, 19). A number of groups have studied the role of ROS in cytokine signaling pathways. More specifically, it has been shown that both tumor necrosis factor alpha and LPS-mediated IL-8 production are at least partially reliant on the generation of reactive oxygen intermediates (7, 8, 29, 34, 38). Here we demonstrate that a rapid oxidative burst in cells exposed to highly purified LPS is dependent on the presence of TLR4 protein. Most likely, higher LPS concentrations were required to induce ROS in TLR4-transfected HEK cells due to the limited number of cells that could express the transiently transfected accessory molecules MD2 and CD14. It has been reported that only approximately 30% of cells will become transfected with the Fugene reagent (44).
By using a panel of antioxidants, we observed that the subsequent induction of IL-8 is in turn dependent on this oxidative burst. Both of the hydroxyl radical scavengers NAC and DMSO significantly eliminated LPS-induced IL-8 at both the mRNA and protein levels. We did not find that DMSO significantly affected our assay controls, i.e., 18S rRNA for PCR and thymidine kinase-Renilla for transfections. DMSO also inhibited LPS-induced transactivation of both a full-length and truncated (missing an AP-1 binding site) IL-8 promoter construct. Although transactivation of the truncated IL-8 promoter was inhibited by NAC, transactivation of the full-length construct was not. This discrepancy is most likely due to molecular differences in the signaling pathway machinery that lead to intracellular ROS generation between the two cell types used, i.e., THP-1 for ELISA and PCR and HEK293 for the transfection studies.
NAC may have a secondary influence on the cell's redox state in that it is a precursor of glutathione, a molecule that helps to regulate the intracellular redox equilibrium through its reactive cysteinyl moieties. The antioxidant MEG is a selective inducible nitric oxide synthase inhibitor and also a peroxinitrite scavenger. This antioxidant eliminated IL-8 protein secretion, but did not significantly affect LPS-induced IL-8 mRNA. Moreover a peroxinitrite-generating system induced an increase in IL-8 secretion but did not affect IL-8 mRNA levels. Together, these results suggest that the generation of peroxinitrite in a cell may play some role in the eventual secretion of IL-8, possibly through a posttranscriptional mechanism. None of the other antioxidants employed in this study had any significant effect on IL-8 production. It would appear from the fact that MnTBAP did not inhibit either LPS-, sodium nitroprusside-, or xanthine oxidase-induced IL-8 that superoxide induction is not directly involved in IL-8 signaling. In concurrence, we did not observe a superoxide burst in cells treated with LPS in the DHE assay. Finally, L-NAME did not affect LPS-induced IL-8 mRNA and protein levels. Although L-NAME is an inhibitor of nitric oxide synthase, physiological levels of NO may be sufficient for peroxinitrite formation. Thus, nitric oxide synthase would not be required for IL-8 expression.
Filep et al. (11) previously reported that peroxinitrite can mediate IL-8 expression in monocytes. They also reported that L-NAME and a second nitric oxide synthase inhibitor, aminoguanidine, could block LPS-induced IL-8. However, there is no way to tell from this earlier study if the peroxinitrite itself was mediating the signaling changes or if this molecule was being converted to hydroxyl radical which may have been the effector molecule. In fact, Remick and colleagues (33) reported in an earlier study that DMSO can inhibit exogenously added, NO-driven IL-8 secretion in neutrophils, again suggesting a role for hydroxl radical in IL-8 signaling. In a related study, also in neutrophils, Remer et al. recently showed that TLR4 is necessary for a cell killing-associated oxidative burst in LPS-stimulated cells (32). Unfortunately, this study used unpurified LPS that may have contained contaminants capable of signaling through receptors other than TLR4. Earlier studies looking at LPS-stimulated whole blood showed, similar to our own results, that hydroxyl radical scavengers could inhibit IL-8 production, although intracellular ROS levels were not measured and again an unpurified LPS preparation was used (7, 8). Taken together, these results suggest that although LPS may induce more than one type of reactive oxygen intermediate, the hydroxyl radical is the most likely endpoint species important for signaling.
Since the data support the notion that ROS or reactive nitrogen species are involved in LPS TLR4-mediated IL-8 signaling, the question arises of which part of the NF-κB signaling cascade they affect. In the present study, we found that the antioxidant NAC could decrease LPS-induced nuclear translocation of NF-κB. This concurs with our finding that antioxidants also decreased IL-8 promoter transactivation. Thus, it is probable that ROS are altering cellular conditions that inhibit movement of NF-κB to the nucleus, where it can bind target DNA sequences. One interesting model to explain how this may happen suggests that hydrogen peroxide can directly activate IκB kinases through phosphorylation of its activation loops (20). Although this would provide a functional activity for certain ROS, hydrogen peroxide activation of IκB kinases alone was not sufficient for eventual NF-κB transactivation (20). Others (40) have hypothesized that tumor necrosis factor alpha-induced IL-8 expression is controlled by two distinct pathways, only one of which is ROS dependent. They argue that ROS transcriptional activity is distinct from NF-κB translocation and that an independent NF-κB activating pathway may exist. Indeed, our electrophoretic mobility shift assay results show that at longer time points, the effect of NAC on decreasing NF-κB translocation is diminished. This may also be evidence of the presence of a distinct, ROS-independent pathway which becomes activated at a later time point.
A further possibility is ROS induction of the Nrf2 pathway. The IL-8 gene promoter contains an antioxidant response element (34) which can enhance gene expression following binding of the transcription factor Nrf2 (22, 28). The antioxidant response element is typically found in genes associated with maintaining the redox balance in a cell (10, 27) but has yet to be studied in relation to IL-8 gene expression. This potential mechanism provides an intriguing possibility for an ROS-induced antioxidant response element-mediated or -enhanced pathway. Studies to investigate this are currently under way in our laboratory.
Undoubtedly, the intracellular redox balance is a critical determinant that regulates the signaling mechanisms leading to the induction of IL-8. We provide evidence herein that ROS play an important part in innate immune signaling through TLR4. It may well be that altering the redox state of the cell is a simple yet evolutionarily conserved way to enhance a particular response in times of stress.
Acknowledgments
This work was supported by National Institutes of Health grants DK50980, AI48173, and RR00175 (to P.B.E.) and AI34358 (to M.F.S.).
Editor: D. L. Burns
REFERENCES
- 1.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., D. S. Weiss, and A. Zychlinsky. 2001. Toll-like receptor-2 transduces signals for NF-kappa B activation, apoptosis and reactive oxygen species production. J. Endotoxin Res. 7:287-291. [PubMed] [Google Scholar]
- 3.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 4.Carl, V. S., K. Brown-Steinke, M. J. Nicklin, and M. F. Smith, Jr. 2002. Toll-like receptor 2 and 4 (TLR2 and TLR4) agonists differentially regulate secretory interleukin-1 receptor antagonist gene expression in macrophages. J. Biol. Chem. 277:17448-17456. [DOI] [PubMed] [Google Scholar]
- 5.Casola, A., R. P. Garofalo, S. E. Crawford, M. K. Estes, F. Mercurio, S. E. Crowe, and A. R. Brasier. 2002. Interleukin-8 gene regulation in intestinal epithelial cells infected with rotavirus: role of viral-induced IkappaB kinase activation. Virology 298:8-19. [DOI] [PubMed] [Google Scholar]
- 6.Chuang, T., and R. J. Ulevitch. 2001. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 1518:157-161. [DOI] [PubMed] [Google Scholar]
- 7.DeForge, L. E., J. C. Fantone, J. S. Kenney, and D. G. Remick. 1992. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J. Clin. Investig. 90:2123-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeForge, L. E., A. M. Preston, E. Takeuchi, J. Kenney, L. A. Boxer, and D. G. Remick. 1993. Regulation of interleukin 8 gene expression by oxidant stress. J. Biol. Chem. 268:25568-25576. [PubMed] [Google Scholar]
- 9.Denning, T. L., H. Takaishi, S. E. Crowe, S. Boldogh, A. M. Jevnikar, and P. B. Ernst. 2002. Oxidative stress induces the expression of Fas and FasL and apoptosis in intestinal epithelial cells. Free Rad. Biol. Med. 33:1641-1650. [DOI] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova, A. T., W. D. Holtzclaw, R. N. Cole, K. Itoh, N. Wakabayashi, Y. Katoh, M. Yamamoto, and P. Talalay. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99:11908-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filep, J. G., M. Beauchamp, C. Baron, and Y. Paquette. 1998. Peroxynitrite mediates IL-8 gene expression and production in lipopolysaccharide-stimulated human whole blood. J. Immunol. 161:5656-5662. [PubMed] [Google Scholar]
- 12.Foley, E., and P. H. O'Farrell. 2003. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 17:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman, H. J., M. Torres, and J. Fukuto. 2002. Redox signaling. Mol. Cell. Biochem. 234-. 235:49-62. [PubMed] [Google Scholar]
- 14.Haddad, J. J., and S. C. Land. 2002. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br. J. Pharmacol. 135:520-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto, C., K. L. Hudson, and K. V. Anderson. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269-279. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 17.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 19.Hsu, H. Y., and M. H. Wen. 2002. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 277:22131-22139. [DOI] [PubMed] [Google Scholar]
- 20.Kamata, H., T. Manabe, S. Oka, K. Kamata, and H. Hirata. 2002. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 519:231-237. [DOI] [PubMed] [Google Scholar]
- 21.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffman. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifundgal response in Drosphila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 22.McMahon, M., K. Itoh, M. Yamamoto, S. A. Chanas, C. J. Henderson, L. I. McLellan, C. R. Wolf, C. Cavin, and J. D. Hayes. 2001. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 61:3299-3307. [PubMed] [Google Scholar]
- 23.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 26.Mukaida, N., Y. Mahe, and K. Matsushima. 1990. Cooperative interaction of NF-κB and cis-regulatory enhancer binding protein-like factor binding elements in activating the IL-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265:21128-21132. [PubMed] [Google Scholar]
- 27.Ng, D., N. Kokot, T. Hiura, M. Faris, A. Saxon, and A. Nel. 1998. Macrophage activation by polycyclic aromatic hydrocarbons: evidence for the involvement of stress-activated protein kinases, activator protein-1, and antioxidant response elements. J. Immunol. 161:942-951. [PubMed] [Google Scholar]
- 28.Nguyen, T., P. J. Sherratt, and C. B. Pickett. 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43:233-260. [DOI] [PubMed] [Google Scholar]
- 29.Oka, S., H. Kamata, K. Kamata, H. Yagisawa, and H. Hirata. 2000. N-acetylcysteine suppresses TNF-induced NF-kappaB activation through inhibition of IkappaB kinases. FEBS Lett. 472:196-202. [DOI] [PubMed] [Google Scholar]
- 30.Olsen, C. H. 2003. Review of the use of statistics in infection and immunity. Infect. Immun. 71:6689-6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 32.Remer, K. A., M. Brcic, and T. W. Jungi. 2003. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol. Lett. 85:75-80. [DOI] [PubMed] [Google Scholar]
- 33.Remick, D. G., and L. Villarete. 1996. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J. Leukoc. Biol. 59:471-475. [DOI] [PubMed] [Google Scholar]
- 34.Roebuck, K. A. 1999. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 19:429-438. [DOI] [PubMed] [Google Scholar]
- 35.Schubert, S. Y., I. Neeman, and N. Resnick. 2002. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J. 16:1931-1933. [DOI] [PubMed] [Google Scholar]
- 36.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 37.Seo, J. Y., H. Kim, and K. H. Kim. 2002. Transcriptional regulation by thiol compounds in Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. Ann. N. Y. Acad. Sci. 973:541-545. [DOI] [PubMed] [Google Scholar]
- 38.Shimada, T., N. Watanabe, H. Hiraishi, and A. Terano. 1999. Redox regulation of interleukin-8 expression in MKN28 cells. Dig. Dis. Sci. 44:266-273. [DOI] [PubMed] [Google Scholar]
- 39.Smith, M. F. Jr., D. Eidlen, W. P. Arend, and A. Gutierrez-Hartmann. 1994. LPS-induced expression of the human IL-1 receptor antagonist gene is controlled by multiple interacting promoter elements. J. Immunol. 153:3584-3593. [PubMed] [Google Scholar]
- 40.Vlahopoulos, S., I. Boldogh, A. Casola, and A. R. Brasier. 1999. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94:1878-1889. [PubMed] [Google Scholar]
- 41.Werling, D., and T. W. Jungi. 2003. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 91:1-12. [DOI] [PubMed] [Google Scholar]
- 42.Wright, S. D., P. S. Tobias, R. J. Ulevitch, and R. A. Ramos. 1989. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J. Exp. Med. 170:1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, G. D., E. J. Lai, N. Huang, and X. Wen. 1997. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 272:2396-2403. [PubMed] [Google Scholar]
- 44.Young, A. T., J. R. Lakey, A. G. Murray, and R. B. Moore. 2002. Gene therapy: a lipofection approach for gene transfer into primary endothelial cells. Cell Transplant. 11:573-582. [PubMed] [Google Scholar]