Abstract
OBJECTIVE
Mechanical characteristics of high velocity low amplitude spinal manipulations (HVLA-SM) can be variable. Sustained changes in peripheral neuronal signaling due to altered load transmission to a sensory receptor’s local mechanical environment are often considered a mechanism contributing to the therapeutic effects of spinal manipulation. The purpose of this study was to determine whether an HVLA-SM’s thrust amplitude or duration altered neural responsiveness of lumbar muscle spindles to either vertebral movement or position.
METHODS
Anesthetized cats (n=112) received L6 HVLA-SMs delivered to the spinous process. Cats were divided into 6 cohorts depending upon the peak thrust force (25%, 55%, 85% body weight) or thrust displacement (1, 2, 3mm) they received. Cats in each cohort received 8 thrust durations (0–250ms). Afferent discharge from 112 spindles was recorded in response to ramp and hold vertebral movement before and after the manipulation. Changes in mean instantaneous frequency (MIF) during the baseline period preceding the ramps (ΔMIFresting), during ramp movements (ΔMIFmovement), and with the vertebra held in the new position (ΔMIFposition) were compared.
RESULTS
Thrust duration had a small but statistically significant effect on ΔMIFresting at all six thrust amplitudes compared to control (0ms thrust duration). The lowest amplitude thrust displacement (1mm) increased ΔMIFresting at all thrust durations. For all the other thrust displacements and forces, the direction of change in ΔMIFresting was not consistent and the pattern of change was not systematically related to thrust duration. Regardless of thrust force, displacement, or duration, ΔMIFmovement and ΔMIFposition were not significantly different from control.
Conclusion
Relatively low amplitude thrust displacements applied during an HVLA-SM produced sustained increases in the resting discharge of paraspinal muscle spindles regardless of the duration over which the thrust was applied. However, regardless of the HVLA-SM’s thrust amplitude or duration, the responsiveness of paraspinal muscle spindles to vertebral movement and to a new vertebral position was not affected.
Keywords: spinal manipulation, dose, neurophysiology, paraspinal muscles, muscle spindle, Spine, Chiropractic
INTRODUCTION
The classification, training, and clinical application of spinal manipulative techniques involve a number of mechanical considerations including application rate, amplitude and direction, the type of leverage; the specificity of the manual contact site on both the clinician and patient, as well as the magnitude and duration of any preload.1 From the perspective of clinical practice, these parameters may be adapted to the needs of a given patient2, with clinical responses potentially depending upon their characteristics.2 From a research perspective, the extent to which these features can be characterized or measured provides a basis for comparing outcomes from experimental studies because these parameters can be considered elements of the manipulation’s dose. From a training perspective, these parameters identify elements of the clinical skill set that need to be taught and acquired.3
High velocity, low amplitude (HVLA) thrust techniques represent one form of spinal manipulation (HVLA-SM) often used in clinical practice and research.4,5 When HVLA-SM is applied to the lumbar region, preload forces preceding the manipulative thrust generally range between 20 and 180 N, comprise up to 25% of the thrust force6, and last between 0.5 and 2.2s.7 The HVLA-SM’s thrust phase rises to a peak force of 220 to 889N6,8–10 within 150ms.6,9,11 Clinicians performing short-lever manipulations in the low back typically make contact with the patient over the spinous process, lamina, or mammillary process of a specific lumbar vertebra using their hypothenar eminence or pisiform bone.1 It is has been reported that by the time SM is delivered the actual contact point may have migrated up to 10mm from the originally intended contact point.12 The direction in which the thrust force is applied is considered important in that it should be parallel to either the plane of the intervertebral disc or the facet joint space so as to provide the least resistance to vertebral movement.13,14 It has been suggested that in the absence of preload, only forces directed perpendicular to the skin surface are actually transmitted to the vertebra and deep soft tissues because the skin-fascia interface can slide.15
Clearly, mechanical characteristics of an HVLA-SM can be variable. Little is known about the relationship between this variation and clinical outcomes. For example, is it important that the thrust amplitude be kept within a certain range or that the thrust be applied with a minimum speed in order to be effective? Controlling and/or measuring these characteristics in a clinical study is challenging. As an initial step, we have begun approaching this issue using an animal preparation in which the mechanical characteristics of an HVLA-SM can be more easily controlled.16,17 Because spinal manipulation is thought to alter the pattern of sensory input into the central nervous system as part of its therapeutic effect,18–24 we used changes in primary afferent nerve activity as a response measure.
In this study we investigated the response of one type of proprioceptor located in back muscles of the lumbar spine, the muscle spindle. Muscle spindles lie parallel to extrafusal fibers, respond to changes in muscle length, and thereby supply sensory information about joint position.25,26 We investigated whether the thrust amplitude and/or thrust duration of an HVLA-SM alters the responsiveness of muscle spindle afferents to either vertebral position or vertebral movement. Altered responsiveness would suggest that the HVLA-SM can produce changes in the local mechanical environment of the sensory receptor. We hypothesized that as the thrust duration approached that used clinically, the responsiveness of muscle spindles to vertebral position and movement would change.
METHODS
All experiments were approved by the Institutional Animal Care and Use Committee (#20070101). Electrophysiological activity in 112 single primary afferent fibers of the L6 (cats have 7 lumbar vertebrae) dorsal root (92 units from lumbar longissimus muscle and 20 from multifidus muscle) was obtained from cats (n=112) of either sex weighing an average of 3.97kg (SD 0.85). Only one afferent could be investigated per cat because following delivery of the spinal manipulative protocols to the intact lumbar tissues deeper tissues needed to be surgically exposed to confirm that the source of afferent activity was from muscle spindles in the lumbar multifidus or longissimus muscles. Calibrated nylon monofilaments (Stoelting, Ill) were applied to the exposed muscles to verify that the most sensitive potion of the afferent’s receptive field was in fact located in the paraspinal muscles. Muscle spindle afferents were identified based upon their increased discharge to succinylcholine (100 – 400μg/kg, ia) and decreased discharge to electrically induced muscle contraction as described previously.16 In addition, to help differentiate muscle spindle from Golgi Tendon Organ afferents27 we determined whether the afferent was able to produce a sustained response to a fast vibratory stimulus applied to the muscle’s surface close to the afferent’s receptive field.
GENERAL PROCEDURES
The surgical procedures and device used to apply spinal manipulations have previously been described in detail.16,28,29 Under isoflurane anesthesia, catheters were placed in a common carotid artery and an external jugular vein to monitor blood pressure and introduce fluids. Deep anesthesia was then maintained with Nembutal (35mg/kg, iv). The trachea was intubated and the cat ventilated mechanically using a Harvard Respirator (Model 681). The laminae of L5 and of the caudal half of L4 were removed to expose the cranial portion of the L6 dorsal rootlets. Thin filaments from the rootlets were teased using forceps under a dissecting microscope until impulse activity from a single neuron was identified 30. The region of the lumbar spine where the spinal manipulations were applied remained intact, including the skin, muscle, ligament, and bone, beginning at the L6 vertebra and extending caudalward.
SPINAL MANIPULATION
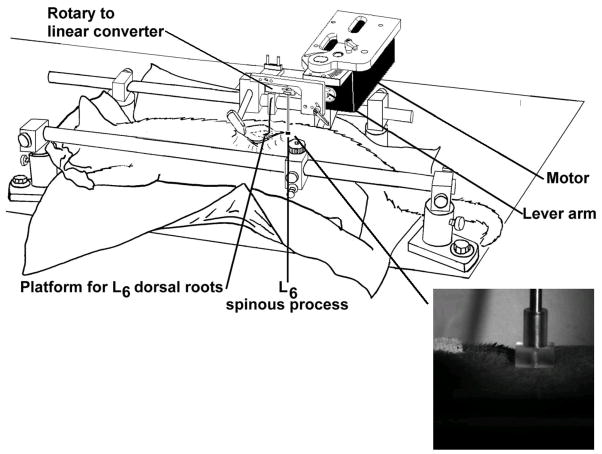
A schematic of the preparation is shown in Figure 1. With the cat lying prone, an L6 spinal manipulation was applied at the spinous process in a posterior to anterior direction using a feedback-controlled motor (Aurora Scientific, Lever System Model 310). The motor was attached to a custom-made manipulandum which terminated in a rectangular-shaped tip (7 mm x 10 mm) with a narrow channel (5 mm wide x 2 mm deep ) designed to cradle the sides of the spinous process and prevent lateral slippage (photographic inset in Fig. 1). The tip made direct contact with the skin overlying the L6 spinous process. Load-time profiles for the spinal manipulations were similar to HVLA-SMs given to human subjects6,9,11 being delivered as triangle waves (Fig. 2). The thrust phase was applied under linear control of either force or displacement and the resolution or recoil phase always occurred faster than the thrust phase to minimize continued contact with the back. The programmable output of the data acquisition system provided the linear signal controlling the applied force or displacement.
Figure 1.
Schematic of the preparation for recording neural activity and delivering spinal manipulation at the lumbar spine. Manipulations were delivered via the motor which was controlled by an electronic feedback system (not shown). Computer-controlled rotation of the motor’s shaft rotated the lever arm. The lever arm was attached to a custom built rotary-to-linear converter, which in turn was attached to a rod terminating in a plexiglass tip (see photographic inset) that contacted the skin overlying the L6 spinous process (see inset). The converter transformed the lever arm’s rotary motion to linear motion of the rod and plexiglass tip.
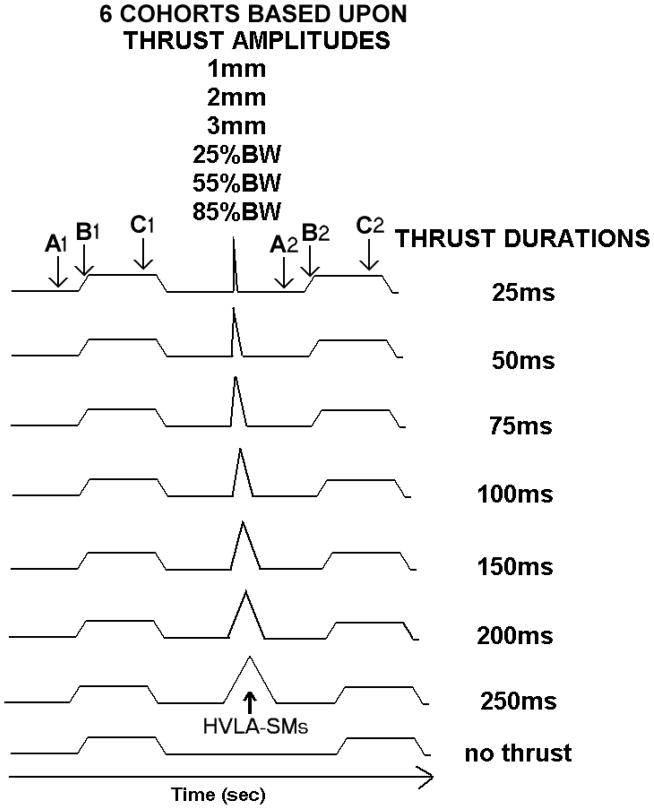
Figure 2.
Schematic of experimental protocols. Six cohorts were established based upon the thrust amplitude each received. Each cohort received all thrust durations. The high velocity, low amplitude spinal manipulations (HVLA-SMs) are represented by the triangular waveform. Trapezoidal waveform represents the ramp and hold displacement of the L6 vertebra. Letters with arrows represent the portion of the ramp and hold where neural activity was quantified determined: A) baseline resting discharge, B) during movement at the end of the ramp, C) at the new position during the last half of the hold. Numbers next to the A, B, and C represent activity before (1) and after (2) the HVLA-SM. See Methods for complete description.
Because the thrust of a spinal manipulation is intended to impart movement to a vertebra1 we determined the minimal applied force beyond which the L6 vertebra would be expected to move ventralward using methods previously described.31 Briefly, we attached a vertical post with markings to the cranial edge of the L6 spinous process and used the motor to slowly actuate the L6 spinous process 4 mm ventralward (1.33 mm/s). We simultaneously captured vertebral movement using a high resolution optical recording system (Motion Pro Digital Image System, Redlake MASD Inc, San Diego, CA) and the forces required to actuate the vertebra. We obtained the relationship between the visual onset of movement and the force at which the movement occurred. Movement occurred at a force represented by the inflection of the force-displacement curve as the spinal segment transitioned from a lower to a higher region of stiffness. This inflection force was termed the contact load and was determined in each cat. Future experiments will determine how manipulative preloads greater than the contact load affect neural activity.
COHORTS AND PROTOCOLS
Cats were grouped into 6 cohorts based upon the manipulative thrust amplitude received by the group (Fig. 2). With the motor in displacement control, a cohort received one of 3 displacement amplitudes (1, 2, or 3 mm) or with the motor in force control, a cohort received one of 3 force amplitudes [25%, 55%, or 85% of the cat’s body weight (BW)]. All cohorts consisted of 20 cats except the 25%BW cohort which consisted of 12 cats. Cats in each cohort received 8 manipulative thrust durations (0, 25, 50, 75, 100, 150, 200, and 250 ms) encompassing durations both longer and shorter compared to that delivered clinically [≤ 150 ms].6,9,11 The 0ms thrust duration served as a time control. These thrust durations and amplitudes were identical to those described and used in previous biomechanical studies31. Experimental protocols were separated by 5 minutes and were presented in random order yielding a randomized complete block experimental design.
Prior to and following each thrust duration, the motor applied a ramp and hold displacement to the L6 vertebra (Fig. 2). The lever arm slowly (0.5mm/s) moved ventralward 1.5mm for the displacement control experiments and 2.0mm for the force control experiments and held the vertebra at this new position for 4 seconds in all experiments. The lever arm was then slowly returned to its original position. During the displacement control experiments the first ramp and hold was applied 30 seconds before the thrust. During the force control experiments the first ramp and hold was applied 5 minutes before the thrust. The second ramp and hold was identical to the first and was always applied 30 seconds following the thrust.
DATA ANALYSIS
Spindle discharge was first quantified as instantaneous frequency (IF) by taking the reciprocal of the time interval between successive action potentials. Mean IF (MIF) was then determined at time intervals during each ramp and hold and represented: 1) resting spindle discharge prior to the onset of the ramp (MIFresting); 2) response to vertebral movement (MIFmovement); and 3) response to a new vertebral position (MIFposition). MIFresting was calculated over the 2s preceding the onset of the ramp (letter A, Fig. 2). MIFmovement was obtained by taking the average of the 3 largest IFs during the last half of the ramp (letter B, Fig. 2). MIFposition was calculated during the last 2s of the 4s hold which minimized mechanical contributions from the effects of tissue creep and spindle receptor adaptation to this metric32 (letter C, Fig. 2). Changes in the responsiveness of muscle spindles due to the manipulation were determined by subtracting MIFs during the 1st ramp and hold from MIFs during the 2nd ramp and hold yielding ΔMIFresting, ΔMIFmovement, and ΔMIFposition.
Neural responses were compared across the 8 levels of thrust duration with an ANOVA for the randomized complete block design using SAS System for Windows (Release 9.2) (SAS Institute Inc., Cary, NC). Cat served as a blocking factor in order to control for the level of spindle activity and intra-animal variability. An overall F-test was used to test whether the means were the same over the thrust durations. For statistically significant overall F-tests, preplanned contrasts using Fisher’s least significant difference tests between the time control (0 ms thrust duration) and each of the 7 other thrust durations were performed. Residual plots were used to confirm the assumptions of normality and homogenous variances. Overall F-tests and pre-planned contrasts were tested at the 0.05 level of significance.
To our knowledge no previous studies of muscle spindle responses before and after an HVLA-SM have been performed. Therefore, sample size calculations were based on previous reports33,34 of muscle spindle responses that occur during the HVLA-SM. Cohort sizes of 20 cats yielded >99% power for the overall F-test and at least 80% power to detect mean differences of 60 impulses per sec (imp/s) or more between adjacent levels of thrust duration. The 25%BW cohort was the last cohort studied. After collecting data from 8 cats in this cohort, we observed little difference among the cohorts in muscle spindle responses during the thrust of the HVLA-SM.29 Following data collection from an additional 4 cats, the pattern and magnitude of spindle responses remained similar. Therefore, we considered it appropriate to reduce the number of cats in the 25%BW cohort without compromising the interpretability of the results.
RESULTS
Stability of the preparation across the 6 cohorts
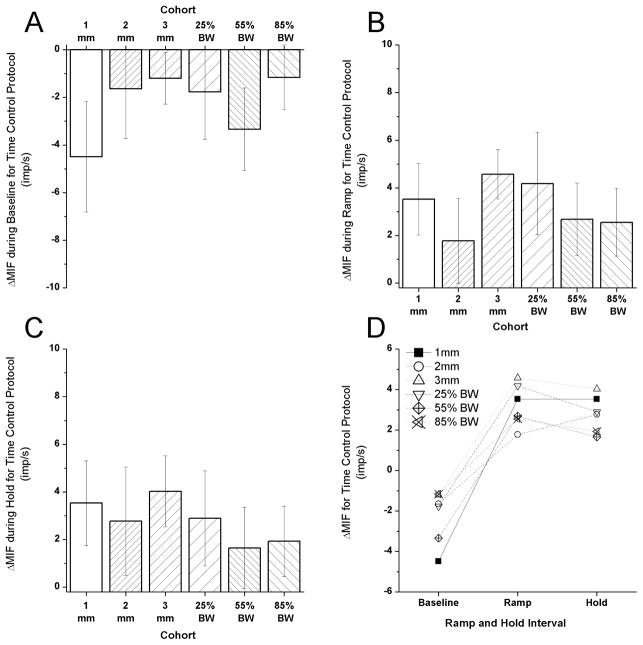
For control protocols where no spinal manipulative thrust was applied, Figure 3 provides comparisons of changes in muscle spindle discharge during the baseline period and during the ramp and hold challenge for all 6 cohorts. All changes in discharge were small and consistent in direction across the cohorts. ΔMIFresting (Fig. 3A) decreased between −1.2 and −4.5 imp/s. ΔMIFmovement (Fig. 3B) increased between 1.8 and 4.6 imp/s. ΔMIFposition (Fig. 3C) increased between 1.6 and 4.0 imp/s. The changes in discharge across the ramp and hold intervals did not show any systematic trends among the cohorts (Fig. 3D).
Figure 3.
Time control protocols. Change in neural activity between 1st and 2nd ramp and hold in the absence of a spinal manipulation. A) change in resting discharge during the baseline period; B) change in responsiveness during vertebral movement; C) change in responsiveness to a new vertebral position; D) pattern of the change in responsiveness across the 6 cohorts. Data are reported as means and 95% confidence intervals (lower, upper 95% CI).
Effect of spinal manipulation on resting spindle activity
Manipulative thrust duration during linear control of thrust displacement (Fig. 4A) had a significant effect on ΔMIFresting for all 3 thrust amplitudes (1mm, p=0.03; 2mm, p=0.02; and 3mm, p<0.001; Table 1). For the 1mm thrust amplitude, preplanned comparisons (see Data Analysis) showed that all manipulative thrust durations significantly increased (p < 0.04) resting spindle discharge ~4 imp/s compared to control. For the 2mm thrust amplitude, the 200ms thrust duration significantly (p = 0.03) increased resting spindle discharge when compared to no thrust. For the 3mm thrust amplitude, the 50ms thrust duration significantly (p=0.006) increased and the 75ms thrust duration significantly (p = 0.002) decreased resting discharge when compared to no thrust control.
Figure 4.
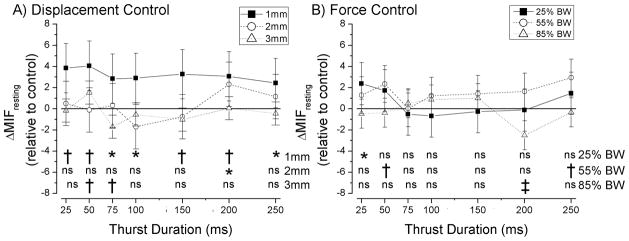
Effect of thrust amplitude and thrust duration of a spinal manipulation on resting responses of lumbar paraspinal muscle spindles. Statistical comparisons are all relative to a time control where no thrust was delivered (data shown in Fig. 3). * p≤0.05; † p≤0.01; ‡ p≤ 0.001. Data are reported as means and 95% confidence intervals (lower, upper 95% CI).
Table 1.
Overall statistical summary from one way ANOVA for the effect of thrust duration at each level of thrust amplitude, controlled for the blocking factor of cat.
| Thrust Amplitude | ΔMIF resting | ΔMIF movement | ΔMIF position | |||
|---|---|---|---|---|---|---|
| F-Value | P | F-Value | P | F-Value | P | |
| 1mm | F7, 133 =2.24 | 0.03 | F7, 133 =0.73 | 0.65 | F7, 133 =1.31 | 0.25 |
| 2mm | F7, 133 =2.57 | 0.02 | F7, 133 =1.03 | 0.41 | F7, 133 =0.54 | 0.80 |
| 3mm | F7, 133 =5.80 | <0.001 | F7, 133 =1.81 | 0.09 | F7, 133 =0.73 | 0.65 |
| 25%BW | F7, 77 =2.73 | 0.01 | F7, 77 = 1.27 | 0.27 | F7, 77 = 1.26 | 0.28 |
| 55%BW | F7, 133 =2.64 | 0.01 | F7, 133 =0.66 | 0.71 | F7, 133 =0.69 | 0.68 |
| 85%BW | F7, 133 =5.10 | <0.001 | F7, 133 =0.80 | 0.59 | F7, 133 =0.35 | 0.93 |
Similarly, thrust duration during linear control of thrust force (Fig. 4B) had a significant effect on ΔMIFresting at all 3 force amplitudes (25%BW, p=0.01; 55%BW, p=0.01; and 85%BW, p<0.001; Table 1). For the 25%BW thrust amplitude preplanned comparisons showed that the 25ms thrust duration significantly (p=0.02) increased resting spindle discharge when compared to no thrust. For the 55%BW thrust amplitude, the 50 and 250 ms thrust duration significantly (p= 0.009 and 0.001, respectively) increased resting spindle discharge when compared to no thrust. For the 85%BW thrust amplitude, the 200ms thrust duration significantly (p < 0.001) decreased resting spindle discharge when compared to no thrust. All significant changes in resting discharge when compared to control were less than 4 imp/s.
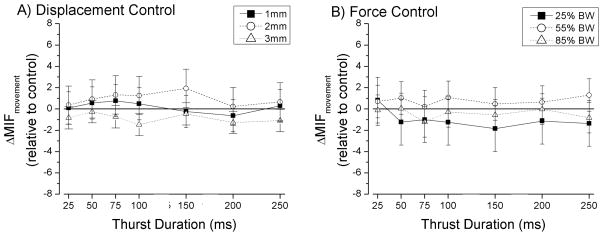
Effect of spinal manipulation on spindle responses to vertebral movement during the ramp
Regardless of the magnitude of thrust displacement (Fig. 5A), thrust duration did not affect muscle spindle responses to movement (ΔMIFmovement) of the L6 vertebra (1mm, p=0.65; 2mm, p=0.41; and 3mm, p=0.09; Table 1). Similarly, regardless of thrust force (Fig. 5B), thrust duration did not affect muscle spindle responses during the change in vertebral position (25%BW, p=0.27; 55%BW, p=0.71; and 85%BW, p=0.59; Table 1).
Figure 5.
Effect of thrust amplitude and thrust duration of a spinal manipulation on the response of lumbar paraspinal muscle spindles to vertebral movement. Data are reported as means and 95% confidence intervals (lower, upper 95% CI).
Effect of spinal manipulation on spindle responses to the new vertebral position during the hold
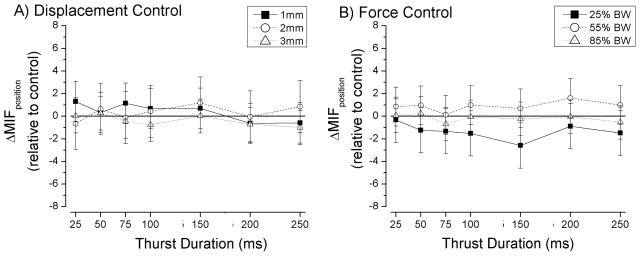
Regardless of the magnitude of thrust displacement (Fig. 6A), thrust duration did not affect muscle spindle responses to the new position of the L6 vertebra (ΔMIFposition) during the hold (1mm, p=0.25; 2mm, p=0.80; and 3mm, p=0.65; Table 1). Similarly, regardless of thrust force (Fig. 6B), thrust duration did not affect muscle spindle responses to the new static position (25%BW, p=0.28; 55%BW, p=0.68; and 85%BW, p=0.93; Table 1).
Figure 6.
Effect of thrust amplitude and thrust duration of a spinal manipulation on the response of lumbar paraspinal muscle spindles to a change in vertebral position. Data are reported as means and 95% confidence intervals (lower, upper 95% CI).
DISCUSSION
Sustained changes in sensory signaling from peripheral paraspinal tissues are often considered a possible mechanism contributing to the therapeutic effects of spinal manipulation.20–23 Such changes have been thought to arise subsequent to improved spinal biomechanics following the manipulation.20–24 The new mechanical behavior would be expected to alter load transmission to the local mechanical environment of sensory nerve endings and/or sensitized peripheral axons.35 Previous clinical and animal studies suggest that following a spinal manipulation, stiffness of the lumbar spine changes31,36 thus altering the extent of tissue strain for any given stress.
The present study is the first to our knowledge to investigate whether changes in peripheral sensory signaling persist beyond the duration of the manipulation itself. Its design enabled us to specifically investigate whether several of the mechanical features that characterize a high velocity low amplitude spinal manipulation (HVLA-SM) change the passive mechanical signaling properties of lumbar muscle spindles. The mechanical features that characterize a spinal manipulation in clinical practice are widely variable6,8–11,37 and their relationship to clinical outcomes unknown. Using a computer-controlled feedback motor we defined and controlled the variation in these features. Thrust duration and amplitude were varied whereas thrust site, thrust direction and preload were kept constant. Previous studies show that the relationship between the duration of a manipulative impulse and the discharge of muscle spindles during the manipulation is non-linear with spindle discharge becoming substantially greater when thrust duration approaches that used clinically (i.e. < 150ms).33,34,38 While thrust amplitude controlled as either force33 or length34 does not affect this pattern, lumbar muscle spindles show more sensitivity to the smaller thrust displacements (1 vs 2mm).34
Our hypothesis that an HVLA-SM’s thrust duration would be related to sustained changes in muscle spindle responsiveness was not supported. Thrust duration did not significantly affect the way spindles responded either to vertebral movement (ΔMIFmovement) or to a new vertebral position (ΔMIFposition). Thrust duration for each thrust amplitude had an overall significant effect on ΔMIFresting, but the direction in which the changes occurred was not consistent and the pattern of changes were not systematically related to thrust duration except with the 1mm thrust displacement. That resting muscle spindle discharge consistently increased at all thrust durations following specifically the 1mm HVLA-SM resembles results from a previous study34 where muscle spindles were found to be more sensitive to smaller thrust displacements (1 vs 2mm) during an HVLA-SM. It was suggested that the latter behavior arises from the non-linear properties of spindles where spindle sensitivity is markedly high to very small muscle stretches.39 Biomechanically, very small muscle stretches load both the parent muscle and spindle in the neutral zone (the start of the force-displacement curve where force increases very little for any change in displacement.)40 With little restoring force after the displacement, tissues don’t necessarily return to their original position. We speculate that following the very low thrust amplitudes of an HVLA-SM regardless of thrust duration, the spindle apparatus did not return to its original position, remaining slightly stretched following the HVLA-SM, resulting in an increase in resting spindle discharge. In contrast, we speculate that the restoring force generated by the higher thrust displacements allowed the spindle apparatus to return to its original position. While the increases in ΔMIFresting for all thrust durations were small in magnitude (less than ~4 imp/s) following the 1mm thrust displacement, lumbar muscle spindles appear to have 3–5 times greater resolution for vertebral position than those in appendicular muscle.41 Thus, even small changes in the discharge of lumbar muscle spindles could represent meaningful changes in sensory input to the central neuronal circuits following spinal manipulation. Thus spinal manipulation, low in amplitude over a wide range of thrust durations, may produce sustained and physiologically-relevant increases in resting spindle discharge to secondary neurons in the spinal cord42 following the manipulation.
The manner in which the HVLA-SM was delivered in the current experiments when compared with previous studies using a similar preparation33,34,38 more closely resembled the manner in which it is given clinically. Previously, contact with the spinous process was made by incising the skin overly the L6 vertebra and rigidly clamping forceps onto the sides of the process. The vertebra and the direction of its movement were obligatorily coupled to movement of the forceps. In the present experiment, the skin overlying the L6 vertebra was left intact and contact was made on the skin as is performed clinically. While we did not vary the preload, contact site or the thrust’s vector in the present study, these variables are being investigated.
LIMITATIONS
While mechanical aspects of the high velocity low amplitude spinal manipulation we used in this study closely resembled its clinical counterpart when delivered to a human patient lying prone, one aspect in particular was not similar. HVLA-SM is typically delivered after positioning the facet joint where slack has been removed from soft tissues and the facet joints are essentially locked or engaged at their end range of motion.1,24 This is thought to most effectively achieve the goal of gapping the facet joint, enabling it to move beyond its normal physiological range of motion without exceeding its anatomic limits.1,24 However, there is no objective measure of which we are aware for determining when this barrier is reached. In the current preparation, the range of preparatory movements needed to be limited otherwise the nerve filament could tear from the electrode. We used a contact load which ensured that the vertebra would begin to move as the manipulative thrust was applied to it (see description in Methods). However, we cannot exclude the possibility that loading toward the end range of motion might have created biomechanical changes that would have affected the passive mechanical signaling properties of the lumbar muscle spindles.
Findings in the present study were obtained using an experimental preparation that might be considered asymptomatic with the cats not clearly demonstrating a musculoskeletal condition for which spinal manipulation might typically be applied clinically. Spinal manipulation is typically delivered to patients who exhibit signs or symptoms reflective of biomechanical dysfunction.20,24,43 While there is as yet no clear measure to identify the location and presence of this dysfunction44,45 no effort was made in the present study to identify any biomechanical abnormality at the level of the manipulated vertebra. While studies show manipulation induces physiological responses, including reduced in H-reflexes46,47 and increased pain thresholds48 in asymptomatic human subjects, it remains possible that the lack of sustained changes in muscle spindle responsiveness to the HVLA reflected the lack of an initial biomechanical abnormality.
FUTURE DIRECTIONS
In addition to muscle spindles, other mechanoreceptors lie embedded in soft tissues of the lumbar spine. Studies are needed to determine whether HVLA-SM changes the signaling behavior of these paraspinal mechanoreceptors. But, muscle spindles are unique sensory structures. Not only can they transduce length changes in muscle, they are the only sensory receptor whose sensitivity can be controlled by the central nervous system. In the present study we evaluated an HVLA-SM’s ability to change their peripheral signaling behavior independent of any changes in efferent gamma-motoneuronal output an HVLA-SM might cause. The study design would not allow us to determine potential changes caused by central mechanisms because our cutting the dorsal roots prevented the inflow of sensory input and the cats were deeply anesthetized. Future work will need to determine in a centrally-intact system, if the high frequency sensory input from muscle spindles known to occur during an HVLA-SM33,34 changes the behavior of gamma motoneurons and produces sustained changes in the proprioceptive signaling from paraspinal muscle spindles.
From the perspective of clinical practice, this study and ones like it, that determine how the biomechanical characteristics of the HVLA-SM affect either clinical outcomes or the physiological systems that we currently think contribute to the therapeutic benefits of spinal manipulation, will lead to improved patient care. We specifically determined how manipulative thrust durations and amplitudes affected the responsiveness of lumbar muscle spindles before and after the manipulation. Over a range of thrust amplitudes, as thrust duration approached that used clinically, we found that an HVLA-SM manipulation did not establish a lasting influence on muscle spindle responsiveness to vertebral movement or to a new vertebral position. However, a low amplitude thrust displacement given over a wide range of thrust durations increased resting muscle spindle discharge and thus was not unique to a clinically relevant, simulated HVLA spinal manipulation. We speculate that clinically, a wide variety of thrust velocities, low in displacement amplitude may be able to change the resting behavior of paraspinal muscle spindles.
Conclusion
This study showed that relatively low amplitude thrust displacements applied during an HVLA-SM produced sustained increases in the resting discharge of paraspinal muscle spindles regardless of the duration over which the thrust was applied. However, regardless of the HVLA-SM’s thrust amplitude or duration, the responsiveness of paraspinal muscle spindles to vertebral movement and to a new vertebral position was not affected.
Acknowledgments
The authors thank Randall Sozio for technical work and Ying Cao for statistical assistance.
FUNDING SOURCES
This work was supported by NIH grant NIH U19 AT004137 to JGP and GNR and conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR15433 from the National Center for Research Resources, NIH.
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest were reported for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Bergmann TF. High-velocity low-amplitude manipulative techniques. In: Haldeman S, Dagenais S, Budgell B, Grunnet-Nilsson N, Hooper PD, Meeker WC, Triano J, editors. Principles and Practice of Chiropractic. New York: McGraw-Hill; 2005. pp. 755–766. [Google Scholar]
- 2.Triano JJ, McGregor M, Skogsbergh DR. Use of chiropractic manipulation in lumbar rehabilitation. J Rehabil Res Dev. 1997;34:394–404. [PubMed] [Google Scholar]
- 3.Triano JJ, Rogers CM, Combs S, Potts D, Sorrels K. Developing skilled performance of lumbar spine manipulation. J Manipulative Physiol Ther. 2002;25:353–361. doi: 10.1067/mmt.2002.126132. [DOI] [PubMed] [Google Scholar]
- 4.Christensen MG, Kerkhoff D, Kollasch MW, Cohn L. Job Analysis of Chiropractic. Greeley, CO: National Board of Chiropractic Examiners; 2005. [Google Scholar]
- 5.Goertz C, Pohlman KA, Vining RD, Brantingham JW, Long CR. Patient-centered outcomes of high-velocity, low-amplitude spinal manipulation for low back pain: a systematic review. J Electromyogr Kinesiol. 2012;22:670–691. doi: 10.1016/j.jelekin.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Hessell BW, Herzog W, Conway PJW, McEwen MC. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J Manipulative Physiol Ther. 1990;13:448–453. [PubMed] [Google Scholar]
- 7.van Zoest GG, Gosselin G. Three-dimensionality of direct contact forces in chiropractic spinal manipulative therapy. J Manipulative Physiol Ther. 2003;26:549–556. doi: 10.1016/j.jmpt.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Conway PJW, Herzog W, Zhang Y, Hasler EM, Ladly K. Forces required to cause cavitation during spinal manipulation of the thoracic spine. Clin Biomech. 1993;8:210–214. doi: 10.1016/0268-0033(93)90016-B. [DOI] [PubMed] [Google Scholar]
- 9.Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM. Forces exerted during spinal manipulative therapy. Spine. 1993;18:1206–1212. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Triano J, Schultz AB. Loads transmitted during lumbosacral spinal manipulative therapy. Spine. 1997;22:1955–1964. doi: 10.1097/00007632-199709010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J. 2001;1:121–130. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 12.Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed low-amplitude thoracic manipulation. Spine. 2001;26:2105–2110. doi: 10.1097/00007632-200110010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Triano J. The mechanics of spinal manipulation. In: Herzog W, editor. Clinical Biomechanics of Spinal Manipulation. New York: Churchill Livingstone; 2000. pp. 92–190. [Google Scholar]
- 14.Peterson DH. Principles of Adjustive Technique. In: Bergmann TF, Peterson DH, Lawrence DJ, editors. Chiropractic Technique. New York: Churchill Livingstone; 1993. pp. 123–196. [Google Scholar]
- 15.Bereznick DE, Ross JK, McGill SM. The frictional properties at the thoracic skin-fascia interface: implications in spine manipulation. Clin Biomech (Bristol, Avon ) 2002;17:297–303. doi: 10.1016/s0021-9290(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 16.Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 17.Vaillant M, Pickar JG, Kawchuk GN. Performance and reliability of a variable rate, force/displacement application system. J Manipulative Physiol Ther. 2010;33:585–593. doi: 10.1016/j.jmpt.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korr IM. The Neurobiologic Mechanisms in Manipulative Therapy. New York: Plenum Press; 1978. [Google Scholar]
- 19.Haldeman S. Spinal manipulative therapy; a status report. Clin Orthop. 1983;179:62–70. [PubMed] [Google Scholar]
- 20.Leach RA. The Chiropractic Theories. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 21.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2:357–371. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 23.Henderson CNR. Three neurophysiologic theories on the chiropractic subluxation. In: Gatterman MI, editor. Foundations of chiropractic: Subluxation. St. Louis: Elsevier Mosby; 2005. pp. 296–303. [Google Scholar]
- 24.Greenman PE. Principles of Manual Medicine. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- 25.Burgess PR, Wei JY. Signaling of kinesthetic information by peripheral sensory receptors. Ann Rev Neurosci. 1982;5:171–187. doi: 10.1146/annurev.ne.05.030182.001131. [DOI] [PubMed] [Google Scholar]
- 26.Gandevia SC. Kinethesia: roles for afferent signals and motor commands. I. Vol. 12. Bethesda, MD: Am. Physiol. Soc Handbook of Physiology; 1996. pp. 128–172. chapt. 4. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement Rowell, L. B. and Shephard, J. T. [Google Scholar]
- 27.Brown MC, Engberg I, Matthews PBC. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192:773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J Manipulative Physiol Ther. 2001;24:2–11. doi: 10.1067/mmt.2001.112017. [DOI] [PubMed] [Google Scholar]
- 29.Reed WR, Cao DY, Long CR, Kawchuk GN, Pickar JG. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration, and thrust rate. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2013/492039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd IA. The isolated mammalian muscle spindle. Trends Neurosci. 1980;3:258–265. [Google Scholar]
- 31.Vaillant M, Edgecombe T, Long CR, Pickar JG, Kawchuk GN. The effect of duration and amplitude of spinal manipulative therapy on the spinal stiffness. Manual Therapy. 2012 doi: 10.1016/j.math.2012.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd IA, Ward J. The response of isolated cat muscle spindles to passive stretch. J Physiol. 1969;200:104P–105P. [PMC free article] [PubMed] [Google Scholar]
- 33.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29:22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Pickar JG, Sung PS, Kang YM, Ge W. Response of lumbar paraspinal muscle spindles is greater to spinal manipulative loading compared with slower loading under length control. Spine J. 2007;7:583–595. doi: 10.1016/j.spinee.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–1955. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- 36.Fritz JM, Koppenhaver SL, Kawchuk GN, Teyhen DS, Hebert JJ, Childs JD. Preliminary investigation of the mechanisms underlying the effects of manipulation: exploration of a multivariate model including spinal stiffness, multifidus recruitment and clinical findings. Spine (Phila Pa 1976 ) 2011;36:1772–1781. doi: 10.1097/BRS.0b013e318216337d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog W. The mechanical, neuromuscular, and physiological effects produced by spinal manipulation. In: Herzog W, editor. Clinical Biomechanics of Spinal Manipulation. New York: Churchill Livingstone; 2000. pp. 191–207. [Google Scholar]
- 38.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine. 2005;30:115–122. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 39.Matthews PBC, Stein RB. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969;200:723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panjabi MM, Crisco JJ, Vasavada A, Oda T, Cholewicki J, Nibu K, Shin E. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine. 2001;26:2692–2700. doi: 10.1097/00007632-200112150-00012. [DOI] [PubMed] [Google Scholar]
- 41.Cao DY, Pickar JG, Ge W, Ianuzzi A, Khalsa PS. Position sensitivity of feline paraspinal muscle spindles to vertebral movement in the lumbar spine. J Neurophysiol. 2009;101:1722–1729. doi: 10.1152/jn.90976.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durbaba R, Taylor A, Ellaway PH, Rawlinson SR. Spinal projection of spindle afferents of the longissimus lumborum muscles of the cat. J Physiol. 2007;580:659–675. doi: 10.1113/jphysiol.2006.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatterman MI. What’s in a word? In: Gatterman MI, editor. Foundations of Chiropractic. St. Louis: Mosby; 1995. pp. 6–17. [Google Scholar]
- 44.Hestbaek L, de Leboeuf YC. Are chiropractic tests for the lumbo-pelvic spine reliable and valid? A systematic critical literature review. J Manipulative Physiol Ther. 2000;23:258–275. doi: 10.1067/mmt.2000.106097. [DOI] [PubMed] [Google Scholar]
- 45.Seffinger MA, Najm WI, Mishra SI, Adams A, Dickerson VM, Murphy LS, Reinsch S. Reliability of spinal palpation for diagnosis of back and neck pain: a systematic review of the literature. Spine. 2004;29:E413–E425. doi: 10.1097/01.brs.0000141178.98157.8e. [DOI] [PubMed] [Google Scholar]
- 46.Dishman JD, Dougherty PE, Burke JR. Evaluation of the effect of postural perturbation on motoneuronal activity following various methods of lumbar spinal manipulation. Spine J. 2005;5:650–659. doi: 10.1016/j.spinee.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J. 2011;11:440–446. doi: 10.1016/j.spinee.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu X, Wang X, Zhang J, Wang Y. Changes in pressure pain thresholds and Basal electromyographic activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: a randomized controlled trial. J Manipulative Physiol Ther. 2012;35:437–445. doi: 10.1016/j.jmpt.2012.07.001. [DOI] [PubMed] [Google Scholar]