Abstract
Context
Recent neuroimaging research has revealed functional abnormalities in the anterior cingulate cortex, amygdala and hippocampus in posttraumatic stress disorder (PTSD).
Objective
To determine whether resting functional abnormalities found in PTSD are acquired characteristics or familial risk factors.
Design
Cross-sectional design including identical twins discordant for trauma exposure.
Setting
Academic medical center.
Participants
Combat-exposed veterans with PTSD (n=14) and their identical, combat-unexposed co-twins (n=14), as well as combat-exposed veterans without PTSD (n=19) and their identical, combat-unexposed co-twins (n=19).
Main Outcome Measures
We used positron emission tomography and [18F]-fluorodeoxyglucose to examine resting regional cerebral metabolic rates for glucose (rCMRglu).
Results
Veterans with PTSD and their co-twins had significantly higher resting rCMRglu in dorsal anterior cingulate/mid cingulate cortex (dACC/MCC) compared to non-PTSD veterans and their co-twins. Resting rCMRglu in dACC/MCC in the combat-unexposed co-twins was positively correlated with combat exposure severity, PTSD symptom severity, and alcohol use in their exposed twins.
Conclusions
Enhanced resting metabolic activity in dACC/MCC appears to represent a familial risk factor for developing PTSD after exposure to psychological trauma.
Keywords: stress disorders, post-traumatic, twins, monozygotic, positron-emission tomography, fluorodexoyglucose F18, metabolism, cingulate gyrus
Neuroimaging studies of post-traumatic stress disorder (PTSD) have reported functional abnormalities in several brain regions, including anterior cingulate cortex (ACC), amygdala, and hippocampus. The ACC is a medial prefrontal structure consisting of several functional subdivisions.1, 2 In healthy individuals, rostral regions of the ACC (rACC) are activated during emotional states and tasks that involve interference from emotional stimuli.3–8 In contrast, dorsal regions of the ACC (dACC) have traditionally been thought to be involved in multiple cognitive processes such as performance monitoring, response selection, error detection, and decision making,5, 6, 9, 10 although a role for the dACC in fear learning has recently been reported.11 In PTSD, the rACC is hyporesponsive to trauma-related and other emotionally negative stimuli12–23 and is hypoactive at rest.24 In addition, rACC activation appears to be inversely related to PTSD symptom severity.19, 21, 25 In contrast, the dACC appears to be hyperresponsive during fear conditioning, interference, and auditory oddball tasks in PTSD.23, 26–28
The amygdala is a medial temporal lobe structure that is involved in the detection of potential threat or biologically relevant predictive ambiguity in the environment.29–31 In PTSD, the amygdala appears to be hyperresponsive during exposure to both trauma-related stimuli32–38 and trauma-unrelated, emotional stimuli,19, 21, 23, 39 as well as during the performance of neutral tasks,24, 27 and even at rest.40 Amygdala activation has been shown to be positively correlated with PTSD symptom severity36, 37, 39, 41 and self-reported anxiety.34
The hippocampus is involved in explicit memory processes, as well as memory for fear extinction and context in Pavlovian fear conditioning.42–45 Diminished hippocampal activation in PTSD has been observed during symptomatic states,12, 13 administration of the alpha-2-antagonist yohimbine,46 and memory tasks involving words, passages, or spatial locations.47–50
Most functional neuroimaging studies of PTSD have involved the examination of brain activation during symptom provocation or cognitive tasks. Fewer studies have examined resting brain activity in PTSD,24, 40, 46, 51–53 and their findings have been inconsistent. Nearly all previous resting-state studies have measured regional cerebral blood flow using single photon emission computed tomography (SPECT) or positron emission tomography (PET). Only two previous PET studies have examined regional cerebral metabolic rates for glucose (rCMRglu) at rest in PTSD. One such study reported diminished rCMRglu in the temporal cortex in PTSD.46 The other study found diminished rCMRglu in cingulate gyri, hippocampus, and insula among other regions, and increased rCMRglu in cerebellum, fusiform, temporal, and occipital cortices.53
The origin of functional neuroimaging abnormalities in PTSD is largely unknown. It is tempting to conclude that because PTSD is defined as a result of traumatic life event, all abnormalities associated with it were also caused by the event. However, PTSD is moderately heritable.54–56 We studied identical twins who are discordant for combat exposure to determine whether resting rCMRglu abnormalities found in PTSD represent acquired signs of the disorder or familial risk factors for developing it upon traumatic exposure. Vietnam combat veterans with and without PTSD, as well as their combat-unexposed identical co-twins (without PTSD) were studied. We reasoned that resting rCMRglu abnormalities found in the combat veterans with PTSD but not in their identical co-twins would reflect acquired characteristics of PTSD, whereas resting rCMRglu abnormalities present in both the combat veterans with PTSD and their co-twins would represent familial risk factors. Based on the studies reviewed above, we hypothesized that combat veterans with PTSD would show lower rCMRglu in rACC and hippocampus, and higher rCMRglu in dACC and amygdala compared to veterans without PTSD. However, given the dearth of informative research, we had no hypotheses regarding whether any rCMRglu abnormalities found to be associated with PTSD would represent acquired signs or risk factors. We chose to use PET over SPECT due to its superior spatial resolution. Measures of rCMRglu are closely coupled to neuronal function.57
Methods
Participants
Participants were drawn from a pool of identical twins who had participated in a previous study of physiological responses to loud tones. A description of the recruitment strategy and characteristics of the participant population has been reported elsewhere.58 Thirty-three pairs of male monozygotic twins participated (66 participants in total). One “exposed” (Ex) twin had served in the Vietnam combat theater, whereas his “unexposed” (Ux) co-twin had not. Of the Ex twins, 14 developed current combat-related PTSD (P+), and 19 never did (P−), as determined by the Clinician Administered PTSD Scale (CAPS)59 using criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).60 Thus, there were four participant cells as follows: ExP+: combat-exposed veteran with current, combat-related PTSD (n=14), and UxP+: his combat-unexposed co-twin (without PTSD) (n=14); as well as ExP−: combat-exposed veteran who never had combat-related PTSD (n=19), and UxP−: his combat-unexposed co-twin (also without PTSD) (n=19). The study was approved by the Partners Healthcare System (Boston, MA) Institutional Review Board. Written informed consent was obtained from each participant after a full explanation of the procedures.
Demographics and Psychometrics
Fifty-six participants were right-handed, and 3 (1 ExP+; 2 ExP−) were left-handed. (Handedness information was missing for 1 ExP+, 2 ExP−, 2 UxP+, 2UxP−.) None of the participants reported a history of major head injury involving loss of consciousness for more than 10 minutes, tumor, epilepsy, cerebrovascular accident, or other neurological disorder.
According to the Structured Clinical Interview for DSM-IV (SCID),61 participants in the ExP+ group met criteria for the following current comorbid diagnoses: major depression (n=4), dysthymia (n=2), panic disorder (n=1), social phobia (n=3), specific phobia (n=1), GAD (n=1), eating disorder (n=1), alcohol dependence (n=1), and substance use disorder (n=2). Participants in the other groups met criteria for the following current diagnoses: major depression (n=1 in UxP+), dysthymia (n=1 in ExP− and n=2 in UxP−) social phobia (n=1 in UxP+), specific phobia (n=1 in UxP+ and n=1 in ExP−), eating disorder (n=1 in ExP−), alcohol dependence (n=1 in UxP+; n=2 in UxP−).
Thirteen participants (5 ExP+, 3 UxP+, 2 ExP−, 3 UxP−) were taking antidepressants at the time of study. Two (1 ExP+ and 1 UxP+) were taking benzodiazepines. These medications were included among the potentially confounding medications or drugs that were excluded in a sub-analysis reported below. Potentially confounding drugs or medications were defined as: antihistamines, sympathomimetics, sympatholytics, parasympathomimetics, parasympatholytics, skeletal muscle relaxants, hypotensive agents, vasodilating agents, pressor agents, beta-blockers, antiarrhythmics, calcium channel blockers, narcotics, anticonvulsants, antidepressants, neuroleptics, benzodiazepines, other psychotherapeutic agents, cerebral stimulants, sedatives, and hypnotics.
Participants completed the Beck Depression Inventory (BDI),62 the Michigan Alcohol Screening Test (MAST),63 the Childhood Trauma Questionnaire (CTQ),64 the Positive and Negative Affect Schedule (PANAS),65 and a measure of the severity of combat exposure.66 The latter scale, which has good reliability and validity, assesses the extent to which the veteran had experienced a variety of different situations in combat, including being wounded, ambushed, captured, etc.66 (See Table 1 for demographic and clinical information.)
Table 1.
Group means (sd) of combat-exposed Vietnam veterans with and without PTSD and their combat-unexposed, identical co-twins
| PTSD Pairs* | Non-PTSD Pairs† | Mixed Model ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed (n=14) |
Unexposed (high risk) (n=14) |
Exposed (n=19) |
Unexposed (low risk) (n=19) |
Diagnosis F(1,31) |
p |
Exposure F(1,32) |
p |
Interaction F(1,31) |
p |
||
| Age (years)‡ | 57.8 (2.8) | 57.1 (2.2) | 0.6 | ns | |||||||
| Education (years) | 14.1 (2.4) | 13.5 (2.3) | 14.0 (1.9) | 13.4 (1.8) | 0.0a | ns | 1.6b | ns | 0.0a | ns | |
| MAST# | 9.3 (5.7) | 8.4 (5.5) | 6.3 (2.3) | 5.6 (2.9) | 4.7c | 0.04 | 1.1d | ns | 0.00c | ns | |
| Combat everity¶ | 8.8 (2.0) | 4.2 (3.0) | 24.2 | <.0001 | |||||||
| CAPS§-Current | 66.0 (25.8) | 6.6 (9.3) | 86.4 | .0001 | |||||||
| CAPS§- Lifetime | 90.9 (25.5) | 10.6 (12.0) | 145.2 | .0001 | |||||||
| BDI¥ | 12.2 (11.7) | 2.9 (3.8) | 3.1 (3.6) | 4.4 (5.5) | 3.7e | ns | 7.3f | .02 | 12.8e | .002 | |
| CTQ❷ | 58.6 (8.7) | 55.2 (17.8) | 61.3 (8.3) | 61.2 (2.9) | 2.6 | ns | 0.5 | ns | 0.5 | ns | |
| PANAS£-Positive Affect | 21.7 (7.9) | 22.4 (5.4) | 23.9 (8.9) | 21.8 (5.7) | 0.2b | ns | 0.2g | ns | 0.8b | ns | |
| PANAS£-Negative Affect | 17.0 (8.7) | 11.5 (2.3) | 11.8 (3.4) | 10.9 (1.2) | 6.5b | .02 | 7.4g | .01 | 3.7b | .07 | |
As determined by the presence of current, combat-related PTSD in the combat-exposed twin
As determined by the absence of current or past, combat-related PTSD in the combat-exposed twin
As of January 1, 2005
Michigan Alcoholism Screening Test (range 0–25)
18-item measure (range 0–18)
Clinician-Administered PTSD Scale (range 0–136)
Beck Depression Inventory (range 0–63)
Childhood Trauma Questionnaire
Positive and Negative Affect Schedule
df=1,29;
df=1,30;
df=1,28;
df=1,29;
df=1,27;
df=1,28;
df=1,31
PET-FDG Procedures
The PET equipment and procedures have been described previously.67 Participants were instructed to fast at least 6 hours prior to PET scanning. Blood glucose levels were checked immediately before intravenous administration of [18F]-fluorodeoxyglucose (FDG) (approximately 185 MBq, 5 mCi). Then each participant was instructed to sit quietly with eyes closed in a dedicated waiting room for a 40-minute uptake period. The participant was then escorted to an adjacent room that housed the HR+ PET scanner (CTI/Siemens Medical Solutions), which had an in-plane and axial resolution of 4.5 mm FWHM (full-width at half-maximum intensity), 63 contiguous slices with 2.5 mm separation, and a sensitivity of 200,000 cps/microcurie/mL [two dimensional (2D)] and 900,000 cps/microcurie/mL (3D). After entering the scanner, each participant’s head was fitted with an inflatable cushion to minimize movement and aligned in the scanner relative to the canthomeatal line.
Magnetic Resonance Imaging (MRI) Procedures
Structural MRI scans were obtained from a Symphony/Sonata 1.5 Tesla whole body high-speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Iselin NJ) with a 3 axis gradient head coil. Head movement was restricted using expandable foam cushions. After an automated scout image was acquired and shimming procedures were performed to optimize field homogeneity, high-resolution structural MRI images (3D MPRAGE; TR/TE/flip angle=2.73sec/3.31ms/7°) with a 1.33 mm slice thickness were collected. Functional MR images were subsequently collected for separate studies, the results of which are to be reported elsewhere. PET scans preceded MRI scans by one day.
Data Analysis
Two types of analyses were employed: (1) those conducted on whole-brain voxelwise PET-FDG data and (2) those conducted on PET-FDG data extracted from functional regions of interest (ROIs). These two different types of data required somewhat different but parallel, two-factor analytic strategies. Conceptually speaking, in both types of analyses, we treated Exposed versus Unexposed co-twins as a repeated measure (i.e., Exposure). The twin pairs in which the combat-exposed twin had PTSD (P+) were treated as a separate group from the twin pairs in which the exposed twin never had PTSD (P−). We reasoned that a significant difference between these two groups (i.e., Main effect of PTSD Diagnosis) would be consistent with a familial risk factor (as long as there was also no interaction between PTSD Diagnosis and Exposure); in this case, the combat-exposed twin with PTSD (ExP+) would have the same functional abnormality as his unexposed co-twin without PTSD (UxP+). (Follow-up analyses were conducted to confirm differences between ExP+ and ExP− subgroups, and between UxP+ and UxP− subgroups.) A significant PTSD Diagnosis by Exposure interaction (reflecting an abnormality in the exposed twins with PTSD only) would indicate an acquired sign of PTSD. Lastly, a difference between all combat-exposed twins as compared to all combat-unexposed twins (i.e., a main effect of Exposure) in the absence of an interaction would suggest that a functional abnormality is associated with exposure to combat and not PTSD per se.
Voxelwise Analyses
The whole-brain voxelwise analyses were conducted using the Statistical Parametric Mapping 2 (SPM2) software package (Wellcome Department of Cognitive Neurology, London, UK). Within SPM2, each participant’s PET image was coregistered to his high-resolution structural MRI image. The resulting images were spatially normalized in a standard stereotactic space (Montreal Neurological Institute, MNI) and then smoothed (6mm FWHM). At each voxel, the rCMRglu data were normalized by the global mean and fit to a linear statistical model by the method of least squares. Hypotheses were tested as contrasts in which linear compounds of the model parameters were evaluated using t statistics, which were then transformed to z-scores.
We used an approach that consisted of two hierarchical levels of analysis, in which the second level’s random-effects analysis absorbed the random effects from the first level. For the purpose of examining the main effect of PTSD Diagnosis, for each pair, the rCMRglu values of the Ex and Ux participants were averaged (first level), and then the P+ and P− pairs were contrasted (second level). For the purpose of examining the PTSD Diagnosis x Exposure interaction, for each pair, the rCMRglu values of the Ex and Ux participants were subtracted one from the other (first level), and then the P+ and P− pairs were contrasted (second level). For the purpose of examining the main effect of combat Exposure, the rCMRglu values of the Ex and Ux subjects were contrasted (first level only).
The statistical parametric maps resulting from the above voxelwise analyses were inspected for the main effect of PTSD Diagnosis, main effect of Exposure, and the PTSD Diagnosis x Exposure interaction in our a priori structures of interest (dACC, rACC, amygdala, hippocampus). The amygdala and hippocampus were defined by their anatomical boundaries, as visualized on the MNI structural MRI (T1) template within SPM. The superior and lateral boundaries of the ACC were also defined anatomically. The dACC was defined as the portion of the anterior cingulate gyrus superior to the corpus callosum, between y= 0 and y= +30mm.68 The rACC was defined as the portions of the anterior cingulate gyrus that are anterior to the genu of the corpus callosum and where y>30mm. (Most, although not all, previous findings of diminished function in ACC in PTSD have occurred at y> 30mm.) Given our strong hypotheses, we applied a significance threshold of uncorrected, two-tailed p< 0.001 (z-score>3.29) to rCMRglu differences found in these structures. (Because the procedure of correcting p values based upon region size is biased toward finding significance in small structures, we chose to employ the above stated constant significance threshold.) To regions about which we had no a priori prediction, we applied a more conservative constant significance threshold of uncorrected, two-tailed p< 0.00001 (z-score>4.42).
Region of Interest Analyses
We extracted rCMRglu data from clusters surrounding significant voxels identified the SPM analyses. We then analyzed these clusters for the main effect of PTSD Diagnosis, main effect of Exposure, and their interaction using a mixed model that treated combat Exposure as a within-pairs repeated measure, Diagnosis as a between-pairs measure, and twin pairs as a random effect,69 including the covariates described under Results. Additional correlational analyses were performed on the ROI data as appear below.
Results
Voxelwise Analyses
No voxels met significance thresholds for a main effect of Exposure or a PTSD Diagnosis x Exposure interaction. However, there were significant main effects of PTSD Diagnosis in the dACC, midcingulate cortex, and left inferior parietal cortex (Table 2). In each case, combat-exposed veterans with PTSD (ExP+) and their unexposed co-twins (UxP+) (combined) exhibited greater rCMRglu than combat-exposed veterans without PTSD (ExP−) and their unexposed co-twins (UxP−) (combined). With one exception, these results remained significant when we temporarily removed from the voxelwise analyses data from (1) participants with current mood disorders or substance use disorders (all z-scores > 3.80) (2) participants taking potentially confounding medications (as defined above) (all z-scores > 3.77), or (3) participants who were left-handed or with missing handedness information (all z-scores > 4.41). The exception was that the z-score of the left inferior parietal finding dropped below threshold after the above participant exclusions; for this reason, this brain region is not considered further below. No voxels exhibited significantly lower rCMRglu in the PTSD twin pairs (i.e., ExP+ and UxP+ groups) relative to the non-PTSD twin pairs (i.e., ExP− and UxP− groups). Comparisons between subgroups (ExP+ vs. ExP− and UxP+ vs. UxP−) are presented in Table 3 and are consistent with the main effect of PTSD Diagnosis.
Table 2.
Main Effect of Diagnosis
| Region | Z-score | MNI Coordinates (x, y, z) |
|
|---|---|---|---|
| PTSD Pairs > non-PTSD Pairs | |||
| dACC | 4.70 | +10, +2, +42 | |
| midcingulate cortex | 5.03 | +16, −2, +46 | |
| 4.65 | −10, −4, +38 | ||
| left inferior parietal cortex | 4.78 | −46, −50, +28 | |
| Non-PTSD Pairs > PTSD Pairs | |||
| none | |||
Note: MNI = Montreal Neurological Institute.
Table 3.
| Exposed Twins | Unexposed Twins | |||||
|---|---|---|---|---|---|---|
| Region | Z-score | MNI Coordinates (x, y, z) |
Region | Z-score | MNI Coordinates (x, y, z) |
|
| PTSD > non-PTSD: | ||||||
| dACC | 3.16 | +16, +8, +46 | dACC | 4.16 | +8, +2, +44 | |
| 3.54 | −6, 0, +38 | |||||
| 3.35 | −8, +14, +36 | |||||
| midcingulate cortex | 3.49 | +16, −4, +46 | ||||
| Non-PTSD > PTSD: | ||||||
| none | none | |||||
Note: MNI = Montreal Neurological Institute.
Region of Interest Analyses
Dorsal Anterior Cingulate/Midcingulate Cortex
Inspection of the statistical parametric maps revealed that the most significant voxels in dACC and midcingulate cortex were part of a common cluster of k=109 voxels, henceforth designated the “dorsal anterior cingulate/midcingulate cortex” (dACC/MCC) ROI and shown in Figure 1, along with a bar graph of group means and standard errors. Individual subjects’ values from this ROI were extracted and plotted by pairs in Figure 2. The within-pair correlation across PTSD and non-PTSD groups was r=0.73, p<10−6, indicating a high degree of familiality of the measure. (For non-PTSD subjects alone, r=0.71, p<0.001; for PTSD subjects alone, r=0.41, p=0.07. These correlations were not significantly different from each other, p=0.25)
Figure 1.
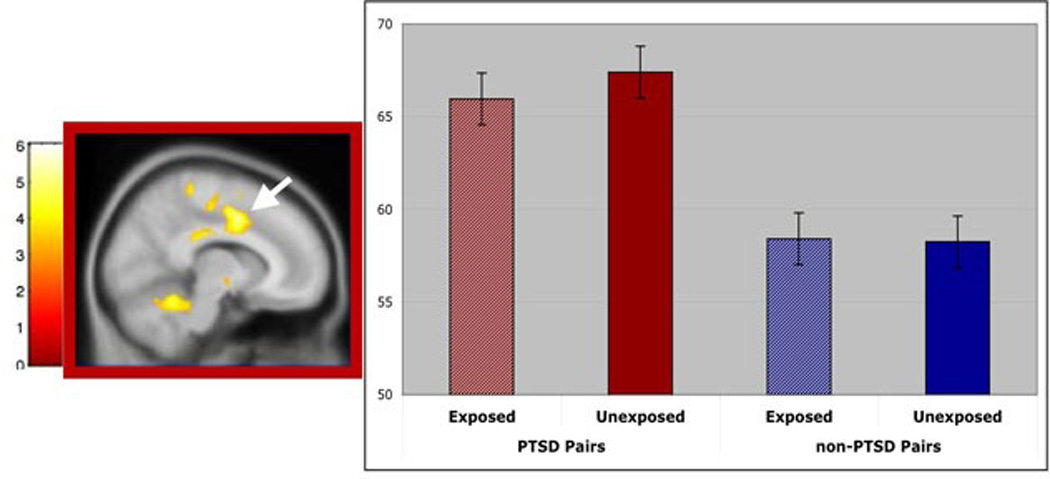
Main effect of PTSD Diagnosis on regional cerebral metabolic rates for glucose (rCMRglu). This image shows resting rCMRglu in dorsal anterior cingulate/mid cingulate cortex that is greater in trauma-exposed twins with PTSD and their unexposed identical co-twins, compared with trauma-exposed twins without PTSD and their identical co-twins. FDG data are superimposed on a standard SPM2 T1 template and displayed according to neurological convention. The accompanying bar graphs present group means; error bars represent standard error of the mean. MNI=Montreal Neurological Institute
Figure 2.
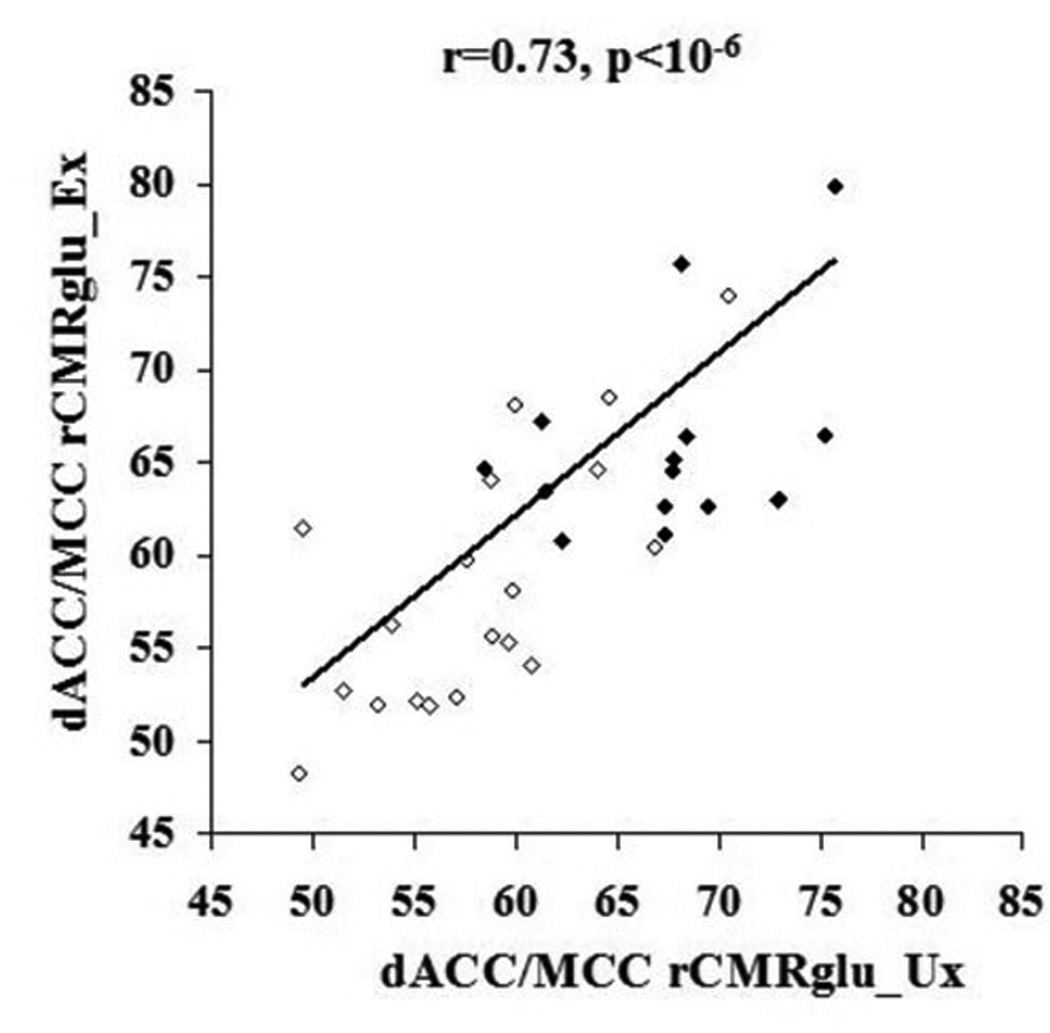
rCMRglu correlation between co-twins. Individual subjects’ rCMRglu values from the dorsal anterior cingulate/mid cingulate cortex cluster (k=109) plotted by pairs, with the value of the combat-unexposed (Ux) twin shown on the x-axis and that of the combat-exposed (Ex) twin on the y-axis; open circles represent non-PTSD pairs and closed circles PTSD pairs.
For the dACC/MCC rCMRglu ROI, the PTSD main effect yielded F(1, 31.2)=18.0, p=0.0002. The following covariates were screened as potential confounders of this result by examining their association with the dependent measure using a screening threshold of p<.0.20: weeks premature, birth weight, age, total score on the CTQ, education, BDI score, MAST score, PANAS scores, and severity of combat exposure (in the Ex twin). Only combat severity met this threshold. Adjusted for combat severity, the PTSD main effect yielded F(1, 30.4)=7.8, p=0.009. Parallel analyses in combat-exposed participants (ExP+ vs. ExP−) alone indicated that only birth weight and combat severity passed screening as potential confounders. Unadjusted, the PTSD main effect yielded F(1,31)=11.5, p=0.002; adjusted for birth weight, F(1,27)=9.2, p=0.005; adjusted for combat severity, F(1,30)=5.0, p=0.03. Parallel analyses in combat-unexposed participants (UxP+ vs. UxP−) alone indicated that only MAST score and combat severity passed screening as potential confounders. Unadjusted, the PTSD main effect yielded F(1,31)=28.2, p<0.0001; adjusted for MAST score, F(1,27)=28.2, p=0.0001; adjusted for combat severity F(1,30)=10.1, p=0.004.
Correlational Analyses with Clinical Variables
Significant correlations between dACC/MCC rCMRglu in the Ux co-twins and other variables of interest included: their own MAST scores: r=0.53, p=.003; their Ex twins’ MAST scores: r=0.38, p=0.04; their Ex twins’ combat severity scale scores: r=0.49, p=0.004; and their Ex twins’ lifetime CAPS scores: r=0.64, p=0.0001 (Figure 3). The last of these correlations adjusted for Ex twins’ MAST and combat severity scores yielded: partial r=0.53, p=0.003.
Figure 3.
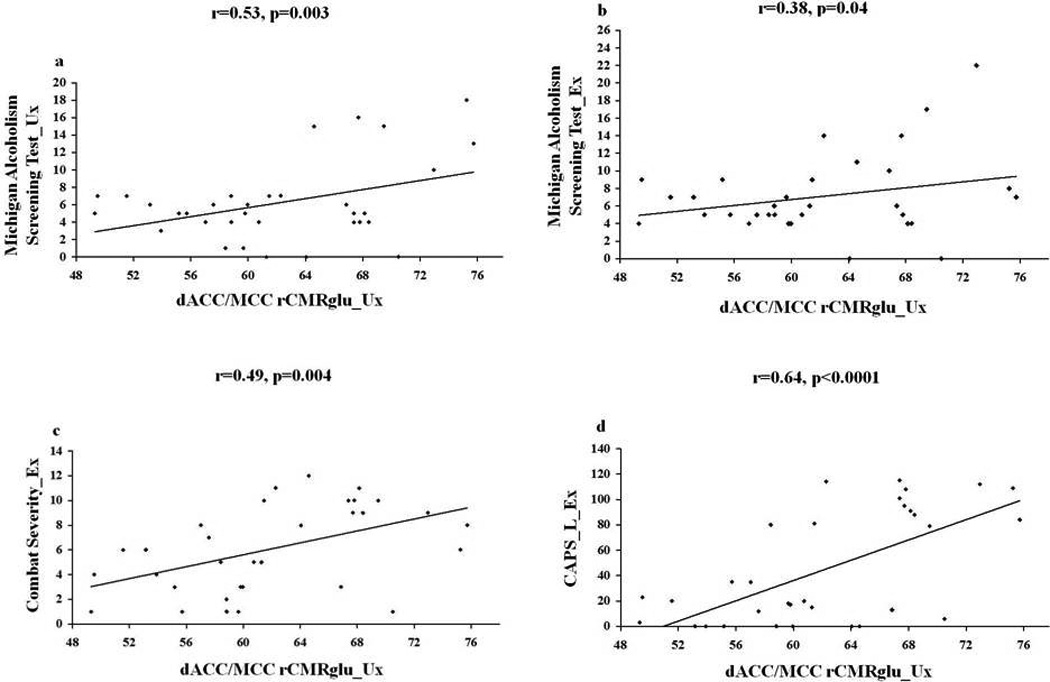
rCMRglu correlations with clinical variables. The scatterplots show the zero-order correlations between rCMRglu values extracted from the dorsal anterior cingulate/midcingulate cortex for combat-unexposed (Ux) co-twins and a.) their own Michigan Alcoholism Screening Test scores, b.) their combat-exposed (Ex) twins’ Michigan alcoholism Screening Test scores; c.) their Ex twins’ combat severity scores, and d.) their Ex twins’ lifetime Clinician-Administered PTSD Scale scores.
Discussion
The results presented here showed greater resting rCMRglu, indicative of greater resting metabolic activity, in the dorsal anterior cingulate/mid cingulate cortex of the combat exposed veterans with PTSD and their identical, combat-unexposed co-twins, compared with the combat-exposed veterans without PTSD and their co-twins. This finding remained significant after adjusting for potentially confounding factors. The finding of dACC/MCC hypermetabolism in combat veterans with PTSD is consistent with previous findings of increased activation in these structures in PTSD singletons,23, 26–28 and further suggests that this functional abnormality may be a risk factor rather than an acquired characteristic of PTSD. The current finding appears to be inconsistent with that of a previous PET FDG study53 that reported rCMRglu decreases in anterior cingulate in PTSD; however, because coordinates were not reported in that study, it is unclear whether those decreases occurred in rostral or dorsal portions of the anterior cingulate. One other previous PET FDG study46 reported no rCMRglu difference in the cingulate between 10 Vietnam combat veterans with PTSD and 10 healthy trauma-unexposed participants. However, unlike the current study, the previous study used a structural region-of-interest approach, which involved extracting PET FDG data from manually traced brain structures. The “cingulate” region in that study did not appear to distinguish between different subdivisions of this structure (i.e., anterior vs. posterior, dACC vs. rACC). Extracting and analyzing PET FDG data from the entire cingulate gyrus could easily obscure possible group differences in specific subregions of the cingulate, such as the dACC.
We did not find evidence of resting rCMRglu main effects or interactions in the rACC, amygdala, or hippocampus. Most of the previous findings of abnormal function in these regions have occurred in neuroimaging studies that utilized emotional or cognitive tasks; perhaps abnormalities in these brain structures are more likely to be manifest when participants are engaged in such tasks. Furthermore, amygdala responses are known to habituate70–72 over seconds to minutes, even in PTSD.19, 37 It is possible that such habituation occurred during the 40-minute FDG uptake period, thus obscuring any possible group differences that may have existed early in the uptake period. Two previous resting PET-FDG studies46, 53 reported no group differences between PTSD and comparison groups with regard to rCMRglu in the amygdala, although one of those studies reported diminished rCMRglu in the hippocampus.53 The fact that some of our a priori brain regions of interest did not show abnormal glucose metabolic rates in PTSD at rest does not preclude their involvement in the pathophysiology of the disorder. In future research, we plan to use cognitive and emotional tasks during fMRI to further probe these structures using the present twin design.
The dACC (also referred to as the dorsal anterior mid cingulate cortex 2, 73) appears to be involved in many cognitive processes, such as conflict monitoring, response selection, and error detection.5, 10 However, it also appears to be involved in aversive conditioning,11, 74 the anticipation and perception of pain,75, 76 and task/stimulus-related heart rate responses.77 In rhesus monkeys, increased dACC metabolism is positively correlated with increased freezing behavior in response to a human intruder.78 In humans, dACC activation is positively correlated with neuroticism and interoceptive accuracy79 and emotional awareness.80 Increased rCMRglu in the dACC recently has been reported in individuals with the short (s/s) allele of the serotonin transporter gene,81 the frequency of which has been found to be increased in PTSD.82,83
The Michigan Alcoholism Screening Test and the measure of combat severity were originally included in the design for use as covariates to control for potentially confounding variables. Additionally, we found that hypermetabolism in dACC/MCC in the combat-unexposed co-twins positively and significantly correlated with their own and their exposed twins’ alcoholism histories, as well as their exposed twins’ combat exposure severity and PTSD severity. Although not predicted, these results are of substantial interest in view of a study of 4072 male–male twin pairs, both of whom were in military service during the Vietnam War, that found that the same additive genetic influences that affect the level of combat exposure also influence the level of alcohol use and the level of avoidance/arousal and reexperiencing PTSD symptoms.84 The authors concluded that the genetic influences that lead to exposure to combat also lead to increased alcohol use and PTSD symptoms, and further that some genetically transmitted personal characteristics, possibly including impulsivity and sensation seeking, influence the veteran’s probability of being exposed to a high level of combat, to PTSD symptoms, and to alcohol use. The results of the present study suggest that resting dACC/MCC hypermetabolism may be an endophenotypic manifestation of these genetic influences and personality characteristics. Confidence in this conclusion, however, is limited by the lack of relevant personality measures in this twin sample, as well as the dearth of prior studies regarding the relationship between dACC/MCC glucose metabolism, alcoholism, and personality characteristics such as impulsivity. However, one functional magnetic resonance imaging (fMRI) study reported exaggerated dACC/MCC activation in detoxified alcoholics in response to alcohol-related vs. neutral pictures.85 Another fMRI study that employed a perceptual face processing task found that activation of the dACC was positively correlated with impulsivity.86
If replicated in further twin or prospective singleton studies, the current findings could have specific theoretical implications. The finding of hypermetabolism in the dACC in PTSD is consistent with conditioning and extinction neurocircuitry models of PTSD,87 which implicate the dACC in fear learning.11 More generally, the identification of regional brain metabolic activity as a familial risk factor challenges the notion that the traumatic event is the sole etiologic factor in the development of PTSD (see88) and is broadly consistent with many previous findings suggesting that certain psychological and biological factors appear to increase risk for PTSD following exposure to trauma.89 For example, smaller hippocampal volumes,90 diminished neurocognitive function,91, 92 and increased neurological soft signs 93 have been shown to be familial risk factors for the development of PTSD after psychological trauma. In contrast, diminished gray matter density in rostral ACC appears to be an acquired sign of PTSD.94
In summary, we found hypermetabolism in the dACC/MCC in individuals with PTSD and in their trauma-unexposed identical co-twins without PTSD. Enhanced resting metabolic activity in the dACC/MCC therefore appears to represent a familial risk factor for the development of PTSD after exposure to psychological trauma. The current study is limited by the presence of disorders other than PTSD, medication use in some participants, and missing handedness data in 7 of 66 participants; however, the finding of hypermetabolism in the dACC/MCC in the P+ pairs remained even when the above participants’ data were temporarily excluded from the analyses. It is important to note that, in the absence of dizygotic twin participants, the current twin design cannot distinguish between genetic and environmental contributions to familial risk. Future research examining the relationship between dACC hypermetabolism and specific genotypes should help to address this issue. Future longitudinal studies will be needed to confirm that dACC hypermetabolism identified before trauma exposure increases the risk of PTSD after trauma exposure. Finally, despite the fact that PTSD and non-PTSD pairs differed significantly on rCMRglu values in the dACC/MCC, there was both variability within groups and overlap between groups. Although this pattern of findings is typical in functional neuroimaging studies of psychiatric patient groups, it limits the ability to use rCMRglu in the dACC/MCC as a sole predictor of vulnerability to PTSD following psychological trauma. In future studies, factoring in other measures (such as genotypes) may increase separation between groups and the predictive power of the rCMRglu measure.
Acknowledgments
This work was supported by USPHS Grant #R01MH54636 to Dr. Pitman. The U.S. Department of Veterans Affairs provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Through their support of the VET Registry, numerous other U.S. organizations also provided invaluable assistance, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; and Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry, and of the other participants, without whose contribution this research would not have been possible. The authors also thank Mary Foley and Lawrence White for technical assistance. Drs. Pitman and Shin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Although no direct conflict is anticipated, the financial disclosures of one author (SLR) are as follows: he received funded research through MGH for Brain Stimulation Therapy from Medtronics, Inc.; funded research through MGH for VNS from Cyberonics; and funded research through MGH on anxiolytic action from Cephalon. He also received honoraria from Novartis for consultation on emerging treatments; Neurogen for his participation as a consultant on emerging trends in anxiety associated with insomnia; Sepracor for his consultation on fear/conditioning/extinction and from Primedia for his participation in developing a CE activity and Medtronics, Inc for his attendance of the Advisory Board meeting on the Anatomy and Neuroscience of anxiety and depression. Dr. Rauch is a trustee at McLean Hospital and also serves on the Board at Massachusetts Society for Medical Research (MSMR) as well as on the National Foundation of Mental Health (NFMH) Board. All other authors have no competing financial interests. Correspondence and requests for materials should be addressed to L.M.S. (lisa.shin@tufts.edu).
Footnotes
The data described herein were presented at the annual meeting of the Society of Biological Psychiatry, May 2008, Washington DC and the annual meeting of the American College of Neuropsychopharmacology, December 2008, Scottsdale, AZ.
References
- 1.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 2.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush G, Luu P, Posner MI. Cognitive and emotional influence in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 7.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 8.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 14.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 15.Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Hou C, Liu J, Wang K, Li L, Liang M, He Z, Liu Y, Zhang Y, Li W, Jiang T. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res. 2007;1144:165–174. doi: 10.1016/j.brainres.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 19.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 20.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 21.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon SJ, Jeong DU, Lyoo IK. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2008;42:268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 25.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- 26.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 27.Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, Gordon E, Williams LM. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20:701–712. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;6:178–188. [Google Scholar]
- 32.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 33.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 34.Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 35.Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Marzol Peters P, Metzger L, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 37.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, Ledoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- 39.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 40.Chung YA, Kim SH, Chung SK, Chae JH, Yang DW, Sohn HS, Jeong J. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 42.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 43.Schacter DL. The cognitive neuroscience of memory: perspectives from neuroimaging research. Philos Trans R Soc Lond B Biol Sci. 1997;352:1689–1695. doi: 10.1098/rstb.1997.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- 46.Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 47.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 48.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 49.Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko NB, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 50.Astur RS, St Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 51.Sachinvala N, Kling A, Suffin S, Lake R, Cohen M. Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Mil Med. 2000;165:473–479. [PubMed] [Google Scholar]
- 52.Bonne O, Gilboa A, Louzoun Y, Brandes D, Yona I, Lester H, Barkai G, Freedman N, Chisin R, Shalev AY. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol Psychiatry. 2003;54:1077–1086. doi: 10.1016/s0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- 53.Molina ME, Isoardi R, Prado MN, Bentolila S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World J Biol Psychiatry. 2007:1–9. doi: 10.3109/15622970701472094. [DOI] [PubMed] [Google Scholar]
- 54.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 55.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 56.Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: informing clinical conceptualizations and promoting future research. Am J Med Genet C Semin Med Genet. 2008;148:127–132. doi: 10.1002/ajmg.c.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 58.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- 59.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 60.APA. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington DC: American Psychiatric Press; 2000. Text-Revision. [Google Scholar]
- 61.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. New York: New York State Psychiatric Institute, Biometrics Research Department; 1995. [Google Scholar]
- 62.Beck AT, Steer RA. Manual for the revised Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 63.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 65.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 66.Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. J Clin Psychol. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 67.Deckersbach T, Miller KK, Klibanski A, Fischman A, Dougherty DD, Blais MA, Herzog DB, Rauch SL. Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depress Anxiety. 2006;23:133–138. doi: 10.1002/da.20152. [DOI] [PubMed] [Google Scholar]
- 68.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Littlell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 70.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 71.Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 72.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 73.Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- 74.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 75.Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- 76.Derbyshire SW. Exploring the pain "neuromatrix". Curr Rev Pain. 2000;4:467–477. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- 77.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 78.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- 80.McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. Neuroimage. 2008;41:648–655. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graff-Guerrero A, De la Fuente-Sandoval C, Camarena B, Gomez-Martin D, Apiquian R, Fresan A, Aguilar A, Mendez-Nunez JC, Escalona-Huerta C, Drucker-Colin R, Nicolini H. Frontal and limbic metabolic differences in subjects selected according to genetic variation of the SLC6A4 gene polymorphism. Neuroimage. 2005;25:1197–1204. doi: 10.1016/j.neuroimage.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 82.Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, Kim YH, Kim YK, Kim JB, Yeon BK, Oh KS, Oh BH, Yoon JS, Lee C, Jung HY, Chee IS, Paik IH. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 83.Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: a comprehensive review. Curr Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- 84.McLeod DS, Koenen KC, Meyer JM, Lyons MJ, Eisen S, True W, Goldberg J. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. J Trauma Stress. 2001;14:259–275. doi: 10.1023/A:1011157800050. [DOI] [PubMed] [Google Scholar]
- 85.Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 86.Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- 87.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Yehuda R, McFarlane AC. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. Am J Psychiatry. 1995;152:1705–1713. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]
- 89.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, Orr SP. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- 92.Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits TV, Shenton ME, Pitman RK, Orr SP. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol Psychiatry. 2007;62:513–520. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gurvits TV, Metzger LJ, Lasko NB, Cannistraro PA, Tarhan AS, Gilbertson MW, Orr SP, Charbonneau AM, Wedig MM, Pitman RK. Subtle neurologic compromise as a vulnerability factor for combat-related posttraumatic stress disorder: results of a twin study. Arch Gen Psychiatry. 2006;63:571–576. doi: 10.1001/archpsyc.63.5.571. [DOI] [PubMed] [Google Scholar]
- 94.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


