Abstract
The neuron-specific K+-Cl− cotransporter SLC12A5, also known as KCC2, helps mediate the electrophysiological effects of GABA. The pattern of KCC2 expression during early brain development suggests that its upregulation drives the postsynaptic switch of GABA from excitation to inhibition. We previously found decreased expression of full-length KCC2 in the postmortem hippocampus of patients with schizophrenia, but not in the dorsolateral prefrontal cortex (DLPFC). Using PCR and rapid amplification of cDNA ends, we discovered several previously unrecognized alternative KCC2 transcripts in both human adult and fetal brain in addition to the previously identified full-length (NM_020708.3) and truncated (AK098371) transcripts. We measured the expression levels of four relatively abundant truncated splice variants, including three novel transcripts (ΔEXON6, EXON2B, and EXON6B) and one previously described transcript (AK098371), in a large human cohort of nonpsychiatric controls across the lifespan, and in patients with schizophrenia and affective disorders. In SH-SY5Y cell lines, these transcripts were translated into proteins and expressed at their predicted sizes. Expression of the EXON6B transcript is increased in the DLPFC of patients with schizophrenia (p = 0.03) but decreased in patients with major depression (p = 0.04). The expression of AK098371 is associated with a GAD1 single nucleotide polymorphism (rs3749034) that previously has been associated with GAD67 expression and risk for schizophrenia. Our data confirm the developmental regulation of KCC2 expression, and provide evidence that KCC2 transcripts are differentially expressed in schizophrenia and affective disorders. Alternate transcripts from KCC2 may participate in the abnormal GABA signaling in the DLPFC associated with schizophrenia.
Introduction
The neuron-specific transmembrane K+-Cl− cotransporter SLC12A5, also known as KCC2, lowers the intracellular chloride concentration below the electrochemical equilibrium potential to mediate the fast hyperpolarizing actions of inhibitory neurotransmitters such as GABA (Gamba, 2005; Blaesse et al., 2009). The rise in KCC2 expression early in postnatal development mediates the GABA switch from excitatory to inhibitory postsynaptic effects (Ben-Ari et al., 1989, 2002; Cherubini et al., 1991; Rivera et al., 1999).
Recent studies implicate KCC2 in both the genetic and neurodevelopmental etiologies of schizophrenia (Hyde et al., 2011; Kalkman, 2011). Consistent with basic studies showing that GABA receptor activity regulated expression of KCC2 (Leitch et al., 2005; Wang et al., 2006), GAD1 allelic variation associated with GAD1 expression and risk for schizophrenia also predicts the level of KCC2 expression in human brain (Hyde et al., 2011). These data suggest that the developmental aspects of GABA dysfunction linked with psychiatric disorders may involve regulation of KCC2 activity. Moreover, SLC12A5, the gene for KCC2, is located in chromosome 20q13.12, a region that shows linkage with schizophrenia (Freedman et al., 2001) and bipolar disorder (Park et al., 2004). We previously reported that KCC2 mRNA expression is significantly decreased in the hippocampus of adult patients with schizophrenia compared with nonpsychiatric controls. In contrast to the hippocampus, prefrontal cortical expression levels did not differ between diagnostic groups (Hyde et al., 2011).
Over 90% of multiexon genes, including KCC2 with 26 exons, undergo alternative splicing (Wang et al., 2008; Nilsen and Graveley, 2010). We have undertaken an extensive series of experiments in the dorsolateral prefrontal cortex (DLPFC) to identify alternative transcripts of KCC2 to delineate its potential role in brain development and psychiatric disorders. Because GABA activity plays a role in the regulation of the expression of KCC2 (Galanopoulou et al., 2003; Lee et al., 2011), we also hypothesized that genetic variation at rs3749034 in GAD1, the gene encoding the GABA synthetic enzyme GAD67, might also be associated with expression of alternative transcripts for KCC2. Genetic variation at rs3749034 in GAD1 has been associated with GAD67 expression and risk for schizophrenia in our previous studies (Addington et al., 2005; Straub et al., 2007).
Materials and Methods
Human postmortem tissue, RNA extraction, and cDNA generation.
Postmortem infant, child, adolescent, and adult brains were collected at the Clinical Brain Disorders Branch, National Institute of Mental Health (NIMH) with informed consent from the legal next of kin under NIMH protocol 90-M-0142 and processed as described previously (Lipska et al., 2006). Postmortem fetal and additional infant and child brain tissue samples were provided by the Brain and Tissue Bank for Developmental Disorders of the National Institute of Child Health and Human Development under contracts NO1-HD-4-3368 and NO1-HD-4-3383, approved by the institutional review board of the University of Maryland at Baltimore, and the tissue was donated to the NIMH under the terms of a material transfer agreement. The DLPFC gray matter was dissected using a hand-held dental drill. This tissue was dissected from the middle frontal gyrus from a 1-cm-thick coronal slab immediately anterior to the genu of the corpus callosum. Seventy-six schizophrenia patients, 20 bipolar disorder patients, 43 major depressive disorder (MDD) patients, and 127 nonpsychiatric controls were used to measure the expression of KCC2 transcripts in the DLPFC (Table 1). An additional cohort of 137 nonpsychiatric controls, spanning ages from gestational weeks 14 to 20 and from birth up to 78 years of age, was used to measure expression of selected KCC2 transcripts in DLPFC across the human life span (Table 1). Diagnoses were determined by independent reviews of comprehensive clinical materials by two psychiatrists applying DSM-IV (1994) criteria. Toxicological analysis was conducted on every subject. The nonpsychiatric controls and fetal samples had no known history of significant substance abuse, and had negative toxicological results. In addition, none of the nonpsychiatric controls had a significant neurological or psychiatric history. Positive toxicology was not an exclusion criterion for the patients with psychiatric disorders.
Table 1.
Demographic information of our human postmortem samples
| Group | Number | Sex | Race | Age (years) | PMI (h) | pH | RIN |
|---|---|---|---|---|---|---|---|
| Controls | 127 | 83M/44F | 75AA/47C/4AS/1H | 45 ± 14 | 33.2 ± 15.2 | 6.51 ± 0.28 | 8.19 ± 0.87 |
| SZ patients | 76 | 50M/26F | 43AA/31C/2H | 51 ± 17 | 39.5 ± 22.9 | 6.39 ± 0.24 | 8.10 ± 0.92 |
| BP patients | 20 | 10M/10F | 2AA/17C/1AS | 43 ± 12 | 28.0 ± 14.6 | 6.21 ± 0.17 | 8.17 ± 0.72 |
| MDD patients | 43 | 22M/21F | 9AA/32C/1AS/1H | 41 ± 17 | 37.7 ± 25.6 | 6.23 ± 0.22 | 8.16 ± 0.77 |
| Life span | 137 | 82M/55F | 78AA/54C/2AS/3H | 29 ± 23 | 25.7 ± 17.4 | 6.51 ± 0.29 | 8.34 ± 0.97 |
SZ, schizophrenia; BP, bipolar disorder; MDD, major depressive disorder; M, male; F, female; AA, African American; C, Caucasian; AS, Asian; H, Hispanic.
Dissected tissue was pulverized and stored at −80°C. RNA extraction and the reverse transcription reaction were performed as described previously (Lipska et al., 2006). In general, total RNA was extracted from 300 mg of tissue with TRIZOL Reagent (Life Technologies). The yield of total RNA was measured by the absorbance at 260 nm. RNA quality was evaluated by high-resolution capillary electrophoresis (Agilent Technologies), generating an RNA Integrity Number (RIN). Using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) and following the manufacture's protocol, 4 μg total RNA was reverse-transcribed to cDNA in a 50 μl reaction system.
Rapid amplification of 5′ cDNA ends (5′RACE) and 3′ cDNA ends (3′ RACE) for detection of KCC2 splice variants.
To identify the 5′ and 3′ ends of KCC2 transcripts in human brain, we performed 5′ RACE and 3′ RACE, using fetal and adult brain poly A+ RNA with KCC2 gene-specific antisense primers binding at exons 1, 3, 7, 9, or 18, and sense primers at exons 9, 18, 19, or 25. We used the SMART RACE cDNA Amplification Kit (Clontech) and Advantage 2 PCR Kit (Clontech) for these assays. Commercial human fetal and adult brain poly A+ RNAs (Clontech) were reverse-transcribed to cDNA by MMLV reverse transcriptase (Clontech) according to the manufacturer's protocol. The PCR amplification profile was 94°C for 2 min, 45 cycles of 94°C for 30 s, 68–72°C for 30 s, 68°C for 2–6 min, and 68°C for 10 min after the last cycle. When necessary, nested PCR was done using nested gene-specific primers and a nested universal primer A. All RACE products were cloned into pCR4-TOPO vector (Invitrogen) and sequenced.
Exon-to-exon PCR and end-to-end PCR for KCC2 splice variants.
Based on known KCC2 gene exons (GenBank: BC132668) and RACE sequence results, we designed primer pairs to amplify full-length and portions of KCC2 transcripts using Platinum TaqDNA polymerase (Invitrogen). The PCR conditions were 94°C for 2 min, 45 cycles of 94°C for 30 s, 54–60°C for 30 s, 72°C for 1–6 min, and 72°C for 10 min after last cycle. All PCR products were cloned into a pCR4-TOPO vector (Invitrogen) and sequenced.
Sequencing.
The sequencing reaction was performed using the Big Dye Terminator 3.1 (Applied Biosystems) on ABI PerkinElmer 9700 Thermal Cyclers according to the manufacturer's protocol. The sequencing reaction products were purified by SDS washing and Sephadex G-50 superfine columns (Sigma-Aldrich), and then lyophilized, resuspended in Hi-Dye formamide, and analyzed on Applied Biosystems 3100 Genetic Analyzers. The final data were read using the Applied Biosystems Sequence Scanner V1.0.
Quantitative real-time PCR and genotyping.
The expression levels of four truncated KCC2 transcripts were measured in the postmortem DLPFC samples, including 249 nonpsychiatric control subjects, 76 schizophrenia patients, 43 major depression patients, and 20 bipolar disorder patients by quantitative real-time PCR on an ABI Prism 7900 sequence detection system (Applied Biosystems). The primers and probes were designed using PRIMER EXPRESS software (Applied Biosystems) according to the unique mRNA sequence of each transcript. rs3749034 genotypes were generated as described by Straub et al. (2007).
Transfection of cultured SH-SY5Y cells with plasmid KCC2 cDNA variants.
Human neuroblastoma cells (SH-SY5Y) were cultured in RPMI 1640 medium (Invitrogen) with 10% fetal bovine serum, 2 mm l-glutamine, and 1% penicillin-streptomycin in a humidified 5% CO2 incubator at 37°C. For Western blot analysis, cells were seeded into 6-well plates at 6 × 105/well for 24 h before the transfection with antibiotic-free RPMI 1640 medium. KCC2 cDNA variants were subcloned into the mammalian expression vector pCMV-SC-NM containing an N-terminus Myc tag (Stratagene) to make c-myc fusion constructs. After the DNA was extracted from KCC2 clones, all the KCC2 cDNA variants templates were verified by direct sequencing reactions. The transfections were performed using 4 μg of plasmid DNA and 10 μl of Lipofectamine 2000 (Invitrogen) per well in 6-well plates according to the manufacturer's instructions. All four KCC2 truncated variants were transfected independently into SH-SY5Y cells. Supernatants were collected and protein concentrations were assayed using the Bradford Method (Bio-Rad). For immunocytochemistry, the transfections were done in 12-well plates with one 18-mm-diameter cover glass by 1.6 ng DNA and 2 μl Lipofectamine 2000.
Western blotting.
Transfected cells were harvested 48 h post-transfection. Cold PBS-washed cells were lysed by Mammalian Protein Extraction Reagent (M-PER, Thermo Scientific). The homogenates were centrifuged at 10,000 × g for 10 min at 4°C. Supernatants were collected and protein concentrations were measured by the Bradford Method (Bio-Rad). Twenty micrograms of each protein sample plus 4× lithium dodecyl sulfate sample buffer (Invitrogen) containing β-mercaptoethanol were boiled for 2 min and resolved on NuPage 12% Bis-Tris gels (Invitrogen). Gels were transferred to nitrocellulose membranes (Invitrogen) and then blocked in TBS containing 0.05% Tween 20 and 5% nonfat, dry milk (TBSTM) for 1 h. To detect c-Myc fusion proteins, an anti-Myc antibody (Calbiochem, Merck Chemicals) conjugated to HRP was diluted in TBSTM at a 1:5000 concentration and incubated overnight at 4°C with medium agitation. Blots were washed three times in TBST for 10 min and developed using chemiluminescence (SuperSignal West Femto, Pierce Biotechnology).
Statistical analysis.
Statistical analyses were done using STATISTICA version 7.1 (StatSoft). The outliers were defined as lying >2 SDs from the group mean for each transcript, and were removed from all subsequent analyses. Comparisons between groups were made by ANCOVA for each mRNA with diagnosis as an independent variable and sex, pH, postmortem interval (PMI), age, and RNA quality as covariates. Comparisons between patients and controls following overall ANCOVA were conducted by ANOVA on residuals that were generated by removing the variations due to the covariates sex, pH, PMI, age, and RIN. To calculate association between genotype and expression, the GAD1 single nucleotide polymorphism (SNP) at rs3749034 was defined as an independent variable and sex, pH, PMI, age, and RIN as covariates.
Results
Alternative transcripts of KCC2 in human brain
5′ RACE sequencing results showed three new start points, at exon 8, exon 9, and intron 8. Each had a similar consensus ribosome binding site (AGGAGGA) in the 5′ untranslated region followed by a potential start codon. We also observed two novel exons in the 5′ RACE experiments, including an exon between exons 6 and 7, and another exon composed of original exon 2, plus intron 2 and exon 3. The 3′ RACE experiments revealed two novel termination points, truncating at exons 8 and 10 separately with a poly A+ tail (Fig. 1). All RACE findings were confirmed by replication in at least three separate RACE reactions.
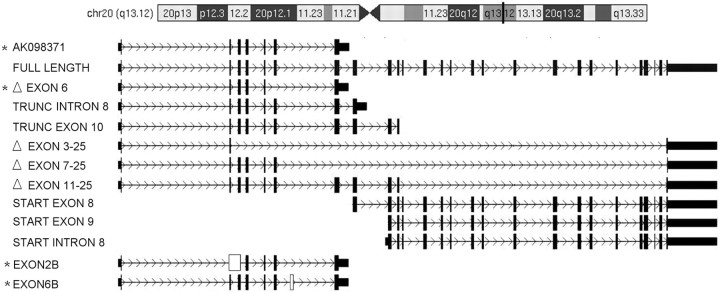
Figure 1.
Identification of alternative transcripts from SLC12A5 (KCC2). We observed three novel start points, two novel end points, four deletions, and two new exons in both adult and fetal human brain. In addition to the confirmation of the previously reported full-length KCC2 transcript (NM_020708.3) and a truncated KCC2 transcript (AK098371), we found 11 novel alternative transcripts. White rectangles, new exons; black rectangles, known exons. Stars mark the 4 truncated transcripts measured with real-time quantitative PCR. Exon deletion is indicated by Δ; for example, ΔEXON6 means deletion of exon 6. TRUNC, truncation; for example, TRUNC INTRON 8 denotes the transcript is truncated at intron 8. START, start point; for example, START EXON8 means the transcript starts at exon 8. Transcripts with a new exon were represented by its former exon name plus the letter “B.” For example, EXON2B means a transcript with a new exon 2.
To add an additional level of confirmation of the RACE results, we then performed end-to-end PCR with paired sense and antisense primers designed for each initial exon and terminal exon, using the cDNA generated from human fetal or adult brain poly A+ RNAs as a template. The PCR products were cloned into a plasmid vector and sequenced. We then aligned all sequences with the KCC2 genomic sequence (NM_020708.3) to determine gene organization and splicing patterns. In addition to confirming all RACE findings, we also observed four exon deletions, including an exon 6 deletion (ΔEXON6), exon 3–25 deletion (ΔEXON 3–25), exon 7–25 deletion (ΔEXON 7–25), and an exon 11–25 deletion (ΔEXON 11–25; Fig. 1). In total, we identified three novel start points, two novel end points, four deletions, and two new exons in human adult and fetal brain, yielding 11 novel alternative transcripts (Fig. 1). Among the 11 novel transcripts, the transcript truncated at exon 10 and the 2 transcripts with new start points (EXON 8 or EXON 9) are questionable because their sequences are completely synonymous with segments of the full-length KCC2 transcripts. All of the end-to-end PCR results were confirmed in separate PCRs and observed in at least three clones in each PCR. Among all alternative transcripts, the previously identified truncated KCC2 transcript, AK098371, showed the highest frequency in our clones. In our next experiment, we focused on AK098371 and three additional alternative transcripts of a similar structure (ΔEXON6, EXON2B, and EXON6B). Theoretically, AK098371, ΔEXON6, EXON2B, and EXON6B should code proteins with a predicted size of 285, 281, 52, and 262 aa, respectively.
Expression of proteins in transfected SH-SY5Y cells
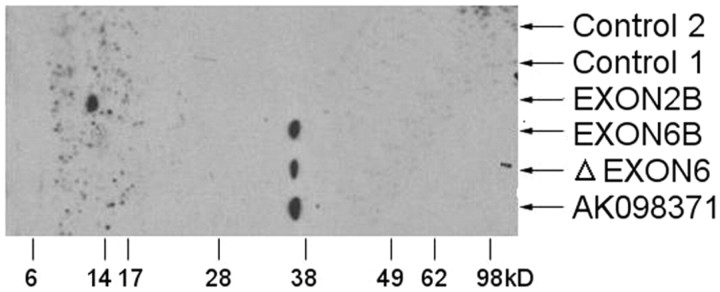
Bioinformatics suggest that the four selected truncated transcripts share the same ATG start points. EXON2B has an early stop codon TAG, and should generate a truncated 5.48 kDa protein. The other three alternative transcripts were predicted to produce truncated ∼30 kDa proteins. In our experiments, SH-SY5Y cells were transfected with a Myc-fusion construct containing AK098371, ΔEXON6, EXON2B, and EXON6B. By Western blotting with an anti-Myc antibody, we observed proteins derived from all four selected variants. The EXON2B variant was found at ∼10 kDa, while AK098371, ΔEXON6, and EXON6B were found at ∼35 kDa (Fig. 2). Their sizes were ∼5–7 kDa bigger than the theoretical molecular weight (5.48 kDa, 30.41 kDa, 30.13 kDa, and 28.06 kDa, respectively), possibly due to post-translational glycosylation and N-terminal triple Myc tags. Of note, the full-length KCC2 is a ∼140 kDa glycoprotein, which is ∼17 kDa bigger than its theoretical molecular weight (Williams et al., 1999).
Figure 2.
Four truncated KCC2 transcripts expressed in transfected SH-SY5Y cell line. The Western blot results indicate that the four alternative transcripts were expressed in transfected SH-SY5Y cells at close to their predicted size. Control 1 is the protein extracted from the nontransfected SH-SY5Y cells. Control 2 is the protein extracted from the DNA-free transfected SH-SY5Y cells.
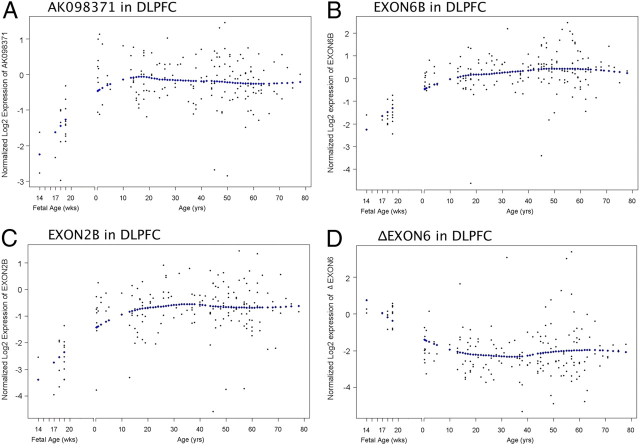
The lifespan expression pattern of the truncated alternative KCC2 transcripts
Prefrontal cortical samples from nonpsychiatric controls, ranging from the 14th through 20th gestational weeks in the fetus and from birth through 80 years of age, were used to investigate the developmental expression profile of the four selected transcripts across the human lifespan. Similar to the lifespan pattern of the full-length KCC2 transcript NM_020708.3 (Hyde et al., 2011), the expression of AK098371 (Fig. 3A), EXON6B (Fig. 3B), and EXON2B transcripts (Fig. 3C) were low in the fetal period, then increased around birth and remained expressed at relatively higher levels throughout postnatal life. However, the ΔEXON6 transcript (Fig. 3D) showed an inverse expression profile, as its expression was highest in the fetal period and then declined during development.
Figure 3.
Expression of alternative KCC2 transcripts in human DLPFC across the lifespan. Each panel shows the expression pattern of four selected alternative KCC2 transcripts across the lifespan in the human DLPFC: A, AK098371; B, EXON6B; C, EXON2B; D, ΔEXON6. ΔEXON6 shows a pattern different from the other three alternative KCC2 transcripts, as it is highest in the fetus and then declines. The x-axis before birth gestational age is in weeks (wks) and after birth the x-axis shifts to years (yrs). The expression levels on the y-axis were Log2 normalized. Each dot represents an individual subject. The diamonds indicate the best fit curve for lifetime expression.
mRNA expression of KCC2 splice variants in schizophrenia, bipolar disorder, and major depression
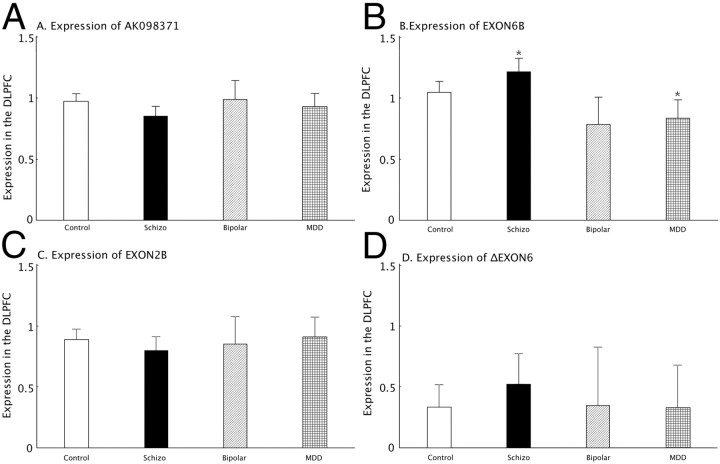
mRNA expression levels of the four selected KCC2 alternative transcripts (AK098371, ΔEXON6, EXON2B, and EXON6B) were normalized to a geometric mean of ACTB (actin, β), B2M (β-2-microglobulin), and GUSB (glucuronidase, β) and covaried by age at death, PMI, pH, RIN, and sex in an ANOVA model to test for association with psychiatric diagnosis. The geometric mean of the three housekeeping genes, used as a normalization factor, did not show diagnostic group differences (F(3,264) = 1.27, p = 0.94).
By ANCOVA, the expression of transcript EXON6B showed an overall effect of diagnosis (F(3,242) = 6.91 and p = 0.0007 after Bonferroni correction; Fig. 4B). Further analysis by ANOVA on residuals revealed that the expression of transcript EXON6B was significantly increased (16%) in schizophrenia patients (F(1,188) = 5.05, p = 0.03) and decreased (20%) in MDD patients (F(1,159) = 4.39, p = 0.04) compared with nonpsychiatric controls. There was no effect of diagnosis on AK098371 expression: (F(3,248) = 1.99; p = 0.12; Fig. 4A), EXON2B expression: (F(3,264) = 0.64; p = 0.59; Fig. 4C), or ΔEXON6 expression: (F(3,264) = 0.53; p = 0.66; Fig. 4D). We also tested whether potential confounding factors contributed to the differences in the expression between psychiatric patients and nonpsychiatric controls. In the psychiatric groups, there were no significant effects of history of substance abuse, smoking, age at the onset of the disease, age at the first hospitalization, duration of illness, and the estimated daily, lifetime, or last dose of neuroleptics on the expression of any of the four KCC2 transcripts (all F values <3.00; all p > 0.10).
Figure 4.
Alternative KCC2 transcript expression in four diagnostic groups. The expression of each alternative transcript was studied across four cohorts of subjects (nonpsychiatric controls, schizophrenia, major depressive disorder, and bipolar disorder). Each panel shows the comparison for each transcript across the groups: A, AK0983171; B, EXON6B; C, EXON2B; D, ΔEXON6. The x-axis shows the different diagnostic groups: Control, nonpsychiatric control group; Schizo, patients with schizophrenia; Bipolar, patients with bipolar disorder; MDD, the patient group with major depression. The y-axis represents the relative expression quantities in the DLPFC. The y-axis is least-squares mean of expression, computed for covariates at their means; error bars indicate 95% CI. Asterisks mark statistically significant differences between psychiatric groups and controls.
Effect of GAD1 schizophrenia risk SNP on expression of KCC2 variants
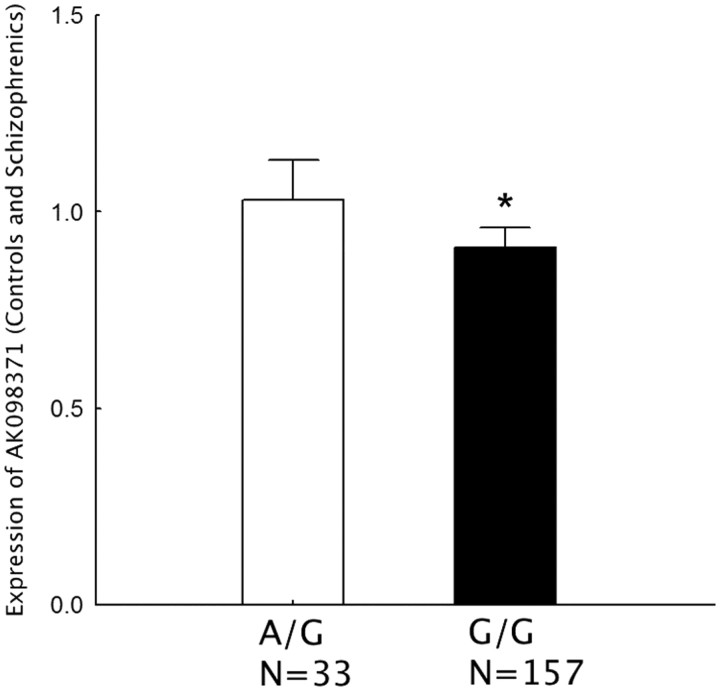
The schizophrenia risk associated SNP rs3740934 in GAD1 was significantly associated with the expression of AK098371 (F(1,182) = 5.44 and p = 0.04 after Bonferroni correction; Fig. 5) in the combined cohort of controls and schizophrenia subjects. The GG genotype of rs3749034 predicted decreased expression of AK098371 mRNA (14%) in human DLPFC in a combined cohort of the schizophrenia patients and controls, compared with the A/G genotype. On the contrary, the genotypes of rs3749034 were not associated with the expression of AK098371 in human DLPFC in the combined cohorts of affective disorders and controls (p > 0.1).
Figure 5.
Schizophrenia-associated GAD1 risk SNP rs3749034 and expression of AK097371 in human DLPFC. The different genotypes are on the x-axis. The schizophrenia-associated risk genotype is G/G. The y-axis represents relative expression levels of AK098371 in the DLPFC of a cohort combining schizophrenia patients and controls.
Discussion
Our study has identified several novel alternative transcripts in human brain derived from SLC12A5 (KCC2) with new start sites, and deletions and/or exons. We examined the expression of a subset of the truncated alternative transcripts across the lifespan in the human DLPFC and showed developmental regulation of their expression. Selected truncated transcripts were successfully translated into proteins of predicted molecular weight in cell systems. Finally, we found that the expression of selected KCC2 mRNA variants differed significantly between patients with psychiatric disorders and nonpsychiatric controls. In a previous study of the full-length KCC2 transcript in the DLPFC of patients with schizophrenia, there was no effect of diagnosis (Hyde et al., 2011). However, in the current analysis of transcript variants, the expression level of EXON6B was upregulated in the DLPFC of schizophrenia subjects. Moreover, EXON6B was expressed at lower levels in MDD subjects. These results suggest a previously unknown level of complexity of KCC2 mRNA variants both in development and in illness, with differential expression across psychiatric disorders.
Function predictions
In addition to the K+-Cl− cotransporter activity, KCC2 also plays a crucial role in the formation of mature dendritic spines and functional excitatory synapses independent of its K+-Cl− cotransporter activity (Li et al., 2007). PEST (proline, glutamic acid, serine, threonine) domains at KCC2's cytoplasmic C terminus are critical for constitutive K+-Cl− cotransport under isotonic conditions (Mercado et al., 2006). In contrast to the full-length KCC2 protein, the predicted proteins AK098371 and EXON6B lack a C terminus PEST domain, suggesting that they probably play a morphogenic role in dendrites and synapses, rather than serving as transporters. The full-length transcript from KCC2 is a factor in the maturation of dendritic spines in addition to its biophysical activities, interacting with the dendritic cytoskeleton to promote spine development (Li et al., 2007).
We cannot exclude the possibility that these alternate transcripts might exert significant effects through interference with the expression or translation of other KCC2 transcripts. Additional study of these alternate transcripts will define their role in brain development and psychiatric disorders. Follow-up studies are needed to assess the localization and levels of expression of the alternate KCC2 transcripts and proteins in human brain using in situ histochemistry hybridization and immunohistochemistry, respectively. We also plan to identify possible binding partners in cultured neurons and human brain tissue with coimmunoprecipitation assays, using validated and specific antibodies. In addition, the overexpression of these alternate KCC2 transcripts in cultured human neurons will help define their role in the morphogenesis of dendritic spines.
AK098371 and rs3749034
Current studies suggest that diminished GABA activity is involved in the pathology of schizophrenia (Costa et al., 2004; Lewis et al., 2005). We originally hypothesized that altered expression of KCC2 transcript variants in patients with schizophrenia might play a role in the pathogenesis of this disorder. This was based upon our previous study that reported lower expression of full-length KCC2 in the hippocampus of patients with schizophrenia (Hyde et al., 2011). Our whole genome expression microarray data showed a common phenomenon: different probes for the same gene had different lifespan patterns in the human DLPFC (Colantuoni et al., 2011). This study strongly suggested that multiple transcripts derived from each gene expressed in the human DLPFC frequently have expression patterns that differ across the lifespan. Initial analyses showed that expression of the full-length transcript for KCC2 was not differentially expressed in the DLPFC in schizophrenia. Nevertheless, we pursued KCC2 as a candidate in the molecular pathology of schizophrenia in the DLPFC by characterizing its full transcriptome. The next step is to examine these KCC2 splice variants in the human hippocampus.
Our original experiments compared patients with schizophrenia and nonpsychiatric controls because our initial hypothesis focused on the expression changes of splice variants in schizophrenia. The preliminary data showed that expression levels of AK098371 and EXON6B were downregulated and upregulated, respectively, in the DLPFC of patients with schizophrenia (p < 0.05, data not shown). After adding two additional diagnostic groups, bipolar disorder and MDD, into this study, the overall ANCOVA results suggested that there was no effect of diagnosis on AK098371 expression: (F(3,248) = 1.99; p = 0.12; Fig. 4A). We still tested the difference between patients with schizophrenia and nonpsychiatric controls by ANOVA on residuals because of our original hypothesis. The results showed AK098371 had lower expression in the patients with schizophrenia (12% decrease) compared with nonpsychiatric controls (p = 0.02 by ANOVA on residuals).
Abnormalities of GABA neurotransmission have been associated with mental disorders, including schizophrenia (Akbarian and Huang, 2006; Straub et al., 2007; Thompson et al., 2009) and mood disorders (Luscher et al., 2011; Sibille et al., 2011). Decreased GAD67 is one of the most highly replicated postmortem findings in patients with schizophrenia (Akbarian et al., 1995; Guidotti et al., 2000; Dracheva et al., 2004; Fatemi et al., 2005). Although levels of full-length KCC2 mRNA expression are not significantly altered in the DLPFC in schizophrenia, the levels of the truncated transcript of KCC2 AK098371 are reduced. This finding may be contextualized as an immature pattern of expression in patients with schizophrenia based on the developmental profile of AK098371 expression (low in early life stages). Moreover, the expression of the AK098371 transcript was associated with the GAD1 schizophrenia risk SNP rs3749034, as the risk genotype (G/G) predicted lower expression of AK098371 in DLPFC. A similar GAD1 SNP-expression relationship also was seen in the hippocampus for the full-length KCC2 transcript (Hyde et al., 2011). These results suggest that downregulation of AK098371 in the DLPFC may be involved in the pathology of genetic risk for schizophrenia. Therefore, it is conceivable that the GAD1 risk SNP may be linked to dysfunction of the GABA system through its role in GAD25 and GAD67 transcript expression as well as in KCC2 expression, both through the full-length KCC2 transcript (Hyde et al., 2011) and through the alternative KCC2 transcript AK098371.
Opposite changes of EXON6B
A recent meta-analysis suggested that schizophrenia and bipolar disorder have distinct, largely nonoverlapping, genomic architectures (Tang et al., 2011). Postmortem studies have shown differential expression of genes encoding neuronal ion-channel subunits in the nucleus accumbens (Str-NAc) of patients with schizophrenia, MDD, and bipolar disorder (Smolin et al., 2011). The truncated transcript EXON6B had a significantly increased expression level in the DLPFC of patients with schizophrenia compared with nonpsychiatric controls, but a decreased expression level in patients with MDD. In addition, we observed a trend toward decreased expression of this transcript in the patients with bipolar disorder compared with nonpsychiatric controls (F(1,134) = 3.33, p = 0.07). The opposite changes in expression of EXON6B suggested that this transcript behaves differently in the pathology of schizophrenia and affective disorders. However, it is also possible that these different directions in expression were driven by extraneous environmental factors, for example, different medication histories. EXON6B is a truncated transcript with a unique C-terminal; the function of the EXON6B protein is unknown. Our future experiments will focus on EXON6B protein and protein-binding partners in the CNS.
The notion that there is diagnostic specificity for expression of KCC2 expression is not just based on the EXON6B expression data. The association of the SNP rs3749034 with expression of the AK098371 transcript in patients with schizophrenia and controls was not seen in affective disorders. One possibility is that this represents an example of epistasis where the genetic background of the affective disorders is different, leading to a loss of this association of the transcript with this SNP.
Unlike in nonpsychiatric controls, positive toxicology was not an exclusion criterion for the patients with psychiatric disorders. Of course, the majority of positive toxicology reports in psychiatric cases were due to psychotropic medications. Toxicology studies also revealed the presence of a variety of illicit substances in a small subset of psychiatric cases. We cannot exclude the possibility that the exposure to these illicit substances contributed to the significant expression changes in the patients. Notably, the most important psychoactive substance discovered on toxicology was nicotine/cotinine, and this was not an exclusion criterion in any diagnostic group. Nicotine/cotinine toxicology was positive in 47.2% of psychiatric patients (17% in MDD, 68% in schizophrenia, and 53% in bipolar disorder) and 27% in controls. We consequently considered nicotine exposure as a potential confound for expression changes of EXON6B. There was no significant effect of nicotine exposure on EXON6B expression among the four groups (F(1,238) = 0.35, p = 0.56), or among the three psychiatric diagnostic groups (F(1,124) = 0.32, p = 0.57). Nonpsychiatric controls were excluded if toxicology was positive for psychotropic medications or illicit substances not including nicotine/cotinine (Hyde et al., 2011).
Accumulated evidence suggests that the GABAergic system is associated with neurodevelopmental psychiatric disorders such as schizophrenia (Levitt et al., 2004; Lewis et al., 2005; Sanacora and Saricicek, 2007; Craddock et al., 2010; Breuer et al., 2011). Our results highlight the complexity of KCC2 transcription in the human DLPFC across normal human development and suggest that two truncated transcripts may be involved in the pathophysiology of schizophrenia, and perhaps affective disorders.
Footnotes
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health. We thank Amy Deep-Soboslay, M.Ed., and Llewellyn B. Bigelow, M.D., of the Clinical Brain Disorders Branch, Genes, Cognition and Psychosis Program, Intramural Research Program, and NIMH for their efforts in clinical diagnosis and demographic characterization, and Vesna Imamovic, Yeva Snitkovsky, Jewell King, Jonathan Sirovatka, and Liqin Wang, M.D., for their excellent technical assistance. Special gratitude also is extended to H. Ronald Zielke, Ph.D., Robert D. Vigorito, M.S., P.A., and Robert M. Johnson, B.S., of the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders at the University of Maryland for their provision of fetal, child, and adolescent brain specimens for this study.
References
- Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Rapoport JL, Straub RE. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Ed 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Breuer R, Hamshere ML, Strohmaier J, Mattheisen M, Degenhardt F, Meier S, Paul T, O'Donovan MC, Mühleisen TW, Schulze TG, Nöthen MM, Cichon S, Craddock N, Rietschel M. Independent evidence for the selective influence of GABA(A) receptors on one component of the bipolar disorder phenotype. Mol Psychiatry. 2011;16:587–589. doi: 10.1038/mp.2010.67. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT, Colantuoni EA, Elkahloun AG, Herman MM, Weinberger DR, Kleinman JE. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L, Veldic M. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, Caesar S, Gordon-Smith K, Fraser C, Russell E, Norton N, Breen G, St Clair D, Collier DA, Young AH, Ferrier IN, et al. Strong genetic evidence for a selective influence of GABAA receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76:581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Hossein Fatemi S, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Olincy A, Kaufmann CA, Malaspina D, Cloninger CR, Svrakic D, Faraone SV, Tsuang MT. Evidence for the multigenic inheritance of schizophrenia. Am J Med Genet. 2001;105:794–800. doi: 10.1002/ajmg.10100. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, DiGiorgi Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkman HO. Alterations in the expression of neuronal chloride transporters may contribute to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:410–414. doi: 10.1016/j.pnpbp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABA(A) receptor-mediated currents. Nat Neurosci. 2011;14:736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch E, Coaker J, Young C, Mehta V, Sernagor E. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J Neurosci. 2005;25:4801–4805. doi: 10.1523/JNEUROSCI.0172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li H, Khirug S, Cai C, Ludwig A, Blaesse P, Kolikova J, Afzalov R, Coleman SK, Lauri S, Airaksinen MS, Keinänen K, Khiroug L, Saarma M, Kaila K, Rivera C. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado A, Broumand V, Zandi-Nejad K, Enck AH, Mount DB. A C-terminal domain in KCC2 confers constitutive K+-Cl- cotransport. J Biol Chem. 2006;281:1016–1026. doi: 10.1074/jbc.M509972200. [DOI] [PubMed] [Google Scholar]
- Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, Nee J, Grunn A, Kanyas K, Lerer B, Endicott J, Gilliam TC, Baron M. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolin B, Karry R, Gal-Ben-Ari S, Ben-Shachar D. Differential expression of genes encoding neuronal ion-channel subunits in major depression, bipolar disorder and schizophrenia: implications for pathophysiology. Int J Neuropsychopharmacol. 2011;19:1–14. doi: 10.1017/S1461145711001428. [DOI] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Tang B, Thornton-Wells T, Askland KD. Comparative linkage meta-analysis reveals regionally-distinct, disparate genetic architectures: application to bipolar disorder and schizophrenia. PLoS One. 2011;6:e19073. doi: 10.1371/journal.pone.0019073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang H, Gong N, Xu TL. Changes of K+ -Cl- cotransporter 2 (KCC2) and circuit activity in propofol-induced impairment of long-term potentiation in rat hippocampal slices. Brain Res Bull. 2006;70:444–449. doi: 10.1016/j.brainresbull.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem. 1999;274:12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]