Abstract
Objectives
The effect of antiviral therapy on clinical outcomes in chronic hepatitis B virus (HBV) is not established. We aimed to assess the effects of interferon and/or nucleos(t)ide analogues versus placebo or no intervention on prevention of hepatocellular carcinoma (HCC) and mortality in chronic HBV.
Design
Random-effects pairwise meta-analysis of randomised trials and observational studies.
Setting
Electronic and manual searches were combined. Randomised controlled trials (RCTs) were included in the primary analyses. Observational studies were included in sensitivity analyses.
Primary and secondary outcome measures
The primary outcome measures were HCC incidence and mortality. The secondary outcome measure was HCC mortality.
Results
We included 8 RCTs, 8 prospective cohort studies and 19 case–control studies with a total of 3433 patients allocated to antiviral therapy and 4625 controls. The maximum duration of follow-up was 23 years. Randomised trials found no effect of antiviral therapy on HCC or mortality. Cohort studies found that antiviral therapy increased the risk of HCC (risk ratio 1.43; 95% CI 1.06 to 1.95), whereas case–control studies found a decreased risk of HCC in the intervention group (risk ratio 0.69; 95% CI 0.54 to 0.88). There was a clear difference between the results of RCTs and observational studies (test for subgroup differences, p<0.001). Antiviral therapy did not affect mortality in cohort studies, but reduced mortality in case–control studies (relative risk 0.71; 95% CI 0.54 to 0.93; test for subgroup differences, p=0.406).
Conclusions
The effect of antiviral therapy on clinical outcomes in HBV remains to be established. Although there was a positive effect in the sensitivity analyses, the strength of the evidence does not allow for extrapolation to clinical practice as research design plays an essential role in the overall assessment.
Trial registration number
Prospero number CRD42013003881.
Article summary.
Article focus
The effect of antiviral treatment for chronic hepatitis B has been assessed using surrogate markers.
An evaluation of the effect on hepatocellular carcinoma and mortality is missing.
Key messages
Research design plays an essential role on the hepatocellular carcinoma incidence estimates. As prospective cohorts and case–control series show opposing results, the reports from such trials should be interpreted with caution.
Sensitivity analyses show a positive effect of treatment on mortality.
Strengths and limitations of this study
A large number of observational studies were included that allowed for detailed sensitivity analyses with tests for subgroup differences.
Only eight randomised controlled trials were included.
The effect of modern nucleos(t)ides could not be assessed as newer trials do not include placebo-treated or untreated patients in the control groups.
Introduction
Worldwide, two billion people have been infected with hepatitis B virus (HBV). Chronic HBV may lead to hepatocellular carcinoma (HCC), cirrhosis and liver failure, and each year about 600 000 people die due to hepatitis.1–3 Globally, HCC is the fifth most common cause of cancer deaths in men, and the sixth in women.4–6 Vaccine programmes have decreased the incidence of HBV,7 8 but mortality from HBV-related HCC and cirrhosis is increasing due to the high prevalence of chronically infected patients.9 10 The aim of antiviral treatment is to prevent progression to these clinical outcome measures.11–13 Recommended treatments include interferon and nucleos(t)ide analogues (NA).14 15 A viral response may reduce the risk of HCC,12 but the results of clinical studies and meta-analyses on antiviral therapy are not consistent.16–24 One meta-analysis25 found that antiviral therapy decreased liver-related mortality, whereas a cohort series found decreased overall mortality in patients with a viral response to interferon.26 On the other hand, randomised controlled trials (RCTs) have failed to show an effect on HCC or mortality.27 28 We therefore conducted a systematic review of the evidence on antiviral treatment for prevention of HCC and mortality in patients with HBV.
Methods
Scope
This systematic review evaluates the effects of antiviral therapy versus placebo or no intervention on prevention of HCC and mortality in patients with HBV. The review is based on a registered written protocol (Prospero number CRD42013003881) according to the methods specified in the Cochrane Handbook for Reviews on Interventions29 and the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies.30 For a more detailed description of the methods, please see the MOOSE checklist.
Data sources
Eligible trials were identified through electronic and manual searches. Electronic searches were performed in MEDLINE (1966–2012), EMBASE (1928–2012) and Web of Science (1900–2012). Literature searches included keywords for HCC, chronic HBV and antiviral treatment. Manual searches included scanning of reference lists in relevant papers and conference proceedings and the International Clinical Trials Registry Platform.
Study selection
Our primary analyses included RCTs (primary analyses) on antiviral interventions (interferon and/or NA) versus placebo or no intervention for patients with HBV who had not previously received antiviral therapy (treatment naïve). Owing to the expected prognosis and the duration of follow-up necessary to evaluate intervention effects on clinical outcome measures in HBV, observational studies were included in sensitivity analyses. The primary outcome measures were HCC diagnosed using recommended criteria31 32 and all-cause mortality. To avoid prevalent cases of HCC, the outcomes were assessed after at least 12 months of follow-up. Some studies did not perform screening ultrasonography and would therefore not detect small HCC present at inclusion. Hence, 12 months was chosen as a limit. The secondary outcome measure was HCC-related mortality.
Data extraction and quality assessment
Two authors extracted data independently. When data were not available in the published reports, additional information was retrieved through correspondence with the primary investigators.
The Cochrane Collaboration's Tool for Assessing Risk of Bias was used to evaluate bias control in RCTs. The assessment included the randomisation methods (allocation sequence generation and allocation concealment), blinding (of participants, personnel and investigators), completeness of outcome data, reporting of data and other biases.33 All observational studies were classed as having a high risk of bias. Based on the MOOSE guidelines, the assessment of potential sources of bias within observational studies included documentation of how data were classified and coded (multiple raters, blinding and inter-rater reliability), assessment of confounding (comparability of cases and controls in studies where appropriate) and blinding of quality assessors, stratification or regression on possible predictors of study results.
Data synthesis and analysis
Statistics were performed using Stata V.12 (Statacorp, College Station, Texas, USA) and Trial Sequential Analysis (CTU, Copenhagen, Denmark). Meta-analyses were performed with results expressed as risk ratios, 95% CI and I2 as a marker of heterogeneity. For meta-analyses showing a statistically significant effect, the number needed to treat was calculated based on the risk difference. Initial sensitivity analyses included repeating all meta-analyses using both random and fixed effect models. The results of these analyses were only reported if the conclusions differed. Regression analyses were performed to assess for publication bias and other small-study effects (Egger's test). Sequential analyses were performed for meta-analyses showing an intervention effect after adjusting for the risk of bias associated with cumulative testing.34 The sequential analysis was performed using a random effects model, α (5%), power (80%) and the incidence rates and the intervention effects identified in the meta-analyses. Preplanned sensitivity analyses were performed with the inclusion of observational studies. These analyses were performed stratified by study design (RCT, prospective cohort or case–control study) and with fixed-effect inverse variance models that compared the results of subgroups. The result of the subgroup comparisons was expressed as p values (test for subgroup differences). Additional sensitivity analyses were performed to evaluate the influence of bias control (limiting the analysis to trials with adequate randomisation), the type of antiviral therapy (comparing interferon, NA or both) and the effect of HCC screening (comparing the results of trials with or without screening). Finally, subgroup analyses including only patients with cirrhosis were performed.
Results
Literature searches and study inclusion
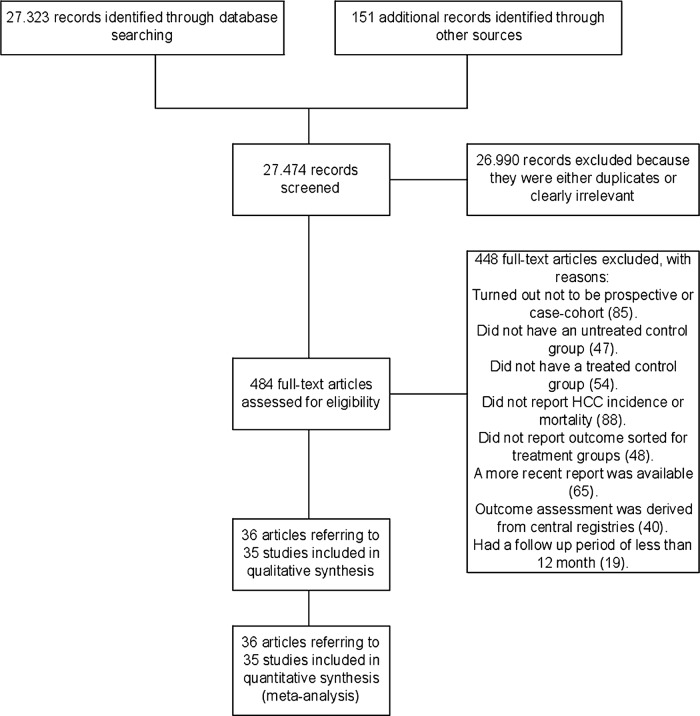
The electronic and manual searches identified 27 474 potentially relevant records (figure 1). After excluding duplicates and studies that did not fulfil our inclusion criteria, 36 references referring to 8 RCTs, 8 prospective cohort studies and 19 case–control studies were included.26–28 35–67
Figure 1.
Flow diagram of the study.
Characteristics of the included RCTs and observational studies
The RCTs were conducted in Europe (n=4), Asia (n=2) and Africa (n=2). The duration of follow-up ranged from 1 to 11 years. One trial performed HCC screening. Six trials assessed interferon and two trials focused on NA (table 1). A total of 840 patients received antiviral therapy and 447 patients received placebo or no intervention. The proportion of men ranged from 70% to 100% and the mean age ranged from 33 to 44 years. The proportion of patients with cirrhosis at inclusion ranged from 0% to 66% (table 2). The proportion of patients with a virological response ranged from 7% to 58% in the treatment group and from 1% to 22% in controls. A biochemical response was achieved in 14–66% of patients in the treatment group and in 1–20% of controls. The randomisation methods were described as adequate in three trials (table 3).
Table 1.
Characteristics of trials
| Study, year (reference) | Country of origin | Intervention (dose) | Number of patients | Follow-up (mean/median year) | HCC screening (yes/no) | Outcomes reported |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Anderson et al, 198735 | England | IFN (2.5–7.5 MU/m2/d) | I 14 C 16 |
I 1.0 C 1.0 |
No | Overall mortality |
| Chan et al, 200739 | China | Lamivudine (100 mg/d) | I 89 C 47 |
I 2.5 C 2.5 |
No | HCC incidence |
| Farci et al, 200443 | Italy | IFN (3–9 MU/×3w) | I 28 C 10 |
I 10.8 C 10.8 |
No | Overall mortality |
| Krogsgaard, 199828 | Europe | IFN (1.5–18 MU/×3w) | I 210 C 98 |
I 1.3 C 1.3 |
No | HCC incidence and mortality |
| Liaw et al, 200427 | Asia | Lamivudine (100 mg/d) | I 436 C 215 |
I 2.7 C 2.7 |
Yes | HCC incidence Overall mortality |
| Mazzella et al, 199953 | Italy | IFN (648 MU total) | I 33 C 31 |
I 7.2 C 6.6 |
No | HCC incidence and mortality |
| Robson et al, 199256 | South Africa | IFN (10 MU/×3w) | I 10 C 10 |
I 1.4 C 1.4 |
No | Overall and HCC mortality |
| Waked et al, 199062 | Egypt | IFN (5 MU/m2/×3w–5 MU/m2/d) | I 20 C 20 |
I 1.3 C 1.3 |
No | Overall and HCC mortality |
| Prospective cohorts | ||||||
| Benvegnu et al, 199836 | Italy | IFN (5–10 MU/×3w) | I 13 C 24 |
I 6.0 C 6.0 |
Yes | HCC incidence Overall mortality |
| Brunetto et al, 200238 | Italy | IFN (9 MU/×3w) | I 103 C 61 |
I 6.0 C 6.0 |
Yes | Overall mortality |
| Chan et al, 201240
Wong et al, 201063 |
China | Nucleos(t)ides IFN (NS) | I 158 C 1271 |
I 10.1 C 10.1 |
Yes | HCC incidence, overall and HCC mortality |
| Di Marco et al, 199942 | Italy | IFN (NS) | I 109 C 193 |
I 7.8 C 7.8 |
Yes | Overall mortality |
| Ma et al, 200849 | China | Nucleos(t)ides (NS) | I 41 C 176 |
I 2.9 C 2.9 |
Yes | Overall mortality |
| Mazzella et al, 199652 | Italy | IFN (10 MU/×3w) | I 34 C 28 |
I 4.1 C 4.0 |
Yes | HCC incidence |
| Papatheodoridis et al, 200155 | Greece | IFN (3 MU/×3w) | I 209 C 152 |
I 6.0 C 6.1 |
Yes | HCC incidence, overall and HCC mortality |
| Tong et al, 200659 | USA | IFN (NS) | I 22 C 378 |
I 7.0 C 7.0 |
Yes | HCC incidence, overall and HCC mortality |
| Case–control series | ||||||
| Bolukbas et al, 200637 | Turkey | Lamivudine (100 mg/d) | I 23 C 15 |
I 1.5 C 2.0 |
Yes | Overall and HCC mortality |
| Das et al, 201041 | India | Lamivudine Adefovir (NS) | I 151 C 102 |
I 4.0 C 3.8 |
Yes | HCC incidence, overall and HCC mortality |
| Fattovich et al, 199744 | Europe | IFN (36 MU to >300 MU total) | I 40 C 50 |
I 6.2 C 6.2 |
No | HCC incidence, overall and HCC mortality |
| IIHCSG, 199845 | Italy and Argentina | IFN (9 MU/w) | I 49 C 97 |
I 5.8 C 6.9 |
Yes | HCC incidence |
| Ikeda et al, 199846 | Japan | IFN (6 MU/×2w) | I 94 C 219 |
I 6.8 C 7.0 |
Yes | HCC incidence |
| Lin et al, 200147 | China | IFN (5 MU/×3w) | I 30 C 28 |
I 2.7 C 2.6 |
No | HCC incidence, overall and HCC mortality |
| Lin et al, 200748 | China | IFN (6–9 MU/m2/×3w) | I 233 C 233 |
I 6.8 C 6.1 |
Yes | HCC incidence and mortality |
| Mahmood et al, 200550 | Japan | IFN (6 MU/d) | I 23 C 68 |
I 7.0 C 7.0 |
Yes | HCC incidence |
| Manolakopoulos et al, 200426 | Greece | Lamivudine (100 mg/d) | I 30 C 30 |
I 1.5 C 1.8 |
Yes | HCC incidence, overall and HCC mortality |
| Matsumoto et al, 200551 | Japan | Lamivudine (100 mg/d) | I 508 C 231 |
I 2.7 C 5.3 |
No | HCC incidence |
| Niederau et al, 199654 | Germany | IFN (2–5 MU/×3w) | I 103 C 53 |
I 4.2 C 3.2 |
No | Overall mortality |
| Romeo et al, 200957 | Italy | Lamivudine (NS) IFN (6–9 MU) |
I 102 C 135 |
I 22.4 C 16.5 |
Yes | HCC incidence, overall and HCC mortality |
| Tangkijvanich et al, 200158 | Thailand | IFN (3–6 MU/×3w) | I 67 C 72 |
I 4.9 C 4.9 |
Yes | HCC incidence |
| Tong et al, 200960 | USA | Lamivudine (NS) | I 27 C 101 |
I 5.3 C 5.3 |
Yes | HCC incidence and mortality |
| Truong et al, 200561 | Japan | IFN (174–687 MU total) | I 27 C 35 |
I 7.0 C 6.2 |
Yes | HCC incidence and mortality |
| Yuen et al, 200164 | China | IFN (2.5–10 MU/m2/×3w) | I 208 C 203 |
I 8.9 C 9.0 |
Yes | HCC incidence and mortality |
| Yuen et al, 200465 | China | IFN (NS) | I 6 C 86 |
I 10.5 C 10.5 |
No | HCC incidence |
| Yuen et al, 200766 | China | Lamivudine (100 mg/d) | I 142 C 124 |
I 7.5 C 9.0 |
Yes | HCC incidence |
| Zampino et al, 200967 | Italy | IFN (5 MU/m2/×3w) | I 41 C 13 |
I 23 C 23 |
No | HCC incidence |
/d, Daily; /×3w, thrice weekly; C, control; HCC, hepatocellular carcinoma; I, intervention; IFN, interferon; MU, million units; NS, not stated.
Table 2.
Patient characteristics in included trials
| Study, year (reference) | Median/mean age (years) | Proportion of men (%) | Proportion with cirrhosis (%) | Proportion with elevated ALT (%) | Proportion positive for HBeAg (%) | HBeAg seroconverters (n, %) |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Anderson et al, 198735 | I 36 C 35 |
100 | 20 | 77 | 100 | I 2, 14 C 0, 0 |
| Chan et al, 200739 | I 39 C 39 |
84 | 16 | 77 | 5 | NS |
| Farci et al, 200443 | I 35 C 38 |
83 | 66 | 100 | 2 | I NA C 1, 100 |
| Krogsgaard, 199828 | I 36 C 36 |
81 | 19 | 100 | 100 | NS |
| Liaw et al, 200427 | I 43 C 44 |
85 | 33 | 78 | 58 | NS |
| Mazzella et al, 199953 | I 36 C 41 |
78 | 0 | 100 | 100 | I 30, 91 C 19, 61 |
| Robson et al, 199256 | I 33 C 31 |
70 | NS | 100 | 100 | I 5, 50 C 1, 10 |
| Waked et al, 199062 | I 35 C 35 |
78 | 40 | 100 | 100 | I 13, 81 C 5, 33 |
| Prospective cohorts | ||||||
| Benvegnu et al, 199836 | I 57 C 60 |
65 | 100 | NS | NS | NS |
| Brunetto et al, 200238 | I 40 C 40 |
80 | 38 | NS | 0 | NA |
| Chan et al, 201240
Wong et al, 201063 |
NS | 67 | 32 | 87 | NS | NS |
| Di Marco et al, 199942 | I 33 C 35 |
71 | 29 | 100 | 29 | I 35, 32 C 29, 15 |
| Ma et al, 200849 | I 54 C 54 |
72 | 100 | NS | 24 | NS |
| Mazzella et al, 199652 | I 48 C 49 |
73 | 100 | NS | NS | NS |
| Papatheodoridis et al, 200155 | I 47 C 49 |
83 | 31 | 100 | 0 | NA |
| Tong et al, 200659 | I 48 C 48 |
71 | 35 | NS | 49 | NS |
| Case–control series | ||||||
| Bolukbas et al, 200637 | I 45 C 46 |
82 | 100 | NS | 0 | NA |
| Das et al, 201041 | I 42 C 46 |
91 | 100 | NS | 45 | I 12, 13 C 3, 10 |
| Fattovich et al, 199744 | I 47 C 45 |
87 | 100 | 100 | 100 | I 27, 68 C 30, 60 |
| IIHCSG, 199845 | I 54 C 54 |
64 | 100 | NS | NS | NS |
| Ikeda et al, 199846 | I 41 C 44 |
79 | 100 | NS | 52 | NS |
| Lin et al, 200147 | I 39 C 41 |
95 | 10 | 100 | 0 | NA |
| Lin et al, 200748 | I 32 C 31 |
94 | 9 | NS | 100 | I 115, 49 C 86, 37 |
| Mahmood et al, 200550 | I 49 C 49 |
69 | 100 | NS | 36 | NS |
| Manolakopoulos et al, 200426 | I 65 C 63 |
80 | 100 | 100 | 0 | NA |
| Matsumoto et al, 200551 | I 42 C 41 |
73 | 18 | NS | 55 | NS |
| Niederau et al, 199654 | I 40 C 41 |
78 | 28 | 100 | 100 | I 53, 51 C 7, 13 |
| Romeo et al, 200957 | NS | 77 | 35 | NS | 27 | NS |
| Tangkijvanich et al, 200158 | I 37 C 40 |
72 | 20 | NS | 100 | I 24, 36 C 7, 10 |
| Tong et al, 200960 | I 46 C 46 |
86 | 100 | 14 | 53 | NS |
| Truong et al, 200561 | I 33 C 37 |
53 | 2 | 100 | 60 | I 9, 53 C 11, 55 |
| Yuen et al, 200164 | I 27 C 28 |
64 | NS | 32 | 100 | I 96, 46 C 93, 46 |
| Yuen et al, 200465 | I 43 C 43 |
71 | NS | NS | 23 | NS |
| Yuen et al, 200766 | I 34 C 33 |
74 | 0 | 48 | 100 | NS |
| Zampino et al, 200967 | NS | 67 | 0 | NS | 54 | I 16, 62 C NS |
ALT, alanine aminotransferase; C, Control; HBeAg, hepatitis B e antigen I, Intervention; NA, not applicable; NS, not stated;.
Table 3.
Risk of bias summary
| Study, year (reference) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Anderson et al, 198735 | ? | ? | + | + | ? | ? |
| Chan et al, 200739 | + | + | + | + | ? | ? |
| Farci et al, 200443 | + | + | + | + | ? | ? |
| Krogsgaard, 199828 | ? | ? | + | + | − | ? |
| Liaw et al, 200427 | + | + | + | + | + | + |
| Mazzella et al, 199953 | ? | ? | + | + | + | ? |
| Robson et al, 199256 | ? | ? | + | + | ? | ? |
| Waked et al, 199062 | ? | ? | + | + | ? | ? |
+, Low risk of bias; −high risk of bias; ?, unknown risk of bias.
The prospective cohorts and case–control studies were conducted in Europe (n=12), Asia (n=13), North America (n=1) and South America (n=1). The duration of follow-up ranged from 2 to 23 years. HCC screening was performed in all prospective cohort studies and in 13 of the case–control studies. In total, 18 studies assessed interferon, 7 assessed NA and 2 combined therapy with interferon and NA (table 1). A total of 2593 patients received antiviral therapy and 4178 patients received no intervention. The proportion of men ranged from 53% to 95% and the mean age ranged from 27 to 65 years. The proportion of patients with cirrhosis ranged from 0% to 100% (table 2). In the prospective cohorts, the proportion of patients with a virological response in the treatment and control groups was 23–69% and 0–23%, respectively. A biochemical response was achieved for 23–69% of patients in the treatment groups and 31% in the control group (only reported in 1 study). In the case–control series, the proportion of patients with a virological response in the treatment and control groups ranged from 7% to 78% and 2% to 100%, respectively. A biochemical response in the two groups was 27–68% and 4–51%, respectively.
Prevention of HCC
HCC was diagnosed in 22 of 768 patients in the treatment group versus 19 of 391 controls (relative risk 0.58, 95% CI 0.32 to 1.07; I2=0%). There was no evidence of small-study effects (Egger's test, p=0.269) and no difference between subgroups of trials assessing interferon or NA (test for subgroup differences, p=0.854). The overall result was confirmed in sensitivity analyses including RCTs with a low risk of bias and trials with HCC screening.
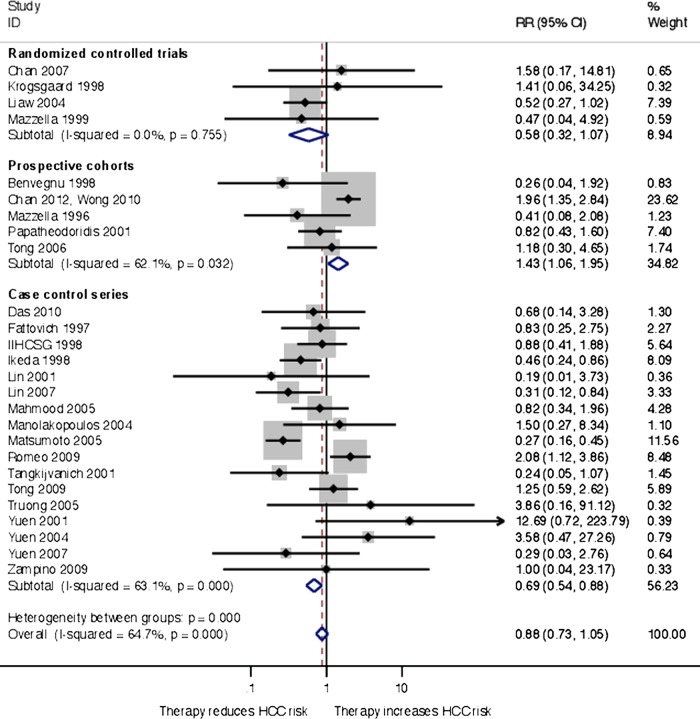
Sensitivity analyses including prospective cohort studies and case–control studies were performed. In the cohort studies, HCC was diagnosed in 51 of 436 patients in the treatment group and 174 of 1853 patients in the control group. In the case–control studies, the numbers were 99 of 1778 and 201 of 1827 patients, respectively. A meta-analysis that combined RCTs and observational studies found no effect of antiviral therapy on HCC (relative risk 0.88, 95% CI 0.73 to 1.05; I2=63%). There was no evidence of small-study effects (Egger's test, p=0.730). Subgroup analyses showed a clear difference between the RCTs, prospective cohorts and case–control studies (test for subgroup differences, p<0.001; figure 2). The prospective cohort studies found that antiviral therapy increased the risk of HCC (relative risk 1.44, 95% CI 1.06 to 1.95), whereas the case–control studies found that antiviral therapy reduced the risk of HCC (relative risk 0.69, 95% CI 0.54 to 0.88). Owing to the high heterogeneity, a post hoc meta-regression analysis was performed. We evaluated the study and patient characteristics not accounted for in the sensitivity analyses, which may have influenced the result. No modifiers were found when adjusting for the following variables: proportion of men (coefficient, −0.074; p=0.08), mean age of treated patients at inclusion (coefficient, 0.020; p=0.94), mean age of untreated patients at inclusion (coefficient, 0.121; p=0.65), proportion with cirrhosis at inclusion (coefficient, −0.001; p=0.76) and region of trial (coefficient −0.394; p=0.55).
Figure 2.
Random-effects inverse variance meta-analysis of antiviral therapy treatment effects on hepatocellular carcinoma in patients with chronic hepatitis B, subgroups according to trial design.
To further evaluate the influence of bias on the overall results, we performed additional subgroup analysis in which trials were stratified for HCC screening. The analysis found 8 trials that did not perform HCC screening (relative risk 0.40, 95% CI 0.26 to 0.63) and 18 trials that did perform HCC screening (relative risk 1.03, 95% CI 0.84 to 1.25). The results of subgroups were clearly different (test for subgroup differences, p<0.001).
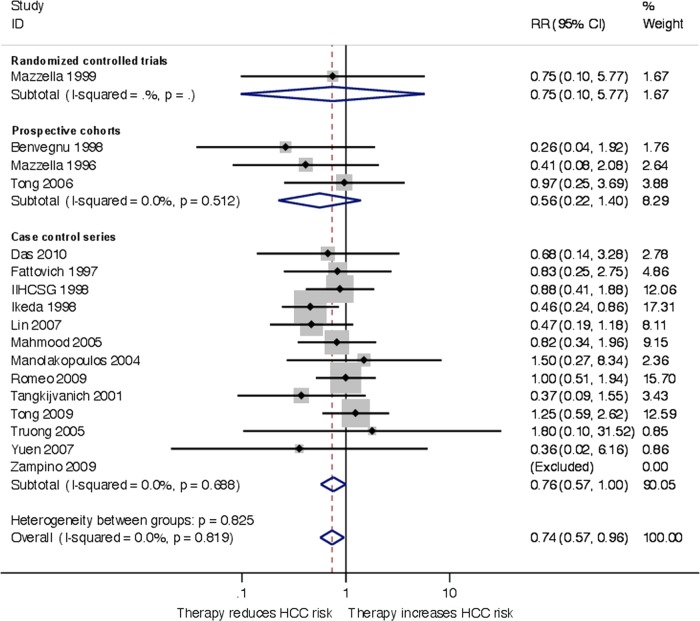
Sensitivity analyses were performed to evaluate the risk of HCC among patients with cirrhosis. In the RCTs, 1 of 20 patients in the treatment group and 2 of 12 controls developed HCC (relative risk 0.75, 95% CI 0.10 to 5.77). In the prospective cohort studies, 32 of 184 vs 142 of 482 patients developed HCC, whereas the numbers were 63 of 680 vs 161 of 955, respectively, for case–control studies. Overall, antiviral therapy reduced the risk of HCC when including data from RCTs and observational studies (relative risk 0.74, 95% CI 0.57 to 0.96, I2=0%, number needed to treat 28 patients; figure 3). The results of RCTs and observational studies were similar (test for subgroup differences, p=0.159). There was no evidence of small-study effects (Egger's test, p=0.890). In the trial sequential analysis, the monitoring and α-spending boundaries did not cross, suggesting that the result was not robust to adjustment for multiple testing.
Figure 3.
Random-effects inverse variance meta-analysis of antiviral therapy treatment effects on hepatocellular carcinoma in patients with chronic hepatitis B and cirrhosis, subgroups according to trial design.
Mortality
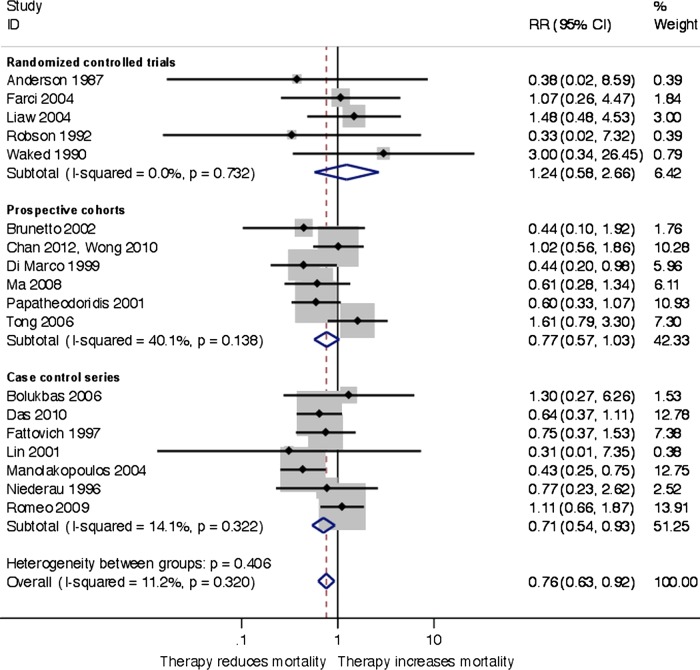
In the RCTs, there was no difference in mortality between the treatment and control groups (21 of 508 vs 9 of 271 patients, relative risk 1.24, 95% CI 0.58 to 2.66; I2=0%). There was no evidence of small-study effects (Egger's test, p=0.783) and no difference between trials stratified by treatment (test for subgroup differences, p=0.668) and HCC screening (p=0.828). In the observational studies, the number of patients in the treatment and control groups who died was 51 of 655 vs 247 of 2231 for prospective cohort studies and 79 of 506 vs 92 of 413 in the cohort studies. When combining RCTs and observational studies, random-effects meta-analysis showed that antiviral treatment decreased mortality (relative risk 0.76, 95% CI 0.62 to 0.95, I2=14%, number needed to treat 77; Egger's test, p=0.487; figure 4). There was no difference between RCTs and observational studies (test for subgroup differences, p=0.406). In the trial sequential analysis, the monitoring boundary crossed the α-spending boundary in 2004, suggesting that the meta-analysis was robust to adjustments for multiple testing.
Figure 4.
Random-effects inverse variance meta-analysis of antiviral therapy treatment effects on mortality in patients with chronic hepatitis B, subgroups according to trial design.
Only observational studies reported mortality in patients with cirrhosis. The number of patients who died in the intervention and control groups was 36 of 298 vs 141 of 499 (relative risk 0.61, 95% CI 0.44 to 0.86, I2=9%; number needed to treat 16 patients). There were no small-study effects (Egger's test, p=0.533) and no differences between the prospective cohort and case–control studies (test for subgroup differences, p=0.292).
HCC-related mortality
Antiviral therapy had no effect on HCC-related mortality (3 of 282 vs 2 of 154, relative risk 0.50, 95% CI 0.10 to 2.44, I2=0%; n=2 RCT). Including data from observational studies had little influence on the overall result (38 of 1233 vs 144 of 2632, relative risk 0.83, 95% CI 0.5 to 1.20, I2=0%; Egger's test, p=0.248). There was no difference between subgroups of trials stratified by design (test for subgroup differences, p=0.481).
Discussion
This systematic review found that the evidence of the effect of antiviral therapy on clinical outcomes in HBV is weak. RCTs found no benefit of treatment on HCC, mortality or HCC-related mortality in HBV. The total number of patients and duration of follow-up may be too small to determine the clinical effects. The inclusion of observational studies did not strengthen the overall findings because there was clear evidence of bias suggesting that the study design was closely related to the estimated treatment effects. The prospective cohort studies found that antiviral therapy increased the risk of HCC and had no effect on mortality. Case–control studies found that antiviral therapy reduced HCC and mortality. These findings suggest that detection and ascertainment bias as well as confounding by indication had a considerable influence on the overall result, which may explain why previous meta-analyses have disagreed in their assessment of the benefit of antiviral therapy.16–18 20 21 23 24 The importance of detection bias was underlined in the subgroup analysis of HCC screening. No intervention effect was found in trials that performed systematic HCC screening.
The main limitation of our review is the limited number of RCTs. Only one of the included trials had prevention of HCC as a primary outcome measure27 and none were designed to evaluate the effect on mortality or HCC-related mortality. Tests to evaluate the robustness of the results (including Egger's test) were difficult to interpret.
The current recommendation to treat patients with HBV is primarily based on surrogate outcomes. At present, the evidence supporting the use of virological markers as surrogate outcomes is weak. The fact that some studies have found a correlation between a virological response and improved liver histology does not necessarily validate their use as surrogate outcomes. Previous evidence has shown that interventions supported by surrogate markers may in fact have no benefit or even harmful effects on clinical outcome measures.68 Still, our findings are not sufficiently convincing and do not allow for changes in clinical practice.
Another limitation of the current review is our failure to extract data for analyses of treatment responders versus non-responders. However, only six cases of HCC were reportedly diagnosed in patients with biochemical or viral treatment response. This suggests that treatment response does not lead to elimination of the HCC risk, but probably decreases HCC incidence compared to non-responders or partial responders. This would be in line with previous findings.19 25 The majority of included trials in the current review assessed first-generation NA and interferon, as reflected by the low response rates. It was, however, not within the scope of the review to investigate modern antiviral treatments, as we included untreated control groups. Newer treatments will most likely result in more patients achieving sustained suppression of HBV–DNA. It is therefore possible that the current review underestimates a potential treatment effect. It would also have been of interest had we been able to adjust for other common risk factors for HCC, such as non-alcoholic steatohepatitis, alcoholic liver disease and coinfection with hepatitis C virus (HCV), hepatitis D and HIV. Although data on these risk factors were extracted, there was not enough data to allow for statistical analyses.
There are several potential explanations for the discrepancies between RCTs and observational studies.69 The fact that only prospective cohort studies found an increased risk of HCC among patients receiving antiviral therapy is in opposition to speculations that the treatment affected HCC development. The findings are more likely to reflect baseline differences in the viral load, genotype and degree of liver disease. The degree of monitoring in the treatment and control groups is also likely to differ and may lead to detection bias. The importance of detection bias is further supported by the subgroup differences observed according to HCC screening. The case–control studies are likely to have an even higher risk of bias, as confounding by indication and ascertainment bias is likely to exist in retrospective studies. Reporting bias should also be considered.33
The subgroup differences with regard to the type of intervention suggest a possible anticarcinogenic effect of interferon, as seen in HCV.70 We also found a decrease in HCC incidence and overall mortality in sensitivity analyses of patients with cirrhosis. This could support the case for continued treatment of patients with cirrhosis.
We found a beneficial effect of interferon and/or NA on mortality in HBV when including RCTs and observational studies. The assessment of mortality is robust to bias.71 Accordingly, our subgroup analysis showed no clear relation between the results and the study design. HCC mortality is more prone to bias. Whether antiviral treatment for HBV decreases mortality except from HCC is unknown.
In conclusion, antiviral treatment for HBV has no proven effect on the clinical outcomes, HCC and mortality. Bias has a paramount impact on the treatment effect estimates in observational studies and we recommend a critical approach to the conclusions drawn in such studies. Future trials on antiviral treatment for HBV should be designed to show an effect on clinical endpoints rather than surrogate markers.
Supplementary Material
Footnotes
Contributors: MT, LLG and AK conceived the idea and design, and analysed and interpreted the data. MT and EKD collected and assembled the data. MT and LLG drafted the manuscript. LLG, EKD and AK revised the manuscript for important intellectual content. All authors discussed and approved the final version of the manuscript. MT is the guarantor.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The dataset is available from the corresponding author.
References
- 1.WHO Position paper: Hepatitis B. WHO Weekly Epidemiological Report. World Health Organization, 2009 [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38 [DOI] [PubMed] [Google Scholar]
- 3.WHO Hepatitis C Fact Sheet. World Health Organization, 2012. http://www.who.int/mediacentre/factsheets/fs164/en/index.html [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer, 2010 [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27 [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–73.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni Y-H, Chang M-H, Wu J-F, et al. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol 2012;57:730–5 [DOI] [PubMed] [Google Scholar]
- 8.Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212–19 [DOI] [PubMed] [Google Scholar]
- 9.Ly KN, Jian X, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156:271–8 [DOI] [PubMed] [Google Scholar]
- 10.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335–52 [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Yang HI, Iloeje UH, et al. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 2009;49(S5):S72–84 [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther 2006;11:669–79 [PubMed] [Google Scholar]
- 13.Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma? J Gastroenterol Hepatol 2011;26:221–7 [DOI] [PubMed] [Google Scholar]
- 14.EASL EASL Clinical Practice Guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167–85 [DOI] [PubMed] [Google Scholar]
- 15.Shamliyan TA, MacDonald R, Shaukat A, et al. Antiviral therapy for adults with chronic hepatitis B: a systematic review for a National Institutes of Health Consensus Development Conference. Ann Intern Med 2009;150:111–24 [DOI] [PubMed] [Google Scholar]
- 16.Yang YF, Zhao W, Zhong YD, et al. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat 2009;16:265–71 [DOI] [PubMed] [Google Scholar]
- 17.Sung JJY, Tsoi KKF, Wong VWS, et al. Meta-analysis: treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2008;28:1067–77 [DOI] [PubMed] [Google Scholar]
- 18.Shen YC, Hsu C, Cheng CC, et al. A critical evaluation of the preventive effect of antiviral therapy on the development of hepatocellular carcinoma in patients with chronic hepatitis C or B: a novel approach by using meta-regression. Oncology 2012;82:275–89 [DOI] [PubMed] [Google Scholar]
- 19.Papatheodoridis GV, Lampertico P, Manolakopoulos S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348–56 [DOI] [PubMed] [Google Scholar]
- 20.Miyake Y, Kobashi H, Yamamoto K. Meta-analysis: the effect of interferon on development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol 2009;44:470–5 [DOI] [PubMed] [Google Scholar]
- 21.Zhang C-H, Xu G-L, Jia W-D, et al. Effects of interferon treatment on development and progression of hepatocellular carcinoma in patients with chronic virus infection: a meta-analysis of randomized controlled trials. Int J Cancer 2011;129:1254–64 [DOI] [PubMed] [Google Scholar]
- 22.Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther 2012;35:674–89 [DOI] [PubMed] [Google Scholar]
- 23.Cammà C, Giunta M, Andreone P, et al. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol 2001;34:593–602 [DOI] [PubMed] [Google Scholar]
- 24.Baffis V, Shrier I. Use of interferon for prevention of hepatocellular carcinoma in cirrhotic patients with hepatitis B or hepatitis C virus infection. Ann Intern Med 1999;131:696–701 [DOI] [PubMed] [Google Scholar]
- 25.Wong GLH, Yiu KKL, Wong VWS, et al. Meta-analysis: reduction in hepatic events following interferon-alfa therapy of chronic hepatitis B. Aliment Pharmacol Ther 2010;32:1059–68 [DOI] [PubMed] [Google Scholar]
- 26.Manolakopoulos S, Karatapanis S, Elefsiniotis J, et al. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol 2004;99:57–63 [DOI] [PubMed] [Google Scholar]
- 27.Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31 [DOI] [PubMed] [Google Scholar]
- 28.Krogsgaard K. The long-term effect of treatment with interferon-alpha 2a in chronic hepatitis B The Long-Term Follow-up Investigator Group. The European Study Group on Viral Hepatitis (EUROHEP). Executive Team on Anti-Viral Treatment. J Viral Hepat 1998;5:389–97 [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Green S.eds Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]: the Cochrane Collaboration, Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011], 2011 [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 31.EASL, EORTC EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43 [DOI] [PubMed] [Google Scholar]
- 32.Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101:513–23 [DOI] [PubMed] [Google Scholar]
- 33.Higgins J, Altman D, Sterne J.eds Chapter 8: assessing risk of bias in included studies: the Cochrane Collaboration, Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. 2011 [Google Scholar]
- 34.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75 [DOI] [PubMed] [Google Scholar]
- 35.Anderson MG, Harrison TJ, Alexander G, et al. Randomised controlled trial of lymphoblastoid interferon for chronic active hepatitis B. Gut 1987;28:619–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benvegnù L, Chemello L, Noventa F, et al. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer 1998;83:901–9 [DOI] [PubMed] [Google Scholar]
- 37.Bolukbas C, Bolukbas FF, Kendir T, et al. The effectiveness of lamivudine treatment in cirrhotic patients with HBV precore mutations: a prospective, open-label study. Dig Dis Sci 2006;51:1196–202 [DOI] [PubMed] [Google Scholar]
- 38.Brunetto MR, Oliveri F, Coco B, et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol 2002;36:263–70 [DOI] [PubMed] [Google Scholar]
- 39.Chan HL, Wang H, Niu J, et al. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. Antivir Ther 2007;12:345–53 [PubMed] [Google Scholar]
- 40.Chan SL, Mo FKF, Wong VWS, et al. Use of antiviral therapy in surveillance: impact on outcome of hepatitis B-related hepatocellular carcinoma. Liver Int 2012;32:271–8 [DOI] [PubMed] [Google Scholar]
- 41.Das K, Das K, Datta S, et al. Course of disease and survival after onset of decompensation in hepatitis B virus-related cirrhosis. Liver Int 2010;30:1033–42 [DOI] [PubMed] [Google Scholar]
- 42.Di Marco V, Iacono OL, Cammà C, et al. The long-term course of chronic hepatitis B. Hepatology 1999;30:257–64 [DOI] [PubMed] [Google Scholar]
- 43.Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon α therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740–9 [DOI] [PubMed] [Google Scholar]
- 44.Fattovich G, Giustina G, Realdi G, et al. Long-term outcome of hepatitis B e antigen-positive patients with compensated cirrhosis treated with interferon alfa. Hepatology 1997;26:1338–42 [DOI] [PubMed] [Google Scholar]
- 45.IIHCSG Effect of interferon-α on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. Lancet 1998;351:1535–9 [PubMed] [Google Scholar]
- 46.Ikeda K, Saitoh S, Suzuki Y, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus. Cancer 1998;82:827–35 [DOI] [PubMed] [Google Scholar]
- 47.Lin CC, Wu JC, Chang TT, et al. Long-term evaluation of recombinant interferon α2b in the treatment of patients with hepatitis B e antigen-negative chronic hepatitis B in Taiwan. J Viral Hepat 2001;8:438–46 [DOI] [PubMed] [Google Scholar]
- 48.Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45–52 [DOI] [PubMed] [Google Scholar]
- 49.Ma H, Wei L, Guo F, et al. Clinical features and survival in Chinese patients with hepatitis B e antigen-negative hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol 2008;23(8pt 1):1250–8 [DOI] [PubMed] [Google Scholar]
- 50.Mahmood S, Niiyama G, Kamei A, et al. Influence of viral load and genotype in the progression of Hepatitis B-associated liver cirrhosis to hepatocellular carcinoma. Liver Int 2005;25:220–5 [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto A, Tanaka E, Rokuhara A, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: a multicenter retrospective study of 2795 patients. Hepatol Res 2005;32:173–84 [DOI] [PubMed] [Google Scholar]
- 52.Mazzella G, Accogli E, Sottili S, et al. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol 1996;24:141–7 [DOI] [PubMed] [Google Scholar]
- 53.Mazzella G, Saracco G, Festi D, et al. Long-term results with interferon therapy in chronic type B hepatitis: a prospective randomized trial. Am J Gastroenterol 1999;94:2246–50 [DOI] [PubMed] [Google Scholar]
- 54.Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996;334:1422–7 [DOI] [PubMed] [Google Scholar]
- 55.Papatheodoridis GV, Manesis E, Hadziyannis SJ. The long-term outcome of interferon-alpha treated and untreated patients with HBeAg-negative chronic hepatitis B. J Hepatol 2001;34:306–13 [DOI] [PubMed] [Google Scholar]
- 56.Robson SC, Brice E, Van Rensburg C, et al. Safety and efficacy of interferon alpha-2b following prednisone withdrawal in the treatment of chronic viral hepatitis B. A case-controlled, randomised study. S Afr Med J 1992;82:317–20 [PubMed] [Google Scholar]
- 57.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Δ infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629–38 [DOI] [PubMed] [Google Scholar]
- 58.Tangkijvanich P, Thong-ngam D, Mahachai V, et al. Long-term effect of interferon therapy on incidence of cirrhosis and hepatocellular carcinoma in Thai patients with chronic hepatitis B. Southeast Asian J Trop Med Public Health 2001;32:452–8 [PubMed] [Google Scholar]
- 59.Tong MJ, Blatt LM, Tyson KB, et al. Death from liver disease and development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection: a prospective study. Gastroenterol Hepatol 2006;2:41–7 [PMC free article] [PubMed] [Google Scholar]
- 60.Tong MJ, Hsien C, Song JJ, et al. Factors associated with progression to hepatocellular carcinoma and to death from liver complications in patients with HBsAg-positive cirrhosis. Dig Dis Sci 2009;54:1337–46 [DOI] [PubMed] [Google Scholar]
- 61.Truong BX, Seo Y, Kato M, et al. Long-term follow-up of Japanese patients with chronic hepatitis B treated with interferon-alpha. Int J Mol Med 2005;16:279–84 [PubMed] [Google Scholar]
- 62.Waked I, Amin M, Abd el Fattah S, et al. Experience with interferon in chronic hepatitis B in Egypt. J Chemother 1990;2:310–18 [DOI] [PubMed] [Google Scholar]
- 63.Wong VW-S, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010;28:1660–5 [DOI] [PubMed] [Google Scholar]
- 64.Yuen M-F, Hui C-K, Cheng C-C, et al. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: the effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 2001;34:139–45 [DOI] [PubMed] [Google Scholar]
- 65.Yuen M-F, Wong DK-H, Sablon E, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology 2004;39:1694–701 [DOI] [PubMed] [Google Scholar]
- 66.Yuen MF, Seto WK, Chow DH, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther 2007;12:1295–303 [PubMed] [Google Scholar]
- 67.Zampino R, Marrone A, Merola A, et al. Long-term outcome of hepatitis B and hepatitis C virus co-infection and single HBV infection acquired in youth. J Med Virol 2009;81:2012–20 [DOI] [PubMed] [Google Scholar]
- 68.Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA 1999;282:786–90 [DOI] [PubMed] [Google Scholar]
- 69.Odgaard-Jensen J, Vist GE, Timmer A, et al. Randomisation to protect against selection bias in healthcare trials. Cochrane Database Syst Rev 2011;(4):MR000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimer N, Dahl EK, Gluud LL, et al. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta-analysis of randomised controlled trials. BMJ Open 2012;2:e001313 doi:10.1136/bmjopen-2012-001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savovic J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429–38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.