Abstract
The lung maintains an elevated level of glutathione (GSH) in epithelial lining fluid (ELF) compared to serum. The mechanism(s) by which the lung maintains high levels of ELF GSH and factors that modulate them are largely unexplored. We hypothesized that lung cystic fibrosis transmembrane conductance regulator protein (CFTR) modulates GSH efflux in response to extracellular stress, which occurs with lung infections. Mice were challenged intratracheally with Pseudomonas aeruginosa, and on the third day of infection bronchoalveolar lavage fluid was obtained and analyzed for cytokines and antioxidants. Lung tissue antioxidants and enzyme activities were also assessed. P. aeruginosa lung infection increased levels of inflammatory cytokines and neutrophils in the ELF. This corresponded with a marked threefold increase in GSH and a twofold increase in urate levels in the ELF of P. aeruginosa-infected wild-type mice. A twofold increase in urate levels was also observed among lung tissue antioxidants of P. aeruginosa-infected wild-type mice. There were no changes in markers of lung oxidative stress associated with the P. aeruginosa lung infection. In contrast with wild-type mice, the CFTR knockout mice lacked a significant increase in ELF GSH when challenged with P. aeruginosa, and this correlated with a decrease in the ratio of reduced to oxidized GSH in the ELF, a marker of oxidative stress. These data would suggest that the lung adapts to infectious agents with elevated ELF GSH and urate. Individuals with lung diseases associated with altered antioxidant transport, such as cystic fibrosis, might lack the ability to adapt to the infection and present with a more severe inflammatory response.
The lung maintains a 140-fold elevation of reduced glutathione (GSH) in epithelial lining fluid (ELF) compared to serum (3). Many pulmonary diseases, such as idiopathic pulmonary fibrosis and cystic fibrosis (CF), are associated with lower levels of GSH in the ELF than those in healthy individuals (2, 27). Very little is known about the mechanisms by which the lung maintains reduced GSH in the ELF and whether this changes in response to lung inflammation. Our laboratory recently reported that mice deficient in CF transmembrane conductance regulator protein (CFTR) have decreased lung ELF GSH (34), and other studies have shown that CFTR can regulate apical GSH transport (11, 22, 23). It is reasonable to assume that there must be other apical GSH transporters, since CTFR knockout (KO) mice have only diminished lung ELF GSH. CFTR is one member of the ABC cassette protein family that includes the multidrug resistance-associated proteins (MRP), which also have been shown to regulate GSH transport in the liver (1).
The CF lung is particularly susceptible to Pseudomonas aeruginosa, and this organism is thought to play a critical role in the development and progression of the pulmonary disease associated with CF (25). CFTR KO mice have an exaggerated inflammatory response and mortality to lung infections with P. aeruginosa (16). Many CF patients' lungs are continuously colonized by P. aeruginosa that forms antibiotic-resistant biofilms (4, 29). This process is thought to set up a chronic inflammatory and oxidative stimulus in the lung. Neutrophilic infiltration into the lung is a hallmark of infections with P. aeruginosa and a significant source of oxidative stress (9, 31, 38). Neutrophils respond to inflammatory stimuli by releasing reactive oxygen species, such as superoxide, hydrogen peroxide, and hypochlorous acid. Hypochlorous acid is produced by cytokine-mediated activation of neutrophil myleoperoxidase (10) and is a significant source of tissue nitration (8). GSH, ascorbate, and urate in the ELF are the lung's first line of defense against these reactive species. Among these ELF antioxidants, GSH has the fastest rate constant for reacting with hypochlorous acid (37).
The effects of P. aeruginosa infection on ELF and lung tissue antioxidants and the oxidation of DNA and lipids were investigated. A model of bronchopulmonary infection by inoculating mice with agarose beads embedded with a clinical isolate of mucoid P. aeruginosa was used. The inflammatory host response to P. aeruginosa was assessed by measuring cytokines and neutrophils in bronchoalveolar lavage fluid (BALF). P. aeruginosa infection increased levels of inflammatory cytokines and neutrophils in the ELF. There was also a marked threefold induction in ELF GSH levels in the P. aeruginosa-infected wild-type mice that was greatly attenuated in the CFTR KO mice. Although there was increased inflammation and neutrophil influx, it appeared that the adaptive antioxidant response protected lung cellular macromolecules from excessive oxidation in wild-type mice but not in the CFTR KO mice. These data suggest that the maintenance of GSH and perhaps urate in the ELF is an important adaptive response during infection and ensuing inflammation.
MATERIALS AND METHODS
Animals.
Wild-type male C57BL/6J mice (5 weeks old) purchased from Jackson Laboratories (Bar Harbor, Maine) were fed autoclaved Purina mouse chow 5010 and autoclaved tap water, which was available at all times. Mice were allowed to acclimate in the vivarium for at least 1 week before use. All mice were maintained in static isolator units on combination-size corncob bedding (The Andersons, Maumee, Ohio) and placed in fresh cages immediately after surgical instillation of agarose beads. To test the role of CFTR in the GSH adaptive response, homozygous congenic C57BL/6J CFTR KO mice (B6.129P2-Cftrtm1Unc) were utilized (30). CFTR KO mice require a liquid elemental diet (Peptamen, Glendale, Calif.); however, previous studies have shown that this does not affect ELF antioxidant levels (34).
Experimental model of endobronchial infection with P. aeruginosa.
An agarose bead model of P. aeruginosa endobronchial infection was used as previously described (16). Briefly, agarose was mixed with tryptic soy broth containing mucoid P. aeruginosa strain M57-15, a clinical isolate from a CF patient, grown to late log phase. The agarose-broth mixture was added to mineral oil that was equilibrated at 50 to 55°C, rapidly stirred for 6 min at room temperature, and then cooled over 10 min. The agarose beads were washed once with 0.5% deoxycholic acid in phosphate-buffered saline, pH 7.4 (PBS), once with 0.25% deoxycholic acid in PBS, and four times with PBS. The beads were measured to be 90 μm in diameter by inverted light microscopy (range, 18 to 204 μm). Quantitative bacteriology was performed on an aliquot of homogenized bead slurry to determine the number of CFU per milliliter (3.4 × 106 CFU/ml of slurry).
Groups of mice were anesthetized with intraperitoneal injections of 2.5% Avertin (2,2,2-tribromoethanol and tert-amyl alcohol in 0.9% NaCl administered at a dose of 0.015 ml/g of body weight). A transverse skin incision was made, and the trachea was visualized by blunt dissection. P. aeruginosa infection was achieved by transtracheal insertion of a 22-gauge 1-in. needle and instillation of an agarose bead slurry (50 μl) with or without P. aeruginosa (1.9 × 104 CFU/mouse). After P. aeruginosa infection, most mice displayed clinical signs of infection with scruffy coat, moderate dehydration, and decreased activity. Mice did not appear severely ill before the third day after infection. Case Western Reserve University's Institutional Animal Care and Use Committee approved all animal protocols. The Animal Resource Center at Case Western Reserve University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Bronchoalveolar lavage and necropsy.
Mice were killed on the third day after infection by carbon dioxide narcosis and exsanguinated by cardiac puncture. Blood plasma was collected for urea analysis (Sigma Diagnostics, St. Louis, Mo.). Bronchoalveolar lavage was performed by cannulating the trachea in situ with a 22-gauge 1.5-in. bead-tipped feeding needle, and three aliquots of 1.0 ml of sterile PBS were instilled and collected by gentle aspiration. The average time of the procedure was about 4 min. The total lavage fluid recovered from all animals was pooled and was always greater than 2 ml. A 500-μl aliquot of BALF was removed for GSH analysis (see below). The remainder of the BALF was treated with 100 μM phenylmethylsulfonyl fluoride and 5 mM EDTA, after which time the fluid was centrifuged at 100 × g for 10 min at 4°C. Supernatants were sterile filtered and stored at −70°C until used. The cell pellet was suspended in 1.0 ml of PBS, and a total leukocyte count was performed using a hemacytometer. A differential cell count was performed on cytocentrifuge preparations (Cytospin 3; Shandon, Pittsburgh, Pa.) stained with hematoxylin and eosin using standard morphometric methods. Following lavage, the right and left lungs were then removed and snap-frozen in liquid nitrogen. The lungs were ground into a fine powder using a liquid nitrogen-cooled mortar and pestle and then stored at −70°C until analysis. Aliquots of the ground tissue were carefully removed under liquid nitrogen as needed for subsequent analyses.
Analysis of cytokines in BALF.
Protease inhibitors, phenylmethylsulfonyl fluoride, and EDTA were added to the BALF samples after collection, as described above. Murine tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, macrophage inflammatory protein 2 (MIP-2), and keratinocyte chemoattractant (KC) were measured using commercially available enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, Minn.). The BALF supernatants were assayed in duplicate and compared to known standards, and the values were dilution corrected for the respiratory ELF volume by the urea dilution method (26). Values that fell below the limits of detection were assigned a value equivalent to the lowest standard dilution detected.
Glutathione analysis in BALF and lung tissue.
A 500-μl cell-free aliquot of BALF was acidified with 5% metaphosphoric acid (150 μl/ml), cooled on ice, and centrifuged (10,000 × g for 10 min at 4°C) to remove precipitated proteins. Ground lung tissue (∼20 mg) was dissolved in 600 μl of PBS, acidified with 5% metaphosphoric acid, cooled, and centrifuged to remove precipitated proteins. Antioxidants in BALF and tissues were analyzed by high-performance liquid chromatography (HPLC) coupled with coulometric electrochemical detection (CoulArray model 5600; ESA Inc., Chelmford, Mass.). Sample analysis was done using a 7- by 53-mm C18 reverse phase (Platinum EPS C18 100A 3 μm; Alltech Associates Inc., Deerfield, Ill.) and a mobile phase of 125 mM potassium acetate in 1% acetonitrile at pH of 3.0. The electrode potentials in a four-channel electrode array were set at 100 (channel 1), 215 (channel 2), 485 (channel 3), and 650 mV (channel 4). Under these conditions, ascorbate, GSH, oxidized GSH (GSSG), and urate exhibited retention times of 3.1, 3.4, 4.4, and 5.1 min, respectively. The GSH signal was distributed across electrodes 2 to 4, with the dominant signal on channel 3. Ascorbate, GSSG, and urate produced signals on channels 1, 3, and 1, respectively. Concentrations of these antioxidants were determined from a 5-μl injection and quantified from a five-point calibration curve generated from standards that were prepared fresh daily.
Lung GR activity.
Ground lung tissue (10 to 25 mg) was dissolved in 800 μl of cold homogenization buffer (50 mM potassium phosphate, 1 mM EDTA; pH 7.5) and centrifuged (8,500 × g for 10 min at 4°C), and the supernatant was retained for analysis. GSH reductase (GR) activity in the lung homogenate was determined spectrophotometrically (340 nm) from the rate of NADPH consumption by GR in the reduction of GSSG using a commercially available kit (GR-340; Oxis International Inc., Portland, Oreg.). GR activity was expressed as units per milligram of sample protein (Coomassie Plus; Pierce, Rockford, Ill.). A unit was defined as 1 micromole of NADPH consumed in a minute. Pellets from these homogenates were utilized for the determination of γ-glutamyltransferase (GGT) activity.
Lung GPx activity.
Ground lung tissue (10 to 35 mg) was dissolved in 1.0 ml of cold homogenization buffer (50 mM Tris-HCl, 5 mM EDTA, 1 mM 2-mercaptoethanol; pH 7.5) and centrifuged (7,500 × g for 15 min at 4°C), and the supernatant was retained for analysis. The GSH peroxidase (GPx) activity in the homogenate was determined using a commercially available kit (GPX-340; Oxis International Inc.) in which tert-butyl hydroperoxide was used as a GPx substrate to generate GSSG. The rate of NADPH consumption by GR in the subsequent reduction of GSSG was used to calculate GPx activity. GPx activity was expressed as units per milligram of sample protein (Coomassie Plus; Pierce). A unit was defined as 1 micromole of NADPH consumed in a minute.
Lung GGT activity.
Ground lung tissue (10 to 25 mg) was dissolved in 800 μl of cold homogenization buffer (50 mM potassium phosphate, 1 mM EDTA; pH 7.5) and centrifuged (8,500 × g for 10 min at 4°C), the pellets were resuspended in homogenization buffer (100 mM Tris-HCl, 10 mM serine, 0.1 mM EDTA; pH 7.6) and centrifuged (5,500 × g for 10 min at 4°C), and the supernatant was retained for GGT analysis. Pellets obtained from the homogenates for GR analysis were resuspended in homogenization buffer (100 mM Tris-HCl, 10 mM serine, 0.1 mM EDTA; pH 7.6) and centrifuged (5,500 × g for 10 min at 4°C), and the supernatant was retained for GGT analysis. A 200-μl aliquot of the supernatant was mixed with 1.0 ml of reagent solution (3.2 mM γ-glutamyl-3-carboxy-4-nitroanilide, 110 mM glycine-glycine, 110 mM Tris-HCl; pH 8.3). GGT activity was calculated from the rate of 3-carboxy-4-nitroaniline production at 405 nm (7). GGT activities were normalized to the supernatant protein concentrations (Coomassie Plus; Pierce).
Lung TBARS.
Oxidation of tissue lipids produces various aldehydes that can be measured colorimetrically by their reaction with thiobarburturic acid. Approximately 25 mg of ground lung tissue was dissolved in 50 mM phosphate buffer (pH 7.4) containing 1 mM butylated hydroxytoluene. An aliquot of the sample was then acidified with an equal volume of phosphoric acid. Thiobarburturic acid (0.1 M) was added, and the mixture was heated at 90°C for 35 min. The thiobarburturic acid-reactive substances (TBARS) in the sample were extracted with n-butanol, and the absorbance at 535 nm was measured using a plate reader (SpectraMax 340PC; Molecular Devices, Sunnyvale, Calif.). TBARS were calculated from a malondialdehyde standard curve and normalized for protein content (6).
HPLC analysis for 8OH2dG in lung DNA.
DNA from mouse lung tissue was obtained by a chloroform-isoamyl alcohol extraction of proteinase K-digested lung homogenates (34). The purified DNA was then hydrolyzed to nucleosides with nuclease P1 and alkaline phosphatase. Samples were analyzed for 8-hydroxy-2-deoxyguanosine (8OH2dG) and 2-deoxyguanosine (2dG) by HPLC coupled with in-line coulometric electrochemical and UV detection (CoulArray model 5600; ESA Inc., Chelmford, Mass.) for 8OH2dG and 2dG, respectively (39). Sample analysis was done using a 4.6- by 150-mm, C18 reverse-phase column (YMCbasic; YMC Inc., Wilmington, N.C.) with a mobile phase of 100 mM sodium acetate in 5% methanol at pH 5.2 (34). 2dG was detected at 265 nm, while 8OH2dG was detected electrochemically with electrode potentials of 285, 365, and 435 mV. Under these conditions, 2dG and 8OH2dG had retention times of approximately 7.4 and 9.5 min, respectively. Nucleoside concentrations were calculated from a five-point standard curve.
Statistical analysis.
Data are expressed as the mean ± standard error of the mean. Statistical analyses were performed using an unpaired t test and, in instances where the variances were not equal between groups, Welch's unpaired t test was utilized using the statistical package in Prism version 3 (GraphPad, San Diego, Calif.). The criterion for statistical significance was a P value of ≤0.05.
RESULTS
Effect of P. aeruginosa infection on inflammatory indices in the BALF.
Wild-type mice inoculated with P. aeruginosa had significant weight loss (22%) from their initial body weight by the third day of infection (Table 1). Some of the infected mice displayed mild physical signs of ill health, manifested as an unkempt or scruffy hair coat. There was no mortality in the mice after inoculation with infected beads. Mice were assessed for evidence of lung inflammation after P. aeruginosa infection by measuring changes in BALF cytokines and chemokines that are commonly associated with inflammatory responses, namely TNF-α, IL-1, IL-6, MIP-2, and KC (16). In addition, differential cell counts were performed on cells recovered from the BALF. As shown in Table 1, there were significant increases in all BALF cytokines and chemokines examined from the P. aeruginosa-infected mice that ranged from 7- to 70-fold (MIP-2 [73-fold] ≫ IL-6 [29-fold] > IL-1 [19-fold] ≫ KC [7-fold] = TNF-α [7-fold]). The large 70-fold increase in MIP-2 correlated with the massive cellular infiltration of neutrophils that accounted for 89% of the total BALF leukocytes.
TABLE 1.
Inflammation markers
| Parameter | Control | PA | P valuee |
|---|---|---|---|
| Weight changea | 1.5 ± 0.6 | −21.8 ± 1.9 | 0.001 |
| PMNb | 0.0 ± 0.0 | 1.1 ± 0.3 | 0.01 |
| % PMNc | 0.5 ± 0.3 | 89.8 ± 2.6 | 0.001 |
| TNF-αd | 1.4 ± 0.1 | 11.1 ± 3.6 | 0.03 |
| IL-1βd | 0.5 ± 0.0 | 10.2 ± 3.3 | 0.02 |
| IL-6d | 0.9 ± 0.1 | 26.6 ± 8.5 | 0.02 |
| MIP-2d | 0.5 ± 0.0 | 37.2 ± 13.1 | 0.03 |
| KCd | 0.9 ± 0.1 | 7.3 ± 2.2 | 0.02 |
Data are percent change from initial body weight at the start of the study.
Data are percent presented as 106 PMN/ml of BALF.
Data are presented as percentage of PMNs of the total cells recovered in the BALF.
Data are presented as nanograms per milliliter of ELF.
P values were determined using an unpaired t test with Welch's correction for unequal variances.
Effect of P. aeruginosa infection on lung antioxidant levels.
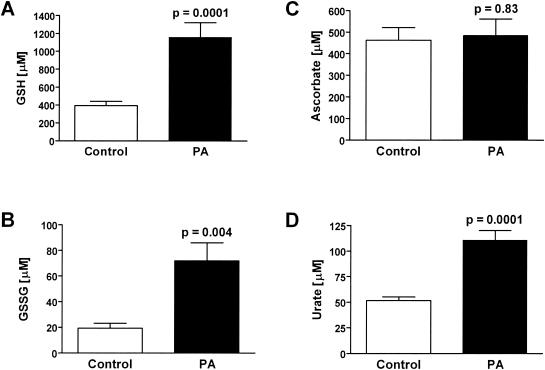
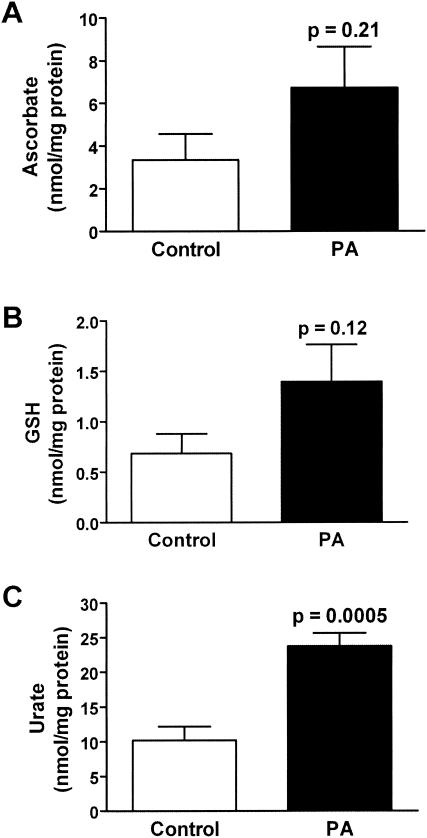
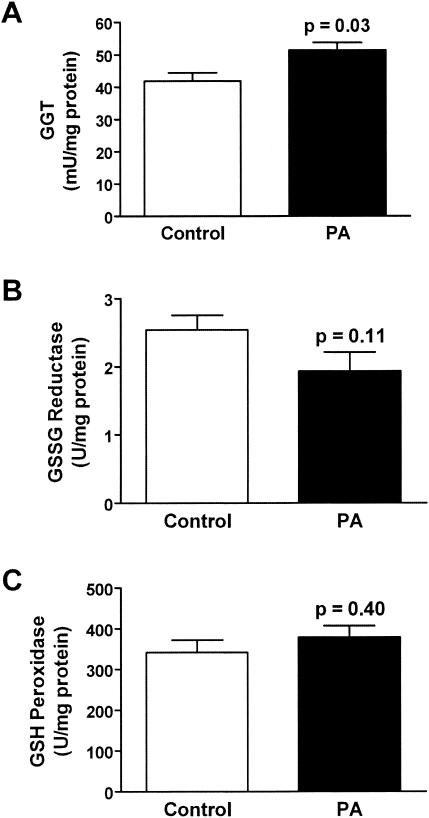
The effect of P. aeruginosa lung infection on ELF antioxidant levels was determined. P. aeruginosa infection had differential effects on ELF ascorbate, GSH, and urate. Whereas P. aeruginosa infection produced no change in ELF ascorbate levels, urate and GSH levels were increased two- and threefold, respectively (Fig. 1). The ratio of reduced to oxidized GSH in the ELF of P. aeruginosa-infected wild-type mice was similar to that in their uninfected counterparts despite a threefold elevation in the ELF levels of GSSG (Table 2). Changes in the ELF antioxidant concentrations were mirrored in the lung tissue of the P. aeruginosa-infected wild-type mice; however, only changes in urate levels reached statistical significance (Fig. 2). Only minor changes were seen in lung tissue enzymes involved in GSH catabolism and recycling (Fig. 3). The activity of GGT, the cell membrane protein involved in the breakdown of extracellular GSH, was increased 15% in the lungs of P. aeruginosa-infected wild-type mice. In contrast GR, the enzyme involved in recycling intracellular GSSG back to GSH, was decreased 30% in the lungs of P. aeruginosa-infected wild-type mice; however, this decrease failed to reach statistical significance. Finally, the activity of GPx, which is a family of enzymes that utilize GSH to detoxify cellular peroxides, was unchanged in the lungs of P. aeruginosa-infected mice. The increase in GGT activity suggests that the lung epithelial cells are actively recycling the extracellular GSH in the ELF.
FIG. 1.
Differential effects of P. aeruginosa infection on ELF antioxidant levels. Groups of six to eight wild-type mice were instilled with agarose beads with and without P. aeruginosa, and bronchoalveolar lavage was performed on the third day of infection. Values were corrected for lavage dilution using the urea method and expressed as ELF concentrations. P. aeruginosa infection increased GSH (A) and urate (D) ELF levels three- and twofold, respectively. P. aeruginosa infection had similar effects on the level of GSSG (B) but no effect on ascorbate (C) levels.
TABLE 2.
Oxidative stress markers
| Parameter | Control | PA | P valued |
|---|---|---|---|
| GSH/GSSGa | 10.1 ± 2.8 | 7.9 ± 2.1 | NS |
| 8OHdG/dGb | 9.5 ± 1.1 | 7.4 ± 0.7 | NS |
| TBARSc | 1.25 ± 0.08 | 1.14 ± 0.09 | NS |
Data are presented as a ratio of GSH to GSSG in the ELF.
Data are presented as the number of 8OHdG per 105 2dG in lung DNA.
Data are presented as malondialdehyde equivalences (nanomole per milligram of lung protein).
P values were determined using an unpaired t test with Welch's correction for unequal variances. NS, the P value was greater than 0.05.
FIG. 2.
P. aeruginosa infection produces small changes in lung tissue antioxidant levels. Groups of six to eight wild-type mice were instilled with agarose beads with and without P. aeruginosa, and lung tissue homogenate antioxidant levels were assessed by HPLC analyses on the third day of infection. P. aeruginosa infection produced a slight increase in lung tissue ascorbate (A) and GSH (B) levels while increasing urate (C) levels twofold.
FIG. 3.
Differential effects of P. aeruginosa infection on lung GSH-utilizing enzymes. Groups of six to eight wild-type mice were instilled with agarose beads with and without P. aeruginosa, and lung tissue homogenate enzyme activities were assessed on the third day of infection. (A) P. aeruginosa infection increased the enzyme activities of GGT, which is involved in recycling GSH from the ELF, by 15%. (B) P. aeruginosa infection decreased the activity of glutathione reductase, which is involved in recycling intracellular GSH, by 30%. (C) P. aeruginosa infection had no effect on glutathione peroxidase, which is involved in the detoxification of lipid peroxides.
Effect of P. aeruginosa infection on lung markers of oxidative stress.
Oxidative stress occurs when there is an imbalance between the steady-state levels of oxidants and antioxidants. Since the levels of both oxidants and antioxidants can change in response to each other, markers of lipid and DNA oxidation in the lungs of mice 3 days postinfection were determined. The formation of TBARS in lung tissue homogenates was used as an index of lipid oxidation. No significant differences in TBARS concentrations were observed between the lungs from uninfected and P. aeruginosa-infected wild-type mice (Table 2). To assess oxidation of DNA, the formation of 8OH2dG was used. Again, no significant differences in lung 8OH2dG levels were found between the control and P. aeruginosa-infected wild-type mice (Table 2). It is apparent that, under the conditions employed in these studies, there was no evidence to indicate that the lung tissue of P. aeruginosa-infected animals was subjected to any significant oxidative stress.
Role of CFTR in GSH efflux in response to P. aeruginosa lung infection.
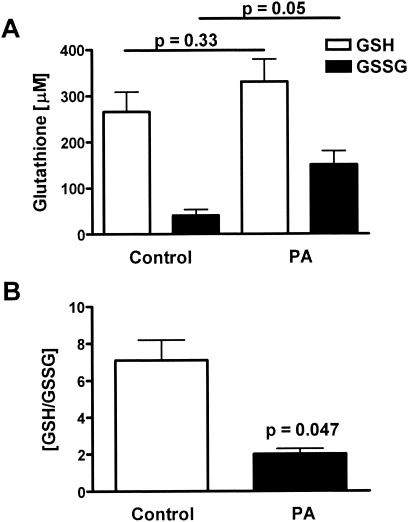
To investigate the mechanism behind the P. aeruginosa-stimulated increase in ELF GSH, cogenic homozygous C57BL/6J CFTR KO mice were infected with P. aeruginosa, and their lungs were lavaged 3 days postinfection. In contrast to wild-type mice, the CFTR KO mice lacked a significant increase in reduced GSH in their ELF (Fig. 4A). In addition, the P. aeruginosa-infected CFTR KO mice had an increase in the levels of oxidized GSSG in their ELF. Consequently, the ratio of reduced to oxidized glutathione (GSH/GSSG) dramatically decreased in the CFTR KO P. aeruginosa-infected mice (Fig. 4B). A decrease in the GSH/GSSG ratio is often used as an index of oxidative stress (18). These data suggest that a functional CFTR is required for the lung to mount an adaptive GSH response to P. aeruginosa infection.
FIG. 4.
Lack of a GSH adaptive response and oxidative stress in P. aeruginosa-infected CFTR KO mice. Groups of four to eight CFTR KO mice were instilled with agarose beads with and without P. aeruginosa, and bronchoalveolar lavage was performed on the third day of infection. Values were corrected for lavage dilution using the urea method and are expressed as ELF concentrations. (A) P. aeruginosa infection significantly increased the levels of GSSG by threefold but did not affect the levels of reduced GSH. (B) These changes in GSSG resulted in a substantial decrease in the ratio of GSH to GSSG, which is an index of oxidative stress.
DISCUSSION
The mechanism(s) by which the lung maintains high levels of GSH in the ELF and factors that modulate them are largely unexplored. In these studies, we sought to determine the lung's antioxidant response to a mucoid P. aeruginosa infection. Mice inoculated with P. aeruginosa-laden agarose beads have elevations in proinflammatory mediators and chemokines compared to uninfected controls. This response peaks between 2 and 3 days after inoculation with P. aeruginosa-infected beads (16). This study also demonstrated that the P. aeruginosa infection provokes a strong inflammatory response in the lungs by the third day of infection. This response is characterized by elevations in proinflammatory cytokines, chemokines, and a pronounced influx of neutrophils. Concomitant with this inflammatory response was an adaptive response by the lung of increasing levels of ELF antioxidants, namely, a twofold increase in urate and a threefold increase in GSH levels. This extracellular antioxidant adaptive response in wild-type mice appears to have minimized any P. aeruginosa-induced oxidative damage to lung tissue, as evidenced by the lack of increased tissue lipid or DNA oxidation and no change in the GSH/GSSG ratio in the ELF. In contrast, the CFTR KO mice lacked an adaptive GSH response to P. aeruginosa infection and had a decreased ELF GSH/GSSG ratio, an index of oxidative stress (18). These results suggest that the lung defends itself against oxidative damage from inflammatory stimuli by elevating the levels of extracellular antioxidants, most likely through stimulation of apical transport, and for GSH this requires a functional CFTR protein.
Urate is an important lung antioxidant that has high rates of reaction with air pollutants such as ozone (20). Urate is the final product of purine degradation in humans, and it is likely that the increase in urate in the ELF and lung tissue observed after P. aeruginosa infection is due to increased purine degradation. It is interesting that xanthine oxidase is a key enzyme in the purine degradation pathway that paradoxically produces both the antioxidant urate and the reactive oxygen species superoxide and hydrogen peroxide. Furthermore, an elevated xanthine oxidase activity has been reported in CF patients (15). However, further studies are necessary to determine which enzyme(s) in purine degradation is being stimulated, the mechanism, and the importance of these findings in the pathophysiology associated with lung infection.
CF is a common recessive genetic disorder involving over 700 mutations that produce a defective CFTR protein (28). CFTR is a member of the ABC cassette family of transporter proteins and is thought to predominately regulate chloride conductance in secretory epithelial cells (21, 36). Defective function of CFTR in airway epithelial cells and submucosal glands results in a chronic state of pulmonary disease that is manifested by airway obstruction and recurrent lung infections (5). Despite 20 years of investigation, it is still unclear how impairment in CFTR function contributes to CF lung disease. Adult CF patients are reported to have diminished levels of GSH in their ELF (27) that are associated with an excessive inflammatory response during recurrent endobronchial P. aeruginosa infections (24). It has been debated whether the changes in ELF GSH observed in CF patients are a consequence of the recurrent endobronchial infections or a result of CFTR dysfunction (33).
Our investigators recently reported that uninfected mice deficient in CFTR have decreased lung ELF GSH (34), and other studies have shown that CFTR can regulate GSH transport (11, 22, 23). CFTR KO mice are known to have an exaggerated cytokine response and a higher rate of mortality in response to lung infection with P. aeruginosa (16). The present studies demonstrated that wild-type mice responded to P. aeruginosa infection with elevated ELF GSH levels, whereas this response was greatly attenuated in the CFTR KO mice. These data suggest that the lung may adapt to increased oxidation of reduced GSH in the ELF by stimulating apical transport of reduced GSH through CFTR to maintain redox balance in the ELF. Recent studies have suggested that this GSH in the ELF can protect the epithelium against hypochlorous acid, a product of stimulated neutrophils, and its oxidation of membrane proteins that alter membrane currents (35). In addition, GSH can react with reactive nitrogen species to form S-nitrosoglutathione (GSNO), which has bronchodilatory properties (12) and is deficient in CF patients (14). GSNO is also important in the maturation of functional CFTR (39). It is interesting that GGT is a key enzyme in GSNO metabolism (17) and is elevated during P. aeruginosa infection. Together, these findings support the notion that individuals with CF may have a diminished adaptive GSH response towards P. aeruginosa lung infections, and this plays a role in the excessive inflammatory response and increased mortality previously reported (24).
It is interesting to speculate whether CFTR and/or MRP polymorphisms may be associated with other lung diseases and contribute to diminished GSH adaptive responses during infection or environmental exposures that may increase one's risk for greater morbidity and mortality. Within the ABC cassette family, both CFTR and MRP2 are known to be expressed apically and transport GSH (1, 22). Genetic variations are common in ABC cassette proteins, and 779 single nucleotide polymorphisms (SNPs) have been recently reported in a Japanese population for eight genes in the ABC cassette family. In this study, 58 SNPs in the CFTR gene and 41 in the MRP2 gene were reported (28). One out of every 20 persons in the United States Caucasian population is a heterozygous carrier of a CFTR mutation (19). Some studies have already revealed that patients with pulmonary diseases, such as disseminated bronchiectasis (13), asthma, and chronic obstructive pulmonary disease, have a higher frequency of carrying a CFTR mutation (32). It is currently unknown whether SNPs in the MRP genes occur at higher frequencies in these or other pulmonary diseases. Further studies are needed to determine whether any of these SNPs produce functional changes in GSH adaptive responses in the lung.
In summary, this is the first report to our knowledge that lung CFTR modulates GSH ELF levels in response to P. aeruginosa lung infections. These data support the concept that individuals who lack functional CFTR and/or apically expressed MRPs may be at increased risk for lung damage associated with P. aeruginosa infectious processes and that diminished GSH adaptive responses may actively contribute to the progressive lung dysfunction and destruction that occurs in CF.
Acknowledgments
This work was supported in part through funding by the National Institutes of Health (HL75523, DK48996, DK43999, DK48994, HL50527, and DK27651) and funding provided by the Cystic Fibrosis Foundation (Core Center grant and research grants).
We express appreciation to Chris Statt, Heidi Carroll, Lisa Shyjka, Jerry Chipuk, Lisa Hogue, Christiaan van Heeckeren, Merle Fleischer, and Alma Wilson for providing their expert technical support.
Editor: F. C. Fang
REFERENCES
- 1.Ballatori, N., and J. F. Rebbeor. 1998. Roles of MRP2 and oatp1 in hepatocellular export of reduced glutathione. Semin. Liver Dis. 18:377-387. [DOI] [PubMed] [Google Scholar]
- 2.Cantin, A. M., R. C. Hubbard, and R. G. Crystal. 1989. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 139:370-372. [DOI] [PubMed] [Google Scholar]
- 3.Cantin, A. M., S. L. North, R. C. Hubbard, and R. G. Crystal. 1987. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 63:152-157. [DOI] [PubMed] [Google Scholar]
- 4.Costerton, J. W. 2001. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 9:50-52. [DOI] [PubMed] [Google Scholar]
- 5.Davis, P. B., M. Drumm, and M. W. Konstan. 1996. Cystic fibrosis. Am. J. Respir. Crit. Care Med. 154:1229-1256. [DOI] [PubMed] [Google Scholar]
- 6.Day, B. J., I. Batinic-Haberle, and J. D. Crapo. 1999. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med. 26:730-736. [DOI] [PubMed] [Google Scholar]
- 7.Day, B. J., G. P. Carlson, and D. B. DeNicola. 1990. γ-Glutamyltranspeptidase in rat bronchoalveolar lavage fluid as a probe of 4-ipomeanol and α-naphthylthiourea-induced pneumotoxicity. J. Pharmacol. Methods 24:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich, J. P., M. Hristova, C. E. Cross, A. D. Jones, B. A. Freeman, B. Halliwell, and A. van der Vliet. 1998. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391:393-397. [DOI] [PubMed] [Google Scholar]
- 9.Fick, R. B., Jr., F. Sonoda, and D. B. Hornick. 1992. Emergence and persistence of Pseudomonas aeruginosa in the cystic fibrosis airway. Semin. Respir. Infect. 7:168-178. [PubMed] [Google Scholar]
- 10.Furtmuller, P. G., C. Obinger, Y. Hsuanyu, and H. B. Dunford. 2000. Mechanism of reaction of myeloperoxidase with hydrogen peroxide and chloride ion. Eur. J. Biochem. 267:5858-5864. [DOI] [PubMed] [Google Scholar]
- 11.Gao, L., K. J. Kim, J. R. Yankaskas, and H. J. Forman. 1999. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am. J. Physiol. 277:L113-L118. [DOI] [PubMed] [Google Scholar]
- 12.Gaston, B., J. M. Drazen, A. Jansen, D. A. Sugarbaker, J. Loscalzo, W. Richards, and J. S. Stamler. 1994. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J. Pharmacol. Exp. Ther. 268:978-984. [PubMed] [Google Scholar]
- 13.Girodon, E., C. Cazeneuve, F. Lebargy, T. Chinet, B. Costes, N. Ghanem, J. Martin, S. Lemay, P. Scheid, B. Housset, J. Bignon, and M. Goossens. 1997. CFTR gene mutations in adults with disseminated bronchiectasis. Eur. J. Hum. Genet. 5:149-155. [PubMed] [Google Scholar]
- 14.Grasemann, H., B. Gaston, K. Fang, K. Paul, and F. Ratjen. 1999. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J. Pediatr. 135:770-772. [DOI] [PubMed] [Google Scholar]
- 15.Hamelin, B. A., K. Xu, F. Valle, L. Manseau, M. Richer, and M. LeBel. 1994. Caffeine metabolism in cystic fibrosis: enhanced xanthine oxidase activity. Clin. Pharmacol. Ther. 56:521-529. [DOI] [PubMed] [Google Scholar]
- 16.Heeckeren, A., R. Walenga, M. W. Konstan, T. Bonfield, P. B. Davis, and T. Ferkol. 1997. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Investig. 100:2810-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg, N., R. J. Singh, E. Konorev, J. Joseph, and B. Kalyanaraman. 1997. S-Nitrosoglutathione as a substrate for gamma-glutamyl transpeptidase. Biochem. J. 323:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, D. P. 2002. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 348:93-112. [DOI] [PubMed] [Google Scholar]
- 19.Kane, K. 1988. Cystic fibrosis: recent advances in genetics and molecular biology. Ann. Clin. Lab. Sci. 18:289-296. [PubMed] [Google Scholar]
- 20.Kelly, F. J., A. Blomberg, A. Frew, S. T. Holgate, and T. Sandstrom. 1996. Antioxidant kinetics in lung lavage fluid following exposure of humans to nitrogen dioxide. Am. J. Respir. Crit. Care Med. 154:1700-1705. [DOI] [PubMed] [Google Scholar]
- 21.Knowles, M. R., M. J. Stutts, A. Spock, N. Fischer, J. T. Gatzy, and R. C. Boucher. 1983. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221:1067-1070. [DOI] [PubMed] [Google Scholar]
- 22.Kogan, I., M. Ramjeesingh, C. Li, J. F. Kidd, Y. Wang, E. M. Leslie, S. P. Cole, and C. E. Bear. 2003. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 22:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linsdell, P., and J. W. Hanrahan. 1998. Glutathione permeability of CFTR. Am. J. Physiol. 275:C323-C326. [DOI] [PubMed] [Google Scholar]
- 24.Pier, G. B. 1985. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J. Infect. Dis. 151:575-580. [DOI] [PubMed] [Google Scholar]
- 25.Pitt, T. L. 1986. Biology of Pseudomonas aeruginosa in relation to pulmonary infection in cystic fibrosis. J. R. Soc. Med. 79(Suppl. 12):13-18. [PMC free article] [PubMed] [Google Scholar]
- 26.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 27.Roum, J. H., R. Buhl, N. G. McElvaney, Z. Borok, and R. G. Crystal. 1993. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 75:2419-2424. [DOI] [PubMed] [Google Scholar]
- 28.Saito, S., A. Iida, A. Sekine, Y. Miura, C. Ogawa, S. Kawauchi, S. Higuchi, and Y. Nakamura. 2002. Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR). J. Hum. Genet. 47:147-171. [DOI] [PubMed] [Google Scholar]
- 29.Sharma, G. D., M. F. Tosi, R. C. Stern, and P. B. Davis. 1995. Progression of pulmonary disease after disappearance of Pseudomonas in cystic fibrosis. Am. J. Respir. Crit. Care Med. 152:169-173. [DOI] [PubMed] [Google Scholar]
- 30.Snouwaert, J. N., K. K. Brigman, A. M. Latour, N. N. Malouf, R. C. Boucher, O. Smithies, and B. H. Koller. 1992. An animal model for cystic fibrosis made by gene targeting. Science 257:1083-1088. [DOI] [PubMed] [Google Scholar]
- 31.Suntres, Z. E., A. Omri, and P. N. Shek. 2002. Pseudomonas aeruginosa-induced lung injury: role of oxidative stress. Microb. Pathog. 32:27-34. [DOI] [PubMed] [Google Scholar]
- 32.Tzetis, M., A. Efthymiadou, S. Strofalis, P. Psychou, A. Dimakou, E. Pouliou, S. Doudounakis, and E. Kanavakis. 2001. CFTR gene mutations—including three novel nucleotide substitutions—and haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary disease. Hum. Genet. 108:216-221. [DOI] [PubMed] [Google Scholar]
- 33.van der Vliet, A., J. P. Eiserich, B. Halliwell, and C. E. Cross. 1997. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 272:7617-7625. [DOI] [PubMed] [Google Scholar]
- 34.Velsor, L. W., A. van Heeckeren, and B. J. Day. 2001. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Physiol. Lung Cell Mol. Physiol. 281:L31-L38. [DOI] [PubMed] [Google Scholar]
- 35.Venglarik, C. J., J. Giron-Calle, A. F. Wigley, E. Malle, N. Watanabe, and H. J. Forman. 2003. Hypochlorous acid alters bronchial epithelial cell membrane properties and prevention by extracellular glutathione. J. Appl. Physiol. 95:2444-2452. [DOI] [PubMed] [Google Scholar]
- 36.Widdicombe, J. H., M. J. Welsh, and W. E. Finkbeiner. 1985. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc. Natl. Acad. Sci. USA 82:6167-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winterbourn, C. C., and S. O. Brennan. 1997. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochem. J. 326:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witko-Sarsat, V., C. Delacourt, D. Rabier, J. Bardet, A. T. Nguyen, and B. Descamps-Latscha. 1995. Neutrophil-derived long-lived oxidants in cystic fibrosis sputum. Am. J. Respir. Crit. Care Med. 152:1910-1916. [DOI] [PubMed] [Google Scholar]
- 39.Zaman, K., M. McPherson, J. Vaughan, J. Hunt, F. Mendes, B. Gaston, and L. A. Palmer. 2001. S-Nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem. Biophys. Res. Commun. 284:65-70. [DOI] [PubMed] [Google Scholar]