Abstract
In the Streptococcus pneumoniae genome, stkP, encoding a membrane-associated serine/threonine kinase, is not redundant (L. Novakova, S. Romao, J. Echenique, P. Branny, and M.-C. Trombe, unpublished results). The data presented here demonstrate that StkP belongs to the signaling network involved in competence triggering in vitro and lung infection and bloodstream invasion in vivo. In competence, functional StkP is required for activation of comCDE upstream of the autoregulated ring orchestrated by the competence-stimulating peptide. This is the first description of positive regulation of comCDE transcription in balance with its repression by CiaRH.
The human pathogen Streptococcus pneumoniae is highly versatile. Its response to environment determines the bacterium's virulence and transformability as shown by mutational studies targeting metabolic (1, 16), regulatory (4, 11), transport (5, 19), and signaling (6, 8) functions. The factors so far identified which influence the fate of the bacterium include mainly the concentrations of divalent cations (5, 19, 20) and H+ and dioxygen (6). Signaling involves two-component signal-transducing systems (6, 8, 13), an oxidase (7), and a global regulator (4). In the present work, we provide evidence for the role of the serine/threonine kinase StkP in competence signaling and in experimental virulence. StkP is membrane associated, carries the PASTA signature (22), and has been shown to avoid LytA-dependent autolysis induced by growth at alkaline pH and by low concentrations of cell wall-directed antibiotics. It is proposed that virulence expression and competence development represent population responses to cell wall stress induced in specific growth conditions.
The rough S. pneumoniae RX derivatives (19) carrying the lytA mutation (17) and the smooth serotype 2 (2) strain D39 and serotype 6 strain 23477 (1) were used for competence tests and virulence studies, respectively. Bacterial growth and storage were as previously described (12, 17, 19). An insertion mutation in stkP was obtained in vitro with the pBluescript derivative (15) plasmid pPHK29 carrying a 2.96-kbp EcoRI/SalI amplimer containing stkP from the RX chromosome. The 1.3-kbp BamHI fragment from pPJ1 (14) containing the aphA3 cassette was inserted into a BglII site of stkP to give the mutagenic plasmid pPKB3. The mutated stkP::aphA3 allele was introduced by genetic transformation into the relevant genetic backgrounds, and strains carrying the allelic exchange were selected on kanamycin (50 mg/liter) plates as described previously (6). Recombinant clones were verified by PCR, and it has been verified that the stkP::aphA3 mutation did not affect bacterial growth of the different strains in vitro and also that the insertion mutation was very unlikely to impact the expression of downstream genes in the region. Indeed, with the software described in reference 9, it is predicted that genes SP1731 and SP1732 following the stkP stop codon show no relationship with stkP. Moreover, in the 118-bp intergenic region between stkP and SP1731, we found a stem-loop with free energy of −6.7 kcal/mol. This DNA structure shows the features of a putative transcription terminator for stkP.
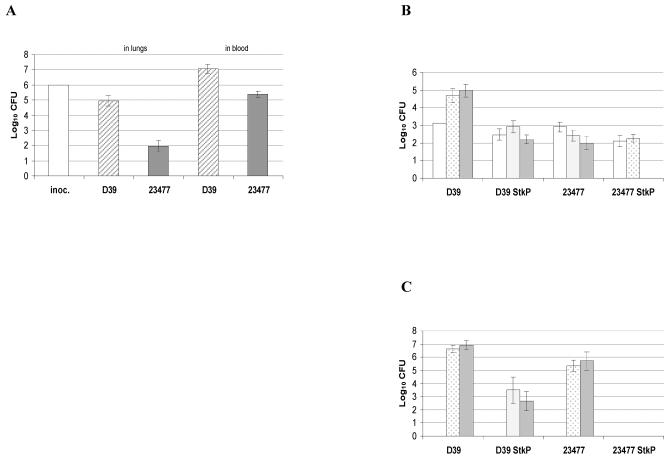
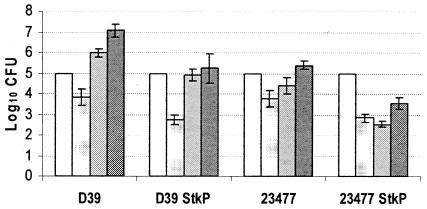
In order to assess the importance of StkP in the virulence of the pneumococcus, mice were intranasally challenged with strains D39 and 23477 and their corresponding stkP::aphA3 derivatives. Figure 1A illustrates the fate of both D39 and 23477 strains after intranasal challenge of the mice, notably with regards to recovery of CFU from lungs and blood at 48 h postinfection. Data presented in Fig. 1B and C clearly indicate that the stkP::aphA3 mutation significantly reduces the virulence of both strains, as shown by analysis of variance giving a P value of <0.05. The numbers of CFU of each of the two stkP::aphA3 mutant strains were significantly lower than those of the corresponding wild-type strains, with a striking elimination of the 23477 stkP::aphA3 bacteria from the lungs and the blood at 48 and 24 h postinfection, respectively. This strong effect of the stkP mutation on bloodstream invasion incited us to evaluate the role of StkP specifically during growth in the bloodstream. Results presented in Fig. 2 indeed show a requirement for StkP for optimal growth in blood after intravenous injection (P < 0.05); however, comparison of the numbers obtained in both models for each couple of strains indicates that StkP increased invasion efficiency by 2 orders of magnitude in the intravenous challenge and by 4 to 6 orders of magnitude in the intranasal challenge. This suggests an important role for the protein specifically in successful access into the bloodstream in addition to the requirement for StkP for optimal growth in blood.
FIG. 1.
Positive impact of StkP on growth in lungs and bloodstream invasion. (A) Bacteria from the wild-type strains D39 and 23477 were used for intranasal infection of female MF1 outbred mice as described in reference 12. At 48 h, CFU were recovered from the lungs and blood as described in reference 12. inoc., inoculum. (B) CFU recovery from lungs after intranasal challenge with the wild-type strains and their stkP::aphA3 mutant derivatives. Infection was as described for panel A. CFU were recovered from lungs at 15 min (white bars), 24 h (dotted bars), and 48 h (grey bars). (C) CFU recovery from blood. The conditions of infection were as described for panel A, and CFU recovery was at 24 h (dotted bars) and 48 h (grey bars). Five mice were used at each time point, and suspensions of 106 CFU in 50 μl of phosphate-buffered saline were used for infection. The results are expressed as numbers of CFU recovered per milliliter of blood (C) and per lung (B), and the standard error of the mean (SEM) is given.
FIG. 2.
StkP and bloodstream invasion. Suspensions of 105 bacteria in 50 μl of phosphate-buffered saline were injected intravenously into a tail vein, and growth in blood was determined by counting CFU at 15 min (white bars), 2 h (dotted bars), 24 h (grey bars), and 48 h (dark grey bars). Five mice were used at each time point. Data are expressed as numbers of viable CFU per milliliter of blood, and the SEM is given.
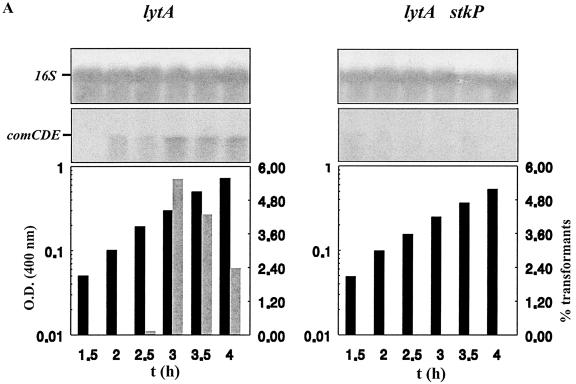
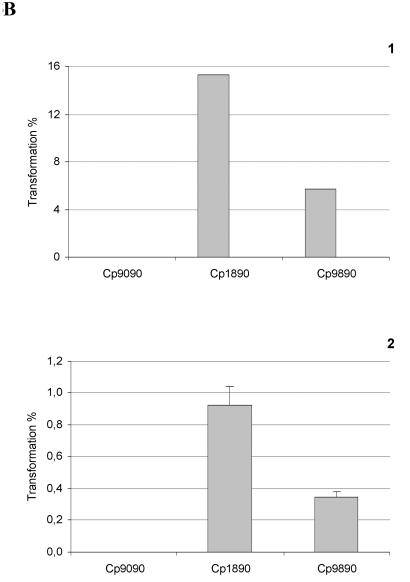
The role of StkP in the development of competence during growth has been assessed using the RX derivative, strain Cp9090 carrying the stkP::aphA3 mutation in the lytA genetic background, in order to avoid autolysis at alkaline pH (Novakova et al., unpublished). Expression of the central competence operon comCDE and subsequent expression of bacterial transformability have been investigated by measuring the cellular levels of comCDE transcripts and transformant recovery in cultures. Results of Northern blotting of comCDE mRNA and the yields of transformants in cultures correlated and revealed central and positive control of comCDE expression by StkP (Fig. 3A). The level of comCDE mRNA in aerobic cultures is modulated by negative control due to CiaRH, as shown by uniform expression in strains defective for CiaRH compared to that in the wild type (6). Results presented in Fig. 3B show that the ciaR::spc mutation restored transformability of bacteria carrying the stkP::aphA3 mutation, and this finding was correlated with the cellular level of comCDE transcripts (data not shown). Measurements of transformant yields for strains Cp9090 (stkP::aphA3), Cp1890 (ciaR::spc), and Cp9890 (stkP::aphA3 ciaR::spc) clearly indicate that the ciaR::spc mutation induced only partial reversion of the competence defect due to the stkP::aphA3 mutation (Fig. 1 and B2). These results provide credence to the idea of an antagonistic role of CiaRH and StkP in comCDE regulation and demonstrate for the first time positive control of comCDE expression by StkP. In the absence of StkP and CiaRH, residual competence in strain Cp1890 may either correspond to basal comCDE expression or reveal additional inputs upstream of the competence-stimulating peptide autoregulated ring.
FIG. 3.
Positive control of comCDE mRNA cellular levels by StkP and impact on transformability in the wild-type and ciaR::spc genetic backgrounds. (A) Strains Cp1095 (lytA stkP+) and Cp9090 (lytA stkP) were grown aerobically in static flasks, and samples were withdrawn at 30-min intervals and analyzed for biomass increase (black bars), transformability (grey bars), and cellular levels of comCDE mRNA. Methods and material were as described in reference 8. The level of specific mRNA in cultures was evaluated by Northern blotting of total cellular RNA with the appropriate probes, and 16S rRNA was used as an internal qualitative and quantitative control. The columns representing growth, measured by comparing optical densities at 400 nm [O.D. (400 nm)], and transformability as the percentage of transformants (transformed by rifR chromosomal DNA) recovered relative to the total population are aligned with the Northern blot signals corresponding to the same culture. The experiment was repeated with independent cultures to test reproducibility. t, time. (B) The ciaR::spc mutation partially restores transformability of the stkP::aphA3 mutant strain. Both in situ transformation tests (4) (B1) and liquid transformation tests (8) (B2) were performed with strains Cp9090 (stkp::aphA3), Cp1890 (ciaR::spc), and Cp9890 (ciaR::spc stkP::aphA3). In panel B1, the data are expressed as the mean of results from two independent experiments, the results of which differed by less than 10%. In panel B2, the data are expressed as the mean of results from three independent experiments and the SEM is given.
Taken together, the results provided demonstrate that in S. pneumoniae, virulence and genetic transformability are positively controlled by the membrane-associated serine/threonine kinase StkP. StkP positively impacts activation of the central competence operon comCDE in balance with CiaRH repression, determining the developmental profile of competence expression in strain RX. In experimental virulence, StkP is crucial for lung infection and bloodstream invasion by both virulent strains D39 and 23477.
Bacterial serine/threonine kinases have been implicated in the control of virulence, notably in Yersinia and pseudomonads, although the inputs and outputs of serine/threonine kinase signaling are not well described (10-21; Novakova et al., unpublished). The recent identification of PASTA motifs in penicillin binding proteins and serine/threonine kinases from gram-positive bacteria led researchers to propose a role for these PASTA motifs in the interaction between these proteins and the cell wall (22). Indeed, in S. pneumoniae the protein is membrane associated and is required to bypass LytA-dependent autolysis triggered by growth at alkaline pH and by the cell wall-directed inhibitors penicillin G and vancomycin (Novakova et al., unpublished). Now we have shown additional phenotypes related to StkP deficiency. On the one hand, StkP is required for the expression of the central competence operon comCDE. Interestingly, competence development occurs during exponential growth in alkaline medium, conditions requiring StkP to avoid bacterial autolysis. It is possible that pH stress constitutes the trigger for comCDE expression, culminating in competence development. On the other hand, StkP deficiency dramatically reduced virulence of strains D39 and 23477. Protection against autolysis may account for the positive role of StkP in bacterial virulence. It must be noticed, however, that a LytA loss-of-function mutation attenuates virulence (3). Alternatively, StkP may constitute a checkpoint determining the autolysis rate and liberation of appropriate levels of inflammatory molecules, allowing successful infection by a given population.
To conclude, we propose a pivotal role for StkP in pneumococcal virulence and in horizontal genetic transfer in addition to its role in the response to pH and to low concentrations of cell wall-directed antibiotics. This membrane-associated protein belongs to the signaling network allowing the pneumococcus to sense and respond to its environment both in vivo and in vitro. StkP is required to overcome low concentrations of cell wall-directed inhibitors, suggesting involvement of StkP in the response to cell wall stress (Novakova et al., unpublished). Although other kinds of StkP-mediated signaling have not been excluded, it is likely that cell wall defects induced in specific growth conditions play a central role in the fate of S. pneumoniae in vitro and in vivo.
Acknowledgments
The work in Toulouse was supported by Université Paul Sabatier Toulouse. J.E. was supported by a postdoctoral fellowship from Aventis Pharma France, and S.R. was an ERASMUS program student. The work in Leicester was supported by the Wellcome Trust.
Editor: A. D. O'Brien
REFERENCES
- 1.Auzat, I., S. Chapuy-Regaud, G. le Bras, D. Dos Santos, D. Ogunniyi, I. le Thomas, J.-R. Garel, J. Paton, and M.-C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. [DOI] [PubMed] [Google Scholar]
- 2.Avery, O. T., C. M. McLeod, and M. McCarthy. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., R. A. Lock, D. Hansman, and J. Paton. 1989. Contribution of autolysis to virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapuy-Regaud, S., A. D. Ogunniyi, N. Diallo, Y. Huet, J. F. Desnottes, J. C. Paton, S. Escaich, and M.-C. Trombe. 2003. RegR, a global LacI/GalR family regulator, modulates virulence and competence in Streptococcus pneumoniae. Infect. Immun. 71:2615-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 6.Echenique, J. R., S. Chapuy-Regaud, and M.-C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36:688-696. [DOI] [PubMed] [Google Scholar]
- 7.Echenique, J. R., and M.-C. Trombe. 2001. Competence modulation by the NADH oxidase of Streptococcus pneumoniae involves signal transduction. J. Bacteriol. 183:768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echenique, J. R., and M.-C. Trombe. 2001. Competence repression under oxygen limitation through the two-component MicAB signal-transducing system in Streptococcus pneumoniae and involvement of the PAS domain of MicB. J. Bacteriol. 183:4599-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermolaeva, M. D., O. White, and S. L. Salzberg. 2001. Prediction of operons in microbial genomes. Nucleic Acids Res. 29:1216-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730-732. [DOI] [PubMed] [Google Scholar]
- 11.Giaraminaro, P., and J. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadioglu, A., J. Echenique, S. Manco, M.-C. Trombe, and P. W. Andrew. 2003. The MicAB two-component signaling system is involved in virulence of Streptococcus pneumoniae. Infect. Immun. 71:6676-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters, B. P., J. H. de Boer, S. Bron, and G. Venema. 1988. Structural plasmid instability in Bacillus subtilis: effect of direct and inverted repeats. Mol. Gen. Genet. 212:450-458. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsh, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idänpään-Heikkilä, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 17.Tomasz, A., P. Moreillon, and G. Pozzi. 1988. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J. Bacteriol. 170:5931-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trombe, M.-C. 1993. Characterization of a calcium porter of Streptococcus pneumoniae involved in calcium regulation of growth and competence. J. Gen. Microbiol. 139:433-439. [DOI] [PubMed] [Google Scholar]
- 19.Trombe, M.-C., C. Clave, and J. M. Manias. 1992. Calcium regulation of growth and differentiation in Streptococcus pneumoniae. J. Gen. Microbiol. 138:77-84. [DOI] [PubMed] [Google Scholar]
- 20.Trombe, M.-C., V. Rieux, and F. Baille. 1994. Mutations which alter the kinetics of calcium transport alter the regulation of competence in Streptococcus pneumoniae. J. Bacteriol. 176:1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, J., C. H. Li Yang, A. Mushegian, and S. Jin. 1998. A novel serine/threonine protein kinase homologue of Pseudomonas aeruginosa is specifically inducible within the host infection site and is required for full virulence in neutropenic mice. J. Bacteriol. 180:6764-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeats, C., R. D. Finnand, and A. Bateman. 2002. The PASTA domain: a beta-lactam-binding domain. Trends Biochem. Sci. 27:438-440. [DOI] [PubMed] [Google Scholar]